Introduction
Gestational diabetes mellitus (GDM) is the most
common medical complication of pregnancy and is known as different
degrees of glucose intolerance that are initially identified during
pregnancy (1). Owing to the
increase in the rate of obesity and the increase in maternal age,
the morbidity of gestational diabetes is currently increasing
(2). However, the pathogenesis of
GDM remains to be fully elucidated and early-mid pregnancy
diagnostic markers may facilitate progress in this area.
Controversy persists regarding GDM screening, but current consensus
recommendations support screening for GDM during mid-pregnancy
(24-28 weeks of gestation) (3). In
addition to its detrimental effects on maternal health, GDM has
also been demonstrated to impede foetal health and amplify the
offspring's tendency to develop metabolic diseases, such as obesity
and type 2 diabetes mellitus (T2D) later in life (4-6).
Therefore, the identification of novel signatures or biomarkers
that enhance the determination of clinical behaviours is essential
for the treatment of GDM.
Several studies have reported that there are
multiple genes associated with diabetes. Over the past decades,
several types of non-coding RNA (ncRNA) species, including
microRNAs (miRNAs/miRs), long ncRNAs (lncRNAs) and circular RNAs,
have emerged as pivotal regulators of multiple cellular functions,
including cell proliferation, apoptosis, cellular differentiation,
tumorigenesis and metastasis (7).
LncRNAs are defined as RNAs with a length of >200 nucleotides
and no protein-coding ability (8,9).
Numerous lncRNAs have been reported to be involved in GDM
progression. Among them, the lncRNA metastasis associated in lung
adenocarcinoma transcript 1 (MALAT1) was increased in endothelial
cells after high levels of glucose stimulation. Mechanistic
investigations suggested that MALAT1 promotes insulin resistance by
increasing the stability of the nuclear transcription factor sterol
regulatory element-binding protein 1C (10). However, to the best of our
knowledge, only a small number of studies have been performed on
the utility of circulating lncRNAs for the dynamic monitoring of
patients for GDM (11).
miRNAs are known to post-transcriptionally regulate
the expression of their target genes by binding to the 3'
untranslated region of the respective mRNA (12). The identification of stable
circulating miRNAs in plasma and serum has provided promise for
minimally invasive biomarkers for disease prediction, diagnosis and
prognosis (13). According to a
previous review, an increasing number of studies have identified
that certain miRNAs are associated with insulin secretion,
inflammation and insulin resistance (14). Differences have also been detected
in circulating levels of miRNAs when comparing T2D cases to
controls (15).
In the present study, bioinformatics analysis was
used to explore significantly differentially expressed genes during
GDM progression and the lncRNA paired box 8 antisense 1 (PAX8-AS1)
and miR-4646 were identified as the most downregulated ncRNAs in
GDM. Therefore, the study further endeavoured to investigate
PAX8-AS1 and miR-4646 in the blood leukocytes of patients with GDM
compared to those of healthy pregnant females as biomarkers for the
diagnosis and monitoring of GDM.
Materials and methods
Retrieval of RNA sequencing data and
analysis
RNA sequencing datasets from the gene expression
omnibus (GEO) database were used to analyse the expression profiles
of lncRNAs and miRNAs in GDM (www.ncbi.nlm.nih.gov/geo). The dataset no. GSE92772
was used, containing the expression data of 8 subjects with normal
glucose tolerance (NGT) and 8 patients with GDM who were matched in
terms of body mass index (BMI) and age (16). The RNA sequencing of the leukocytes
of these samples was performed on an Illumina HiSeq 2500 platform
(Illumina, Inc.).
Participants and maternal blood
collection
To validate the expression levels of lncRNAs and
miRNAs in patients with GDM and normal controls, a group of 35
pregnant females with NGT (age, 32±3 years; BMI, 27.2±3.8
kg/m2) and 35 patients with GDM (age, 34±4 years; BMI,
28.5±4.1 kg/m2) were selected from a pregnancy cohort.
Maternal blood samples were from the second trimester (24-28
weeks). Patients with other pregnancy-associated diseases,
including chronic hypertension, multiple pregnancies or any other
diseases, including liver or kidney disease, cancer and
gynaecological diseases, were excluded from the present study.
Serum and blood samples of the pregnant females in the second
trimester (24-28 weeks of gestation) were collected at the Yiwu
Women's and Children's Hospital (Jinhua, China) between June and
November 2019. Patients who had other pregnancy-associated
diseases, such as gynaecological diseases, or carcinomas, chronic
hypertension or liver diseases, were excluded from the present
study.
