Introduction
Skin aging is caused by a variety of factors,
including hormone imbalance and some metabolic pathway disorders,
especially ultraviolet radiation (1). Photoaging refers to premature aging of
skin due to long-term exposure of the skin to ultraviolet radiation
(2). Of the three wavelengths of
ultraviolet rays, ultraviolet B (UVB) (280-320 nm) is considered
the root cause of skin photoaging. It can penetrate the epidermis
to the dermis (3). Long-term
ultraviolet radiation, especially UVB radiation, would give rise to
the reduction of skin collagen fibers and abnormal elastic fiber
deposition. The manifestations are capillary dilatation,
erythrocyte sedimentation, pachulosis, dermatochalasis, and wrinkle
(4).
Human dermal fibroblasts maintain skin thickness and
elasticity by producing extracellular matrix (ECM) (5). UV radiation, especially UVB radiation,
can result in skin photoaging. It can induce apoptosis of
keratinocytes (3,6) and reactive oxygen species
(ROS)-mediated inflammatory response (7). UV radiation upregulates the activity
of matrix metalloproteinases (MMPs) via mitogen-activated protein
kinase (MAPK) and NF-κB signaling pathways (8). MMPs can specifically degrade almost
all types of ECM, such as Col-I, and Col-III, which play a pivotal
role in skin photoaging (9). In
many studies on skin photoaging, excessive generation of ROS is
considered as the most important reason and therapeutic target
(10). Excessive ROS activates
NF-κB, leading to the release of inflammatory factors including
tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and
interleukin-6 (IL-6) (11,12). It also induces the expression of
ECM-degrading enzyme, MMPs, which finally results in oxidative
damage to cells (13).
Dual oxidase 2 (DUOX2) is an important NADPH oxidase
catalytic subunit that regulates the production of ROS (14,15).
It causes cellular inflammatory response by mediating epidermal
keratinocytes by promoting the activation of NF-κB signals and
inducing the upregulation of IL-8 and CCL20(15). This suggests that DUOX2 may play a
crucial role in UVB-induced photoaging and inflammatory response.
There are no related reports at present, and the specific mechanism
of action is still under investigation. Therefore, in this study,
UVB was used to irradiate human skin fibroblast lines (HSF2) to
construct cell models of photoaging. Then DUOX2 overexpression and
interference were used in the treatment. The aim of the study was
to explore the function of DUOX2 in the photoaging process and the
role of NF-κB signals in this process.
Materials and methods
Cell culture and UVB radiation
Skin fibroblasts (HSF2) were purchased from the
Shanghai Institute of Biochemistry and Cell Biology (SIBCB; Chinese
Academy of Sciences). The study was approved by the Ethics
Committee of Shanghai East Hospital, Tongji University School of
Medicine.
HSF2 cells were resuscitated and cultured in
Dulbecco's modified Eagle's medium (DMEM; Hyclone) containing 10%
fetal bovine serum (FBS; Life Technologies) under 5% CO2
at 37˚C. The cells were harvested and washed three times with
sterile phosphate-buffered saline (PBS). The cells were evenly
distributed in an uncovered culture dish, and irradiated with a UVB
lamp (Haining Lei Top Lighting Co., Ltd.) in a biosafety cabinet at
room temperature. The ultraviolet irradiation intensity was
verified by an ultraviolet intensity detector (Deshengxing
Technology Co., Ltd.). The irradiation distance was set to 15 cm,
and UVB irradiation doses for different groups were 0 (treated in
the dark), 15, 30 and 60 mj/cm2, respectively. After
each irradiation, the cells were collected by centrifugation at
1100-1200 x g at room temperature for 3 min and resuspended in
sterile PBS. After repeated washing three times, they were finally
resuspended in culture medium and cultured under the aforementioned
conditions. The cells were irradiated with UVB for 3 days, 1 h each
time, once a day. Finally, the cells and supernatant cultures were
collected after irradiation for 0, 24, 48 and 72 h,
respectively.
Radiation dose selection
UVB of different doses (0, 15, 30 and 60
mj/cm2) was, respectively, used to irradiate human skin
fibroblast (HSF2) cell lines. The cells and supernatants were then
collected using the aforementioned methods after UVB irradiation.
Subsequently, the proliferation of cells at 0, 12, 24 and 48 h
after UVB irradiation was determined using a Cell Counting Kit-8
(CCK-8) (SABBiotech) as per the protocol. At 48 h after UVB
irradiation, the ROS content of the cells was detected by flow
cytometry. In addition, the content of hydrogen peroxide in the
cells was determined using a hydrogen peroxide detection kit
(Jiancheng Bioengineering). The expression of TNF-α and IL-6 in the
cells was determined referring to the method of enzyme-linked
immuno-sorbent assay (ELISA) kit (Cell Signaling Technology).
Furthermore, the expression of MMP2, MMP9, Col-I, and α-SMA in the
cells was detected using quantitative PCR (qPCR), and the
expression of DUOX2, p65 and p-p65 was detected using western blot
analysis.
Effects of DUOX2 overexpression on
HSF2 cells
DUOX2 overexpression and interference lentivirus
vectors were constructed by JRDun Biotech, and sequences and
primers are shown in Table I.
