Introduction
Obstructive sleep apnea-hypopnea syndrome (OSAHS) is
characterized by partial or total upper airway obstruction during
sleep, with different degrees of blood oxygen saturation,
hypercapnia and hypoxemia (1).
Adenoid hypertrophy is one of the most common causes of OSAHS in
children (2). According to an
epidemiological investigation, the incidence of children with OSAHS
is 1.2-5.7%, whilst 50% children with OSAHS exhibit moderate and
severe symptoms in China (3). The
incidence of OSAHS among obese children is two times greater
compared with healthy weight children, and OSAHS in obese children
is accompanied by abnormal lipid metabolism, with abnormal high
islet glucose tolerance and hypertension, which are referred to as
the insulin resistance syndrome (4). The association between OSAHS and
insulin resistance has been confirmed by observational and
experimental studies, which demonstrate that OSAHS is an
independent risk factor for insulin resistance in children
(3,5,6).
Adenoid hypertrophy is an important risk factor for OSAHS in
children and the severity of OSAHS is positively associated with
the severity of adenoid (2).
Adenoid hypertrophy can result in the downregulation of the
expression of insulin-like growth factor-1 (IGF-1) and insulin-like
growth factor binding protein-3, and inhibit the growth and
development of children (7). IGF-1
can ameliorate insulin resistance by promoting glucose uptake and
insulin secretion (8,9). Therefore, the present study
hypothesized that there might be an association between adenoid
hypertrophy and insulin resistance.
Several studies have demonstrated that inflammatory
response markers play an important role in glucose tolerance and
insulin resistance, including TNF-α and IL-6. TNF-α induces insulin
resistance by mediating serine phosphorylation of the insulin
receptor substrate, which becomes a tyrosine kinase inhibitor for
the insulin receptor (10).
Furthermore, TNF-α inhibits the insulin-stimulated glucose
transport by downregulating the expression of the glucose
transporter type 4 insulin-responsive (GLUT-4) (11). TNF-α can also promote the
decomposition of adipose tissue and the release of free fatty acids
to regulate glucose metabolism, which indirectly leads to insulin
resistance (12). Clinical studies
have demonstrated that patients with sleep apnea have higher plasma
levels of IL-6 and TNF-α compared with healthy individuals
(13,14). Hotamisligil et al (11) reported that TNF-α is an important
intermediate factor that induces insulin resistance and metabolic
syndrome. In addition, TNF-α has the ability to impair insulin
signaling, and inhibition of TNF-α increases insulin sensitivity
(15,16). Under physiological conditions, NF-κB
binds to IKβ, which is present in the cytoplasm without activity
(17). However, phosphorylation of
IKKβ caused by external stimulation results in phosphorylation of
IKβ, and, following dissociation of NF-κB, IKβ enters the nucleus
to regulate the secretion and expression of inflammatory factors
(18). Previous studies have
demonstrated that obesity and a high-fat diet lead to fat deposits
in the liver that activate the IKKβ/NF-κB pathway, which induces
insulin resistance (19,20).
The present study aimed to investigate TNF-α
expression in adenoid tissues of obese children with OSAHS to
determine how TNF-α regulates insulin resistance via the
TNF-α/IKKβ/IKβ/NF-κB pathway in OSAHS.
Materials and methods
Human specimens
The present study enrolled 30 obese children with
OSAHS (8-12 years old; mean age, 10.13±1.65 years; 15 male/15
female) and 30 non-OSAHS obese children (8-12 years; mean age,
10.38±1.24 years; 15 male/15 female) from the Nantong First
People's Hospital (Jiangsu, China) between March 2018 and October
2019. The obesity criteria was referring to the ‘Screening for
overweight and obesity in school-age children and adolescents’
(WS/T 586-2018). Patient inclusion criteria were as follows: i) One
or more of the following clinical histories of sleep: Mouth
breathing, snoring, frequent waking, suffocating and enuresis; ii)
hypertrophy of the adenoids resulting in oropharyngeal and/or
nasopharyngeal stenosis; and iii) overnight polysomnography
monitoring performed within 1-2 weeks before surgery, with the
results meeting the diagnostic standard of OSAHS (21). The adenoid tissue samples obtained
from biopsy and plasma were stored at -80˚C until subsequent
experimentation. The present study was approved by the Ethics
Committee of Nantong First People's Hospital (grant no. 20180209NT)
and informed consent was provided by the patients or their family
members.
Western blotting
Total protein was extracted from adenoid tissues
with cell lysis buffer (Beyotime Institute of Biotechnology) and
quantified using the BCA protein analysis kit (cat. no. ab102536;
Abcam). Protein samples (50 µg/well) were separated by 10%
SDS-PAGE, transferred onto PVDF membranes and subsequently blocked
with 5% skim milk powder at room temperature for 2 h. The membranes
were incubated with primary antibodies against: TNF-α (cat. no.
ab215188; dilution, 1:1,000; Abcam), IL-1β (cat. no. ab216995;
dilution, 1:1,000; Abcam), IL-6 (cat. no. ab233706; dilution,
1:1,000; Abcam), IFN-γ (cat. no. 8455; dilution, 1:1,000; Cell
Signaling Technology, Inc.), insulin receptor substrate 1 (IRS1;
cat. no. ab40777; dilution, 1:1,000; Abcam), GLUT4 (cat. no.
ab216661; dilution, 1:1,000; Abcam), IKKβ (cat. no. ab124957;
dilution, 1:1,000; Abcam), phosphorylated (p)-IKKβ (cat. no. 5441R;
dilution, 1:500; Shanghai YaJi Biological Technology Co., Ltd.;
https://china.guidechem.com/trade/pdetail22435375.html#f_2),
IKβ (cat. no. ab109509; dilution, 1:1,000; Abcam), p-IKβ (cat. no.
