Introduction
Artificial nerve conduits have recently become used
for peripheral nerve damage with a long nerve defect and damage of
multiple peripheral nerves in which the nerve stumps cannot be
directly sutured (1-3).
However, there are several problems with artificial nerve conduits.
Decreased angiogenesis, a decrease in nerve fibers, and reduction
of axon regeneration were previously reported in artificial nerve
conduits when an artificial nerve conduit was used for a nerve
defect that was long to a certain extent (4).
On the other hand, Biasibetti et al reported
that decreased angiogenesis, a decrease in nerve fibers, and myelin
degeneration are age-associated changes in peripheral nerves
(5). Furthermore, we previously
reported a mechanism to prevent oxidative stress-induced peripheral
nerve degeneration (6). Peripheral
nerves are functionally and morphologically impaired due to aging
(5-7).
Based on the above, the use of artificial nerve
conduits is expected to be limited for the elderly whose peripheral
nerves are considered to have low axon regenerative ability.
However, the influence of aging on the induction of nerve
regeneration in artificial nerve conduits has yet to be clarified.
Thus, in this study, to histologically evaluate the influence of
aging on the induction of peripheral nerve regeneration in
artificial nerve conduits, we performed artificial nerve conduit
transplantation to the sciatic nerve in young and elderly mice. In
addition, histological changes after artificial nerve conduit
transplantation in each mouse were compared using hematoxylin and
eosin (H-E) and immunohistochemical staining.
Materials and methods
Animal model
The present study was approved by the Animal Care
Committee of Juntendo University, Tokyo, Japan (registration no.
1398; approval no. 310212).
Thirty male C57BL/6 mice purchased from Japan SLC,
Inc. were used. To histologically evaluate the influence of aging
on the induction of peripheral nerve regeneration in artificial
nerve conduits, an artificial nerve conduit was surgically
transplanted to the sciatic nerve at 8 (Young group, n=15) or 70
weeks of age (Aged group, n=15), and the sciatic nerve was
collected and histologically evaluated at 1, 4, and 12 weeks after
surgery. The mice were maintained at 5 per cage in a sterile
environment, and had free access to water and 15-kGy
gamma-irradiated feed, CRF-1 (Oriental Yeast Co., Ltd.), at a room
temperature of 22 ± 2˚C and 40-60% humidity under a 12-h light and
12-h dark environment.
Preparation of the nerve conduit
The collagen artificial nerve conduit was prepared
according to the Nipro patent (United States patent US 6953482 B2).
Briefly, enzyme-solubilized collagen (a mixture of collagen types I
and III; Nippon Ham, Ibaragi, Japan) was dissolved in water to
prepare a 5% aqueous solution and extruded in a coagulating liquid
to produce a collagen fiber with a diameter of 50 nm. The collagen
fiber was wound around a metal mandrel to produce a tubular
structure consisting of collagen with an inner diameter of 1 mm,
wall thickness of 0.25 mm, and length of 50 mm. The tube was filled
with 60 collagen filaments (50 nm in diameter) aligned
longitudinally with the 5% aqueous collagen solution. The
constructs were rapidly frozen and then lyophilized in vacuo. The
construct contained 10% v/v collagen filaments under dry
conditions. The collagen artificial nerve conduit was then
subjected to 25-kGy gamma-ray irradiation for sterilization.
Surgical procedures
Under generalized anesthesia with isoflurane
inhalation anesthetic solution (4% isoflurane used for induction
and 2% for maintenance), a skin incision was made on the lateral
side of the right hind limb. For manipulation of the sciatic nerve,
a light microscope (Zeiss Axioskop2; magnification, x40) was used.
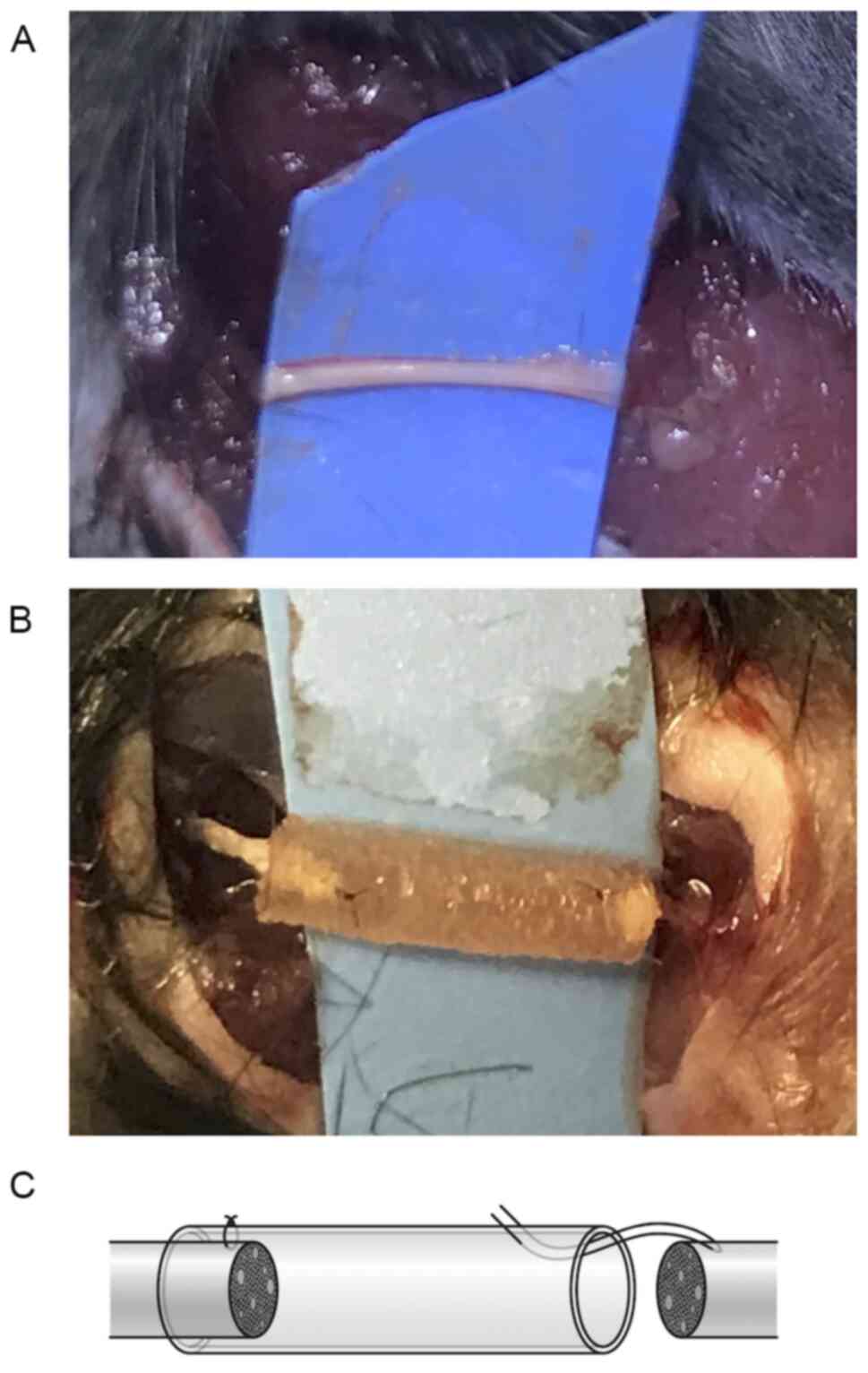
The sciatic nerve was dissected from the surrounding tissue
(Fig. 1A) and cut to prepare a 3-mm
defect. The nerve defect was bridged by the collagen artificial
nerve conduit (Fig. 1B). The
proximal and distal nerve stumps were both placed within the
conduit, and epineural sutures were made using 9-0 nylon, as
described previously by Fields et al (Fig. 1C) (8).