Recombinant lentiviruses were produced and amplified in 293 cell
packaging according to previous methods (16). The virus supernatant was diluted to
required concentration in culture medium and then added to the
monolayer HSF2 cells at the logarithmic phase. After 24-h
incubation in DMEM medium with 10% fetal bovine serum at 37˚C with
5% CO2, the relative mRNA and protein expression of
DUOX2 in ADSCs was detected by reverse transcriptase-quantitative
PCR and western blot analysis.
 | Table IDUOX2 overexpression and interference
primers. |
Table I
DUOX2 overexpression and interference
primers.
| Genes | Primer sequence
(5'-3') |
|---|
| DUOX2
overexpression | Forward:
5'-CCCAAGCTTATGCTCCGTGCAAGACCAG-3' |
| | Reverse:
5'-CGGAATTCTCAGAAGTTCTCATAGTGGTGC-3' |
| DUOX2 interference
(si-1) | Forward:
5'-CGCUAUGACGGCUGGUUUATT-3' |
| | Reverse:
5'-UAAACCAGCCGUCAUAGCGTT-3' |
| DUOX2 interference
(si-2) | Forward:
5'-GGAGUGAUCUCAACCCUAATT-3' |
| | Reverse:
5'-UUAGGGUUGAGAUCACUCCTT-3' |
| DUOX2 interference
(si-3) | Forward:
5'-GGAAUGGGCUGUUCUCCAATT-3' |
| | Reverse:
5'-UUGGAGAACAGCCCAUUCCTT-3' |
| Interference control
(si-NC) | Forward:
5'-UUCUCCGAACGUGUCACGUTT-3' |
| | Reverse:
5'-ACGUGACACGUUCGGAGAATT-3' |
Three groups were established for the experiment:
the empty vector group, the DUOX2 overexpression group, and the
DUOX2 overexpression + NAC group (5 mM, NF-κB inhibitor). Cells in
each group were cultured for 24 h under normal conditions, and then
the cells and supernatant were harvested. Finally, the content of
hydrogen peroxide in the cells was determined using the biochemical
method. The expression of TNF-α and IL-6 in the cells was
determined by ELISA. Furthermore, the expression of MMP2, MMP9,
Col-I and α-SMA in the cells was detected using qPCR, and the
expression of DUOX2, p65 and p-p65 was detected using western blot
analysis.
Effects of DUOX2 interference on
UVB-induced aging of HSF2 cells
Five groups were set up for the experiment: the
control group, the UVB irradiation group, the empty vector group,
the UVB + siDUOX2 group, and the UVB + NAC group. Cells in the
control group were given dark treatment, and cells in the remaining
four groups were irradiated with 30 mJ/cm2 UVB and
transfected with corresponding virus vectors or given NAC
treatment. Cells and supernatant were collected 24 h after
treatment. Finally, the content of hydrogen peroxide in the cells
was determined using the biochemical method. The expression of
Col-I, α-SMA, TNF-α and IL-6 in the cells was determined using
ELISA. In addition, the expression of DUOX2, MMP2, MMP9, p65 and
p-p65 in cells was determined using western blot analysis.
ROS detection
According to a previous report, DCFH-DA analysis was
used to evaluate the ROS content (17). Briefly, HSF2 cells
(1x106/ml) were cultured with 5 µM
2',7'-dichlorofluorescein diacetate (DCFH-DA) at 37˚C for 20 min
(mixed forward and backward every 3 min). DCFH-DA (non-fluorescent)
entered cells and hydrolyzed into cell impermeable and
non-fluorescent DCFH. ROS in the cells oxidized DCFH into highly
fluorescent dichlorofluorescein (DCF). Green fluorescence intensity
was proportional to ROS content. After the cells were stimulated at
480 nm, the DCF fluorescence was detected at 525 nm via a flow
cytometer (Becton Dickinson). The result was expressed as the ratio
of fluorescence intensity of HSF2 cells in other groups to that of
the normal group.
RT-qPCR
The mRNA expression was detected by reverse
transcriptase-quantitative PCR (RT-qPCR). Total RNA (2 µg per
sample) was extracted and reverse transcribed into cDNA using a
reverse transcription kit (Thermo Fisher Scientific, Inc.). The
obtained cDNA was taken as a template and analyzed using RT-qPCR
with an RT-PCR instrument (ABI-7300; Applied Biosystems) and
SYBR-Green reagent (Thermo Fisher Scientific, Inc.).
Primers for RT-qPCR (Table II) were designed in Primer 5.0
software and synthesized by JRDun Biotech. Using
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene expression as
the internal reference, the relative mRNA expression of each sample
was detected by the 2-ΔΔCq method (18) and relative quantitative analysis.
ΔΔCq=(Cq, target gene of the treatment group-Cq,
internal reference gene of the treatment group)-(Cq, target gene of
the control group-Cq, internal reference gene of the control
group).
 | Table IIPrimers used for RT-qPCR. |
Table II
Primers used for RT-qPCR.
