Introduction
Vitiligo is an acquired depigmentation disorder
caused by the progressive destruction of melanocytes (1). Vitiligo pathogenesis involves defects
that are intrinsic to melanocytes and the autoimmune targeting of
these cells (2). Although cytotoxic
CD8+ T cells serve as the effector arm in autoimmunity,
CD4+ T helper cells and relevant cytokines appear to
play important roles in the development of vitiligo (3).
Interleukin (IL) 17A is primarily expressed by the T
helper (Th) 17 subset of CD4+ T cells, which is
characterized by the expression of retinoic acid receptor-related
orphan receptor (ROR) A and RORC genes (4). IL22 is an IL10-family cytokine that is
produced by Th17, γδ T, natural killer T (NKT), innate lymphoid and
Th22 cells (5). Recent studies have
shown the participation of IL17A and IL22 in the development of
vitiligo (6-9).
Studies have also demonstrated that systemic, tissue and cellular
levels of IL17A and IL22 are elevated in vitiligo (10,11).
Although IL17A is widely accepted to be involved in the
pathogenesis of vitiligo, its role in the disease remains
controversial. IL22 has opposing effects, from pro-inflammatory to
protective, but it has been proposed that in acute inflammation
IL22 is protective, while in more chronic settings it is pathogenic
(12-14).
Together, these data imply that modulation of IL17A and IL22 is
beneficial in the treatment of progressive vitiligo and that these
cytokines may represent an effective therapeutic target.
The aryl hydrocarbon receptor (AhR) is a cytosolic
transcription factor expressed in many different cell types and is
a member of the basic helix-loop-helix/Per-Arnt-Sim family
(15). AhR activation, nuclear
translocation and formation of a complex with the AhR nuclear
translocator complex results in the regulation of a number of
target genes (15). AhR activation
is involved in multiple biological processes, including immune
response, endocrine secretion and metabolism of low
molecular-weight chemicals (16).
Previous studies by the authors of the current study have shown
that AhR activation is involved in immune dysregulation in
CD4+ T cells (17) and
inflammation in keratinocytes (18).
In the past decade, the role of AhR in the
pathogenesis of vitiligo has generated significant interest.
Studies have demonstrated that AhR expression is lower in vitiligo
lesions compared with normal skin (19,20).
Therefore, AhR activation has been suggested as a potential therapy
for vitiligo (21). The traditional
herbal supplement Ginkgo biloba (G. biloba) is used for its
purported health benefits. It has been suggested to have beneficial
effects on senile dementia, peripheral arterial occlusive disease
and on various neurosensory disturbances (22). There are multiple reports of
promising results for G. biloba in the treatment of vitiligo
(23-26).
EGb 761 is a standardized extract of G. biloba leaves, with
22-27% flavonoid glycosides (primarily quercetin) and 6% terpene
lactones (2.8-3.4% ginkgolides A, B and C and 2.6-3.2% bilobalide)
(27). However, whether G.
biloba extract can modulate the AhR pathway and, therefore, the
production of IL17A and IL22 in CD4+ T cells in vitiligo
remains to be elucidated.
The aim of the present study was to investigate the
expression of AhR and its role in the regulation of IL17A and IL22
in CD4+ T cells of patients with vitiligo. The effects
of G. biloba extract on AhR activation and the expression of
IL17A and IL22 in CD4+ T cells from patients with
vitiligo were also studied.
Materials and methods
Subjects
Based on the revised vitiligo classification
(1), 20 patients with progressive,
unstable vitiligo (age, 16-58 years; mean age, 23 years; 14 females
and 6 males) and 20 age- and sex-matched healthy controls (age,
18-60 years; mean age, 27 years; 14 females and 6 males) were
recruited from patients at the Department of Dermatology, Shanghai
General Hospital. Progressive, unstable disease was defined
arbitrarily as the appearance of new lesions in the preceding 3
months. Clinical and demographic data were collected; two
dermatologists reviewed all cases. Relevant clinical information
regarding the study subjects is presented in Tables I and SI. The study was approved by the Human
Ethics Committee of Shanghai Jiaotong University and written
informed consent was obtained from each subject.
 | Table IClinical characteristics of the
subjects. |
Table I
Clinical characteristics of the
subjects.
|
Characteristics | Vitiligo
patients | Healthy
controls |
|---|
| Number | 20 | 20 |
| Male/female
ratio | 6/14 | 6/14 |
| Age, years | 23.40±0.99 | 27.44±1.36 |
| Skin type, n
(%) | | |
|
III | 15(75) | 17(85) |
|
IV | 5(25) | 3(15) |
| BSA involved, n
(%) | | |
|
≤10% | 4(20) | N/A |
|
>10% | 16(80) | N/A |
| Mucosa involved, n
(%) | 10(50) | N/A |
| Hair involved, n
(%) | 6(30) | N/A |
Isolation, culture, and treatment of
CD4+ T cells
Blood samples (~15 ml) were collected from all
participants. CD4+ T cells were purified by negative
selection using RosetteSep Human CD4+ T Cell Enrichment
Cocktail (StemCell Technologies, Inc.) followed by density gradient
centrifugation using Histopaque (Sigma-Aldrich; Merck KGaA). The
purity of CD4+ T cells (>95%) was evaluated by flow
cytometry. Briefly, Peripheral blood mononuclear cells (PBMCs) were
washed twice with 200 µl ice-cold fluorescence-activated cell
sorting (FACS) buffer, centrifuged at 4˚C and 200 x g for 5 min and
fixed with 100 µl BD Cytofix/Cytoperm (BD Biosciences) for 20 min
at 4˚C. PBMCs were then washed twice and resuspended in 100 µl ice
cold FACS buffer containing 1% FITC-conjugated anti-human CD4
Antibody (cat. no. 60016FI, Clone OKT4; StemCell Technologies,
Inc.) and incubated at 4˚C for 30 min. PBMCs were again washed
twice, and CD4+ T cells were resuspended in 200 µl FACS
buffer. CD4+ T cell counts were performed by flow
cytometry (Fortessa; BD Biosciences). Data were analyzed with
FlowJo v10 (FlowJo LLC). Cells were cultured in X-VIVO 15 medium
(Lonza Group, Ltd.) supplemented with 10% human AB serum (Valley
Biomedical Products and Services, Inc.) at 37˚C under 5%
CO2. CD4+ T cells (1x106
cells/well) were cultured in 24-well plates with plate-bound
anti-CD3 (eBioscience; cat. no. 16-0037) and anti-CD28
(eBioscience; cat. no. 16-0289; both 2 µg/ml). Where indicated,
cells were treated with 7-aminoactinomycin D (7-AAD; BD
Biosciences), G. biloba extract EGb 761 (Dr. Willmar Schwabe
GmbH), AhR antagonist CH223191 (3 µM; Sigma-Aldrich; Merck KGaA) or
AhR agonist
[4-(3-chloro-phenyl)-pyrimidin-2-yl]-(4-trifluoromethyl-phenyl)-amine
VAF347 (50 nM; Sigma-Aldrich; Merck KGaA).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using the RNeasy Mini kit
(Qiagen, Inc.). RT was performed using the PrimeScript RT-PCR kit
according to the manufacturer's protocol (Takara Bio, Inc.). qPCR
was performed on an Mx3000P qPCR system (Agilent Technologies,
Inc.) using SYBR Premix Ex Taq (Takara Bio, Inc.). The following
thermocycling conditions were used for the qPCR: Initial
denaturation at 95˚C for 30 sec; 40 cycles of 95˚C for 5 sec and
60˚C for 20 sec. Target gene expression was normalized to the
housekeeping gene GAPDH and analyzed using the 2-ΔΔCq
method (28). The primer pairs used
for the qPCR are shown in Table
SII.
