Introduction
Osteoporosis (OP) is a chronic disease characterized
by decreased bone strength, which predisposes affected individuals
to fractures (1). With the aging
population, it represents a growing global public health problem.
In the US, among adults aged ≥50 years, ~10.3% or 10.2 million had
OP of the lumbar spine or femoral neck and 43.9% or 43.4 million
had osteopenia at skeletal sites in 2010(2). The medical expenses for OP management
place a heavy financial burden on society and individuals (3). For instance, >1.7 million patients
were admitted to hospital with fragility fractures in 2011 and the
direct costs associated with OP treatment exceeded $70 billion in
the US (4).
Pharmaceuticals that improve bone mineral density
(BMD) are used as the first-line treatment for OP. However, due to
poor compliance, high medical costs and negative side effects, the
overall outcomes remain limited (5)
and exercise training is one of the proven alternative strategies
that improves multiple skeletal structures and reduces the fall
risk simultaneously (6). Agents
currently approved for OP treatment, such as calcium and vitamin D,
have the major function of inhibiting bone resorption. However,
once OP is developed, substantial increases in bone mass may be
necessary to reverse the disease. Therefore, it is necessary to
develop therapeutics that increase bone formation (7).
To date, various pharmacological treatments for
osteoporosis have been proven to reduce fracture risks, including
bisphosphonates, selective estrogen receptor modulators, the
monoclonal antibody against the receptor activator of nuclear
factor-κB ligand (RANKL) (8).
Numerous herbal medicines derived from natural products have been
used in the treatment of osteoporosis with relatively few negative
effects (9). In addition, several
bioactive components have been identified for alternative
treatments of OP (9) and a large
number of them promote osteogenic differentiation via osteoblast
activation (10). These therapeutic
agents have been proven to promote osteogenesis with osteoblasts as
the target (11,12).
Irisin is a newly discovered myokine produced during
exercise (13). It drives brown
fat-like development of white fat to generate thermal energy.
Previous studies have indicated that irisin exerts the beneficial
effect of exercise on metabolic diseases such as diabetes, obesity
and osteoporosis (14,15). The level of irisin in plasma was
determined to be related to BMD in the elderly (16), implying that it has a role in bone
development. Recently, irisin has been demonstrated to promote
osteoblast differentiation and mineralization in rat osteoblasts
and MC3T3-E1 cells. It upregulates the expression of osteoblastic
transcription regulators, such as runt-related transcription
factor-2 (RUNX2), osterix (OSX)/sp7 and differentiation markers,
such as alkaline phosphatase (ALP) (17). However, the receptor for irisin has
not been identified, although it is suggested that irisin may
activate P38/ERK MAP kinase signaling cascades to promote
osteogenic activity (17). In
addition, Colaianni et al (18) reported that excise increases irisin
levels in mouse myoblasts and that media conditioned with the
myoblasts extracted from exercised mice induces osteoblast
differentiation in vitro, demonstrating that irisin directly
targets osteoblasts. Besides osteoblasts, irisin was also indicated
to promote osteogenic activity of human periodontal ligament cells
by enhancing extracellular matrix formation, indicating its
potential for dental tissue engineering (19). Since irisin is released during
excise and its level is related to BMD, it was hypothesized that it
may be explored for potential use in the prevention and treatment
of OP.
In the present study, the effect of irisin on
osteogenic differentiation was investigated using MC3T3-E1 cells.
This cell line is one of the most common cell lines for studying
osteoblast proliferation and differentiation (20). It was established from newborn mouse
calvaria and selected on the basis of high ALP activity during
differentiation into osteoblasts in vitro. The present
results provide insight into the roles of irisin in osteogenesis
and clues for potential therapeutics for OP.
Materials and methods
Cells
Mouse calvaria-derived pre-osteoblast cell line
MC3T3-E1 (CL-0378) was purchased from Procell Life Science &
Technology Co., Ltd. and maintained in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin in 5% CO2 at 37˚C. Cells were
grown to 85% confluence and subcultured as previously described
(21).