Oral glucose tolerance test and
clinical chemical analyses
The participants underwent a 5-point 75-g oral
glucose tolerance test after overnight fasting. Venous blood
samples were obtained at 0, 60 and 120 min for the determination of
plasma glucose and insulin levels. Blood glucose was determined
using a bedside glucose analyser (Roche Diagnostics). NGT was
defined as fasting glucose ≤5.11 mmol/l, 1-h glucose ≤10.00 mmol/l
and 2-h glucose ≤8.50 mmol/l. GDM patients were diagnosed according
to the recommendations of the International Association of the
Diabetes and Pregnancy Study Groups for the diagnosis and
classification of hyperglycaemia during pregnancy, published in
2010(3).
Total RNA extraction and reverse
transcription-quantitative (RT-q)PCR
Firstly, to separate leukocytes from the blood
samples, 200 µl fresh blood was taken, where 600 µl red blood cell
lysate buffer (cat. no. R1010; Beijing Solarbio Science &
Technology Co., Ltd.) was added and mixed at room temperature for
10 min, which were then and centrifuged for 7300 x g for 1 min at
room temperature. Leukocytes were obtained after supernatant was
discarded. Total RNA from maternal blood leukocytes was then
extracted using TRIzol® reagent according to
manufacturer's protocol (Invitrogen; Thermo Fisher Scientific,
Inc.). Complementary (c)DNA was synthesized with
PrimeScript™ IV 1st strand cDNA Synthesis Mix (Takara
Biotechnology, Co., Ltd.) using a LightCycler 480 II (Roche
Diagnostics). The reverse transcription was performed under the
following conditions: 30˚C for 10 min, followed by 42˚C for 15 min.
Real-time PCR analyses were quantified using TB Green®
Fast qPCR Mix (Takara Biotechnology, Co., Ltd.) on a LightCycler
480 II (Roche Diagnostics). The PCR reaction system is as follows:
4 µl qPCR mix, 1 µl Taq enzyme, 4 µl primers, 2 µg cDNA and 10 µl
water. The following candidate ncRNAs were selected: miR-4646,
Pax8-AS1, miR-5196, interleukin 21 receptor-AS1 (IL21R-AS1), signal
recognition particle 14-AS1 (SRP14-AS1), MORC family CW-type zinc
finger 2-AS1 (MORC2-AS1), miR-8061, LIM homeobox 4-AS1 (LHX4-AS1),
Sjogren syndrome/scleroderma autoantigen 1-AS1 (SSSCA1-AS1) and
miR-3679. The sequences of the primers used are listed in Table I. The amplification was performed
under the following conditions: 95˚C for 10 min for initial
denaturation; followed by 35 cycles of 94˚C for 15 sec, annealing
at 56˚C for 25 sec and 72˚C for 30 sec. The expression levels of a
gene were determined by the following formula: ΔCq=Cq (target
gene)-ΔCq (internal reference gene) (17). The levels of the ncRNAs were
normalized to the levels of U6(18).
 | Table IPrimer sequences used for PCR. |
Table I
Primer sequences used for PCR.
| Gene | Forward primer
(5'→3') | Reverse primer
(5'→3') |
|---|
| miR-4646 | ACTGGGAAGAGGAGCT | TGTCGTGGAGTCGGC |
| Pax8-AS1 |
CCCCAAAGCCTAACTCCCTG |
CACTTGGGTTTTGCTGCCTC |
| miR-5196 |
TCTGAGGAGACCTGGGCTGT | TGTCGTGGAGTCGGC |
| IL21R-AS1 |
TGCTGGTTCTTGTAGCTCCG |
CTATGGGGCCACCAGTTGTC |
| SRP14-AS1 |
CTAACTCTGCCACACACGGT |
GCTCAGACCTGCAACCTCTT |
| MORC2-AS1 |
GTTCATAACCGTTGGCTGGT |
GGGTCCACTTTTGTCCCCAA |
| miR-8061 |
GAGAGGATGCCTTAGATTA | TGTCGTGGAGTCGGC |
| LHX4-AS1 |
GCATCTCACCTGTACGACCC |
TGCTGCAGATAGGCCGAAG |
| SSSCA1-AS1 |
CCATCGCCACCCCAGTAATC |
CATGCAGAAGGGGAGTGGTC |
| miR-3679 |
CGTGGTGAGGATATGGCAGG | TGTCGTGGAGTCGGC |
| U6 |
CCCTTCGGGGACATCCGATA |
TTTGTGCGTGTCATCCTTGC |
Statistical analysis
SPSS 18.0 software (SPSS, Inc.) was used to
statistically analyse the data. Receiver operating characteristic
(ROC) curve analysis was applied to determine the best cut-off
values of Pax8-As1 and miR-4646 for diagnosing patients with GDM.
The Youden index is used to determine the best cut-off value
(19). Correlation between two
variables was analyzed using the Pearson's correlation test.
Independent-samples unpaired t-tests were used when a normal
distribution and homogeneity of variance were both satisfied.
P<0.05 was considered to indicate statistical significance.