Western blot analysis
CD4+ T cells were lysed and protein was
extracted using the Complete Lysis-M reagent (Roche Applied
Science). Protein concentration was measured using a BCA Protein
Assay kit (Pierce; Thermo Fisher Scientific, Inc.). Equal amounts
of protein (20 µg) were dissolved in NuPage LDS Sample Buffer
(Invitrogen; Thermo Fisher Scientific, Inc.) and 10% NuPage Sample
Reducing Agent (Invitrogen; Thermo Fisher Scientific, Inc.).
Protein lysates were boiled at 70˚C for 10 min prior to loading.
Electrophoresis was conducted on 4-12% NuPage Bis-Tris gels
(Invitrogen; Thermo Fisher Scientific, Inc.) at 200 V for 40 min.
The separated proteins were transferred onto PVDF membranes
(Invitrogen; Thermo Fisher Scientific, Inc.) and blocked in TBS
containing 2% BSA (Sigma-Aldrich; Merck KGaA) and 0.1% Tween-20
(Sigma-Aldrich; Merck KGaA). Membranes were probed with the
following primary antibodies: Anti-AhR mouse monoclonal IgG
antibody (1:1,000; cat. no. ab2769; Abcam), anti-cytochrome P450
1A1 (CYP1A1) rabbit polyclonal IgG antibody (1:500; cat. no.
ab3568; Abcam) or anti-GAPDH rabbit IgG antibody (1:50; cat. no.
FL-335; Santa Cruz Biotechnology, Inc.) overnight at 4˚C. Following
primary incubation, membranes were incubated with anti-rabbit
(1:5,000; cat. no. ab205718; Abcam) or anti-mouse (1:5,000; cat.
no. ab205719, Abcam) horseradish peroxidase-conjugated IgG
secondary antibodies for 2 h at room temperature. Protein bands
were visualized using the WesternBreeze Chemiluminescent kit
(Invitrogen; Thermo Fisher Scientific, Inc.).
Immunohistochemistry
Skin tissue specimens were obtained from the
biopsies of lesion and nonlesion areas from vitiligo patients.
Normal skin tissue specimens were obtained from healthy volunteers.
All skin tissue samples were fixed with 10% buffered formalin for 1
day at room temperature. The paraffin-embedded tissue blocks were
cut into 4-µm tissue sections, deparaffinized using xylene for 10
min, rehydrated through a graded series of ethanol, followed by
blocking of endogenous peroxidase activity in 0.3%
H2O2 in methanol for 30 min at room
temperature. Antibody-binding epitopes were retrieved by
pressure-cooking the tissue sections in 10 mmol/l sodium citrate
buffer (pH 7.0; LSI Medience Corporation) for 10 min. Non-specific
binding was blocked using 10% goat serum (cat. no. 50062Z; Thermo
Fisher Scientific, Inc.) for 10 min at room temperature. The
sections were then incubated with anti-AhR antibody (1:100; cat.
no. ab2769; Abcam), anti-CD4 antibody (1:100; cat. no. ab67001;
Abcam), anti-IL17A antibody (1:100; cat. no. ab189377; Abcam) or
anti-IL22 antibody (1:100; cat. no. ab134035; Abcam). Biotinylated
anti-mouse antibodies (1:200; cat.no. SP KIT-C3, Fuzhou Maixin
Biotech Co., Ltd.) were applied for 15 min in a humidified chamber
at room temperature. A DAB kit (DAB-0031; Fuzhou Maixin
Biotechnology Development Co., Ltd.) was used to generate
chromogenic signals. PBS was used as the negative control.
Quantification
Quantification of immunohistochemical staining and
western blotting bands was performed using Image J software
(version 1.48 v; National Institutes of Health). For evaluation of
immunohistochemical staining, the epidermis was selected as the
region of interest (ROI) and the staining intensity of the ROI was
quantified. The number of cells in the ROI was then counted. The
relative staining intensity was defined as the intensity of 100
cells and calculated using the formula: Relative staining intensity
(arbitrary units) = intensity of ROI/cell number x 100.
ELISA
IL17A and IL22 in serum and culture supernatants
were measured using commercial immunoassay kits (cat. nos. ab83688
and ab119543; Abcam) according to the manufacturer's
instructions.
Small interfering RNA (siRNA)
transfection
Transfection with AhR-targeted specific siRNA was
performed as previously described (13). Briefly, siRNA targeted against AhR
(si-AhR; cat. no. s1200) and siRNA consisting of a scrambled
sequence (si-control; cat. no. AM4611) were purchased from Ambion
(Thermo Fisher Scientific, Inc.). CD4+ T cells cultured
in 24-well plates were incubated with 3 ml HiPerFect Transfection
Reagent (Qiagen SAS) containing 10 nM siRNA and 0.5 ml culture
medium. Following incubation for 48 h, siRNA-transfected
CD4+ T cells were treated as indicated. Transfection
showed no effect on cell viability, as demonstrated by microscopic
examination (data not shown).
Cell viability
The 7-AAD cell viability assay was performed as
previously described (29). Since
7-AAD is a fluorescent DNA intercalator that binds to double
stranded DNA, live cells with intact membranes are identified by
the exclusion of 7-AAD, which penetrates dead and damaged cells to
label DNA.