Reagents and instruments
Irisin was purchased from Phoenix Pharmaceuticals,
Inc.; DMEM was obtained from Thermo Fisher Scientific, Inc.;
Minimal Essential Media and FBS were obtained from HyClone
(Cytiva); human mesenchymal stem cell osteogenic differentiation
medium (cat. no. PT-3002) was purchased from Lonza Group Ltd.; the
ALP quantification kit and MTT (cat. no. C0009) were obtained from
Beyotime Institute of Biotechnology; the RNA reverse-transcription
kit was a product of Invitrogen (Thermo Fisher Scientific, Inc.);
alizarin red S (ARS) staining solution was purchased from Beijing
Solarbio Science & Technology Co., Ltd.; the High Capacity cDNA
Transcriptase Reverse kit was purchased from Applied Biosystems
(Thermo Fisher Scientific, Inc.); the SYBR Green PCR kit was
obtained from Takara Bio, Inc.; the UV spectrophotometer Evolution
201/220 was purchased from Thermo Fisher Scientific, Inc.; the
fluorescence quantitative (q)PCR instrument LightCycler96 was
purchased from Roche Diagnostics; the PCR thermal cycler PTC-200
was purchased from MJ Research, Inc. (Bio-Rad Laboratories, Inc.);
the inverted microscope (Eclipse TS100) was a product of Nikon
Corp. and the plate reader (Triad LT Multimode Detector) was
obtained from Dynex Technologies, Inc.
Cell culture and osteogenic
differentiation
MC3T3-E1 cells at the third passage were used in the
experiments. At 24 h after the cells were seeded in wells of
24-well plates at 2x104 cells/well, the growth media
were replaced with commercial osteogenic differentiation medium
containing inducers and growth factors (included in the commercial
medium), including dexamethasone, ascorbate, β-glycerophosphate and
mesenchymal cell growth supplement, L-glutamine and 1%
penicillin/streptomycin and cultured in a humidified atmosphere
with 5% CO2 at 37˚C. Irisin was dissolved in sterile
normal saline (NS) at 100 µg/ml and filter-sterilized using a
membrane filter with a pore size of 0.22 µm. It was added to the
medium at final concentrations of up to 100 ng/ml from the
beginning of the experiments. NS was used as a control. Media were
refreshed every 3 days. All of the cultures were grown for 21 days
and then harvested for assessing the osteogenic markers. Cell
cultures were collected and stored at -80˚C prior to RNA
extraction. For each treatment, six wells (samples) were used and
all experiments were performed for a total of three times.
MTT assay
MTT assays were performed according to the
manufacturer's protocols, as described previously (22). In brief, cells were seeded
(1x105 cells/well) in 96-well plates and exposed to
irisin for 48 h at 37˚C. After treatment, 20 µl MTT solution was
added to each well and the plate was incubated for 4 h at 37˚C. The
reaction was stopped by adding 150 µl DMSO to dissolve the purple
formazan dye precipitate, after the media/MTT solution had been
removed. The absorbance was read at 490 nm using a plate reader
(Triad LT Multimode Detector; Dynex Technologies, Inc.). Cell
viability was normalized using NS-treated cells as a control and
calculated as described previously (22).
ALP activity assays
After exposure to medium containing irisin, ALP
activity was measured colorimetrically with a commercial ALP
colorimetric assay kit using p-nitrophenyl phosphate (pNPP) as the
phosphatase substrate according to the supplier's protocols and as
described previously (23). In
brief, cell lysates were prepared using three cycles of
freeze-thawing. Subsequently, 30 µl of the cell lysate was added to
each well of a 96-well plate with 50 µl assay buffer and 50 µl
pNPP. The samples were incubated at room temperature for 1 h. After
this, 20 µl stop solution (3N NaOH) was added to the wells and the
plates were read at a wavelength of 405 nm with the plate reader.
The protein concentrations in the samples were determined using a
bicinchoninic acid assay (Pierce; Thermo Fisher Scientific, Inc.)
according to the supplier's protocol.
ARS staining and quantification of
calcium deposits
ARS staining was used to detect calcium
mineralization according to the manufacturer's protocol. In brief,
cells (1x105) were rinsed with PBS, fixed with 4%
formaldehyde (m/v) in PBS for 30 min, washed with deionized water
and stained for 30 min with 0.5% ARS (w/v in water; pH 6.36-6.4) at
37˚C. Subsequently, the cells were washed with water and viewed
under an inverted microscope (magnification, x100). Calcium
deposits were observed as orange and red spots. For staining
quantification, the deposits were eluted with 20% (v/v) methanol
and 10% (v/v) acetic acid. The absorbance of the extract was
determined at 450 nm wavelength with the plate reader as described
previously (24).