HK6658, dilution, 1:500, Shanghai Hushi Pharmaceutical Technology
Co., Ltd.), NF-κB (cat. no. 8242; dilution, 1:1,000; Cell Signaling
Technology, Inc.), p-NF-κB (cat. no. HK5704; dilution, 1:500;
Shanghai Hushi Pharmaceutical Technology Co., Ltd.) and GAPDH (cat.
no. ab8245; dilution, 1:1,000; Abcam), overnight at 4˚C. Following
the primary antibody incubation, membranes were incubated with
horseradish peroxidase-conjugated secondary antibody (cat. no.
7074; 1:1,000; Cell Signaling Technology, Inc.) for 1-2 h at room
temperature. Protein bands were visualized using ECL reagent (EMD
Millipore) and Image-Pro Plus software (version 6.0; Media
Cybernetics, Inc.) was used for densitometry analysis. Protein
expression was presented as the ratio of the absorbance value of
the targeted protein to the internal reference absorbance value of
the internal control.
Reverse transcription-quantitative PCR
(RT-qPCR)
The adenoid tissues obtained from obese children
with OSAHS and control obese subjects were treated with 1 ml Trizol
(Thermo Fisher Scientific, Inc.) for lysis to extract the total
RNA. RNA level and purity were determined using an ultra-micro
ultraviolet spectrophotometer. cDNA was synthesized by reverse
transcription using total RNA as template with Transcriptor First
Strand cDNA Synthesis kit (Roche Molecular Systems, Inc.) at 42˚C
for 30 min and 85˚C for 5 min. PCR amplification was performed
using cDNA as template and GAPDH as internal reference. The
thermocycling conditions used for the qPCR were as follows: Initial
denaturation at 95˚C for 30 sec; 95˚C for 20 sec, 60˚C for 30 sec
and 72˚C for 40 sec for 35 cycles. The following primer pairs were
used for the qPCR: TNF-α forward, 5'-ACTTTA AGGGTTACCTGGGTTG-3' and
reverse, 5'-TCACATGCG CCTTGATGTCTG-3'; IRS1 forward,
5'-TGTCACCCAGTG GTAGTTGCTC-3' and reverse, 5'-CTCTCAACAGGAGGT
TTGGCATG-3'; GLUT4 forward, 5'-GCCCGAAAGAGT CTAAAG-3' and reverse,
5'-AGAGCCACGGTCATCAAG-3' and GAPDH forward, 5'-GATGCTGGTGCTGAGTATG
TCG-3' and reverse, 5'- TGGTGCAGGATGCATTGCTGA-3'. The expression
levels of TNF-α, IRS1 and GLUT4 were determined using the
SYBR-Green Realtime PCR kit (Beyotime Institute of Biotechnology)
and calculated using the 2-ΔΔCq method (22).
ELISA assay
Plasma samples obtained as aforementioned were taken
from the -80˚C refrigerator and thawed at room temperature.
According to the manual of ELISA kits, the levels of TNF-α, IL-1β,
IL-6 and IFN-γ in plasma of obese children with OSAHS and control
obese subjects were detected by human TNF-α, IL-1β, IL-6 IFN-γ
ELISA kit, respectively.
Immunohistochemistry
Adenoid tissues were fixed in 4% paraformaldehyde
for 48 h at room temperature, dehydrated and embedded in paraffin.
Paraffin-embedded tissue samples were cut into 3-µm-thick sections,
dewaxed in ethyl alcohol solution and washed in xylene solution.
Tissue sections were incubated with 3% hydrogen peroxide for 10 min
at room temperature to inhibit endogenous peroxidase activity, and
antigen retrieval was subsequently performed in EDTA buffer at
75˚C. Tissue sections were blocked with goat serum (Beyotime
Institute of Biotechnology) for 1 h at room temperature and
incubated with primary antibodies against TNF-α (cat. no. ab215188;
dilution, 1:100; Abcam), IRS1 (cat. no. ab40777; dilution, 1:500;
Abcam) and GLUT4 (cat. no. ab654; dilution, 1:1,000; Abcam) at 37˚C
for 30 min. Following the primary antibody incubation, the sections
were incubated with biotinylated goat anti-rabbit IgG antibody
(cat. no. ab150077; dilution, 1:200; Abcam) at 37˚C for 30 min, and
subsequently treated with streptavidin-biotin-peroxidase solution
(Beyotime Institute of Biotechnology). The slides were subsequently
stained with 3,3'-diaminobenzidine and finally observed under a
light microscope (magnification, x200).
Statistical analysis
All experiments were repeated three times.
Statistical analysis was performed using SPSS 19.0 software (IBM
Corp.). Data are presented as the mean ± standard deviation.