Statistical analysis
Data are presented as the mean ± SEM. Data were
analyzed using a Student's t-test or one-way ANOVA followed by
Tukey's or Dunnett's multiple comparison tests. Relationships were
determined by correlation analysis. All analyses were performed
using GraphPad Prism 5.0 (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Identification and purity of
CD4+ T cells
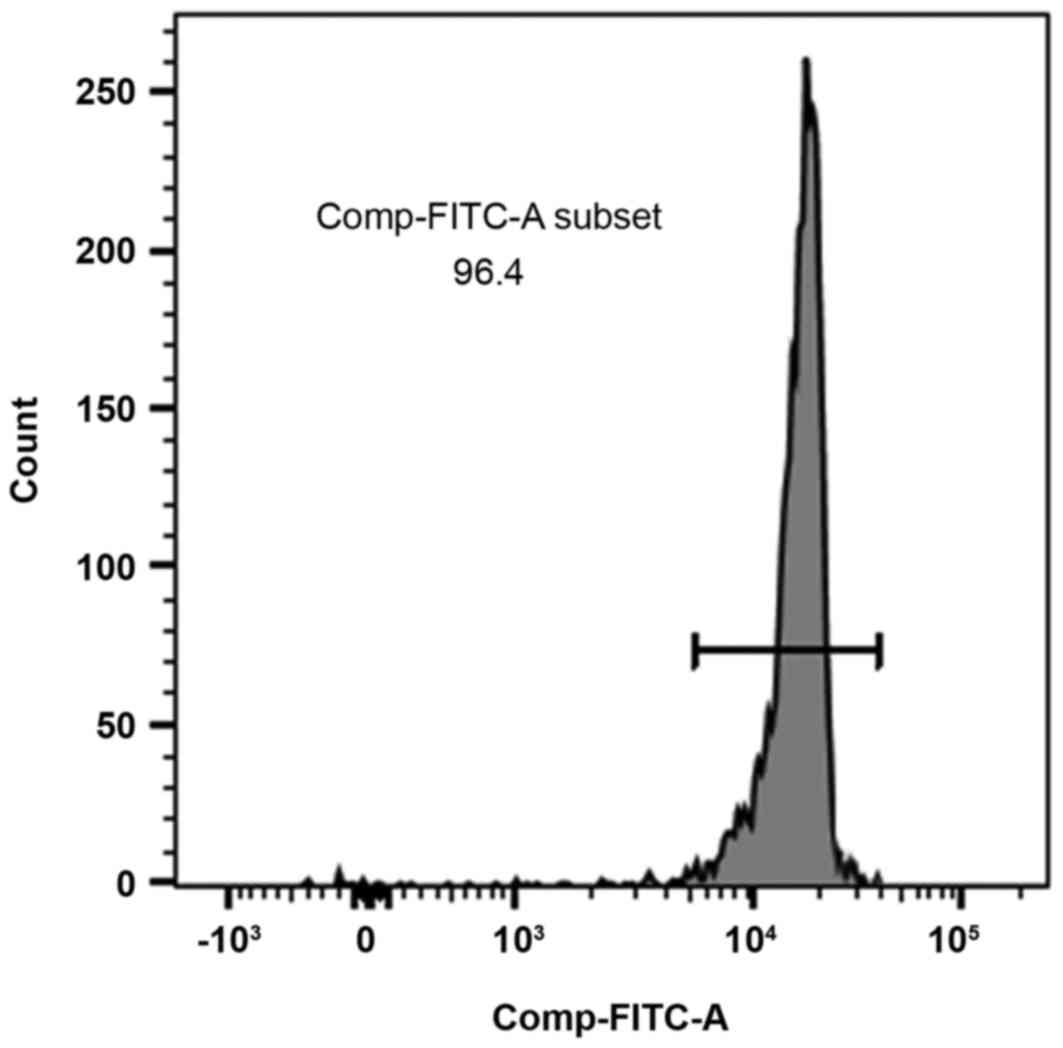
Flow cytometric analysis showed that the purity of
the CD4+ T cells was >95% (Fig. 1).
AhR expression is low in the
CD4+ T cells and the skin of patients with vitiligo
Since different reports have presented contradictory
results (30,31), the expression of AhR in patients
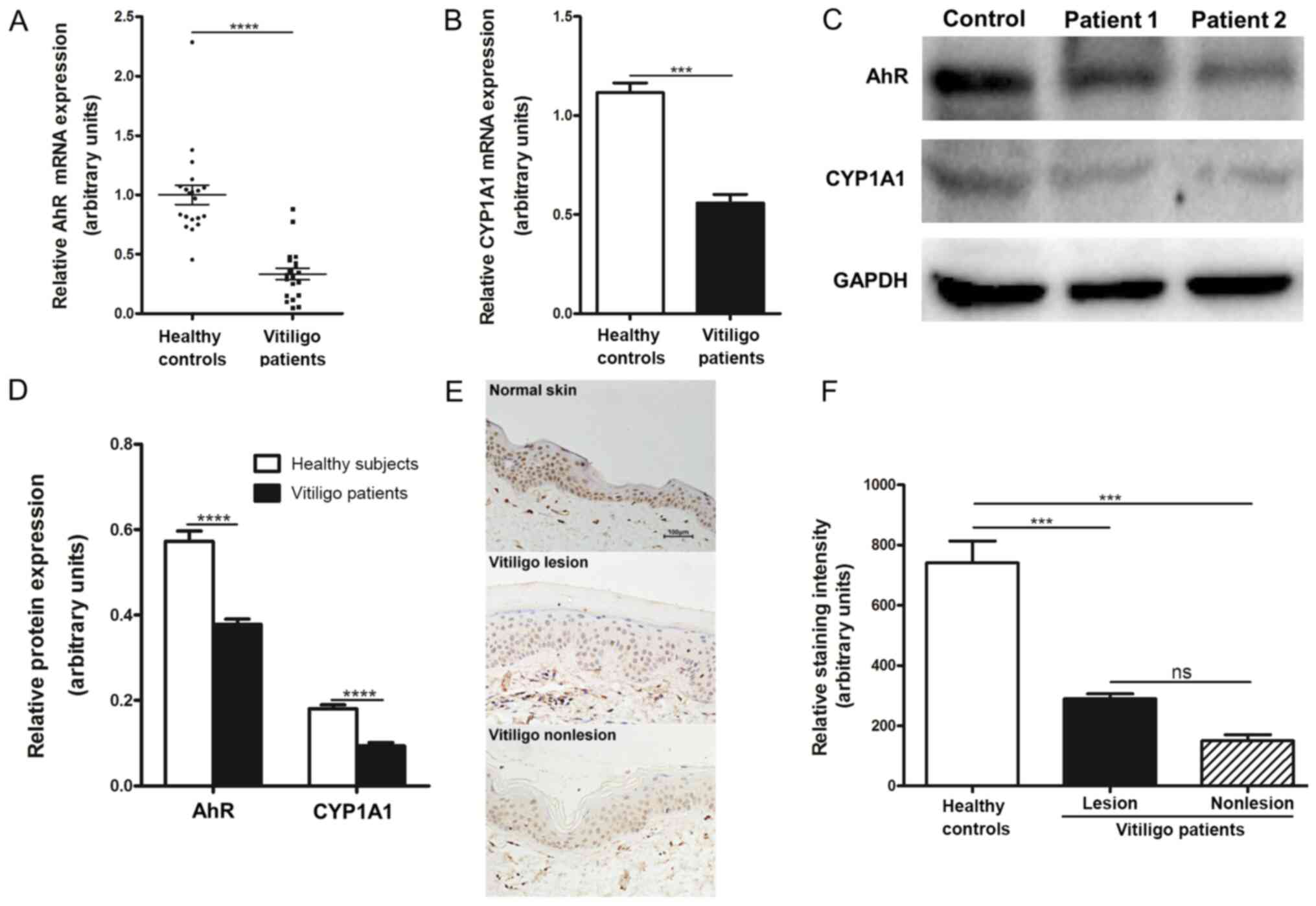
with vitiligo was assayed. AhR mRNA expression levels were
significantly lower in the CD4+ T cells of patients with
vitiligo compared with healthy subjects (Fig. 2A). To determine if the level of AhR
expression was indicative of functional AhR protein, the expression
of CYP1A1, a known AhR-regulated gene, was examined. Low expression
of AhR mRNA was associated with reduced CYP1A1 mRNA expression in
the CD4+ T cells of patients with vitiligo compared with
healthy controls (Fig. 2B).
Consistent with this observation, western blotting revealed that
the protein levels of AhR and CYP1A1 were significantly decreased
in the CD4+ T cells of patients with vitiligo compared
with healthy subjects (Fig. 2C and
D).
The expression of AhR in skin samples from patients
with vitiligo was subsequently investigated by immunohistochemical
analysis. Significantly lower levels of AhR protein in the
nonlesional and lesional skin of patients with vitiligo were found
compared with the normal skin of healthy subjects (Fig. 2E and F). Levels of AhR expression were markedly
lower in nonlesional skin compared with lesional skin in patients
with vitiligo. Collectively, these data suggested decreased
functionality of AhR in patients with vitiligo.