Results
Different expression patterns in blood
leukocytes of patients with GDM
To identify essential ncRNAs in GDM, raw microarray
data were downloaded from GEO (dataset no. GSE92772). To obtain
differentially expressed genes, the signal data were normalized and
z-score-transformed (unpaired t-test according to the experimental
design was used to validate statistical significance; Fig. 1A). It was determined that numerous
lncRNAs and miRNAs were decreased in patients with GDM compared to
normal controls. Among them, miR-4646 and Pax8-AS1 were the most
downregulated and conserved ncRNAs in the GSE92772 dataset
(Fig. 1B).
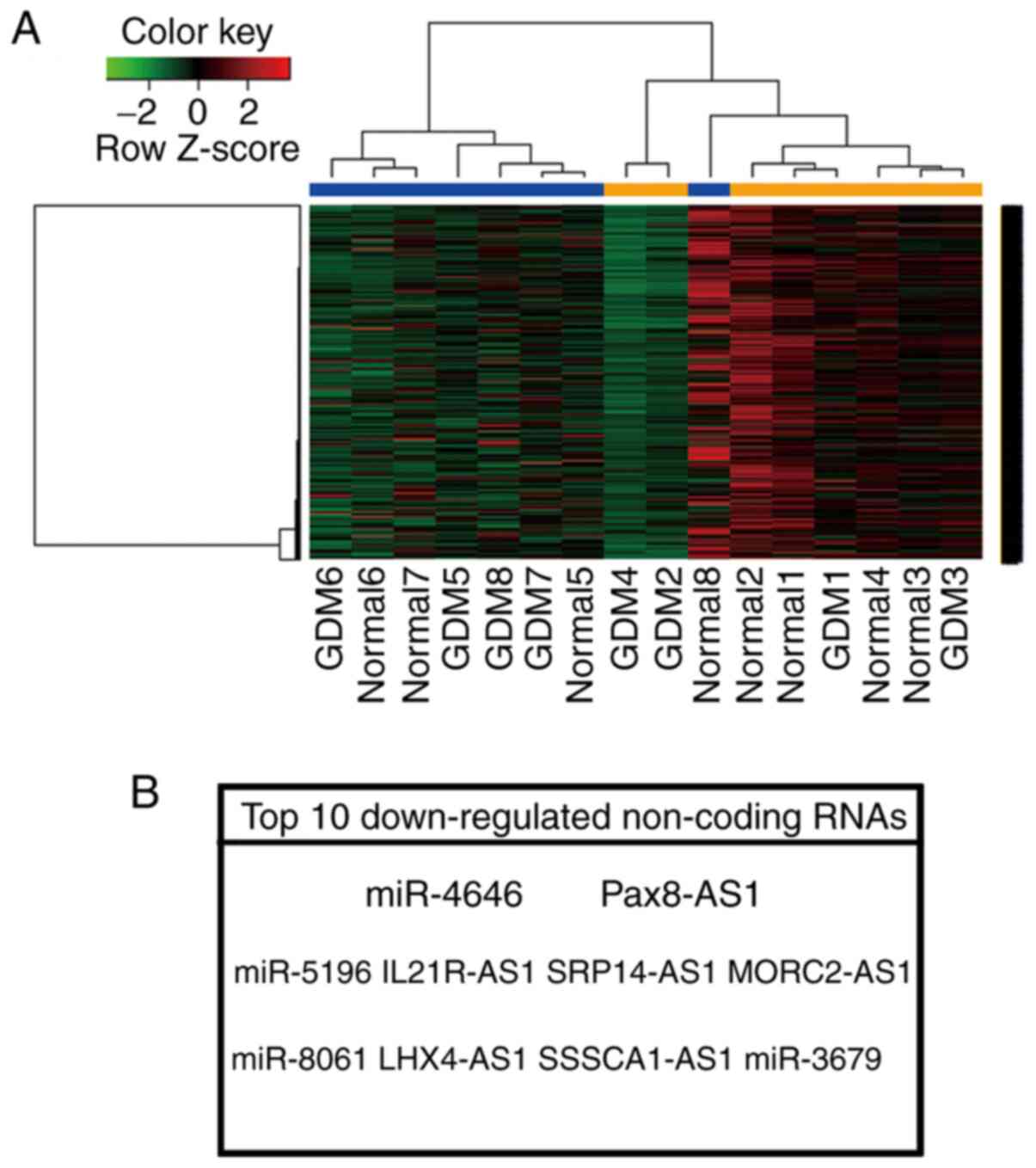 | Figure 1Identification of differentially
expressed genes in GDM. (A) Differentially expressed genes were
detected by analysing the gene expression omnibus dataset GSE92772.