Unpaired Student's t-test was used to compare differences between
two groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
TNF-α expression in adenoid
tissues
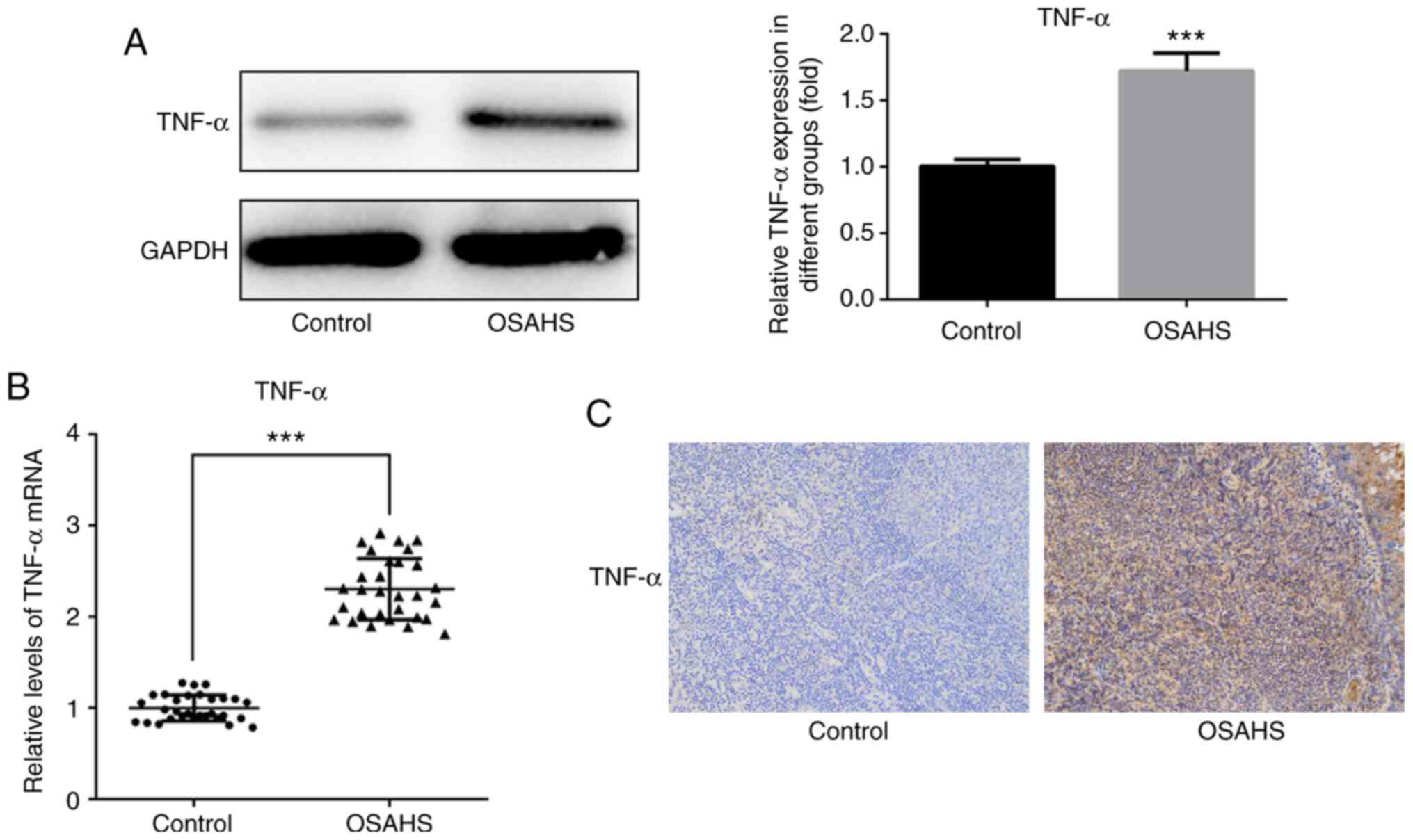
Western blot analysis was performed to detect TNF-α
expression in adenoid tissues. The results demonstrated that TNF-α
expression was notably higher in adenoid tissues of the OSAHS group
compared with that in the control group (Fig. 1A). The results of western blot
analysis were verified via RT-qPCR analysis (Fig. 1B) and immunohistochemistry analysis
(Fig. 1C).
Expression levels of the inflammatory