IL17A and IL22 levels are higher in
patients with vitiligo
Since T cell-mediated autoimmunity and altered
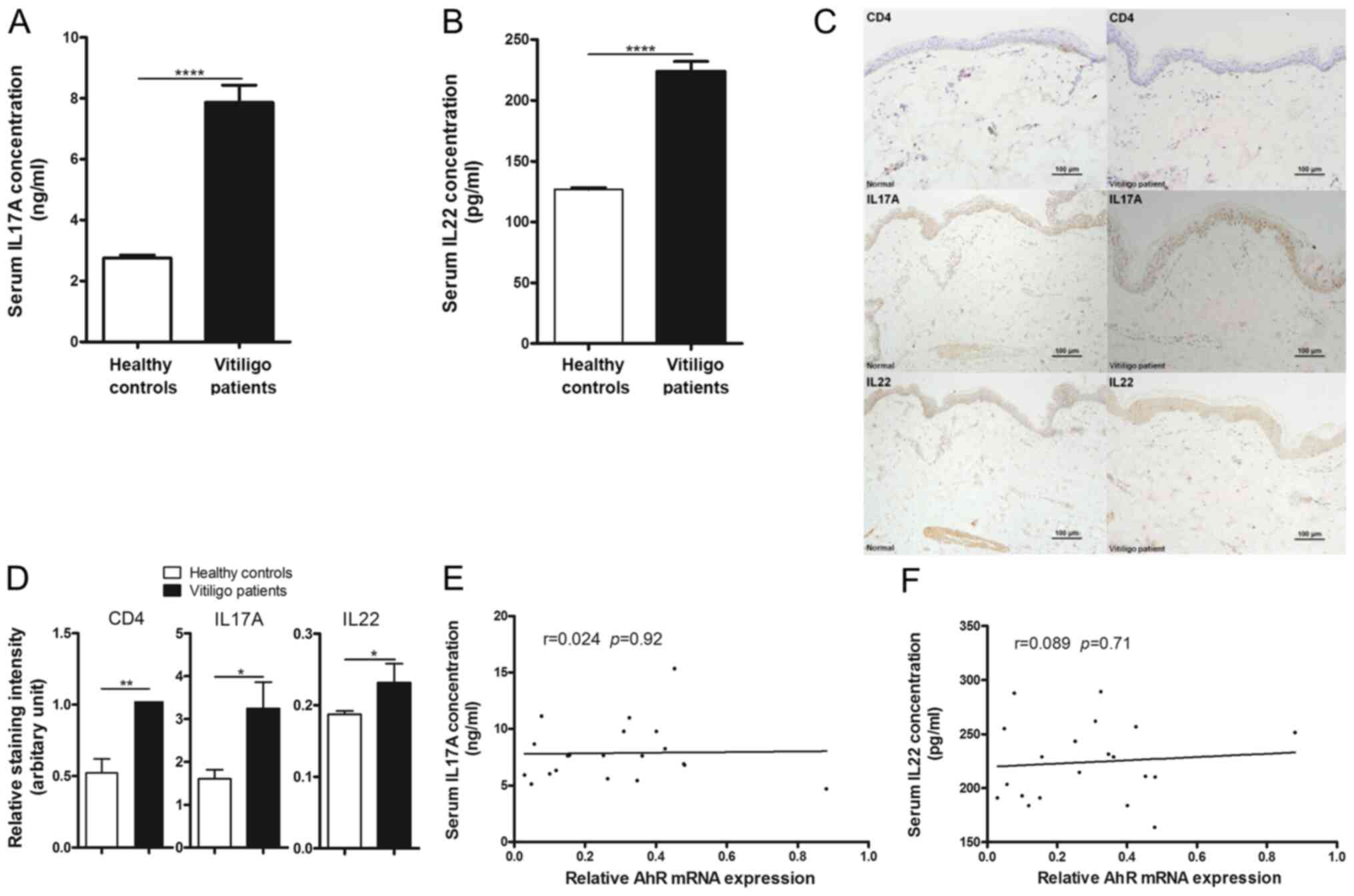
cytokines are involved in vitiligo pathogenesis, cytokine IL17A and
IL22 production in patients with vitiligo and healthy subjects were
investigated. Significantly higher concentrations of IL17A and IL22
were detected in the serum of patients with vitiligo compared with
healthy subjects (Fig. 3A and
B). Similarly, immunohistochemical
data showed that CD4+, IL17A and IL22 expression were
significantly increased in the skin tissues of patients with
vitiligo compared with healthy subjects (Fig. 3C and D).
No correlation was observed between AhR mRNA
expression in CD4+ T cells and serum levels of IL17A or
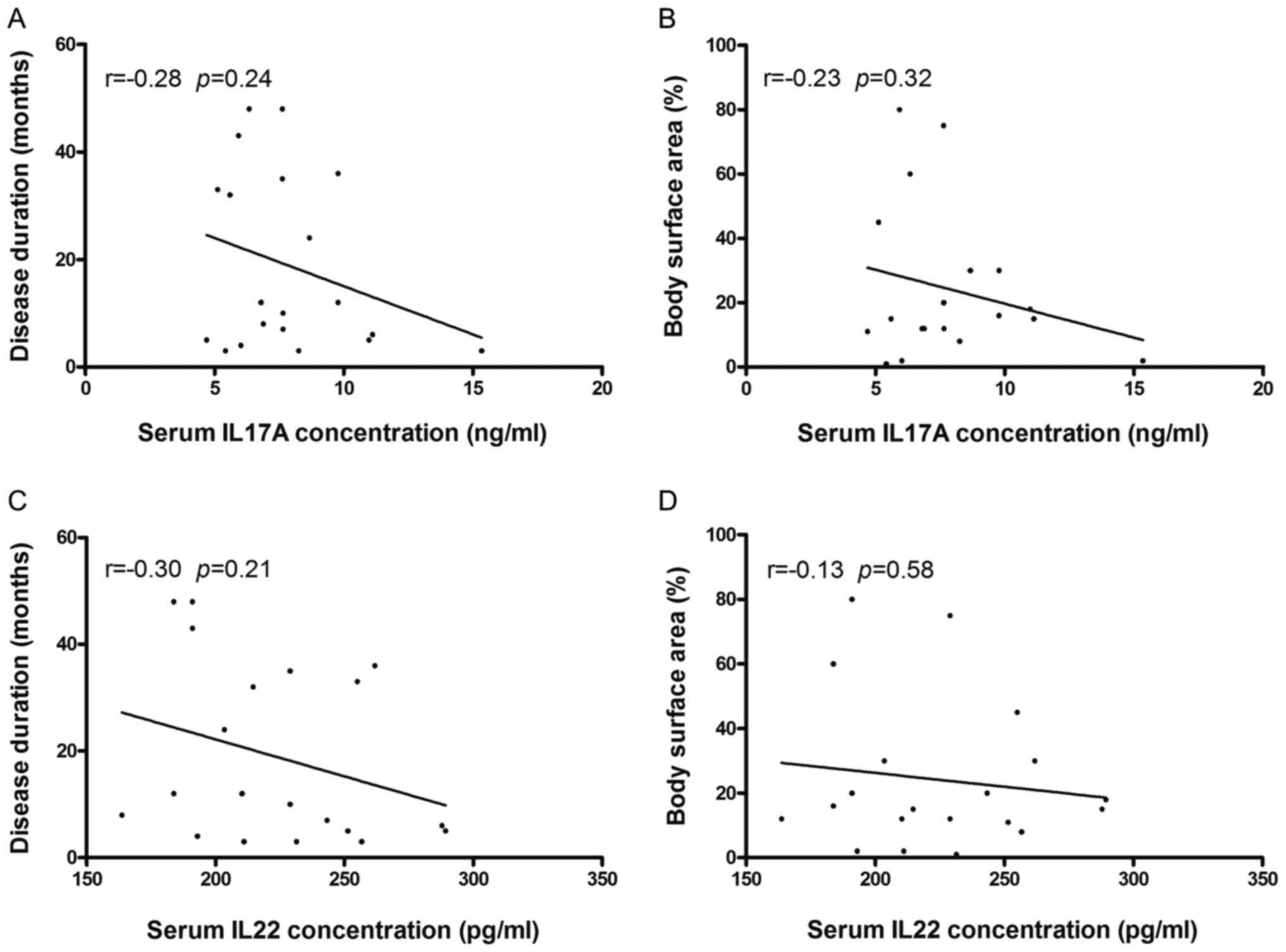
IL22 (Fig. 3E and F). Furthermore, no significant correlation
was observed between clinical manifestations (disease duration and
body surface area involvement) and the serum concentration of
either cytokine (Fig. 4).