The heatmap of all differentially expressed genes is presented. (B)
Top 10 downregulated ncRNAs in GDM. GDM1-6, cases; normal1-8,
controls. GDM, gestational diabetes mellitus; miR, microRNA;
Pax8-AS1, paired box 8 antisense 1; IL21R, interleukin 21 receptor;
SRP14, signal recognition particle 14; MORC2, MORC family CW-type
zinc finger 2; LHX4, LIM homeobox 4; SSSCA1, Sjogren
syndrome/scleroderma autoantigen 1. |
To explore the difference between the GDM and normal
gravidas, a comprehensive metabolic panel in the maternal serum
samples was tested. The results of the oral glucose tolerance test
were significantly higher in the GDM group than in the control
group (P<0.05). However, there was no significant difference in
other laboratory indicators, including uric acid (UA), total bile
acid (TBA), globulin (GLOB), alanine aminotransferase or aspartate
aminotransferase, between the cases and controls (Table II).
 | Table IIVariables of the comprehensive
metabolic panel associated with GDM. |
Table II
Variables of the comprehensive
metabolic panel associated with GDM.
| Variable | Normal range | Normal glucose
tolerance | GDM |
|---|
| ALT (U/l) | 7-45 | 16.83±3.78 | 14.75±2.34 |
| AST (U/l) | 13-40 | 15.83±4.26 | 17.16±5.09 |
| DBIL (µmol/l) | 0.0-8.0 | 1.95±0.59 | 1.75±0.38 |
| TBIL (µmol/l) | 0.0-21.0 | 4.65±1.18 | 4.67±0.94 |
| CREA (µmol/l) | 41-73 | 46.33±5.74 | 48.45±6.15 |
| UA (µmol/l) | 142-339 | 260.08±67.11 | 247.55±69.14 |
| TBA (µmol/l) | 0.0-12.0 | 2.29±1.07 | 2.50±1.32 |
| GLOB (g/l) | 20.0-40.0 | 25.91±1.79 | 25.9±2.47 |