| Genes | Primer sequence
(5'-3') |
|---|
| MMP2
NM_001127891.2 | Forward:
5'-TTGGTGGGAACTCAGAAG-3' |
| | Reverse:
5'-TTGCGGTCATCATCGTAG-3' |
| MMP9
NM_004994.2 | Forward:
5'-GTGGCACCACCACAACATCAC-3' |
| | Reverse:
5'-CGCGACACCAAACTGGATGAC-3' |
| Col-I
NM_000088.4 | Forward:
5'-TCTTTGACCAACCGAACATGAC-3' |
| | Reverse:
5'-TTGATTGCTGGGCAGACAATAC-3' |
| α-SMA
NM_001141945.2 | Forward:
5'-CCGGGACATCAAGGAGAAAC-3' |
| | Reverse:
5'-CGATGAAGGATGGCTGGAAC-3' |
| DUOX2
NM_014080.4 | Forward:
5'-CGAGTTTGCCGAGTCCC-3' |
| | Reverse:
5'-AAGCCATTCTCATCCAGGTC-3' |
| GAPDH
NM_001256799.2 | Forward:
5'-AATCCCATCACCATCTTC-3' |
| | Reverse:
5'-AGGCTGTTGTCATACTTC-3' |
Western blot analysis
Total protein of each sample was extracted using
Cell Protein Extraction Reagent (Thermo Fisher Scientific, Inc.),
and its content was determined by a bicinchoninic acid (BCA)
detection kit (Thermo Fisher Scientific, Inc.). The protein (30
µg/sample) was separated by 10%
sodium-dodecyl-sulfate-polyacrylamide gel-electrophoresis
(SDS-PAGE), and then transferred to a polyvinylidene difluoride
(PVDF) membrane. Subsequently, the membrane was sealed with 5% skim
milk at 25˚C for 1 h, and then cultured with appropriate primary
antibodies: MMP2 (1:1,000; cat. no. ab92536; Abcam), MMP9 (1:1,000;
cat. no. ab76003; Abcam), p65 (1:500; cat. no. sc-71675; Santa Cruz
Biotechnology), p-p65 (1:2,000; cat. no. ab183559; Abcam), DUOX2
(1:400; cat. no. ab170308; Abcam) or GAPDH (1:2,000; no. 5174; Cell
Signaling Technology). The membrane was incubated with
HRP-conjugated IgG secondary antibody (1:1,000; cat no. A0208;
Beyotime Institute of Biotechnology) at 25˚C for 1 h. Finally, the
protein band was detected by an electrochemiluminescence (ECL)
detection kit (Beyotime Institute of Biotechnology). The film was
scanned using a Tanon-5200 chemiluminescent Gel Image System Ver.
4.00 (Tanon Science & Technology Co., Ltd.). GAPDH was used as
control.
Statistical analyses
The data were expressed as mean ± standard deviation
(n=3), and analyzed using the variance analysis and Duncan's
multiple range test. Data differences were analyzed using SPSS 20.0
software (SPSS Inc.). P<0.05 indicates a significant
difference.
Results
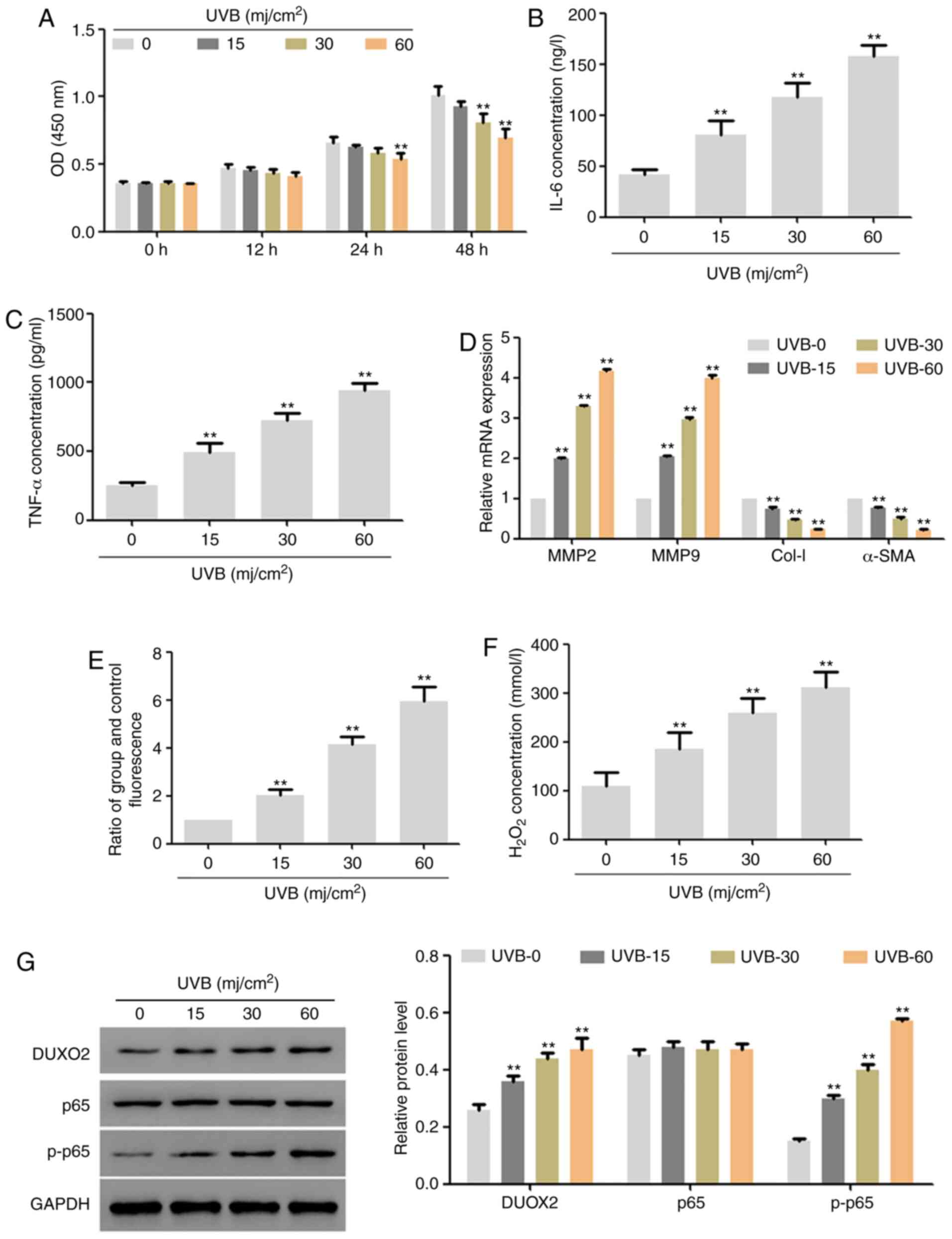
Radiation dose selection
In this part, UVB of different doses (0, 15, 30 and
60 mj/cm2) was used to irradiate human skin fibroblast
lines (HSF2) for 48 h, separately. The results showed that,
low-dose UVB exerted no effect on the proliferation of HSF2 cells,
but with the extension of time and increase of dose, UVB
significantly inhibited proliferation (Fig. 1A). In addition, 48 h after
treatment, UVB significantly upregulated the expression of IL-6 and
TNF-α (Fig. 1B and C) and the mRNA expression of MMP2 and
MMP9, significantly downregulated the mRNA expression of Col-I and
α-SMA (Fig. 1D), and also
upregulated the ROS content and hydrogen peroxide content (Fig. 1E and F). Moreover, UVB upregulated the protein
expression of DUOX2 and p-p65 in a dose-dependent manner (Fig. 1G). According to the above results
and the effects of UVB on cell proliferation, we used 30
mj/cm2 UVB to treat cells for 48 h in the subsequent
experiments.