factors, TNF-α, IL-1β, IL-6 and IFN-γ, in adenoid tissues and
plasma
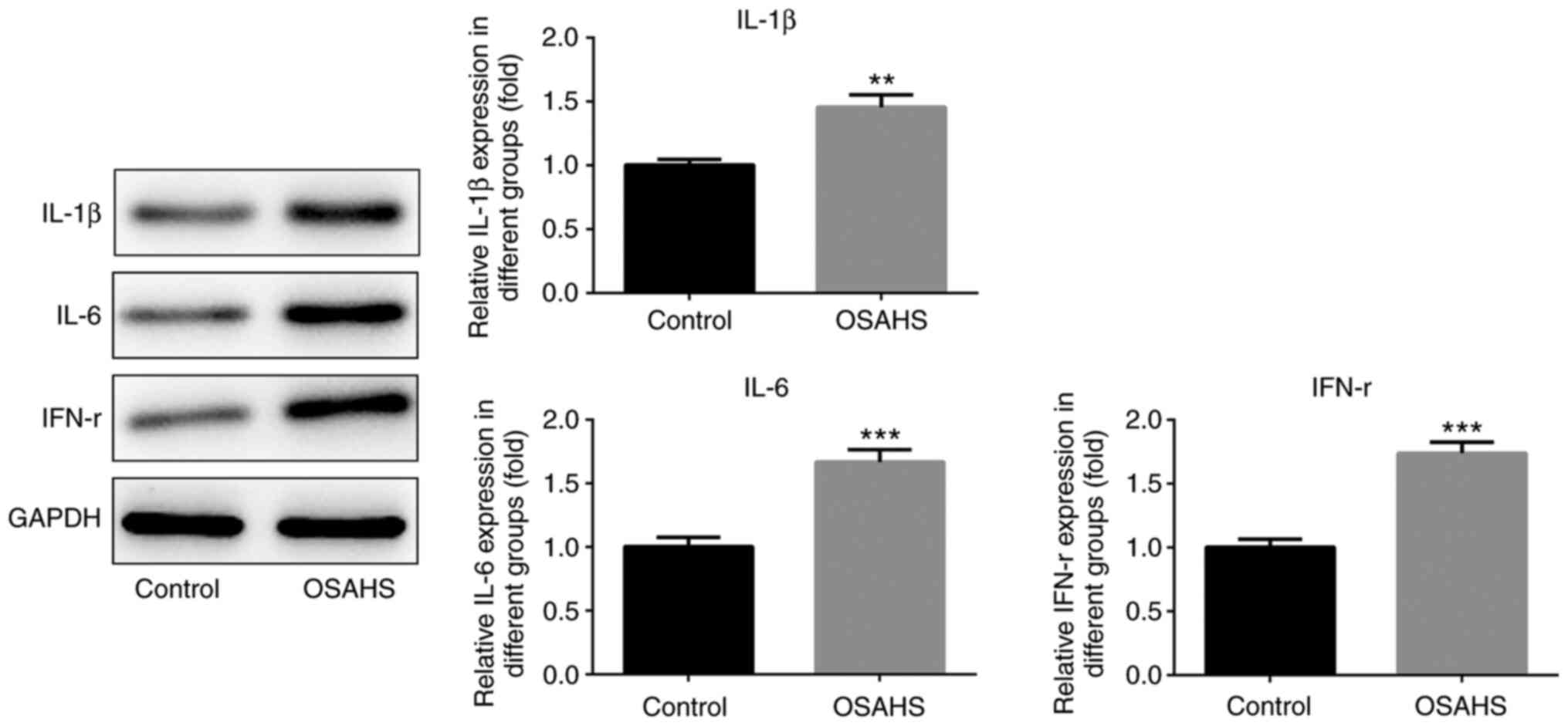
Western blot analysis was performed to detect the
protein expression levels of IL-1β, IL-6 and IFN-γ in adenoid
tissues. As presented in Fig. 2,
the protein expression levels of IL-1β, IL-6 and IFN-γ were higher
in adenoid tissues of the OSAHS group compared with those in the
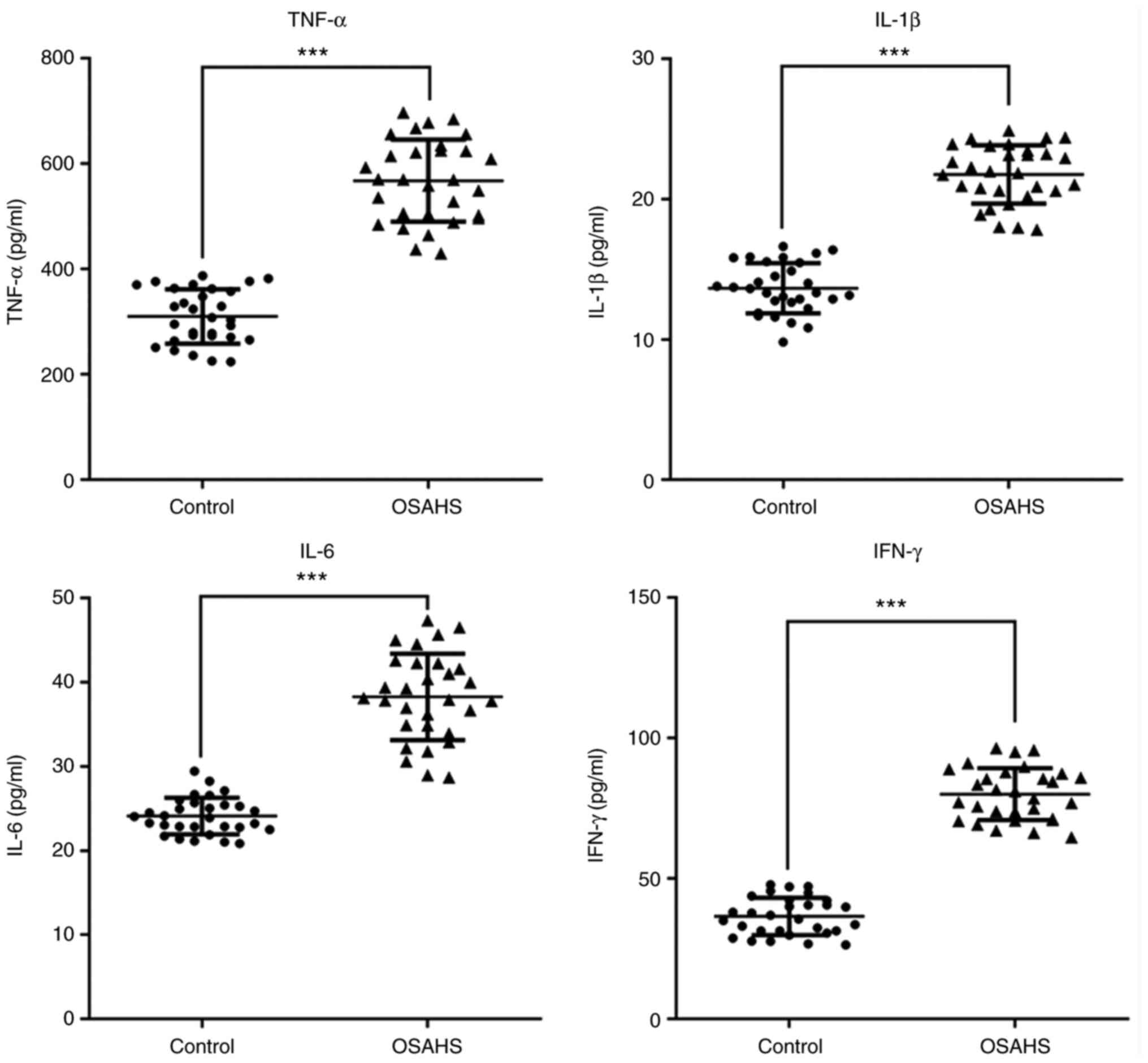
control group. The results presented in Fig. 3 indicated that the levels of TNF-α,
IL-1β, IL-6 and IFN-γ were also increased in plasma of the OSAHS
group compared with those in the control group.