The postoperative activity of the mice was not
limited and they were maintained the same as in the preoperative
environment. Under generalized anesthesia with isoflurane
inhalation anesthetic solution (4% isoflurane used for induction
and 2% for maintenance), the right sciatic nerves including
collagen nerve conduits from both groups were harvested at 1, 4,
and 12 weeks after surgery. Mice were sacrificed by cervical
dislocation on the day the sciatic nerves were harvesting.
H-E staining assessment of the
age-associated change in the peripheral nerve
The collected sciatic nerve was fixed in 4%
paraformaldehyde at room temperature for 72 h and paraffin blocks
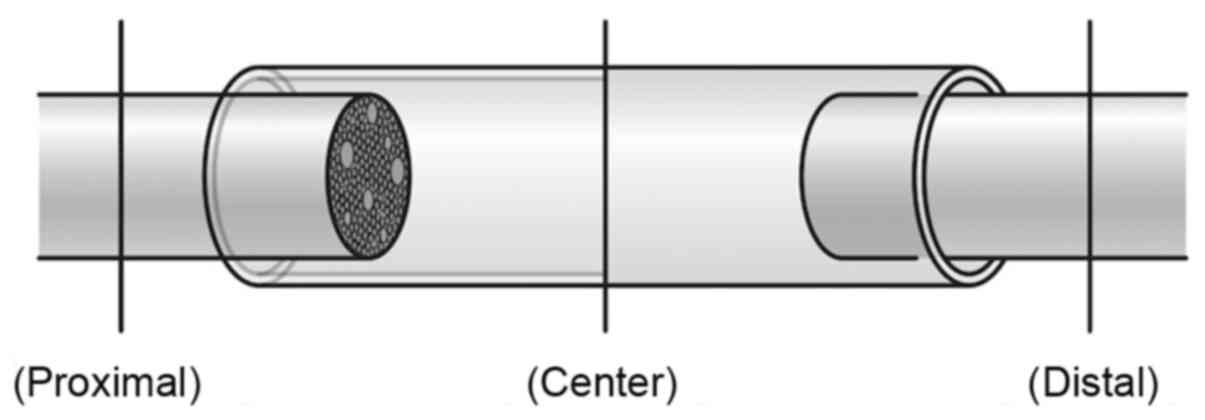
were prepared. Tissue sections were prepared by cutting the sciatic
nerve in the minor axis direction at 3-µm thickness in the
following positions: (Proximal): The sciatic nerve proximal to the
artificial nerve conduit; (Center): The central region of the
artificial nerve conduit; and (Distal): The sciatic nerve distal to
the artificial nerve conduit (Fig.
2). H-E staining was performed to evaluate the state of
absorption of the outer cylinder of the artificial nerve conduit at
Center and state of nerve regeneration induction in the artificial
nerve conduit. Using a light microscope (Olympus, AX73), the
cross-sectional area including the outer cylinder of the artificial
nerve conduit (I), that of the inner lumen of the artificial nerve
conduit in the region filled with nerve fibers (II), and the rate
of the inner lumen of the artificial nerve conduit being filled
with nerve fibers (III=II/I x100) were calculated to evaluate the
state of induction of nerve regeneration in the artificial nerve
conduit.
Histochemical assessment of the
age-related change in the peripheral nerve
Several peripheral nerve-specific proteins were
immunohistochemically stained. The antibodies used were those
against vascular endothelial growth factor A (VEGFA) as an
angiogenic marker, sex-determining region Y-box 10 (SOX10) and S100
calcium-binding protein β (S100β) as Schwann cell markers, and
nerve growth factor (NGF) as a marker for nerve damage. The primary
antibodies and secondary biotinylated antibodies are summarized in
Table I.
 | Table ISummary of primary and secondary
biotinylated antibodies used in the present study. |
Table I
Summary of primary and secondary
biotinylated antibodies used in the present study.
| Antigen | Primary antibody,
cat. no. (supplier) | Host of primary
antibody | Primary antibody
dilution | Secondary
biotinylated antibody, cat. no. (supplier) | Secondary antibody
dilution | Antigen
retrieval |
|---|
| VEGFA | ab51745 (Abcam) | Rabbit (pc) | 1:50 | Anti-rabbit, E0432
(Dako; Agilent Technologies, Inc.) | 1:300 | - |
| SOX10 | AF2864 (R&D
Systems, Inc.) | Goat (pc) | 1:50 | Anti-goat, E0466
(Dako; Agilent Technologies, Inc.) | 1:300 | Citrate buffer (pH
6.0) |
| S100β | ab52642 (Abcam) | Rabbit (mc) | 1:800 | Anti-rabbit, E0432
(Dako; Agilent Technologies, Inc.) | 1:300 | Tris buffer (pH
9.0) |
| NGF | ab6199 (Abcam) | Rabbit (pc) | 1:100 | Anti-rabbit, E0432
(Dako; Agilent Technologies, Inc.) | 1:300 | - |
After deparaffinization, antigen activation
treatment for SOX10 and S100β was performed at 121˚C for 10 min
using an autoclave, whereas VEGFA and NGF did not require antigen
activation treatment. Then, to block endogenous biotin, the
sections were treated with the Avidin/Biotin Blocking Kit
(VectorR, SP-2001) and blocked with 2% bovine serum
albumin (BSA) (Sigma-Aldrich, A2153) in PBS for 30 min at room
temperature, followed by reactions with primary antibodies against
the different proteins at 4˚C for 15 h. After treatment with the
primary antibody, the sections were immersed in 3% hydrogen
peroxide for 5 min to block endogenous peroxidase. Subsequently,
the sections were reacted with the secondary antibodies against the
proteins at room temperature for 40 min. Lastly, the sections were
reacted with peroxidase-conjugated streptavidin (Dako, P397) at
room temperature for 40 min. The level of protein expression in the
nerve was quantitated in each section using the Nuance FX
(PerkinElmer) multispectral camera system. The area stained in
brown was regarded as positive, and the ratio of the area of the
positive section to the area of the short-axis section of the
sciatic nerve was calculated.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD) and were analyzed for significant differences by the
Mann-Whitney U test (Prism 4; GraphPad Software). Differences were
considered significant at P<0.05.