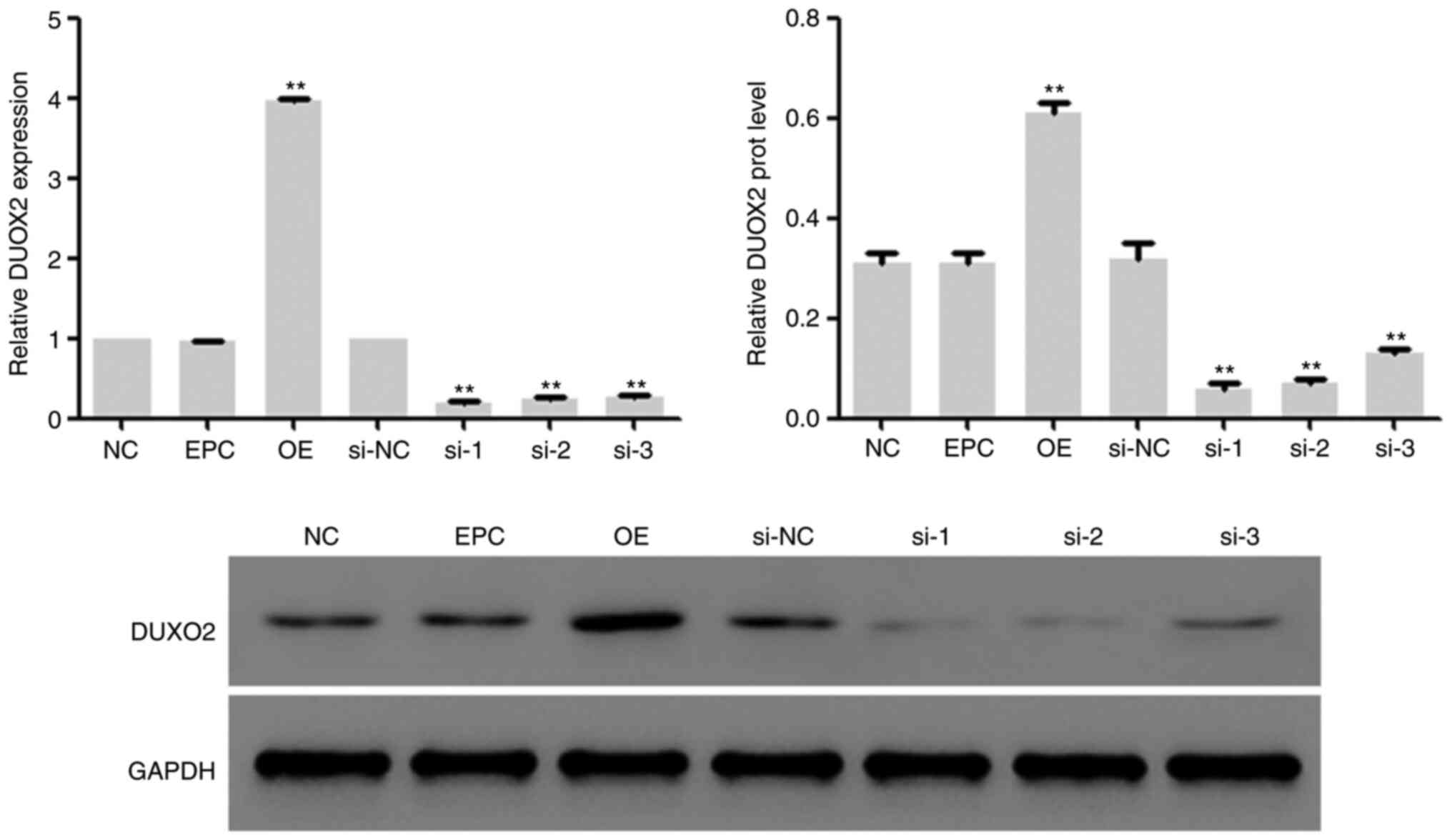
Efficacy identification of DUOX2
interference and overexpression vectors
In the subsequent experiments, lentivirus vectors
with DUOX2 interference and overexpression were constructed, and
transfected into HSF2 cells, followed by 48-h incubation in DMEM
medium with 10% fetal bovine serum at 37˚C with 5% CO2.