Expression levels of the insulin
resistance-associated factors, IRS1 and GLUT, in adenoid
tissues
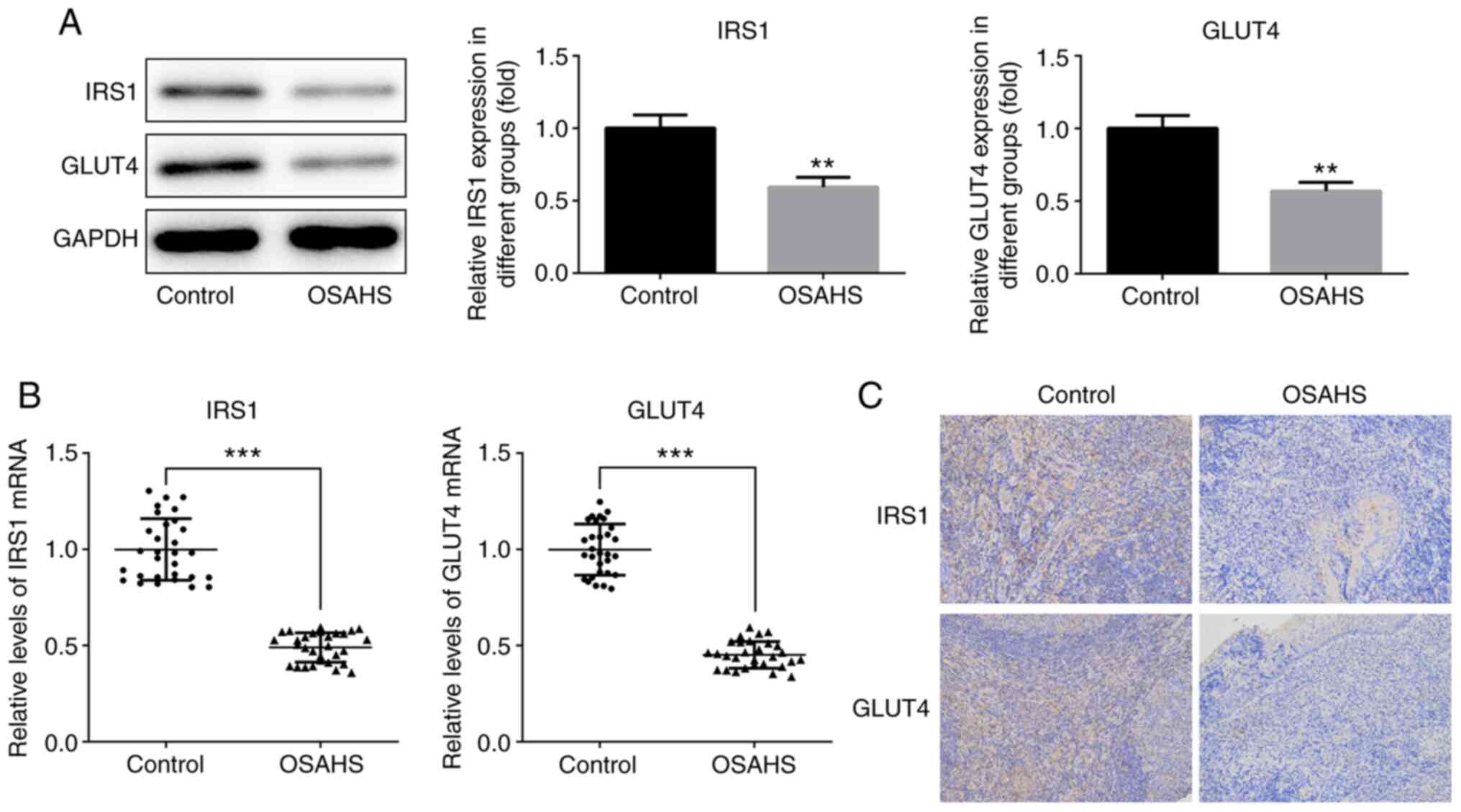
Western blot and immunohistochemistry analyses were
performed to detect IRS1 and GLUT4 expression in adenoid tissues.
The results demonstrated that IRS1 and GLUT4 expression levels were
downregulated in adenoid tissues of the OSAHS group compared with
those in the control group (Fig.
4A), which was consistent with RT-qPCR analysis (Fig. 4B) and immunohistochemistry analysis
(Fig. 4C).
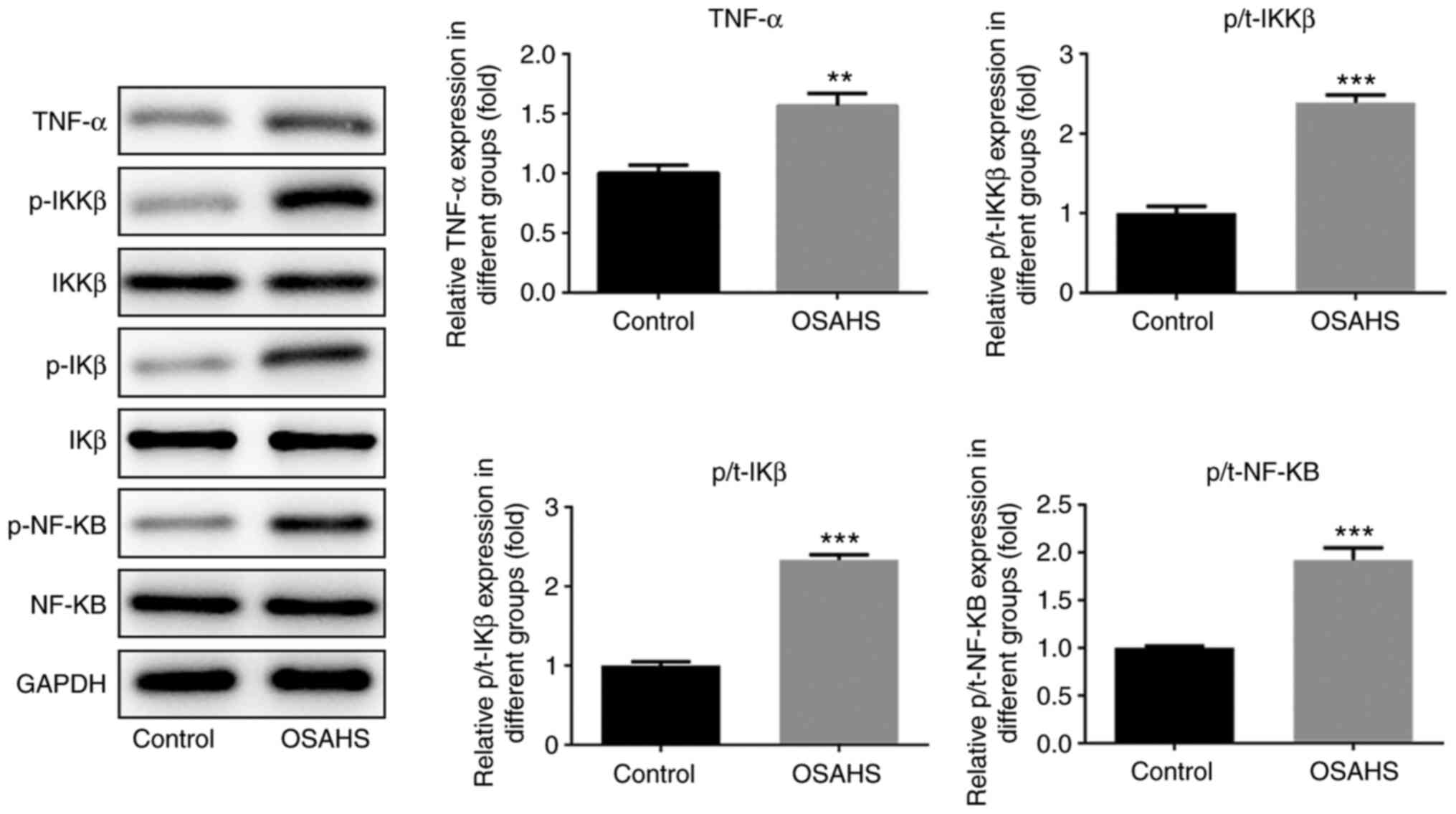
TNF-α may regulate insulin resistance
by activating the TNF-α/IKKβ/IKβ/NF-κB signaling pathway
Western blot analysis was performed to detect the
protein expression levels of TNF-α, IKKβ, p-IKKβ, IKβ, p-IKβ, NF-κB
and p-NF-κB in adenoid tissues. As presented in Fig. 5, the protein expression levels of
TNF-α, p-IKKβ, p-IKβ and p-NF-κB were higher in adenoid tissues of
the OSAHS group compared with the control group, while no
significant changes were observed in the expression levels of IKKβ,
IKβ and NF-κB between the two groups.
Discussion
The present study aimed to investigate whether TNF-α
decreases GLUT4 expression to promote insulin resistance via the
TNF-α/IKKβ/IKβ/NF-κB signaling pathway in OSAHS. The results
demonstrated that TNF-α expression was upregulated in adenoid
tissues of obese children with OSAHS, and TNF-α promote insulin
resistance via the TNF-α/IKKβ/IKβ/NF-κB pathway in OSAHS.
A previous study demonstrated that children with
OSAHS account for 1-5% of all childhood diseases (23). OSAHS can occur in children of all
ages, most of whom are 2-8 years old, because the tonsil and
adenoid hypertrophy account for important parts of the upper airway
in children of this age range (24). OSAHS in children is commonly
associated with impaired neurocognition, behavior, decreased
quality of life, systemic hypertension, increased risk of pulmonary
heart disease and increased health care spending (25-30).
OSAHS also causes metabolic syndrome in children, as well as in
adults (31). Previous studies have
demonstrated that OSAHS wwcan induce insulin resistance
independently of confounding factors, such as the amount of body
fat and age. Furthermore, insulin resistance, as one of the core
components of metabolic syndrome, may play an important role in the
systemic damage caused by OSAHS (32,33).
TNF-α is a small-molecule protein mainly secreted by
macrophages as a key regulator of systemic inflammation and has
several inflammatory biological functions (34,35).
Most patients with OSAHS are affected by pharyngeal inflammation,
which can induce a series of changes in the level of inflammatory
mediators, including increased secretion of TNF-α (36). The serum TNF-α level of patients