| ALB (g/l) | 40.0-55.0 | 38.64±1.96 | 37.58±2.64 |
| ALP (U/l) | 35-100 | 88.75±35.59 | 108.25±46.41 |
| LDH (U/l) | 120-250 | 175.1±33.99 | 174.41±20.147 |
| ADA (U/l) | 0.0-15.0 | 8±1.89 | 7.36±1.51 |
| 2-h glucose
(mmol/l) | <8.5 | 5.40±0.75 |
9.69±0.96a |
| 1-h glucose
(mmol/l) | <10.0 | 6.47±1.02 |
10.23±1.69a |
| Fasting glucose
(mmol/l) | <5.1 | 4.73±0.82 | 4.81±0.82 |
ncRNA expression patterns in blood
leukocytes associated with GDM
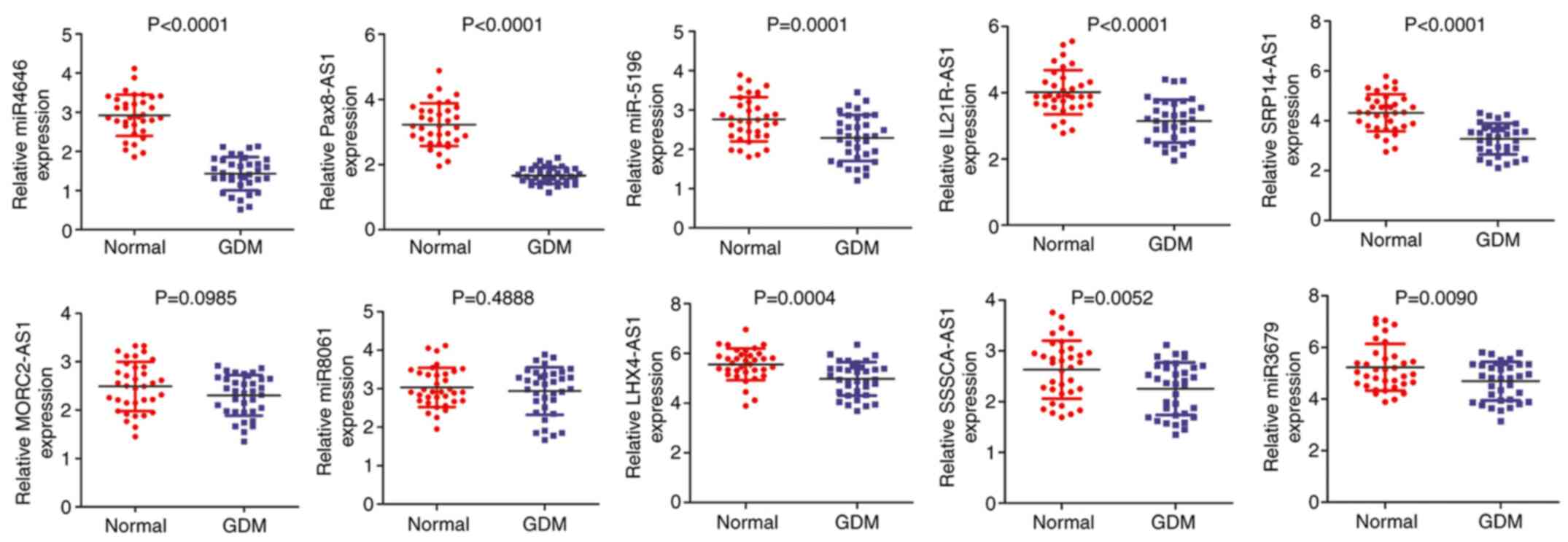
To explore the expression of 10 candidate
GDM-associated ncRNAs in blood leukocytes, the total RNA from whole
blood cells was extracted from 35 subjects with NGT and 35 patients
with GDM. As indicated in Fig. 2,
the levels of miR-4646, Pax8-AS1, miR-5196, IL21R-AS1, SRP14-AS1,
LHX4-AS1, SSSCA1-AS1 and miR-3679 in patients with GDM were
significantly lower than those in the controls. However, there were
no significant differences in the levels of MORC2-AS1 and miR-8061
between the two groups. Furthermore, miR-4646 and Pax8-AS1 were the
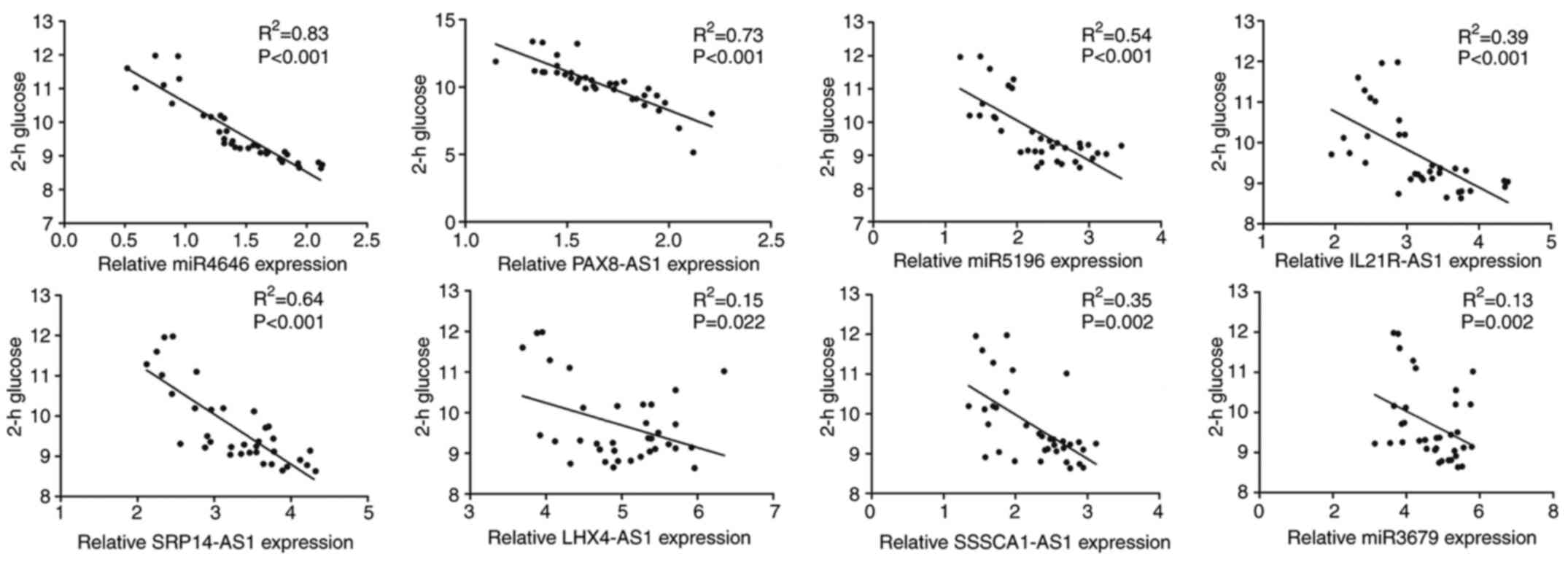
most downregulated ncRNAs among them. Subsequently, the
correlations between the expression levels of the eight candidate
genes (miR-4646, Pax8-AS1, miR-5196, IL21R-AS1, SRP14-AS1,
LHX4-AS1, SSSCA1-AS1 and miR-3679) and 2-h glucose in the
leukocytes of patients with GDM were detected. As presented in
Fig. 3, a significant negative
correlation was observed between miR-4646, Pax8-AS1, miR-5196 and
SRP14-AS1 levels and 2-h glucose in the GDM group (r=0.83, r=0.73,
r=0.54 and r=0.64, respectively; P<0.001).