Then the mRNA and protein expression of DUOX2 in the cells was
determined, and it was found that the expression of DUOX2 in HSF2
cells was inhibited by DUOX2 interference and upregulated by DUOX2
overexpression (Fig. 2). The
lentivirus vectors corresponding to interference sequence 2 (si-2)
was used for subsequent interference experiments.
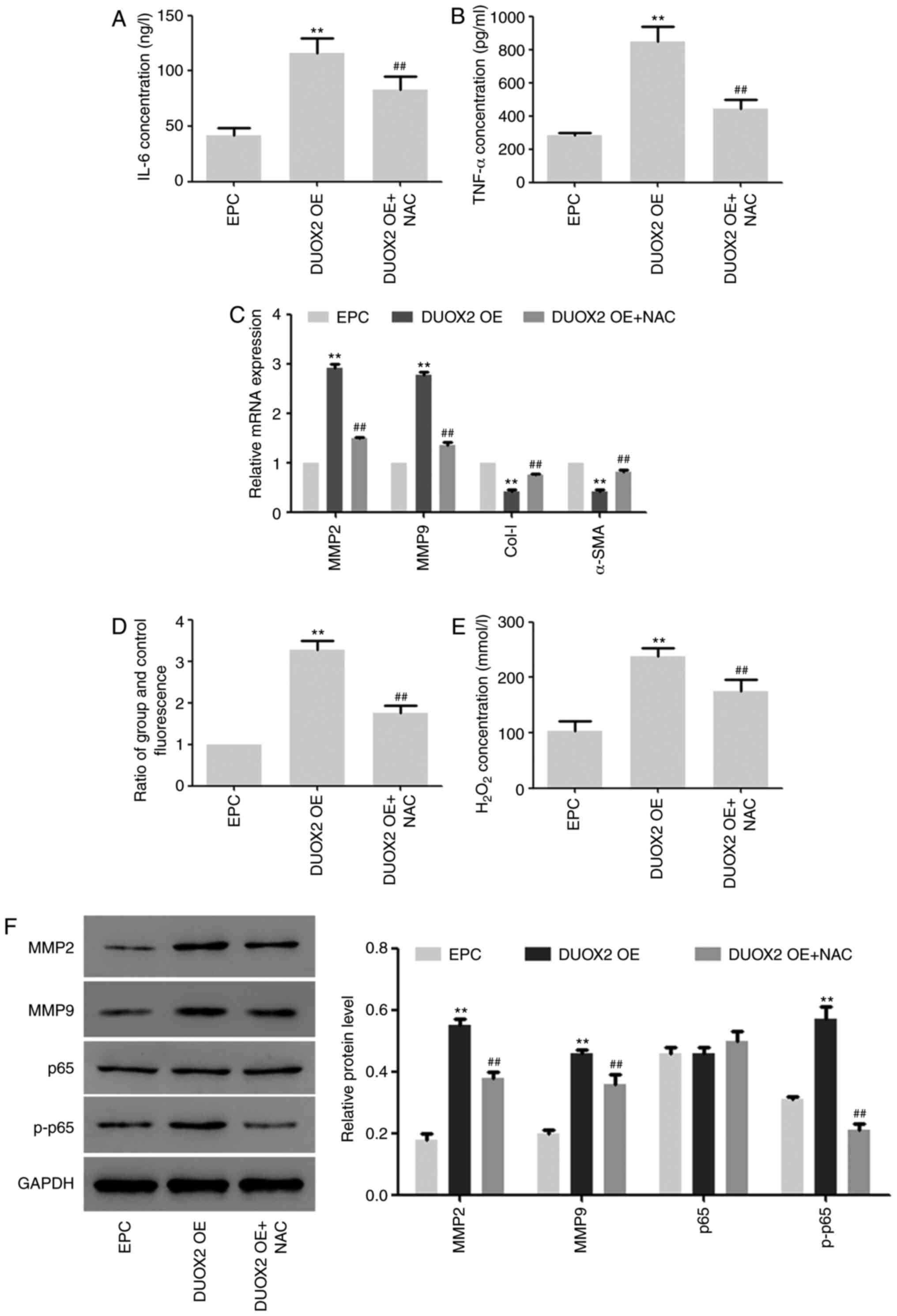
Effects of DUOX2 overexpression on
HSF2 cells
In the subsequent experiments, the effects of DUOX2
overexpression on HSF2 cells were determined, and HSF2 cells were
treated with NF-κB inhibitor NAC to explore the role of NF-κB
signals in this process (19). As
shown in Fig. 3, the expression of
IL-6 and TNF-α was upregulated by DUOX2 overexpression, but the
upregulation was inhibited by NAC (Fig.
3A and B). In addition, the
same trend was found in ROS production and hydrogen peroxide
content in the cells (Fig. 3D and
E). The mRNA and protein expression
of MMP2 and MMP9, as well as the phosphorylation level of p65 were
induced by DUOX2, but downregulated by NF-κB inhibitor NAC
(Fig. 3C and F). The mRNA expression of Col-I and α-SMA
was inhibited by DUOX2 overexpression, but the inhibition was
weakened by NF-κB (Fig. 3C).