with OSAHS significantly increases during the day, which results in
a disordered sleep structure (37).
Ming et al (38) suggested
that TNF-α may be involved in the occurrence and development of
OSAHS, and is closely associated with OSAHS. Thus, TNF-α can be
used to assess the severity of OSAHS. The results of the present
study demonstrated that TNF-α expression was notably higher in
adenoid tissues of obese children with OSAHS, and the levels of
inflammatory factors, IL-1β, IL-6 and IFN-γ, also increased in
adenoid tissues of obese children with OSAHS.
Previous studies have reported that the main
molecular mechanism of insulin resistance caused by several
inflammatory factors is regulated by the association between the
signal transduction of inflammatory factors and the signal
transduction of pancreatic insulin receptors. The IKKβ/NF-κB
pathway is the primary signaling pathway that serves as a link
between inflammatory cytokines and insulin resistance (39,40).
Inflammatory factors stimulate the IKKβ/NF-κB pathway, which
contributes to the amplification and maintenance of the
inflammatory response through NF-κB, and leads to insulin
resistance following inhibition of the insulin signaling pathway
via IKKβ (41-43).
The results of the present study demonstrated that upregulated
TNF-α expression activated the IKKβ/IKβ/NF-κB pathway to
effectively induce insulin resistance, which in turn decreased the
expression levels of IRS1 and GLUT4.
Obesity, especially abdominal obesity, is an
independent risk factor for insulin resistance, and the
accumulation of solid fat has been shown to be highly correlated
with the onset of insulin resistance, type 2 diabetes, metabolic
syndrome and other diseases (44).
Insulin signaling disorder is closely associated with the
development of impaired glucose metabolism and insulin resistance
in cells. In obesity, the effector pathway of insulin will be
affected, resulting in insulin resistance, due to increased free
fatty acids (FFA), lipid deposition, activation of inflammatory
pathways, endoplasmic reticulum stress and mitochondrial
dysfunction (45). In addition, FFA
interferes with the insulin signal transduction pathway by
promoting the expression of inflammatory factors, thereby
interfering with mitochondrial function, the expression of
glucose/lipid metabolism-related genes and related enzymes,
increasing muscle reactive oxygen species levels and reducing
GLUT-4 expression (46). Therefore,
in the present study, it was hypothesized that obesity could affect
the TNF-α/IKKβ/IKβ/NF-κB pathway, which should be verified by
future investigation. The single factor (obesity) could be studied
to see how it could affect the TNF-α/IKKβ/IKβ/NF-κB pathway.