Results
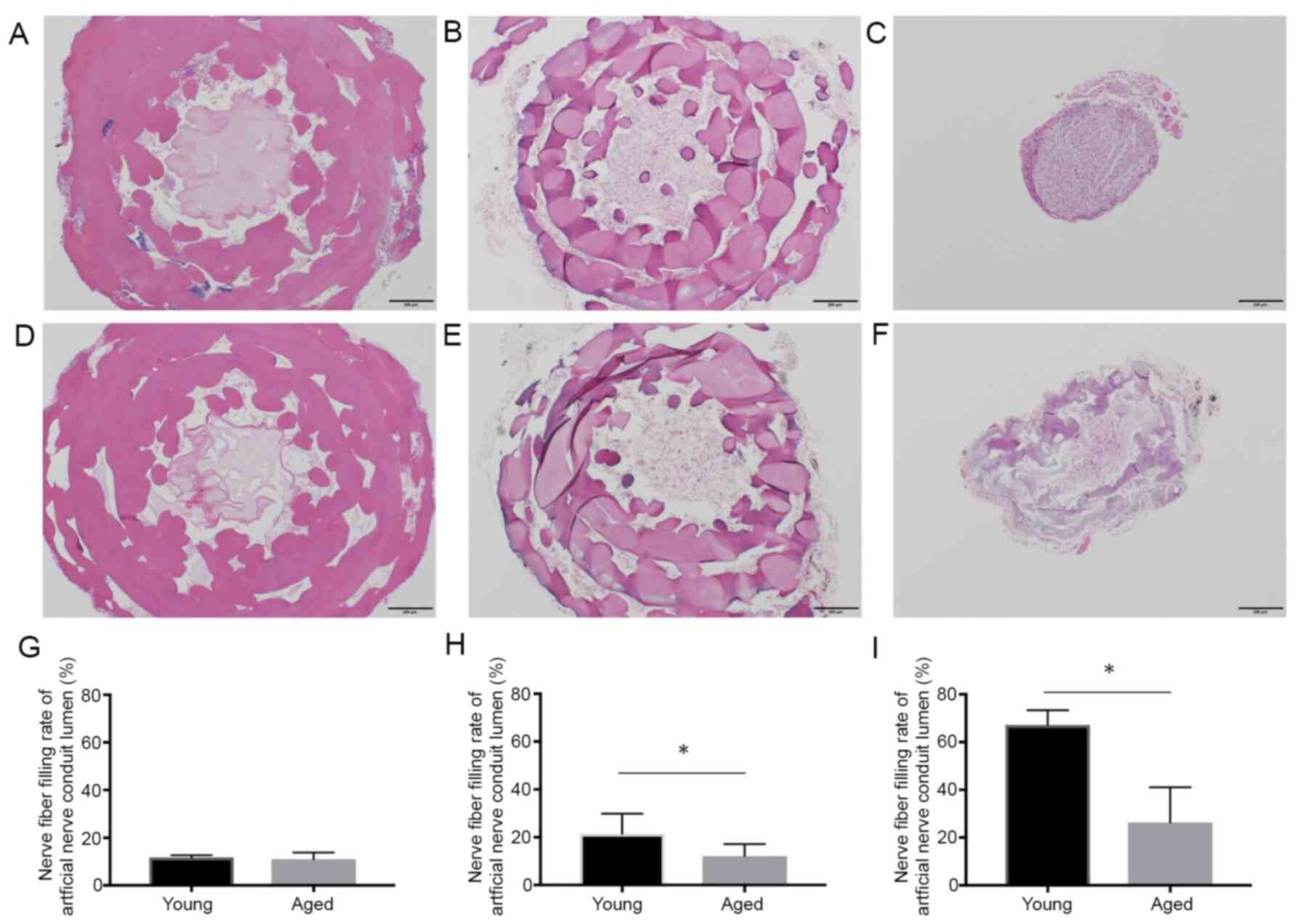
The cross-sectional area (I) including the outer
cylinder of the artificial nerve conduit on H&E staining was
2.19±0.11 mm2 in the Young group and 2.18±0.15
mm2 in the Aged group at 1 week (P>0.99), 1.28±0.21
mm2 in the Young group and 1.75±0.36 mm2 in
the Aged group at 4 weeks (P=0.06), and 0.22±0.06 mm2 in
the Young group and 0.56±0.31 mm2 in the Aged group at
12 weeks (P=0.11). The cross-sectional area (II) of the inner lumen
of the artificial nerve conduit filled with nerve fibers was
0.26±0.02 mm2 in the Young group and 0.24±0.05
mm2 in the Aged group at 1 week (P=0.55), 0.26±0.06
mm2 in the Young group and 0.20±0.05 mm2 in
the Aged group at 4 weeks (P=0.22), and 0.15±0.04 mm2 in
the Young group and 0.11±0.02 mm2 in the Aged group at
12 weeks (P=0.19). The rate of the inner lumen of the artificial
nerve conduit being filled with nerve fibers (III) was 11.7±0.8% in
the Young group and 11.1±2.5% in the Aged group at 1 week (P=0.42),
21.4±7.6% in the Young group and 12.1±4.5% in the Aged group at 4
weeks (P<0.05), and 67.2±5.5% in the Young group and 26.4±12.8%
in the Aged group at 12 weeks (P<0.05). (III) was significantly
higher in the Young group than in the Aged group at 4 and 12 weeks
(Fig. 3).