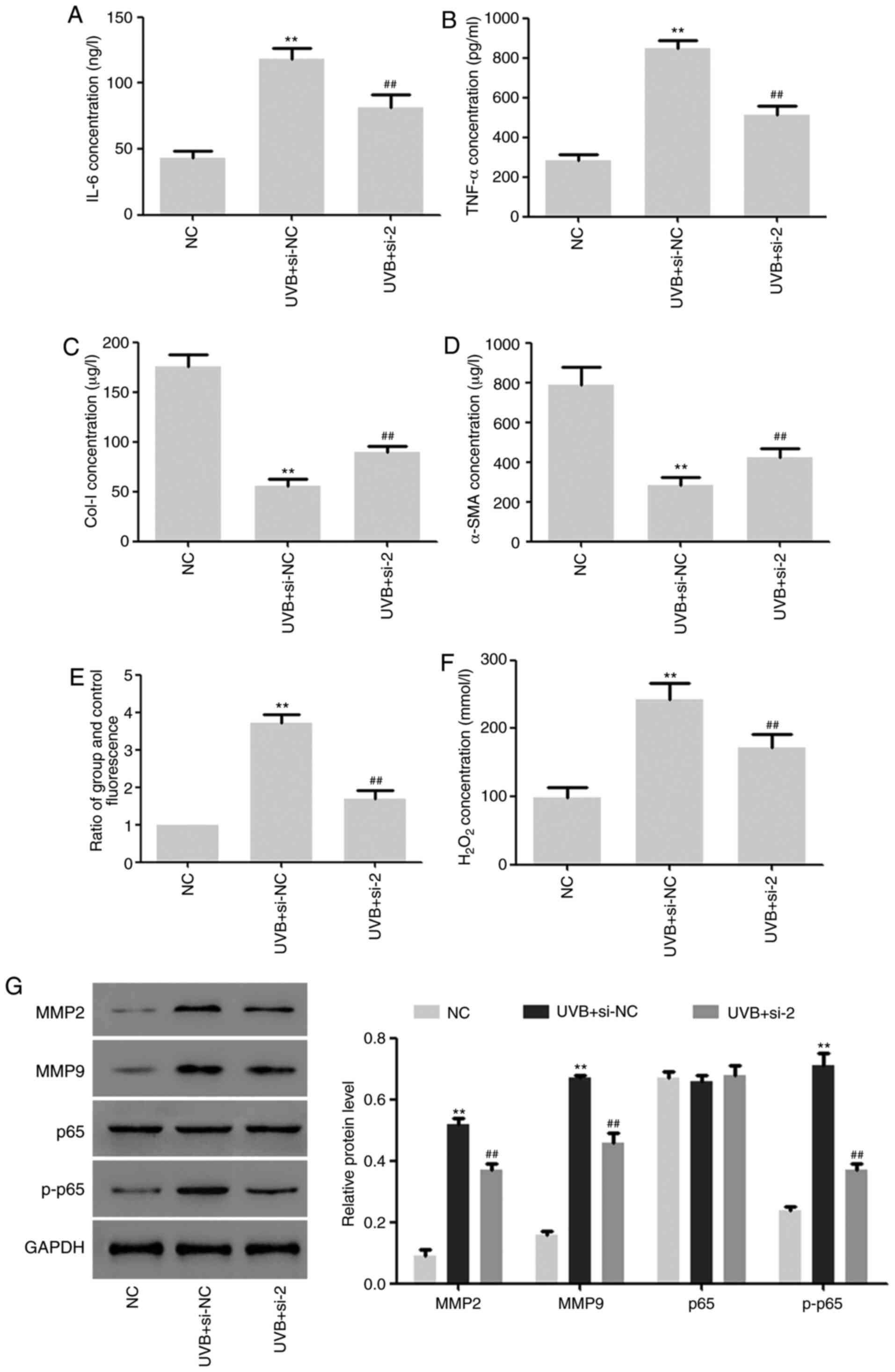
Effects of DUOX2 interference on
UVB-induced aging of HSF2 cells
The effects of DUOX2 interference on HSF2 cells
under UVB (30 mj/cm2) irradiation were further studied.
We found that DUOX2 interference significantly inhibited the
UVB-induced upregulation of TNF-α and IL-6 and downregulation of
Col-I and α-SMA (Fig. 4A-D). ROS
production and hydrogen peroxide content were induced by UVB and
the induction was inhibited by DUOX2 interference (Fig. 4E and F). In addition, the protein expression of
MMP2 and MMP9 and the phosphorylation level of p65 were upregulated
by UVB, but the upregulation was weakened by DUOX2 interference
(Fig. 4G).
Discussion
Long-term ultraviolet irradiation is considered to
be the primary cause of skin damage, and it leads to photoaging
(1). Photoaged skin is clinically
characterized with pachulosis, dermatochalasis, and wrinkle, which
are closely related to the disorder of collagen fibers and the
reduction of collagen content (20,21).
UVB radiation mediates the phosphorylation of protein kinases by
inducing ROS-mediated MAPK and NF-κB signaling pathways, further
inducing cellular inflammatory response (8). Chronic and low-grade inflammation is
also considered a major feature of the aging process. This
phenomenon is called ‘inflamm-aging’. Inflamm-aging plays an
important role in in many age-related diseases such as type 2
diabetes, Alzheimer's disease, cardiovascular disease, weakness,
skeletal muscle reduction, osteoporosis and skin aging (22,23).