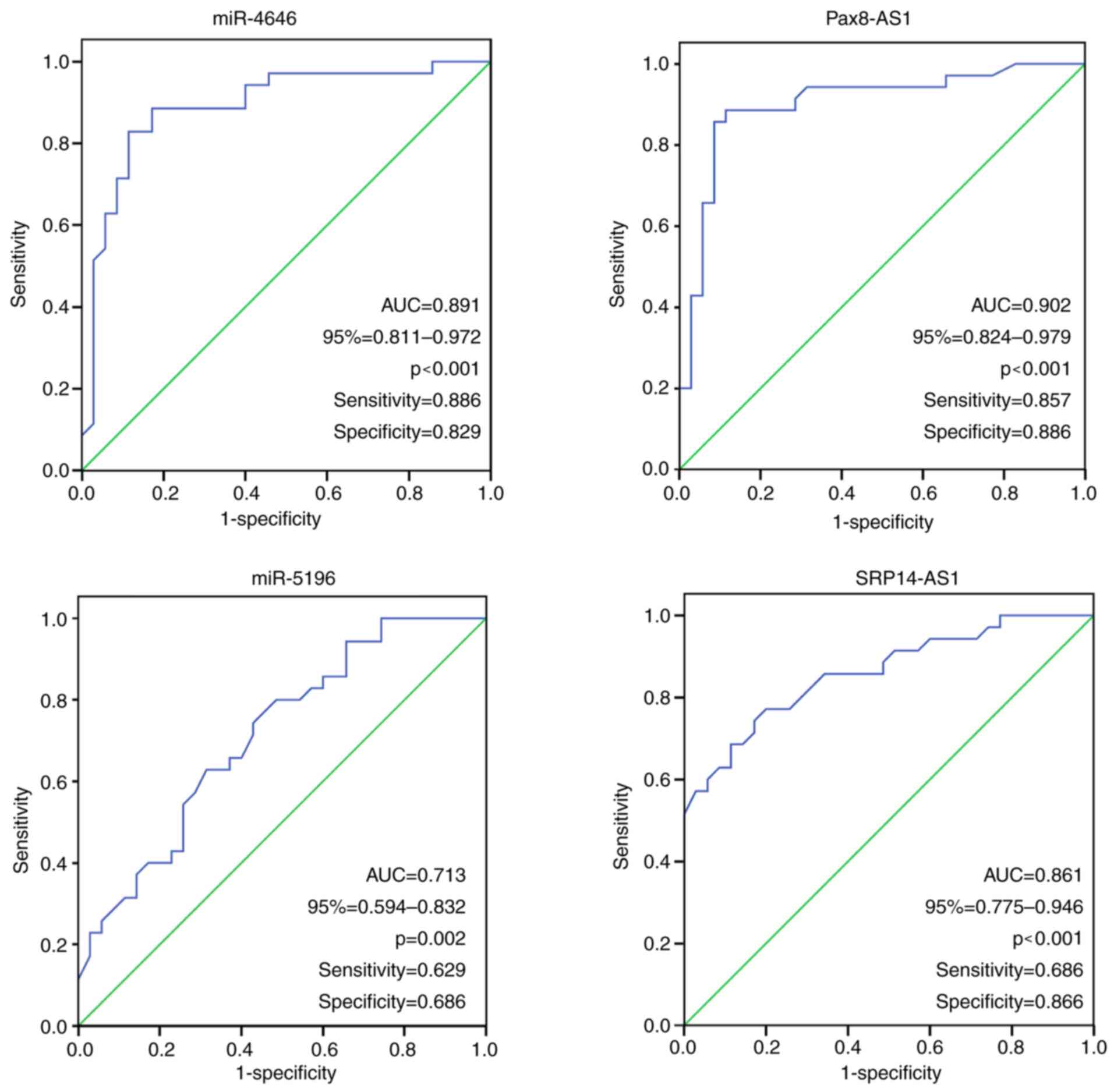
ROC analysis
To determine the diagnostic accuracy of miR-4646,
Pax8-AS1, miR-5196 and SRP14-AS1 in maternal blood leukocytes as
biomarkers for GDM, ROC curves were drawn (Fig. 4). The area under the ROC curve (AUC)
was used to assess the diagnostic value of ncRNAs for GDM. In the
ROC analysis, miR-4646 and Pax8-AS1 achieved an obvious separation
between the GDM and control groups. The AUC of miR-4646 during the
second trimester was 0.891 (95% CI: 0.811-0.972, P<0.001); its
highest sensitivity and specificity were 88.6 and 82.9%,
respectively (cut-off, 1.95). The AUC of Pax8-AS1 for GDM was 0.902
(95% CI: 0.824-0.979, P<0.001), with a sensitivity and
specificity of 85.7 and 88.6%, respectively (cut-off, 2.165).
Discussion
GDM is common and frequently has serious
consequences for mothers and their children. The roles of several
ncRNAs in the occurrence of GDM have recently attracted our
attention, namely miRNAs and lncRNAs. To determine the role of
ncRNAs in GDM, circulating miRNAs and lncRNAs from maternal blood
leukocytes were examined. The present study demonstrated that the
levels of Pax8-AS1 and miR-4646 in the blood leukocytes of patients
with GDM were significantly downregulated compared with those in
pregnant females without GDM. Pax8-AS1 and miR-4646 had the highest
diagnostic value for GDM among the differentially expressed ncRNAs
identified.