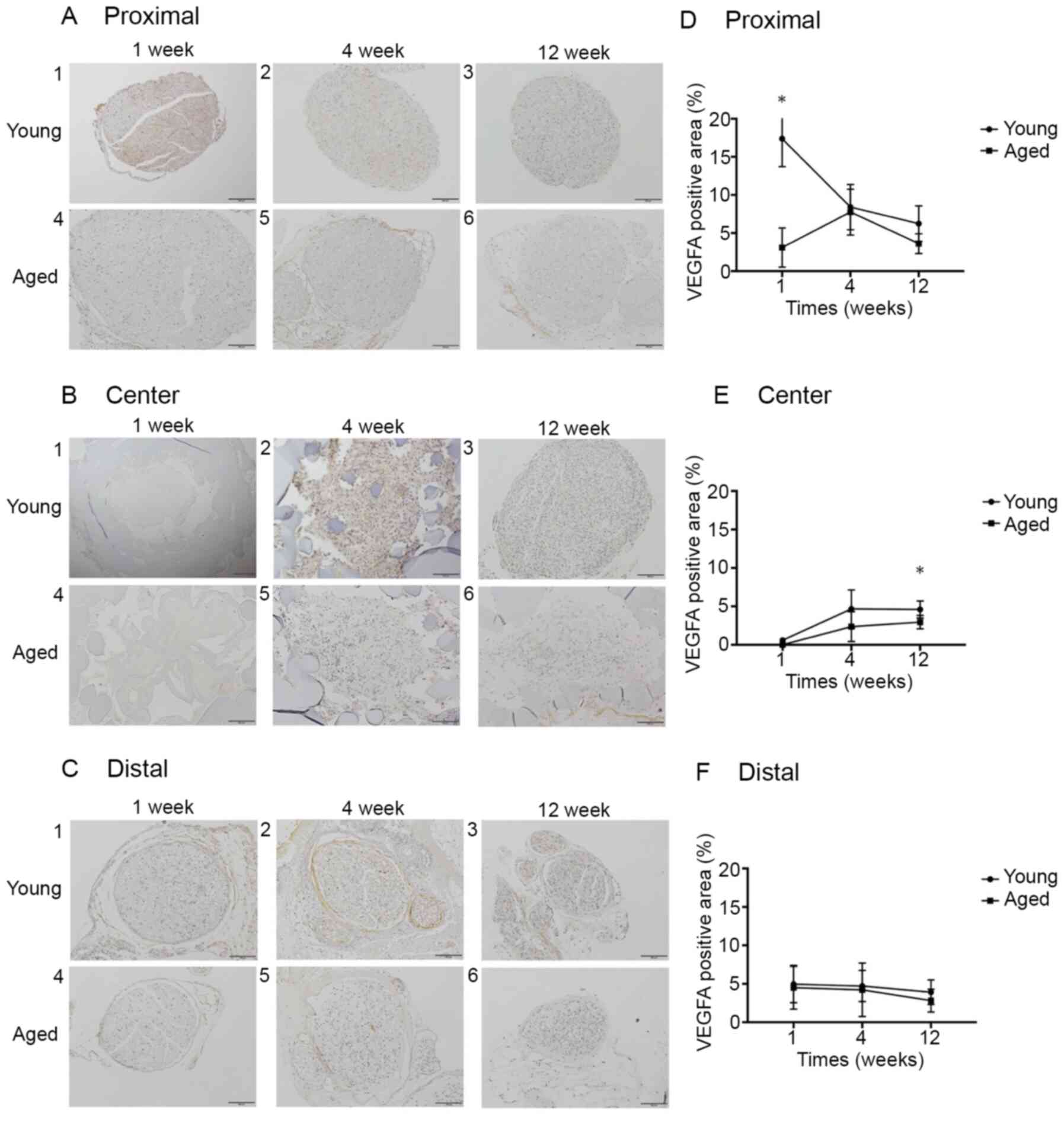
Regarding the expression of each protein in the
peripheral nerve, the anti-VEGFA antibody-positive expression rates
at Proximal in the Young and Aged groups were 17.4±3.3 and
3.1±2.3%, respectively, at 1 week (P<0.05), 8.4±2.7 and
7.8±2.7%, respectively, at 4 weeks (P=0.69), and 6.2±2.1 and
3.6±1.2%, respectively, at 12 weeks (P=0.06). At Center, the rates
were 0.0±0.0 and 0.0±0.0%, respectively, at 1 week (P>0.99),
4.7±2.2 and 2.4±1.7%, respectively, at 4 weeks (P=0.10), and
4.2±0.4 and 2.9±0.8%, respectively, at 12 weeks (P<0.05). At
Distal, the rates were 5.0±2.2 and 4.5±2.5%, respectively, at 1
week (P=0.69), 4.7±1.7 and 4.2±2.8%, respectively, at 4 weeks
(P>0.99), and 3.9±1.3 and 2.8±1.3%, respectively, at 12 weeks
(P=0.25). The VEGFA expression rate at Proximal was significantly
higher in the Young group than in the Aged group at postoperative 1
week, and the rate at Center was significantly higher in the Young
group than in the Aged group at postoperative 12 weeks, whereas no
significant difference was noted in the rate at Distal between the
2 groups at any week (Fig. 4).