In conclusion, the present study investigated the
role of TNF-α in insulin resistance caused by OSAHS in obese
children and demonstrated that TNF-α promoted insulin resistance in
OSAHS. The results presented here provide novel insight for the
effective therapy of insulin resistance in obese children with
OSAHS. However, the present study has certain limitations. No
experiments were included to determine how obesity affected the
TNF-α/IKKβ/IKβ/NF-κB pathway, how this signaling pathway affected
the GLUT4 expression and insulin resistance and how treatment
affected the present factors detected in this study.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW conceived and designed the study. LZ performed
the experiments. LZ and YL analysed, interpretated and
authenticated the data. LZ wrote the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Nantong First People's Hospital (grant no. 20180209NT;
Nantong, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
De Backer W: Obstructive sleep
apnea/hypopnea syndrome. Panminerva Med. 55:191–195.
2013.PubMed/NCBI
|
|
2
|
Shen L, Lin Z, Xu Y and Yang Z: The
relationship between obstructive sleep apnea hypopnea syndrome and
adenoid size as well as tonsil size in children. Lin Chung Er Bi
Yan Hou Tou Jing Wai Ke Za Zhi. 28:381–385. 2014.PubMed/NCBI(In Chinese).
|
|
3
|
Yu M: Relationship between obstructive
sleep apnea hypopnea syndrome and obesity in children. J Clin Nurs.
18:58–60. 2019.
|
|
4
|
Chen J, Duo L and Ye H: Research progress
on obstructive sleep apnea hypopnea syndrome and insulin resistance
in children. Zhongguo Shiyong Erke Zazhi. 78–80. 2008.(In
Chinese).
|
|
5
|
He L, Li S, Shi H and Feng Y: The clinical
investigation of the relationship between obstructive sleep
apnea-hypopnea syndrome and insulin resistance. Lin Chung Er Bi Yan
Hou Tou Jing Wai Ke Za Zhi. 21:353–355. 2007.PubMed/NCBI(In Chinese).
|
|
6
|
Chen Z: The research of the correlation of
obstructive sleep apnea-hypopnea syndrome and insulin resistance.
Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 21:410–412.
2007.PubMed/NCBI(In Chinese).
|
|
7
|
Yang H, Yang Z-b, Zhu B, Zou Y and Tian
T-j: Clinical study on the growth and development of children with
mandibular retrusion and adenoid hypertrophy. J Clin Stomatol.
36:356–359. 2020.
|
|
8
|
Clemmons DR: Role of insulin-like growth
factor iin maintaining normal glucose homeostasis. Horm Res. 62
(Suppl 1):77–82. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Simpson HL, Jackson NC, Shojaee-Moradie F,
Jones RH, Russell-Jones DL, Sönksen PH, Dunger DB and Umpleby AM:
Insulin-like growth factor I has a direct effect on glucose and
protein metabolism, but no effect on lipid metabolism in type 1
diabetes. J Clin Endocrinol Metab. 89:425–432. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Borst SE: The role of TNF-alpha in insulin
resistance. Endocrine. 23:177–182. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hotamisligil GS: Mechanisms of
TNF-α-induced insulin resistance. Experimental and clinical
endocrinology & diabetes: Official journal, German Society of
Endocrinology. Ger Diabetes Assoc. 107:119–125. 1999.PubMed/NCBI View Article : Google Scholar : [and].
|
|
12
|
Galic S, Oakhill JS and Steinberg GR:
Adipose tissue as an endocrine organ. Mol Cell Endocrinol.
316:129–139. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vgontzas AN, Papanicolaou DA, Bixler EO,
Hopper K, Lotsikas A, Lin H-M, Kales A and Chrousos GP: Sleep apnea
and daytime sleepiness and fatigue: Relation to visceral obesity,
insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab.
85:1151–1158. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu H, Liu J, Xiong S, Shen G, Zhang Z and
Xu Y: The change of interleukin-6 and tumor necrosis factor in
patients with obstructive sleep apnea syndrome. J Tongji Med Univ.
20:200–202. 2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mohamad HE, Asker ME, Keshawy MM, Abdel
Aal SM and Mahmoud YK: Infliximab ameliorates tumor necrosis
factor-alpha exacerbated renal insulin resistance induced in rats
by regulating insulin signaling pathway. Eur J Pharmacol.
872(172959)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen FC, Shen KP, Ke LY, Lin HL, Wu CC and
Shaw SY: Flavonoids from Camellia sinensis (L.) O. Kuntze
seed ameliorates TNF-α induced insulin resistance in HepG2 cells.
Saudi Pharm J. 27:507–516. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Scheidereit C: IkappaB kinase complexes:
Gateways to NF-kappaB activation and transcription. Oncogene.
25:6685–6705. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Karin M: How NF-kappaB is activated: The
role of the IkappaB kinase (IKK) complex. Oncogene. 18:6867–6874.
1999.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Alkalay I, Yaron A, Hatzubai A, Orian A,
Ciechanover A and Ben-Neriah Y: I A. Stimulation-dependent I kappa
B alpha phosphorylation marks the NF-kappa B inhibitor for
degradation via the ubiquitin-proteasome pathway. Proc Natl Acad
Sci USA. 92:10599–10603. 1995.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cai D, Yuan M, Frantz DF, Melendez PA,
Hansen L, Lee J and Shoelson SE: Local and systemic insulin
resistance resulting from hepatic activation of IKK-beta and
NF-kappaB. Nat Med. 11:183–190. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Huang H and Huang H: Diagnosis of
obstructive sleep apnea hypopnea syndrome in children. Health Care
(Don Mills). 20:108–109. 2020.