In addition, ROS can lead to the upregulation of expression and
activity of MMPs, which is closely related to collagen degradation
in photoaged skin (24,25). This conclusion is confirmed by our
study. UVB radiation can induce the production of ROS, increase the
hydrogen peroxide content in HSF2 cells and inhibit cell
proliferation. It can also induce the activation of NF-κB signals
and promote the increase of inflammatory factors (TNF-α and IL-6).
Furthermore, UVB radiation can also induce the expression of DUOX2.
Although UVB radiation induces an increase in DUOX2 and p65
phosphorylation levels, the rate of increase in p65 phosphorylation
levels is higher, which indicates that UVB radiation-induced NF-κB
phosphorylation involves multiple factors and may be the result of
multiple cascading signals.
DUOX2 is a subtype of NOX NADPH oxidase, and the
defect in DUOX2 is manifested by congenital hypothyroidism
(26). In addition, it is reported
that DUOX2 could mediate the production ofROSto induce
epithelial-mesenchymal transition in 5-fluorouracil-resistant human
colon cancer cells (27). The
DUOX2-mediated production of hydrogen peroxide, DUOX2-mediated DNA
damage and inflammatory response play important roles in
gastrointestinal tumors and prostate tumors. This effect may be
realized by activating NF-κB signals (28). In addition, findings of correlation
studies between NADPH oxidase ROS and human aging revealed that
properly controlling the activity of physiological redox signals of
NADPH oxidase for cell homeostasis maintenance may be a therapeutic
strategy to promote healthy aging (29). The latest research has found that
dioxygenases (DUOXes), which are usually prominently expressed in
the epithelial lineage, are usually inhibited by epigenetic
mechanisms in epithelial-derived cancers (30). DUOXes also mediate the regulation of
Th1 and Th2 immune cells on the production of respiratory tract ROS
in the host defense response (31).
These mechanisms may directly or indirectly involve the photodamage
mechanism that leads to photoaging and skin cancer. It suggests
that DUOX2 plays an essential role in skin photoaging, especially
UVB-induced photoaging. However, there are no relevant reports thus
far. Results of the present study have shown for the first time, to
the best of our knowledge, the role of DUOX2 in UVB-induced aging
of HSF2 cells. DUOX2 induces the production of ROS, increases the
content of hydrogen peroxide in HSF2 cells. It also promotes the
cellular inflammatory response, the expression of MMP2 and MMP9,
and the degradation of collagens, and activates NF-κB signals. The
correlation between MMP and phosphorylation (p-p65) is
disproportionate. The reason may be that NAC is a specific
inhibitor of NF-κB, and overexpression of DUOX2 may also regulate
the expression of MMP through other signaling pathways such as MAPK
signaling (32). However, this
effect is significantly weakened by NF-κB inhibitor NAC. In
addition, in HSF2 cells irradiated by UVB, interference with DUOX2
can reduce UVB-induced ROS production, cellular inflammatory
response and NF-κB overactivation.
To sum up, the upregulation of DUOX2 expression
plays a crucial role in UVB-induced aging of HSF2 cells, and the
specific mechanism is related to the promotion of ROS production,
cellular inflammatory response, and activation of NF-κB
signals.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XX and FZ conceived, designed the study and drafted
the manuscript. XX, MH and CF collected, analyzed and interpreted
the experimental data. XX revised the manuscript for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Shanghai East Hospital, Tongji University School of Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Krutmann J, Bouloc A, Sore G, Bernard BA
and Passeron T: The skin aging exposome. J Dermatol Sci.
85:152–161. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Huang CY, Lin YT, Kuo HC, Chiou WF and Lee
MH: Compounds isolated from Eriobotrya deflexa leaves
protect against ultraviolet radiation B-induced photoaging in human
fibroblasts. J Photochem Photobiol. 175:244–253. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nichols JA and Katiyar SK: Skin
photoprotection by natural polyphenols: Anti-inflammatory,
antioxidant and DNA repair mechanisms. Arch Dermatol Res.
302:71–83. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Goldman MP, Weiss RA and Weiss MA: Intense
pulsed light as a nonablative approach to photoaging. Dermatol
Surg. 31:1179–1187. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mora Huertas AC, Schmelzer CE,
Hoehenwarter W, Heyroth F and Heinz A: Molecular-level insights
into aging processes of skin elastin. Biochimie. 128-129:163–173.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lei X, Liu B, Han W, Ming M and He YY:
UVB-Induced p21 degradation promotes apoptosis of human
keratinocytes. Photochem Photobiol Sci. 9:1640–1648.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Duncan FJ, Martin JR, Wulff BC, Stoner GD,
Tober KL, Oberyszyn TM, Kusewitt DF and Van Buskirk AM: Topical
treatment with black raspberry extract reduces cutaneous
UVB-induced carcinogenesis and inflammation. Cancer Prev Res
(Phila). 2:665–672. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sharma SD, Meeran SM and Katiyar SK:
Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative
stress and activation of mitogen-activated protein kinases and
nuclear factor-kappaB signaling in in vivo SKH-1 hairless mice. Mol
Cancer Ther. 6:995–1005. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fisher GJ, Datta SC, Talwar HS, Wang ZQ,
Varani J, Kang S and Voorhees JJ: Molecular basis of sun-induced
premature skin ageing and retinoid antagonism. Nature. 379:335–339.
1996.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Poon F, Kang S and Chien AL: Mechanisms
and treatments of photoaging. Photodermatol Photoimmunol Photomed.