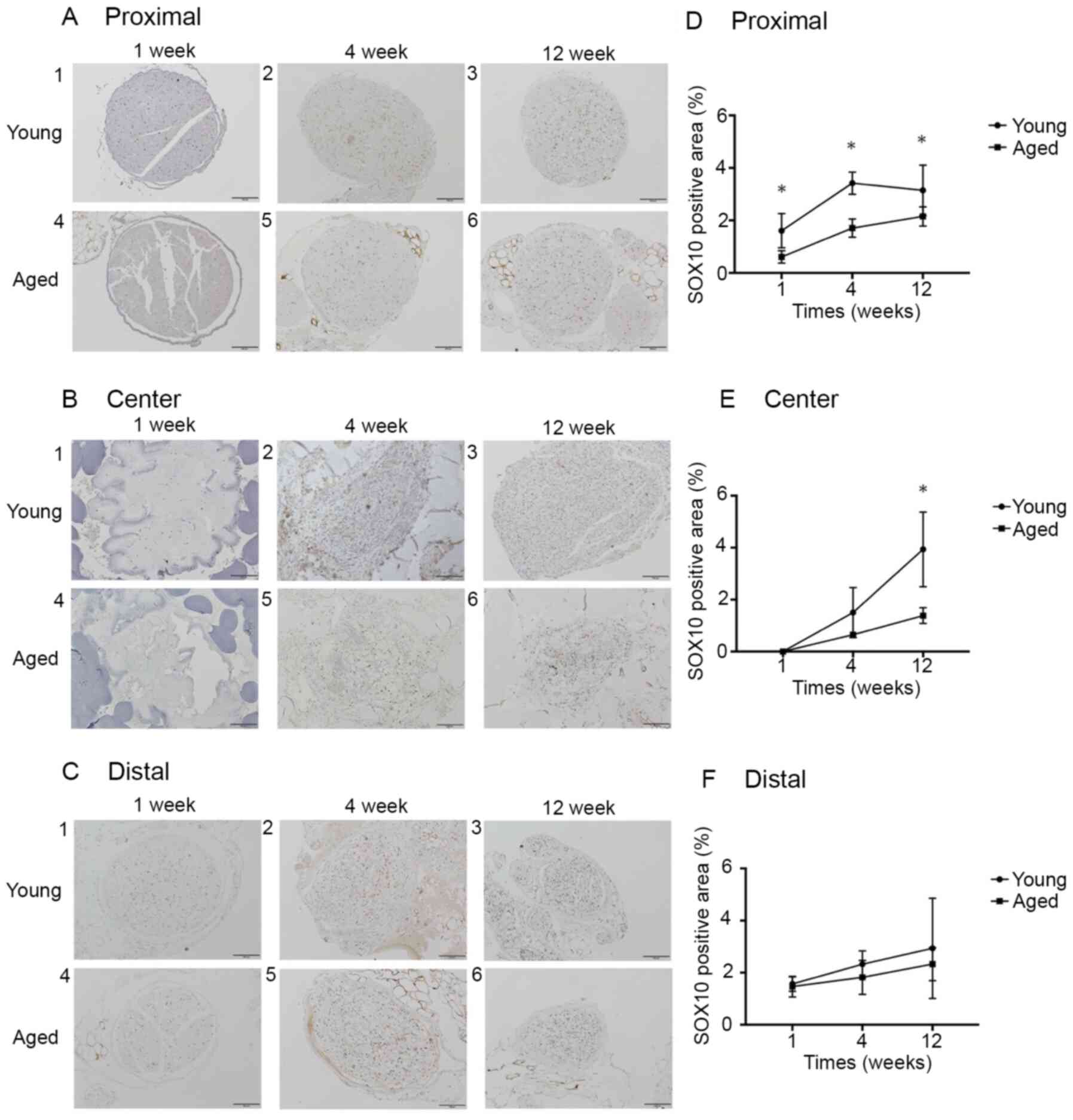
The anti-SOX10 antibody-positive expression rates at
Proximal in the Young and Aged groups were 1.6±0.6 and 0.6±0.2%,
respectively, at 1 week (P<0.05), 3.4±0.4 and 1.7±0.3%,
respectively, at 4 weeks (P<0.05), and 3.1±0.9 and 2.2±0.3%,
respectively, at 12 weeks (P<0.05). The rates at Center were
0.0±0.0 and 0.0±0.0%, respectively, at 1 week (P>0.99), 2.1±1.7
and 0.8±0.4%, respectively, at 4 weeks (P=0.11), and 4.7±1.1 and
1.5±0.6%, respectively, at 12 weeks (P<0.05). The rates at
Distal were 1.6±0.2 and 1.5±0.4%, respectively, at 1 week (P=0.65),
2.3±0.5 and 1.8±0.6%, respectively, at 4 weeks (P=0.41), and
2.9±1.6 and 2.3±0.6%, respectively, at 12 weeks (P>0.99). The
SOX10 expression rate at Proximal was significantly higher in the
Young group than in the Aged group at postoperative 1, 4, and 12
weeks, and the rate at Center was significantly higher in the Young
group than in the Aged group at postoperative 12 weeks. No
significant difference was noted in the SOX10 expression rate at
Distal between the 2 groups at any week (Fig. 5).
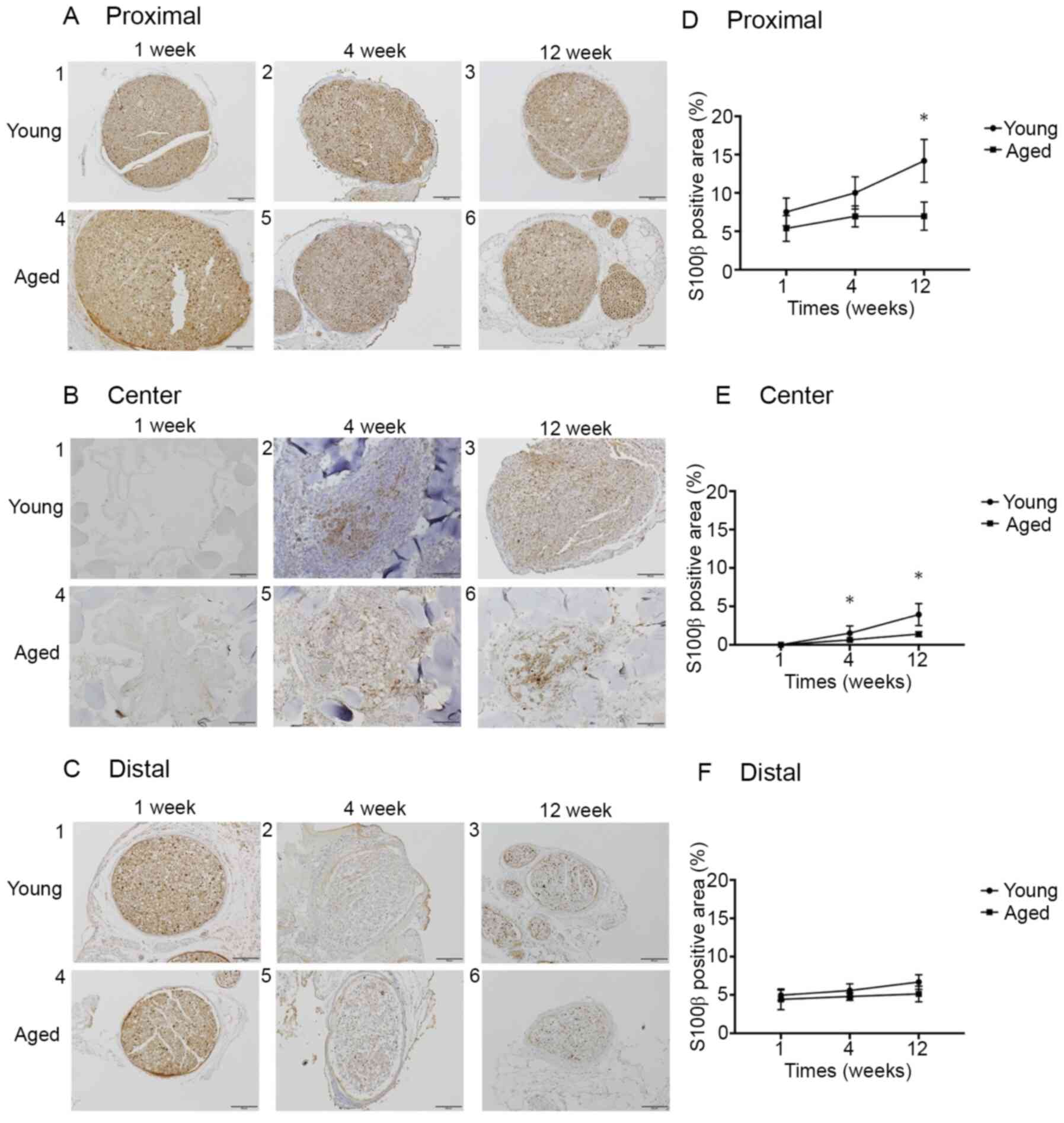
The S100β expression rates at Proximal in the Young
and Aged groups were 7.5±1.6 and 5.4±1.5%, respectively, at 1 week
(P=0.15), 10.0±1.9 and 7.0±1.2%, respectively, at 4 weeks (P=0.06),
and 14.2±2.5 and 7.0±1.6%, respectively, at 12 weeks (P<0.05).