The aberrant expression of lncRNAs is closely
associated with the development of multiple complex diseases,
including cancer, cardiovascular diseases, nervous system diseases
and diabetes (20-22).
However, the clinical significance and biological mechanisms of
lncRNAs in the progression of GDM have remained largely elusive. In
the present study, six lncRNAs were selected as candidate
biomarkers and their diagnostic value for GDM was assessed. It was
indicated that Pax8-AS1 had the highest diagnostic value for GDM.
The lncRNA Pax8-AS1 has been previously reported to participate in
the development and progression of diseases, including childhood
acute lymphoblastic leukaemia, cervical cancer and papillary
thyroid carcinoma (23-25).
However, the association between Pax8-AS1 and GDM has remained
elusive. The present results indicated that Pax8-AS1 was expressed
at low levels in blood leukocytes in the second trimester and was
negatively correlated with postprandial blood glucose. ROC curve
analysis of ncRNAs from maternal blood leukocytes suggested that
the diagnostic value of Pax8-AS1 for GDM was the highest among all
lncRNAs tested, with a sensitivity and specificity of 85.7 and
88.6%, respectively. Compared with known biomarkers, such as plasma
protein profiling in the second trimester, Pax8-AS1 is more
conserved and highly sensitive. The present analysis provided
insight for the development of novel biomarkers for GDM.
miRNAs have been indicated to have functional
relevance in the development of obesity and different types of
diabetes (26-28).
Numerous studies have also reported that miRNAs have a key role
during GDM progression. For instance, miR-517b, which was
associated with GDM among pregnancies with male foetuses only,
regulates the expression of TNF superfamily member 15, a
pro-inflammatory, anti-angiogenic cytokine, which may reflect a
sex-specific placental response to the maternal immune system
(29). Wander et al
(30) observed associations between
plasma levels of miR-21-3p and GDM in overweight/obese but not in
healthy maternal individuals. In the present study, four miRNAs
were selected as candidate biomarkers for GDM in a patient cohort.
Significantly decreased miR-4646 was detected in the leukocytes of
patients with GDM. Further analysis demonstrated that there was a
strong correlation between miR-4646 levels and 2-h plasma glucose
levels in patients with GDM in the second trimester. To assess the
diagnostic value of miR-4646 in GDM, ROC curve analysis was
performed. The results suggested that the diagnostic value of
miR-4646 for GDM was the highest among all miRNAs tested, with a
sensitivity and specificity of 88.6 and 82.9%, respectively. To the
best of our knowledge, the present study was the first to report
that miR-4646 may function as a potential diagnostic biomarker for
GDM.
In conclusion, Pax8-AS1 and miR-4646 were decreased
in GDM in the second trimester of pregnancy and are closely
associated with glycosylation markers. Although the sample size of
the present study was limited, the results enhance the current
knowledge on the relationship between GDM and miRNAs/lncRNAs. The
present results open up possibilities for the diagnosis of GDM or
its treatment by regulating the expression of Pax8-AS1 and
miR-4646. In the future, identification of further ncRNAs will
undoubtedly enhance the current knowledge of new ncRNA functions,
allowing us to better understand the pathogenesis and development
of GDM and ultimately facilitate the development of ncRNA-directed
diagnostics and therapeutics against this potentially fatal
disease.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
JH and QC contributed to the design of the study,
analysis and interpretation of data and prepared all figures and
tables. HM and LG have substantial contributions to the design of
the work. YP, CW, DZ and HD took part in analysing the data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Yiwu Maternal and Child Hospital (Jinhua, China) and all subjects
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Binder AM, LaRocca J, Lesseur C, Marsit CJ
and Michels KB: Epigenome-wide and transcriptome-wide analyses
reveal gestational diabetes is associated with alterations in the
human leukocyte antigen complex. Clin Epigenetics.
7(79)2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhu Y and Zhang C: Prevalence of
gestational diabetes and risk of progression to type 2 diabetes: A
global perspective. Curr Diab Rep. 16(7)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
International Association of Diabetes and
Pregnancy Study Groups Consensus Panel. Metzger BE, Gabbe SG,
Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva A, Hod
M, et al: International association of diabetes and pregnancy study
groups recommendations on the diagnosis and classification of
hyperglycemia in pregnancy. Diabetes Care. 33:676–682.
2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sobngwi E, Boudou P, Mauvais-Jarvis F,
Leblanc H, Velho G, Vexiau P, Porcher R, Hadjadj S, Pratley R,
Tataranni PA, et al: Effect of a diabetic environment in utero on
predisposition to type 2 diabetes. Lancet. 361:1861–1865.