AhR knockdown increases secretion of
IL17A, but not IL22, from CD4+ T cells of patients with
vitiligo
A previous study suggested that AhR expression plays
an essential role in Th17 subset development, and AhR deficiency
increased the expression of IL17A in AhR-/- mice
(32). Additionally, AhR has been
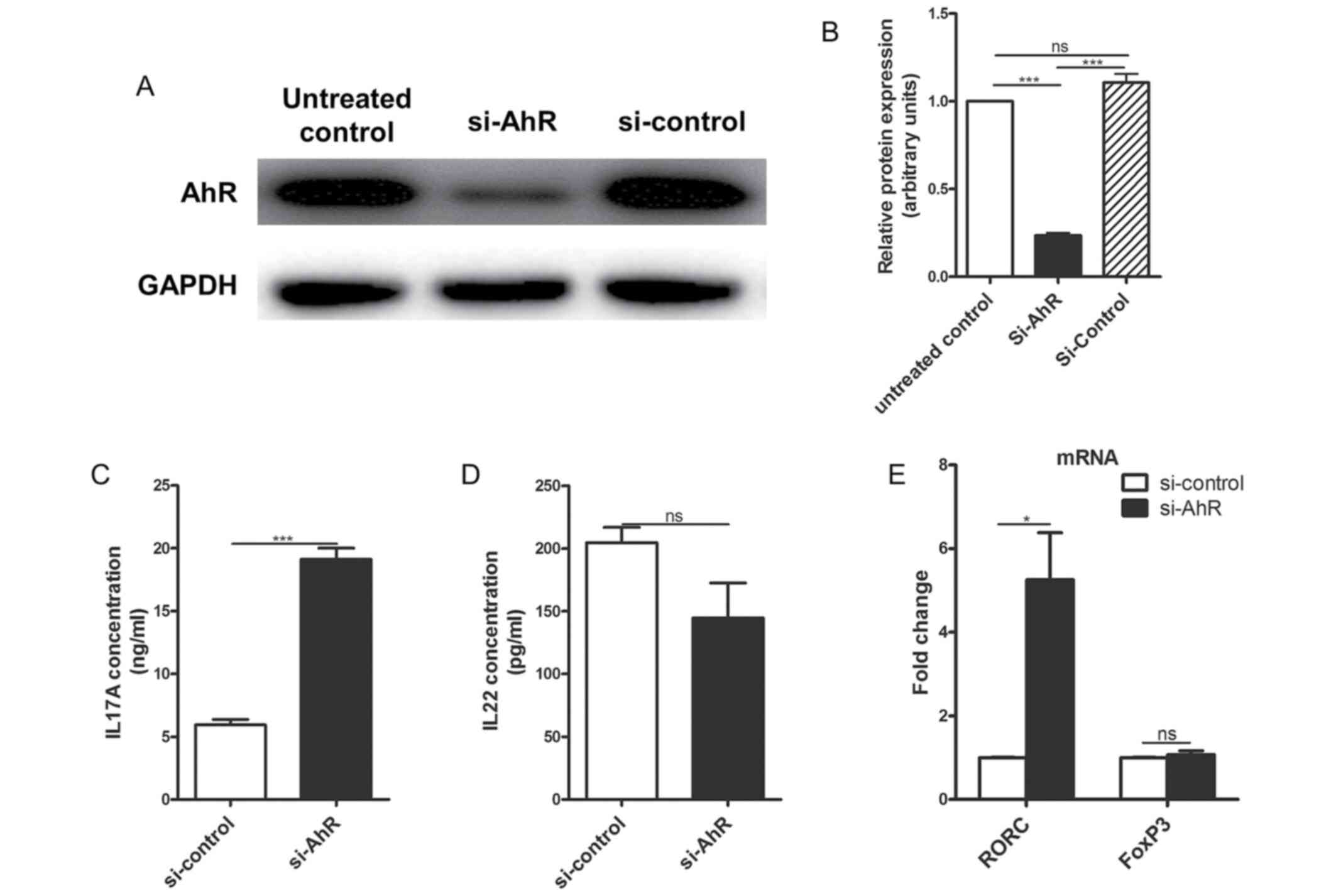
reported to be associated with Th22 subset development (33). Transfection of si-AhR in the
CD4+ T cells of patients with vitiligo reduced AhR
expression at the protein level compared with the non-transfected
and si-control transfected cells (Fig.
5A and B). Furthermore, IL17A
secretion by the si-AhR-transfected CD4+ T cells of
patients with vitiligo significantly increased after 72 h compared
with si-control-transfected cells (Fig.
5C). However, IL22 production was reduced, although not
significantly, after 72 h compared with si-control-transfected
cells (Fig. 5D).
The effects of AhR knockdown on the expression of
RORC and forkhead box protein P3 (FoxP3), which are transcription
factors for the Th17 and Treg subsets, respectively, were examined.
The mRNA expression of RORC was significantly upregulated in the
CD4+ T cells of patients with vitiligo 24 h following
transfection with si-AhR compared with si-control-transfected
cells, while the levels of FoxP3 mRNA expression were similar in
the CD4+ T cells of patients with vitiligo transfected
with si-AhR and si-control (Fig.
5E).
AhR activation by G
biloba extract EGb 761 reciprocally regulates
IL17A and IL22 production in CD4+ T cells of patients
with vitiligo. CD4+ T cells were treated with EGb 761 at
concentrations of 0-200 µg/ml for 24 h. The 7-AAD cell viability
assay indicated that EGb 761 was non-toxic at concentrations ≤100
µg/ml. By contrast, the toxicity of EGb 761 increased in a
concentration-dependent manner at concentrations >120 µg/ml
(data not shown).