The rates at Center were 0.0±0.0 and 0.0±0.0%, respectively, at 1
week (P>0.99), 1.5±0.9 and 0.6±0.1%, respectively, at 4 weeks
(P<0.05), and 3.9±1.2 and 1.4±0.2%, respectively, at 12 weeks
(P<0.05). The rates at Distal were 5.0±0.6 and 4.4±1.2%,
respectively, at 1 week (P=0.41), 5.6±0.7 and 4.8±0.4%,
respectively, at 4 weeks (P=0.19), and 6.7±0.7 and 5.1±0.9%,
respectively, at 12 weeks (P=0.19). The S100β expression rate at
Proximal was significantly higher in the Young group than in the
Aged group at postoperative 12 weeks. The S100β expression rate at
Center was significantly higher in the Young group than in the Aged
group at postoperative 4 and 12 weeks. No significant difference
was noted in the S100β expression rate at Distal between the 2
groups at any week (Fig. 6).
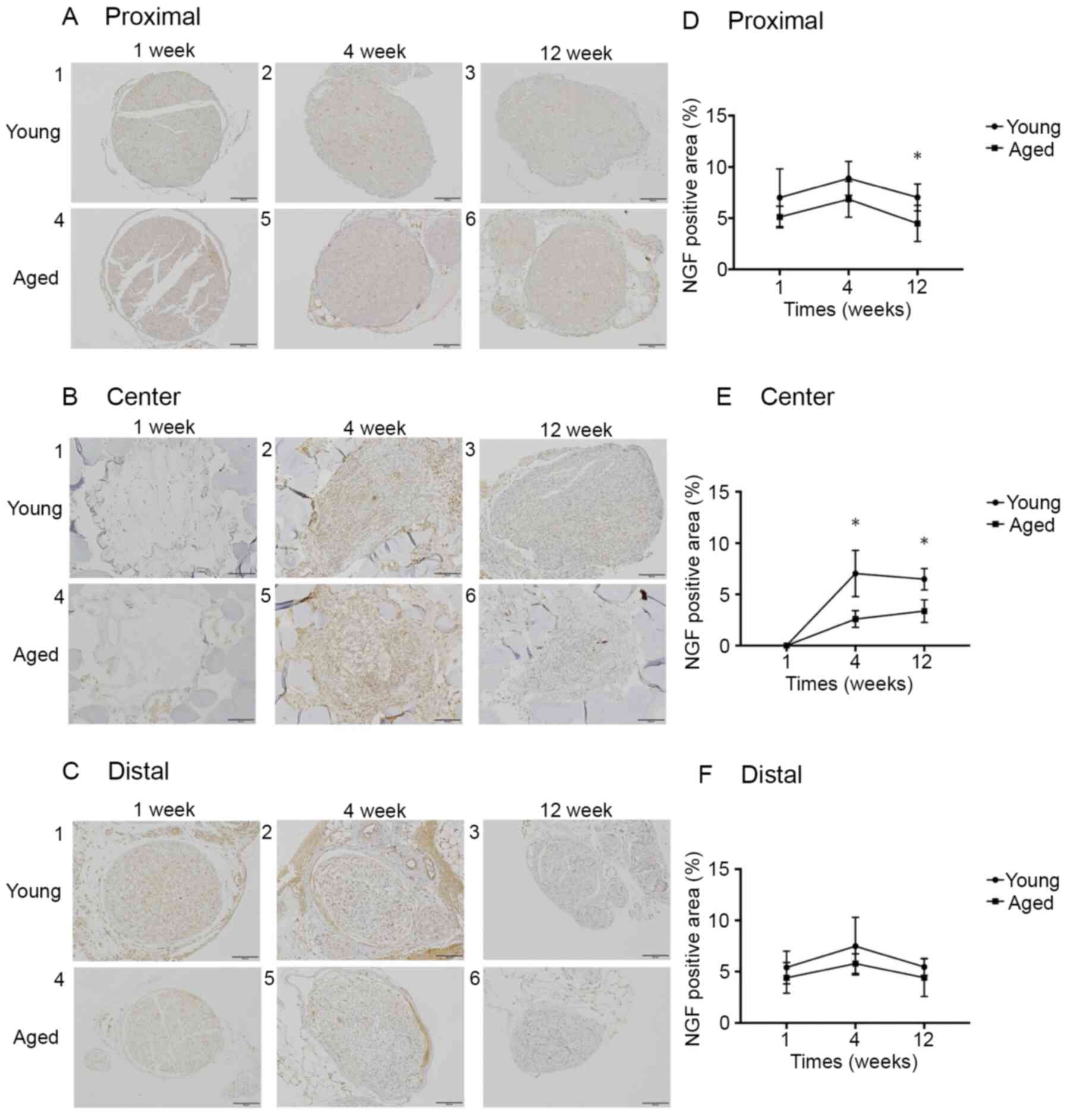
The NGF expression rates at Proximal in the Young
and Aged groups were 7.0±2.5 and 5.1±0.9%, respectively, at 1 week
(P=0.22), 8.9±1.5 and 6.8±1.6%, respectively, at 4 weeks (P=0.25),
and 7.0±1.2 and 4.5±1.6%, respectively, at 12 weeks (P<0.05).