Reverse transcription (RT)-qPCR
To detect the expression of osteogenesis-related
genes, total RNA was extracted from MC3T3-E1 cells using TRIzol
reagent, according to the supplier's protocols, and reverse
transcribed to complementary (c)DNA using the High Capacity cDNA
Transcriptase Reverse kit, according to the manufacturer's
protocols. The transcription levels were determined by qPCR using
the SYBR Green PCR kit on the thermal cycler PTC-200. The PCR
reaction mixtures contained 1 µl cDNA, 10 µl SYBR®
Premix Ex Taq and 0.25 µM each of the primers in a total volume of
25 µl. The PCR started with initial DNA denaturation at 95˚C for 30
sec, followed by 40 cycles of 33 sec at 95˚C and 4 min at 60˚C. The
data were managed using the Applied Biosystems software RQ Manager
v1.2.1 (Thermo Fisher Scientific, Inc.). Relative expression was
calculated by using comparative quantification cycle (Cq) method to
obtain the fold change value (2-ΔΔCq) according to a
previously described protocol (25)
with β-actin as the reference gene. The primers used for PCR are
listed in Table I. Three samples
were measured in each experiment of reverse transcription and PCR
amplification.
 | Table IPCR primers for osteogenic genes. |
Table I
PCR primers for osteogenic genes.
| Gene/primer
direction | Sequence |
|---|
| ALP | |
|
Forward |
5'-CAGACCAGCACACTCCATA-3' |
|
Reverse |
5'-CAGCTCAACACCATCATTC-3' |
| OSX | |
|
Forward |
5'-AGAAGCCATACACTGACCTTTC-3' |
|
Reverse |
5'-GGTGGGTAGTCATTGGCATAG-3' |
| RUNX2 | |
|
Forward |
5'-ACTTCCTGTGCTCCGTGCTG-3' |
|
Reverse |
5'-TCGTTGAACCTGGCTACTTGG-3' |
| ERα | |
|
Forward |
5'-CGCCGTGTTCAACTAC-3' |
|
Reverse | 5'-
AAGCCCCCAGACTATT-3' |
| COL-1 | |
|
Forward |
5'-GGGTCTAGACATGTTCAGCTTTGTG-3' |
|
Reverse |
5'-ACCCTTAGGCCATTGTGTATGC-3' |
| OCN | |
|
Forward |
5'-CAAGCAGGAGGGCAATAAGGT-3' |
|
Reverse |
5'-AGCAGGGTCAAGCTCACATAG-3' |
| OPN | |
|
Forward |
5'-CCCATCTCAGAAGCAGAATCTT-3' |
|
Reverse |
5'-GTCATGGCTTTCATTGGAGTTG-3' |
| OPG | |
|
Forward |
5'-CAAAGGCAGGGCATACTTC-3' |
|
Reverse |
5'-TTCAATGATGTCCAAGAACACC-3' |
| β-actin | |
|
Forward |
5'-CTGTCCCTGTATGCCTCTG-3' |
|
Reverse |
5'-TGATGTCACGCACGATTT-3' |
Statistical analysis
Statistical analysis was performed using SPSS v19.0
(IBM Corp.). All data were expressed as the mean ± standard error
of the mean obtained from at least three independent experiments.
Statistical comparisons between groups were assessed using one-way
ANOVA followed by a Tukey's post-hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