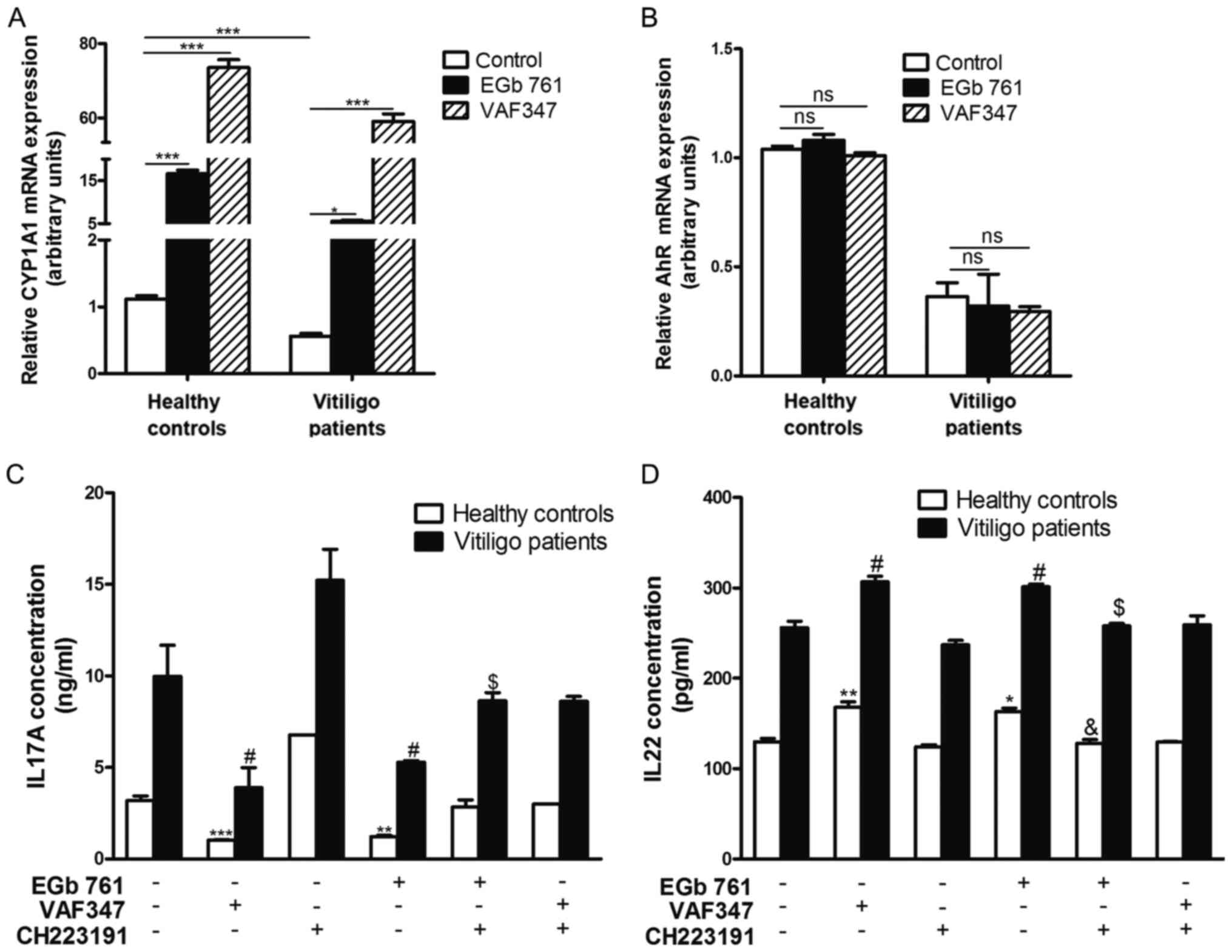
Next, the ability of 100 µg/ml EGb 761 to activate
AhR was assessed. VAF347, a well-established specific AhR agonist,
was used as a positive control to monitor the effects of AhR
activation. Following 6 h of EGb 761 or VAF347 treatment, an
increase in transcript abundance for CYP1A1 was observed in the
CD4+ T cells from both patients with vitiligo and
healthy subjects compared with controls (Fig. 6A). However, the expression of CYP2E1
mRNA, which is not an AhR target gene, was unaffected (data not
shown). The addition of EGb 761 did not influence AhR mRNA
expression in cells either from patients with vitiligo or healthy
subjects compared with controls (Fig.
6B).
The effects of AhR activation on the
production of IL17A and IL22 were then evaluated
CD4+ T cells from patients with vitiligo
and healthy subjects were treated with EGb 761 or VAF347 for 72 h,
followed by measurement of IL17A and IL22 secretion by ELISA. As
shown in Fig. 6C, treatment with
EGb 761 decreased IL17A production by >60% in the
CD4+ T cells of healthy subjects, but only by ~45% in
those patients with vitiligo, compared with the untreated
CD4+ T cells.
Treatment with the AhR antagonist
CH223191 suppressed the inhibitory effects of EGb 761 on IL17A
production
Basal IL22 production was not affected by CH223191
treatment (Fig. 6D). EGb
761-induced IL22 production increased by ~25% in the
CD4+ T cells of healthy subjects and by ~18% in those of
patients with vitiligo, compared with the untreated CD4+
T cells. CH223191 inhibited the upregulation of IL22 induced by EGb
761 in the CD4+ T cells of both patients with vitiligo
and healthy subjects. These findings suggested that AhR activation
by G. biloba extract EGb 761 regulated IL17A and IL22
production in CD4+ T cells from patients with
vitiligo.
Discussion
Recent reports have implicated AhR as an important
factor in the pathogenesis of vitiligo. Consistent with Wang et
al (31), the present data
showed that AhR transcript levels were significantly lower in
patients with vitiligo compared with controls. By contrast,
Behfarjam and Jadali (30) reported
that AhR mRNA levels were significantly elevated in PBMCs of
patients with vitiligo; this difference might be explained by the
fact that patients with inactive vitiligo were examined in the
latter study. It was previously reported that epidermal AhR
expression is significantly decreased in patients with vitiligo
(19,20), consistent with the present results.
The present study demonstrated that AhR protein expression was
significantly lower in the lesional and nonlesional skin of
patients with vitiligo compared with the normal skin of healthy
controls. Furthermore, AhR expression was markedly higher in
lesional compared with nonlesional skin of patients with vitiligo,
which might represent a feedback response to counteract
inflammation (19). Collectively,
the results of the aforementioned in vivo studies suggest
that an intrinsic defect in AhR expression could constitute a major
susceptibility factor for the development of vitiligo.
IL17A and IL22 play roles in various autoimmune
diseases (11,34). IL17A is widely accepted to be
involved in the pathogenesis of several autoimmune diseases, such
as psoriasis and vitiligo, whereas IL22 has been reported to be
protective in acute inflammation, such as in protecting the
intestine from viral infection in an experimental animal disease
model, and pathogenic in chronic settings, such as in atopic
dermatitis and rheumatoid arthritis (11-14).
There are conflicting reports regarding their expression levels in
vitiligo. In line with Elela et al (10), the present study showed that the
serum concentrations of IL17A and IL22 were significantly higher in
patients with vitiligo compared with healthy controls. By contrast,
other studies reported no difference in the serum concentrations of
IL17A and IL22 in vitiligo (19,31).
These discrepancies may be explained by the dependence of
inflammatory cytokine secretion on disease status, as both
progressive and stable patients were included in previous studies
(19,31), while only progressive patients were
assessed in the present study. No obvious expression of either
cytokine was detected in skin by immunohistochemistry (data not
shown), which is inconsistent with results from a previous study
(10). This might be explained by
very mild skin inflammation in the patients with vitiligo in the
present study.