The rates at Center were 0.0±0.0 and 0.0±0.0%, respectively, at 1
week (P>0.99), 7.0±2.0 and 2.6±0.7%, respectively, at 4 weeks
(P<0.05), and 6.5±0.9 and 3.4±1.0%, respectively, at 12 weeks
(P<0.05). The rates at Distal were 5.4±1.4 and 4.4±1.3%,
respectively, at 1 week (P=0.42), 7.5±2.4 and 5.8±0.9%,
respectively, at 4 weeks (P=0.29), and 5.5±0.7 and 4.4±1.6%,
respectively, at 12 weeks (P=0.39). The NGF expression rate at
Proximal was significantly higher in the Young group than in the
Aged group at postoperative 12 weeks. The NGF expression rate at
Center was significantly higher in the Young group than in the Aged
group at postoperative 4 and 12 weeks. No significant difference
was noted in the NGF expression rate at Distal between the 2 groups
at any week (Fig. 7).
Discussion
Several previous studies reported that the
peripheral nerve regeneration ability decreases due to aging
(9,10). In this study, the rate of the inner
lumen of the artificial nerve conduit being filled with nerve
fibers (III) was significantly lower in the Aged group than in the
Young group at 4 and 12 weeks after artificial nerve conduit
transplantation, suggesting aging-induced reduction of peripheral
nerve regeneration ability. ‘Why is nerve regenerative ability
reduced by aging?’ Verdú et al reported that Schwann cell
function declines due to aging, delaying Waller degeneration and
axon regeneration after peripheral nerve damage (10). In addition, the vascular
regeneration ability of nerve tissue after peripheral nerve damage
was recently demonstrated to decrease with age (5,11). On
the other hand, the generation of new blood vessels starting from
the proximal nerve stump after peripheral nerved damage is
important (12,13). Furthermore, Schwann cell migration
along the new blood vessel generated in this manner was clarified
to promote axon regeneration (12).
Thus, we performed immunostaining to elucidate the difference in
the rate of the artificial nerve conduit inner lumen being filled
with nerve fibers (III) between the Young and Aged groups on
H&E staining.
VEGFA promotes new blood vessel generation, playing
an important role in the early step of nerve regeneration, and it
is expressed a few days after peripheral nerve damage (12,13).
In the present study, VEGFA expression at Proximal was
significantly lower in the Aged group than that in the Young group
at postoperative 1 week, and VEGFA expression in the Aged group did
not exceed that in the Young group at postoperative 4 or 12 weeks,
confirming that VEGFA expression ability decreases due to aging. At
Center, VEGFA was gradually expressed from postoperative 1 week to
12 weeks in both groups, and new blood vessels were observed at
postoperative 4 weeks on H&E staining, suggesting that VEGFA
induced new blood vessel generation from the proximal nerve stump
toward the artificial nerve conduit. Generation of these new blood
vessels exhibited the same pattern as that reported by Cattin et
al (12). Based on the above,
aging-induced reduction of VEGFA expression early after peripheral
nerve damage was suggested to delay nerve regeneration.
Regarding the appearance of Schwann cells migrating
along the new blood vessel, immunohistological staining was
performed focusing on SOX10 expressed at all steps of Schwann cell
development from the early step of neural crest cells to S100β
expression by differentiated Schwann cells (14-16).
As SOX10 expression at Proximal increased at postoperative 1, 4,
and 12 weeks in the Young group, Schwann cells may have appeared
with VEGFA at Proximal in the early step of nerve regeneration in
the Young group. The increase in S100β at Proximal at postoperative
12 weeks in the Young group may have been induced by SOX10, which
is consistent with the finding reported by Fujiwara et al
that ‘induction of S100β expression is one of the important
functions of SOX10’ (14), i.e.,
Schwann cell maturation with migration along the new blood vessel
at Proximal was observed in the Young group. On the other hand, in
the Aged group, SOX10 expression at Proximal was lower at
postoperative 1, 4, and 12 weeks than that in the Young group,
confirming that Schwann cell migration is not readily induced at
the proximal nerve stump due to aging. At Center, S100β expression
increased at postoperative 4 weeks in the Young group. This
reaction at Center in the Young group may have been due to
increases in VEGFA and SOX10 expression at Proximal, which induced
Schwann cell migration along the new blood vessel from Proximal to
Center, promoting Schwann cell differentiation. Furthermore, S100β
expression at Center in the Aged group was significantly lower than
that in the Young group at postoperative 4 and 12 weeks. Although
evaluation of aging-associated Schwann cell differentiation ability
is difficult, this study revealed that Schwann cell migration
ability markedly decreased due to aging and mature Schwann cell
development also decreased, suggesting that Waller degeneration
during in the nerve regeneration process and axon regeneration
ability are reduced by aging.
NGF, a marker of nerve damage, has neuroprotective
and nerve/axon regenerative functions, which decline with age
(17-21).
NGF is present at a low level in normal nerve tissue, but upon
nerve damage, NGF production is promoted by Schwann cells (22-24).
Based on this study, NGF expression at Proximal at postoperative 12
weeks, and Center at postoperative 4 and 12 weeks was significantly
higher in the Young group. Such NGF expression was similar to that
of S100β expression, representing mature Schwann cell development,
suggesting that NGF was expressed by mature Schwann cells, i.e.,
mature Schwann cell development decreases in peripheral nerves
damaged by aging, reducing the expression of NGF with nerve/axon
regenerative function and delaying peripheral nerve
regeneration.
In damaged peripheral nerves, Schwann cell migration
along the new blood vessel starting from the proximal nerve stump
promotes axon regeneration. In the peripheral nerve after
artificial nerve conduit transplantation in the Aged group, VEGFA
(new blood vessel), SOX10 and S100β (Schwann cells), and NGF (axon
regeneration) expression representing the axon regeneration process
was significantly lower than that in the Young group, suggesting
that peripheral nerve regeneration is delayed by aging. As no study
of the efficacy and safety of artificial nerve conduits in the
elderly has been performed in Japan, their use for elderly patients
has to be carefully investigated. In addition, based on this study,
the efficacy of the use of an artificial nerve conduit in elderly
patients is inferior to that in young patients. Thus, surgical
treatment using conventional artificial nerve conduits for nerve
injuries in the elderly has limitations; therefore, new additional
therapies aimed at axonal regeneration need to be developed for
elderly patients. Detailed elucidation of age-associated changes in
peripheral nerves in the future may help to prevent and treat
peripheral nerve disorder in an aged society.