2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Catalano PM and Hauguel-De Mouzon S: Is it
time to revisit the Pedersen hypothesis in the face of the obesity
epidemic? Am J Obstet Gynecol. 204:479–487. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
El Hajj N, Schneider E, Lehnen H and Haaf
T: Epigenetics and life-long consequences of an adverse nutritional
and diabetic intrauterine environment. Reproduction. 148:R111–R120.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Anastasiadou E, Jacob LS and Slack FJ:
Non-coding RNA networks in cancer. Nat Rev Cancer. 18:5–18.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Quinn JJ and Chang HY: Unique features of
long non-coding RNA biogenesis and function. Nat Rev Genet.
17:47–62. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yan C, Chen J and Chen N: Long noncoding
RNA MALAT1 promotes hepatic steatosis and insulin resistance by
increasing nuclear SREBP-1c protein stability. Sci Rep.
6(22640)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Leng L, Zhang C, Ren L and Li Q:
Construction of a long non-coding RNA-mediated competitive
endogenous RNA network reveals global patterns and regulatory
markers in gestational diabetes. Int J Mol Med. 43:927–935.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tuna M, Machado AS and Calin GA: Genetic
and epigenetic alterations of microRNAs and implications for human
cancers and other diseases. Genes Chromosomes Cancer. 55:193–214.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Esteves JV, Enguita FJ and Machado UF:
MicroRNAs-mediated regulation of skeletal muscle GLUT4 expression
and translocation in insulin resistance. J Diabetes Res.
2017(7267910)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pordzik J, Jakubik D, Jarosz-Popek J,
Wicik Z, Eyileten C, De Rosa S, Indolfi C, Siller-Matula JM, Czajka
P and Postula M: Significance of circulating microRNAs in diabetes
mellitus type 2 and platelet reactivity: Bioinformatic analysis and
review. Cardiovasc Diabetol. 18(113)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Stirm L, Huypens P, Sass S, Batra R,
Fritsche L, Brucker S, Abele H, Hennige AM, Theis F, Beckers J, et
al: Maternal whole blood cell miRNA-340 is elevated in gestational
diabetes and inversely regulated by glucose and insulin. Sci Rep.
8(1366)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Method. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Iempridee T, Wiwithaphon S, Piboonprai K,
Pratedrat P, Khumkhrong P, Japrung D, Temisak S, Laiwejpithaya S,
Chaopotong P and Dharakul T: Identification of reference genes for
circulating long noncoding RNA analysis in serum of cervical cancer
patients. FEBS Open Bio. 8:1844–1854. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li C, Chen J and Qin G: Partial Youden
index and its inferences. J Biopharm Stat. 29:385–399.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li CH and Chen Y: Targeting long
non-coding RNAs in cancers: Progress and prospects. Int J Biochem
Cell Biol. 45:1895–1910. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Carter G, Miladinovic B, Patel AA, Deland
L, Mastorides S and Patel NA: Circulating long noncoding RNA GAS5
levels are correlated to prevalence of type 2 diabetes mellitus.
BBA Clin. 4:102–107. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bahari G, Hashemi M, Naderi M,
Sadeghi-Bojd S and Taheri M: Long non-coding RNA PAX8-AS1
polymorphisms increase the risk of childhood acute lymphoblastic
leukemia. Biomed Rep. 8:184–190. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Han J, Zhou W, Jia M, Wen J, Jiang J, Shi
J, Zhang K, Ma H, Liu J, Ren J, et al: Expression quantitative
trait loci in long non-coding RNA PAX8-AS1 are associated with
decreased risk of cervical cancer. Mol Genet Genomics.
291:1743–1748. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang Y, Li F and Chen J: MYC promotes the
development of papillary thyroid carcinoma by inhibiting the
expression of lncRNA PAX8-AS1:28. Oncol Rep. 41:2511–2517.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Arner P and Kulyté A: MicroRNA regulatory
networks in human adipose tissue and obesity. Nat Rev Endocrinol.
11:276–288. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Miao C, Chang J, Zhang G and Fang Y:
MicroRNAs in type 1 diabetes: New research progress and potential
directions. Biochem Cell Biol. 96:498–506. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Vienberg S, Geiger J, Madsen S and
Dalgaard LT: MicroRNAs in metabolism. Acta Physiol (Oxf).
219:346–361. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Anton L, Olarerin-George AO, Hogenesch JB
and Elovitz MA: Placental expression of miR-517a/b and miR-517c
contributes to trophoblast dysfunction and preeclampsia. PLoS One.
10(e0122707)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wander PL, Boyko EJ, Hevner K, Parikh VJ,
Tadesse MG, Sorensen TK, Williams MA and Enquobahrie DA:
Circulating early- and mid-pregnancy microRNAs and risk of
gestational diabetes. Diabetes Res Clin Pract. 132:1–9.
2017.PubMed/NCBI View Article : Google Scholar
|