AhR mRNA expression did not exhibit a correlation
with the serum levels of either cytokine. These findings suggest
that CD4+ T cells might not be the only source of serum
IL17A and IL22 in patients with vitiligo, since both cytokines can
be produced by a variety of cells, including NK and γδ T cells
(5,35). Although several studies have
reported that serum IL17A levels are positively correlated with
vitiligo duration and the extent of body area involvement (36,37),
no such correlation was noted in the present study. There are two
potential explanations for these differences. First, the sample
size of the present study was small, with only 20 patients. Second,
all patients in the present study had active disease, which is
significant as it has been reported that IL17A may be better
correlated with active disease than with other clinical
manifestations (38).
In the present study, the suppression of AhR
expression in cultured CD4+ T cells from patients with
vitiligo by AhR-targeted siRNA treatment resulted in a significant
increase in IL17A transcript levels compared with those in
si-control-transfected cells. AhR knockdown also increased the
expression levels of mRNA encoding RORC, the master transcription
factor for Th17 differentiation, but not FoxP3, consistent with a
previous report (32). The
inhibitory effect of AhR knockdown on IL22 transcript abundance was
incomplete in CD4+ T cells from patients with vitiligo,
suggesting the involvement of additional mechanisms. IL22
production has previously been reported to be regulated by
mechanisms involving both AhR-dependent and -independent pathways
(39).
A previous study demonstrated that AhR activation
might be beneficial for the treatment of vitiligo (21). In the present study, treatment of
CD4+ T cells with G. biloba extract EGb 761
activated AhR. This is consistent with findings by Rajaraman et
al (40), which showed that
G. biloba extract activated AhR in MCF-10A human mammary
epithelial cells. EGb 761 primarily contains flavonoid glycosides
(27). Different flavonoids have
different affinities for AhR and the strength of AhR modulation is
dependent on their structures (41). Quercetin, the primary flavonoid
glycoside in EGb 761, is a well-established activator of AhR
(41). Both the expression levels
and activation of AhR have been reported to have important roles in
modulating biological processes. For example, deficiency of AhR
increases the expression of IL17A in CD4+ T cells form
AhR-/- mice (32), and
TCDD, which does not alter AhR expression, has been reported to
affect CD4+ T cell differentiation through activating
AhR (42). In the present study,
AhR mRNA expression was not influenced by EGb 761 treatment in the
present study. Thus, EGb 761 may be hypothesized to exert its
biological function through activating AhR, not by modulating its
expression.
In the present study, EGb 761 inhibited IL17A and
increased IL22 secretion in the CD4+ T cells from
patients with vitiligo and healthy controls. The extent of
regulation of either cytokine in patients with vitiligo was less
compared with that in healthy controls, which might be due to a
decrease in AhR functionality in the CD4+ T cells of
patients with vitiligo. The AhR antagonist CH223191 per se
did not significantly influence the expression of IL22. Plé et
al (39), showed that IL22
expression was reduced in the presence of CH223191, while Rohlman
et al (32), reported that
AhR deficiency had no significant effect on the expression of IL22.
These contradictory results might be associated with the different
cells used in the studies, since Plé et al (39) used PBMCs and IL22 is produced in a
variety of cells including Th17, γδ T, NKT, innate lymphoid and
Th22 cells (5). IL22 expression is
regulated by AhR-independent pathways (39). The changes in IL17A and IL22
secretion induced by EGb 761 in the present study were nearly
suppressed by CH223191, suggesting specific regulation of these two
cytokines by AhR, at least in CD4+ T cells responding to
EGb 761.
In conclusion, the present study demonstrated that
AhR expression is significantly reduced in CD4+ T cells
of patients with vitiligo, and that activation of AhR by G.
biloba extract EGb 761 reciprocally regulates IL17A and IL22
production in CD4+ T cells from patients with
progressive unstable vitiligo, although the exact mechanisms merit
further investigation. The present data also support the hypothesis
that AhR may be a potential therapeutic target for the treatment of
vitiligo.
Supplementary Material
Information for disease duration,
onset age and body surface area of vitiligo patients.
Sequences of primers used for reverse
transcriptionquantitative PCR.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81773310).
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
BL, YX, XM and YS performed the experiments and
analysed the data. BL, YX and XM wrote the manuscript. YS, WS and
ZW designed and supervised the study. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Human Ethics Committee
of Shanghai Jiaotong University. Written informed consent was
obtained from each subject.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ezzedine K, Lim HW, Suzuki T, Katayama I,
Hamzavi I, Lan CC, Goh BK, Anbar T, Silva de Castro C, Lee AY, et
al: Vitiligo Global Issue Consensus Conference Panelists. Revised
classification/nomenclature of vitiligo and related issues: The
Vitiligo Global Issues Consensus Conference. Pigment Cell Melanoma
Res. 25:E1–E13. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Alikhan A, Felsten LM, Daly M and
Petronic-Rosic V: Vitiligo: A comprehensive overview Part I.
Introduction, epidemiology, quality of life, diagnosis,
differential diagnosis, associations, histopathology, etiology, and
work-up. J Am Acad Dermatol. 65:473–491. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rodrigues M, Ezzedine K, Hamzavi I, Pandya
AG and Harris JE: Vitiligo Working Group. New discoveries in the
pathogenesis and classification of vitiligo. J Am Acad Dermatol.
77:1–13. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yang XO, Pappu BP, Nurieva R, Akimzhanov
A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et
al: T helper 17 lineage differentiation is programmed by orphan
nuclear receptors ROR alpha and ROR gamma. Immunity. 28:29–39.
2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang L, Li YG, Li YH, Qi L, Liu XG, Yuan
CZ, Hu NW, Ma DX, Li ZF, Yang Q, et al: Increased frequencies of
Th22 cells as well as Th17 cells in the peripheral blood of
patients with ankylosing spondylitis and rheumatoid arthritis. PLoS
One. 7(e31000)2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Behfarjam F, Mansouri P and Jadali Z:
Imbalance of Peripheral Blood T Helper Type 17 Responses in
Patients with Vitiligo. Iran J Allergy Asthma Immunol. 17:171–178.
2018.PubMed/NCBI
|
|
7
|
Bhardwaj S, Rani S, Srivastava N, Kumar R
and Parsad D: Increased systemic and epidermal levels of IL-17A and
IL-1β promotes progression of non-segmental vitiligo. Cytokine.
91:153–161. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Czarnowicki T, He H, Leonard A, Kim HJ,
Kameyama N, Pavel AB, Li R, Estrada Y, Wen HC, Kimmel GW, et al:
Blood endotyping distinguishes the profile of vitiligo from that of
other inflammatory and autoimmune skin diseases. J Allergy Clin
Immunol. 143:2095–2107. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sushama S, Dixit N, Gautam RK, Arora P,
Khurana A and Anubhuti A: Cytokine profile (IL-2, IL-6, IL-17,
IL-22, and TNF-α) in vitiligo-New insight into pathogenesis of
disease. J Cosmet Dermatol. 18:337–341. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Elela MA, Hegazy RA, Fawzy MM, Rashed LA
and Rasheed H: Interleukin 17, interleukin 22 and FoxP3 expression
in tissue and serum of non-segmental vitiligo: A case- controlled
study on eighty-four patients. Eur J Dermatol. 23:350–355.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Singh RK, Lee KM, Vujkovic-Cvijin I, Ucmak
D, Farahnik B, Abrouk M, Nakamura M, Zhu TH, Bhutani T, Wei M, et
al: The role of IL-17 in vitiligo: A review. Autoimmun Rev.