There is a limitation of this study which is the
lack of investigation of the motor function of the lower limbs. The
mice in both Young and Aged group had motor paralysis with limping
after surgery. However, we had focused on histopathological
evaluation of the peripheral nerve and not investigated the motor
function of the lower limbs.
In damaged peripheral nerves, axon regeneration is
promoted by Schwann cell migration along a new blood vessel
starting from the proximal nerve stump. This study suggested that
these nerve regeneration-inducing functions decrease with age in
the nerve regeneration induction process in artificial nerve
conduits.
Acknowledgements
Not applicable.
Funding
The present study was supported by Nipro
Corporation.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AK mainly wrote the manuscript and acquired,
analyzed and interpretated the data. KN wrote the manuscript and
made substantial contributions to conception and design of the
study, and interpretation of data. SN, KM and YS contributed to
acquisition, analysis and interpretation of data. KG, HO and NN
contributed to acquisition of data. KK made substantial
contributions to conception and design. MI contributed to the
analysis and interpretation of data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
Committee of Juntendo University (Tokyo, Japan; registration no.
1398; approval no. 310212).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Iijima Y, Ajiki T, Murayama A and
Takeshita K: Effect of artificial nerve conduit vascularization on
peripheral nerve in a necrotic bed. Plast Reconstr Surg Glob Open.
4(e665)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kornfeld T, Vogt PM and Radtke C: Nerve
grafting for peripheral nerve injuries with extended defect sizes.
Wien Med Wochenschr. 169:240–251. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rbia N, Bulstra LF, Saffari TM, Hovius SER
and Shin AY: Collagen nerve conduits and processed nerve allografts
for the reconstruction of digital nerve gaps: A single-institution
case series and review of the literature. World Neurosurg.
127:e1176–e1184. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Siemionow M, Cwykiel J, Uygur S, Kwiecien
G, Oztürk C, Szopinski J and Madajka M: Application of epineural
sheath conduit for restoration of 6-cm long nerve defects in a
sheep median nerve model. Microsurgery. 39:332–339. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Biasibetti E, Bisanzio D, Mioletti S,
Amedeo S, Iuliano A, Bianco P and Capucchio MT: Spontaneous
age-related changes of peripheral nerves in cattle: Morphological
and biochemical studies. Anat Histol Embryol. 45:100–108.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Goto K, Naito K, Nakamura S, Nagura N,
Sugiyama Y, Obata H, Kaneko A and Kaneko K: Protective mechanism
against age-associated changes in the peripheral nerves. Life Sci.
253(117744)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Novak CB, Anastakis DJ, Beaton DE,
Mackinnon SE and Katz J: Biomedical and psychosocial factors
associated with disability after peripheral nerve injury. J Bone
Joint Surg Am. 93:929–936. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fields RD, Le Beau JM, Longo FM and
Ellisman MH: Nerve regeneration through artificial tubular
implants. Prog Neurobiol. 33:87–134. 1989.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Painter MW, Lutz AB, Cheng YC,
Latremoliere A, Duong K, Miller CM, Posada S, Cobos EJ, Zhang AX,
Wagers AJ, et al: Diminished schwann cell repair responses underlie
age-associated impaired axonal regeneration. Neuron. 83:331–343.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Verdú E, Ceballos D, Vilches JJ and
Navarro X: Influence of aging on peripheral nerve function and
regeneration. J Peripehr Nerv Syst. 5:191–208. 2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pola R, Aprahamian TR, Bosch-Marcé M,
Curry C, Gaetani E, Flex A, Smith RC, Isner JM and Losordo DW:
Age-dependent VEGF expression and intraneural neovascularization
during regeneration of peripheral nerves. Neurobiol Aging.
25:1361–1368. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cattin AL, Burden JJ, Van Emmenis L,
Mackenzie FE, Hoving JJ, Garcia Calavia N, Guo Y, McLaughlin M,
Rosenberg LH, Quereda V, et al: Macrophage-induced blood vessels
guide Schwann cell-mediated regeneration of peripheral nerves.
Cell. 162:1127–1139. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nishida Y, Yamada Y, Kanemaru H, Ohazama
A, Maeda T and Seo K: Vascularization via activation of VEGF-VEGFR
signaling is essential for peripheral nerve regeneration. Biomed
Res. 39:287–294. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fujiwara S, Hoshikawa S, Ueno T, Hirata M,
Saito T, Ikeda T, Kawaguchi H, Nakamura K, Tanaka S and Ogata T:
SOX10 transactivates S100B to suppress Schwann cell proliferation
and to promote myelination. PLoS One. 9(e115400)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jessen KR and Mirsky R: Signals that
determine Schwann cell identity. J Anat. 200:367–376.
2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kuhlbrodt K, Herbarth B, Sock E,
Hermans-Borgmeyer I and Wegner M: Sox10, a novel transcriptional
modulator in glial cells. J Neurosci. 18:237–250. 1998.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Budni J, Bellettini-Santos T, Mina F,
Garcez ML and Zugno AI: The involvement of BDNF, NGF and GDNF in
aging and Alzheimer's disease. Aging Dis. 6:331–341.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen ZW and Wang MS: Effects of nerve
growth factor on crushed sciatic nerve regeneration in rats.
Microsurgery. 16:547–551. 1995.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hasenöhrl RU, Söderstróm S, Mohammed AH,
Ebendal T and Huston JP: Reciprocal changes in expression of mRNA
for nerve growth factor and its receptors TrkA and LNGFR in brain
of aged rats in relation to maze learning deficits. Exp Brain Res.
114:205–213. 1997.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kemp SW, Webb AA, Dhaliwal S, Syed S,
Walsh SK and Midha R: Dose and duration of nerve growth factor
(NGF) administration determine the extent of behavioral recovery
following peripheral nerve injury in the rat. Exp Neurol.
229:460–470. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lärkfors L, Ebendal T, Whittemore SR,
Persson H, Hoffer B and Olson L: Decreased level of nerve growth
factor (NGF) and its messenger RNA in the aged rat brain. Brain
Res. 427:55–60. 1987.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Grinsell D and Keating CP: Peripheral
nerve reconstruction after injury: A review of clinical and
experimental therapies. Biomed Res Int. 2014(698256)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hall S: Nerve repair: A neurobiologist's
view. J Hand Surg Br. 26:129–136. 2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mokuno K, Sobue G, Reddy UR, Wurzer J,
Kreider B, Hotta H, Baron P, Ross AH and Pleasure D: Regulation of
Schwann cell nerve growth factor receptor by cyclic adenosine
3',5'-monophosphate. J Neurosci Res. 21:465–472. 1988.PubMed/NCBI View Article : Google Scholar
|