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bixler EO, Vgontzas AN, Lin H-M, Liao D,
Calhoun S, Vela-Bueno A, Fedok F, Vlasic V and Graff G: Sleep
disordered breathing in children in a general population sample:
Prevalence and risk factors. Sleep. 32:731–736. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Guilleminault C and Pelayo R:
Sleep-disordered breathing in children. Ann Med. 30:350–356.
1998.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Enright PL, Goodwin JL, Sherrill DL, Quan
JR and Quan SF: Tucson Children's Assessment of Sleep Apnea study.
Blood pressure elevation associated with sleep-related breathing
disorder in a community sample of white and Hispanic children: The
Tucson Children's Assessment of Sleep Apnea study. Arch Pediatr
Adolesc Med. 157:901–904. 2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kohyama J, Ohinata JS and Hasegawa T:
Blood pressure in sleep disordered breathing. Arch Dis Child.
88:139–142. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Leung LC, Ng DK, Lau MW, Chan CH, Kwok KL,
Chow PY and Cheung JM: Twenty-four-hour ambulatory BP in snoring
children with obstructive sleep apnea syndrome. Chest.
130:1009–1017. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li AM, Au C-T, Sung RY, Ho C, Ng PC, Fok
TF and Wing YK: Ambulatory blood pressure in children with
obstructive sleep apnoea: A community based study. Thorax.
63:803–809. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Marcus CL, Greene MG and Carroll JL: Blood
pressure in children with obstructive sleep apnea. Am J Respir Crit
Care Med. 157:1098–1103. 1998.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sateia MJ: International classification of
sleep disorders-third edition: Highlights and modifications. Chest.
146:1387–1394. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sun SS, Grave GD, Siervogel RM, Pickoff
AA, Arslanian SS and Daniels SR: Systolic blood pressure in
childhood predicts hypertension and metabolic syndrome later in
life. Pediatrics. 119:237–246. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Du J, Gu C and Li M: Research progress in
the relationship between obstructive sleep apnea-hypopnea syndrome
and insulin resistance International Journal of. Respiration.
33:1503–1506. 2013.
|
|
33
|
Sarac F, Basoglu OK, Gunduz C, Bayrak H,
Biray Avci C and Akcicek F: Association of osteopontin and tumor
necrosis factor-α levels with insulin resistance in obese patients
with obstructive sleep apnea syndrome. J Endocrinol Invest.
34:528–533. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Wang H, Li J, Gai Z, Kullak-Ublick GA and
Liu Z: TNF-α deficiency prevents renal inflammation and oxidative
stress in obese mice. Kidney Blood Press Res. 42:416–427.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Davizon-Castillo P, McMahon B, Aguila S,
Bark D, Ashworth K, Allawzi A, Campbell RA, Montenont E, Nemkov T,
D'Alessandro A, et al: TNF-α-driven inflammation and mitochondrial
dysfunction define the platelet hyperreactivity of aging. Blood.
134:727–740. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Camacho M, Certal V, Abdullatif J, Zaghi
S, Ruoff CM, Capasso R and Kushida CA: M C: V C, J A, S Z, CM R, R
C and CA K. Myofunctional therapy to treat obstructive sleep apnea:
A systematic review and meta-analysis. Sleep (Basel). 38:669–675.
2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Khalyfa A, Serpero LD, Kheirandish-Gozal
L, Capdevila OS and Gozal D: TNF-α gene polymorphisms and excessive
daytime sleepiness in pediatric obstructive sleep apnea. J Pediatr.
158:77–82. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ming H, Tian A, Liu B, Hu Y, Liu C, Chen R
and Cheng L: Inflammatory cytokines tumor necrosis factor-α,
interleukin-8 and sleep monitoring in patients with obstructive
sleep apnea syndrome. Exp Ther Med. 17:1766–1770. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Shoelson SE, Lee J and Yuan M:
Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity-
and diet-induced insulin resistance. Int J Obes Relat Metab Disord.
27 (Suppl 3):S49–S52. 2003.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Benzler J, Ganjam GK, Pretz D, Oelkrug R,
Koch CE, Legler K, Stöhr S, Culmsee C, Williams LM and Tups A:
Central inhibition of IKKβ/NF-κB signaling attenuates high-fat
diet-induced obesity and glucose intolerance. Diabetes.
64:2015–2027. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jové M, Planavila A, Sánchez RM, Merlos M,
Laguna JC and Vázquez-Carrera M: Palmitate induces tumor necrosis
factor-alpha expression in C2C12 skeletal muscle cells by a
mechanism involving protein kinase C and nuclear factor-kappaB
activation. Endocrinology. 147:552–561. 2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
de Alvaro C, Teruel T, Hernandez R and
Lorenzo M: Tumor necrosis factor alpha produces insulin resistance
in skeletal muscle by activation of inhibitor kappaB kinase in a
p38 MAPK-dependent manner. J Biol Chem. 279:17070–17078.
2004.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Mireia J, Anna P, Carlos LJ and Manuel
Vz-C: Palmitate-induced interleukin 6 production is mediated by
protein kinase c and nuclear-factor κB activation and leads to
glucose transporter 4 down-regulation in skeletal muscle cells.
Endocrinology. 146:3087–3095. 2005.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Czech MP: Insulin action and resistance in
obesity and type 2 diabetes. Nat Med. 23:804–814. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
45
|
Makki K, Froguel P and Wolowczuk I:
Adipose tissue in obesity-related inflammation and insulin
resistance: Cells, cytokines, and chemokines. ISRN Inflamm.
2013(139239)2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ji C, Chen X, Gao C, Jiao L, Wang J, Xu G,
Fu H, Guo X and Zhao Y: IL-6 induces lipolysis and mitochondrial
dysfunction, but does not affect insulin-mediated glucose transport
in 3T3-L1 adipocytes. J Bioenerg Biomembr. 43:367–375.
2011.PubMed/NCBI View Article : Google Scholar
|