31:65–74. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yokoyama T, Komori A, Nakamura M, Takii Y,
Kamihira T, Shimoda S, Mori T, Fujiwara S, Koyabu M, Taniguchi K,
et al: Human intrahepatic biliary epithelial cells function in
innate immunity by producing IL-6 and IL-8 via the TLR4-NF-kappaB
and -MAPK signaling pathways. Liver Int. 26:467–476.
2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Orlando A, Cazzaniga E, Tringali M, Gullo
F, Becchetti A, Minniti S, Taraballi F, Tasciotti E and Re F:
Mesoporous silica nanoparticles trigger mitophagy in endothelial
cells and perturb neuronal network activity in a size- and
time-dependent manner. Int J Nanomedicine. 12:3547–3559.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Matsumura Y and Ananthaswamy HN: Toxic
effects of ultraviolet radiation on the skin. Toxicol Appl
Pharmacol. 195:298–308. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: Physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Song H, Zhao H, Yang L, Li L, Zhang T, Pan
J, Meng Y, Shen W and Yuan Y: Achyranthes bidentata
polypeptides promotes migration of Schwann cells via
NOX4/DUOX2-dependent ROS production in rats. Neurosci Lett.
696:99–107. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Luo J, Tang M, Huang J, He BC, Gao JL,
Chen L, Zuo GW, Zhang W, Luo Q, Shi Q, et al: TGFbeta/BMP type I
receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic
signaling in mesenchymal stem cells. J Biol Chem. 285:29588–29598.
2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Caldefie-Chézet F, Walrand S, Moinard C,
Tridon A, Chassagne J and Vasson MP: Is the neutrophil reactive
oxygen species production measured by luminol and lucigenin
chemiluminescence intra or extracellular? Comparison with DCFH-DA
flow cytometry and cytochrome c reduction. Clin Chim Acta.
319:9–17. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ko EY, Cho SH, Kwon SH, Eom CY, Jeong MS,
Lee W, Kim SY, Heo SJ, Ahn G, Lee KP, et al: The roles of NF-κB and
ROS in regulation of pro-inflammatory mediators of inflammation
induction in LPS-stimulated zebrafish embryos. Fish Shellfish
Immunol. 68:525–529. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hsieh HY, Lee WC, Senadi GC, Hu WP, Liang
JJ, Tsai TR, Chou YW, Kuo KK, Chen CY and Wang JJ: Discovery,
synthetic methodology, and biological evaluation for antiphotoaging
activity of bicyclic[1,2,3]triazoles: In vitro and in vivo studies.
J Med Chem. 56:5422–5435. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wlaschek M, Tantcheva-Poór I, Naderi L, Ma
W, Schneider LA, Razi-Wolf Z, Schüller J and Scharffetter-Kochanek
K: Solar UV irradiation and dermal photoaging. J Photochem
Photobiol B. 63:41–51. 2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhuang Y and Lyga J: Inflammaging in skin
and other tissues-the roles of complement system and macrophage.
Inflamm Allergy Drug Targets. 13:153–161. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fulop T, Larbi A, Dupuis G, Le Page A,
Frost EH, Cohen AA, Witkowski JM and Franceschi C: Immunosenescence
and inflamm-aging as two sides of the same coin: Friends or foes?
Front Immunol. 8(1960)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
PLOS ONE Editors. Retraction: Anti-wrinkle
effect of magnesium lithospermate B from salvia miltiorrhiza BUNGE:
Inhibition of MMPs via NF-κB signaling. PLoS One.
14(e0216473)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Staniforth V, Huang WC, Aravindaram K and
Yang NS: Ferulic acid, a phenolic phytochemical, inhibits
UVB-induced matrix metalloproteinases in mouse skin via
posttranslational mechanisms. J Nutr Biochem. 23:443–451.
2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Buvelot H, Jaquet V and Krause KH:
Mammalian NADPH oxidases. Methods Mol Biol. 1982:17–36.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kang KA, Ryu YS, Piao MJ, Shilnikova K,
Kang HK, Yi JM, Boulanger M, Paolillo R, Bossis G, Yoon SY, et al:
DUOX2-mediated production of reactive oxygen species induces
epithelial mesenchymal transition in 5-fluorouracil resistant human
colon cancer cells. Redox Biol. 17:224–235. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wu Y, Konaté MM, Lu J and Makhlouf H: IL-4
and IL-17A cooperatively promote hydrogen peroxide production,
oxidative DNA damage, and upregulation of dual oxidase 2 in human
colon and pancreatic cancer cells. J Immunol. 203:2532–2544.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ewald CY: Redox signaling of NADPH
oxidases regulates oxidative stress responses, immunity and aging.
Antioxidants (Basel). 7(130)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Little AC, Sulovari A, Danyal K, Heppner
DE, Seward DJ and van der Vliet A: Paradoxical roles of dual
oxidases in cancer biology. Free Radic Biol Med. 110:117–132.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Harper RW, Xu C, Eiserich JP, Chen Y, Kao
CY, Thai P, Setiadi H and Wu R: Differential regulation of dual
NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2
cytokines in respiratory tract epithelium. FEBS Lett.
579:4911–4917. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Moldogazieva NT, Mokhosoev IM, Feldman NB
and Lutsenko SV: ROS and RNS signalling: Adaptive redox switches
through oxidative/nitrosative protein modifications. Free Radic
Res. 52:507–543. 2018.PubMed/NCBI View Article : Google Scholar
|