15:397–404. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mattapallil MJ, Kielczewski JL,
Zárate-Bladés CR, St Leger AJ, Raychaudhuri K, Silver PB,
Jittayasothorn Y, Chan CC and Caspi RR: Interleukin 22 ameliorates
neuropathology and protects from central nervous system
autoimmunity. J Autoimmun. 102:65–76. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Neil JA, Matsuzawa-Ishimoto Y,
Kernbauer-Hölzl E, Schuster SL, Sota S, Venzon M, Dallari S, Galvao
Neto A, Hine A, Hudesman D, et al: IFN-I and IL-22 mediate
protective effects of intestinal viral infection. Nat Microbiol.
4:1737–1749. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zenewicz LA: IL-22: There is a gap in our
knowledge. Immunohorizons. 2:198–207. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hahn ME: Aryl hydrocarbon receptors:
Diversity and evolution. Chem Biol Interact. 141:131–160.
2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Esser C, Rannug A and Stockinger B: The
aryl hydrocarbon receptor in immunity. Trends Immunol. 30:447–454.
2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wu Z, Mei X, Ying Z, Sun Y, Song J and Shi
W: Ultraviolet B inhibition of DNMT1 activity via AhR activation
dependent SIRT1 suppression in CD4+ T cells from systemic lupus
erythematosus patients. J Dermatol Sci. 86:230–237. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wu Z, Uchi H, Morino-Koga S,
Nakamura-Satomura A, Kita K, Shi W and Furue M: Z-Ligustilide
inhibits benzo(a)pyrene-induced CYP1A1 upregulation in cultured
human keratinocytes via ROS-dependent Nrf2 activation. Exp
Dermatol. 23:260–265. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rekik R, Ben Hmid A, Lajnef C, Zamali I,
Zaraa I and Ben Ahmed M: Aryl hydrocarbon receptor (AhR)
transcription is decreased in skin of vitiligo patients. Int J
Dermatol. 56:1509–1512. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Schallreuter KU, Salem MA, Gibbons NC,
Maitland DJ, Marsch E, Elwary SM and Healey AR: Blunted epidermal
L-tryptophan metabolism in vitiligo affects immune response and ROS
scavenging by Fenton chemistry, part 2: Epidermal
H2O2/ONOO(-)-mediated stress in vitiligo hampers indoleamine
2,3-dioxygenase and aryl hydrocarbon receptor-mediated immune
response signaling. FASEB J. 26:2471–2485. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tsuji G, Hashimoto-Hachiya A, Takemura M,
Kanemaru T, Ichihashi M and Furue M: Palladium and platinum
nanoparticles activate AHR and NRF2 in human
keratinocytes-implications in vitiligo therapy. J Invest Dermatol.
137:1582–1586. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
DeFeudis FV: A brief history of EGb 761
and its therapeutic uses. Pharmacopsychiatry. 36 (Suppl 1):S2–S7.
2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Parsad D, Pandhi R and Juneja A:
Effectiveness of oral Ginkgo biloba in treating limited,
slowly spreading vitiligo. Clin Exp Dermatol. 28:285–287.
2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Szczurko O and Boon HS: A systematic
review of natural health product treatment for vitiligo. BMC
Dermatol. 8(2)2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Szczurko O, Shear N, Taddio A and Boon H:
Ginkgo biloba for the treatment of vitilgo vulgaris: An open
label pilot clinical trial. BMC Complement Altern Med.
11(21)2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Whitton ME, Ashcroft DM and González U:
Therapeutic interventions for vitiligo. J Am Acad Dermatol.
59:713–717. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Koltermann A, Hartkorn A, Koch E, Fürst R,
Vollmar AM and Zahler S: Ginkgo biloba extract EGb 761
increases endothelial nitric oxide production in vitro and in vivo.
Cell Mol Life Sci. 64:1715–1722. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wu Z, Uchi H, Morino-Koga S, Shi W and
Furue M: Z-ligustilide ameliorated ultraviolet B-induced oxidative
stress and inflammatory cytokine production in human keratinocytes
through upregulation of Nrf2/HO-1 and suppression of NF-κB pathway.
Exp Dermatol. 24:703–708. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Behfarjam F and Jadali Z: Vitiligo
patients show significant up-regulation of aryl hydrocarbon
receptor transcription factor. An Bras Dermatol. 93:302–303.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang X, Li K, Liu L, Shi Q, Song P, Jian
Z, Guo S, Wang G, Li C and Gao T: AHR promoter variant modulates
its transcription and downstream effectors by allele-specific
AHR-SP1 interaction functioning as a genetic marker for vitiligo.
Sci Rep. 5(13542)2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rohlman D, Pham D, Yu Z, Steppan LB and
Kerkvliet NI: Aryl Hydrocarbon Receptor-Mediated Perturbations in
Gene Expression during Early Stages of CD4(+) T-cell
Differentiation. Front Immunol. 3(223)2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Alam MS, Maekawa Y, Kitamura A, Tanigaki
K, Yoshimoto T, Kishihara K and Yasutomo K: Notch signaling drives
IL-22 secretion in CD4+ T cells by stimulating the aryl hydrocarbon
receptor. Proc Natl Acad Sci USA. 107:5943–5948. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Azizi G, Yazdani R and Mirshafiey A: Th22
cells in autoimmunity: A review of current knowledge. Eur Ann
Allergy Clin Immunol. 47:108–117. 2015.PubMed/NCBI
|
|
35
|
Benghiat FS, Charbonnier LM, Vokaer B, De
Wilde V and Le Moine A: Interleukin 17-producing T helper cells in
alloimmunity. Transplant Rev (Orlando). 23:11–18. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Basak PY, Adiloglu AK, Ceyhan AM, Tas T
and Akkaya VB: The role of helper and regulatory T cells in the
pathogenesis of vitiligo. J Am Acad Dermatol. 60:256–260.
2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bassiouny DA and Shaker O: Role of
interleukin-17 in the pathogenesis of vitiligo. Clin Exp Dermatol.
36:292–297. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tembhre MK, Sharma VK, Sharma A,
Chattopadhyay P and Gupta S: T helper and regulatory T cell
cytokine profile in active, stable and narrow band ultraviolet B
treated generalized vitiligo. Clin Chim Acta. 424:27–32.
2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Plé C, Fan Y, Ait Yahia S, Vorng H,
Everaere L, Chenivesse C, Balsamelli J, Azzaoui I, de Nadai P,
Wallaert B, et al: Polycyclic aromatic hydrocarbons reciprocally
regulate IL-22 and IL-17 cytokines in peripheral blood mononuclear
cells from both healthy and asthmatic subjects. PLoS One.
10(e0122372)2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Rajaraman G, Yang G, Chen J and Chang TK:
Modulation of CYP1B1 and CYP1A1 gene expression and activation of
aryl hydrocarbon receptor by Ginkgo biloba extract in
MCF-10A human mammary epithelial cells. Can J Physiol Pharmacol.
87:674–683. 2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jin UH, Park H, Li X, Davidson LA, Allred
C, Patil B, Jayaprakasha G, Orr AA, Mao L, Chapkin RS, et al:
Structure-dependent modulation of aryl hydrocarbon
receptor-mediated activities by flavonoids. Toxicol Sci.
164:205–17. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Pang C, Zhu C, Zhang Y, Ge Y, Li S, Huo S,
Xu T, Stauber RH and Zhao B: 2,3,7,8-Tetrachloodibenzo-p-dioxin
affects the differentiation of CD4 helper T cell. Toxicol Lett.
311:49–57. 2019.PubMed/NCBI View Article : Google Scholar
|