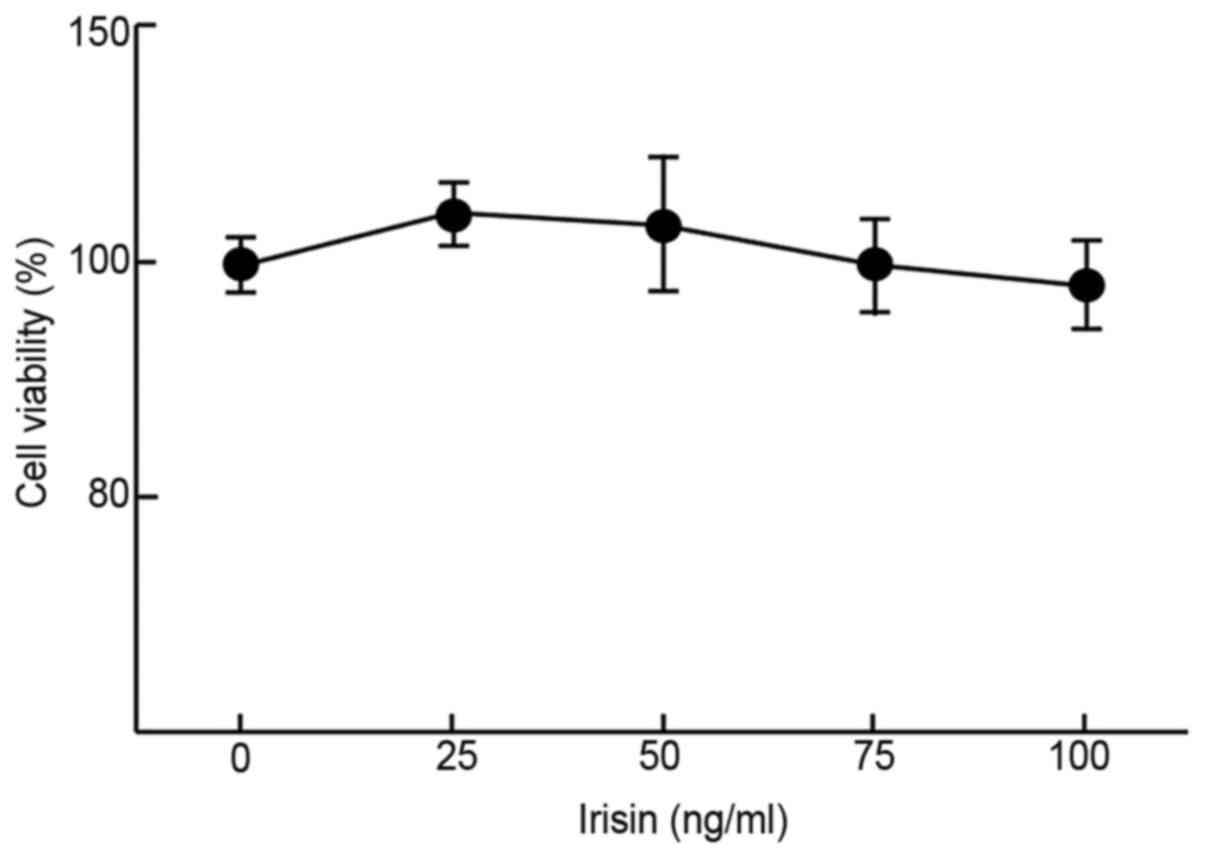
Irisin does not affect the
proliferation of MC3T3-E1 cells
The impact of irisin on the growth/viability of
MC3T3-E1 cells in vitro was examined using MTT assays to
evaluate its possible cytotoxicity. The results suggested that
irisin did not have any significant impact on the viability of
MC3T3-E1 cells at up to 100 ng/ml as compared to the negative
control (99±4.8% at 100 ng/ml vs. 100±2.7% at 0 ng/ml; Fig. 1) after incubation for 48 h. At low
concentrations (50 ng/ml or less), it slightly but insignificantly
increased the cell viability as compared with the control (112±3.1
and 105±7.5% at 25 and 50 mg/ml, respectively, vs. 100% at 0 ng/ml;
Fig. 1).
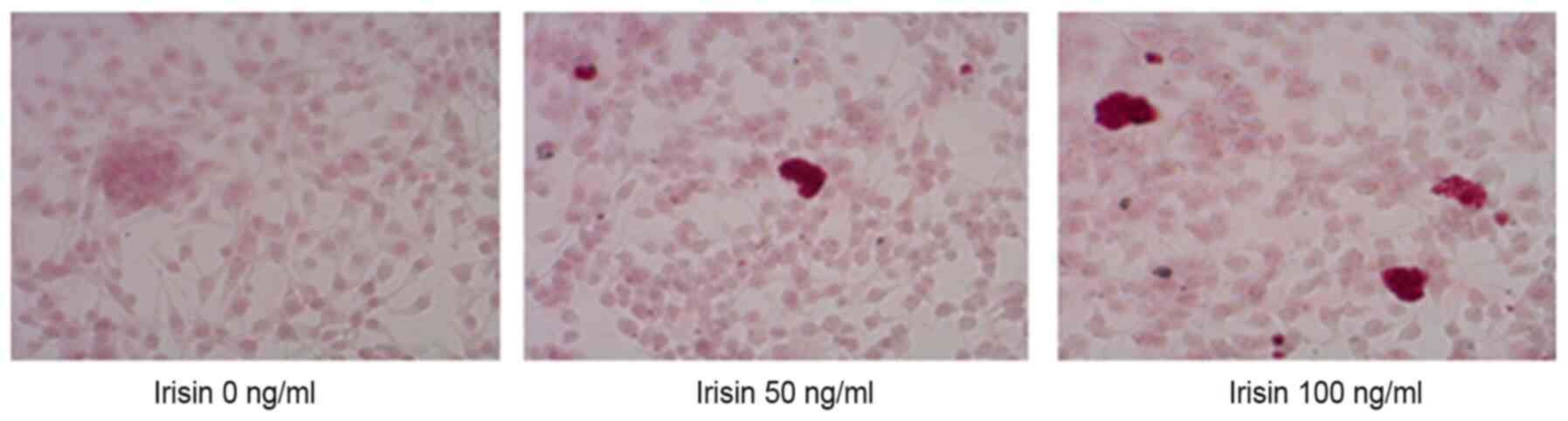
Irisin increases the ALP activity and
promotes the formation of mineralized nodules
ARS staining indicated calcium-rich deposits in the
cells starting after culturing the cells in the osteogenic medium
for one week (Fig. 2). The
formation of mineralized nodules was observed after MC3T3-E1 cells
were cultured in osteogenic medium for one week and became clearly
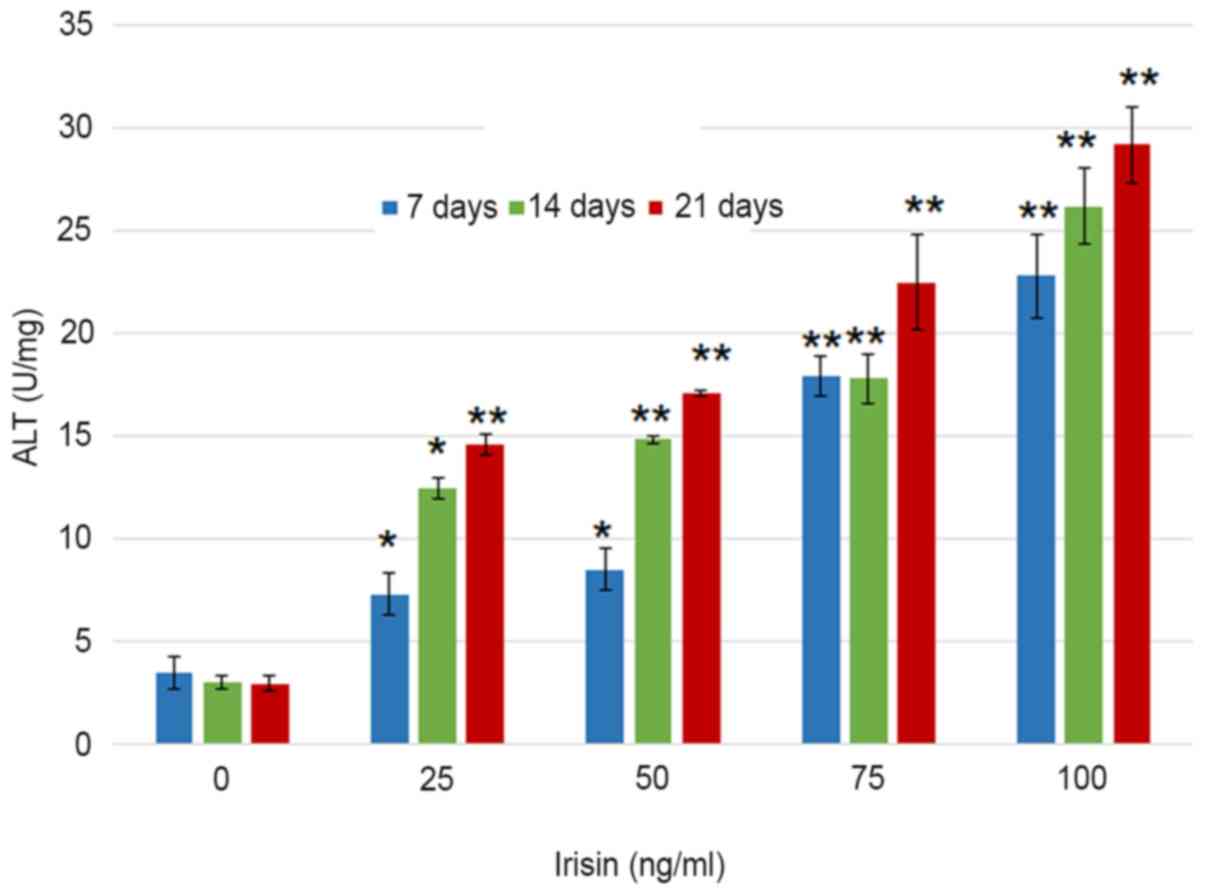
visible at three weeks even with the naked eye (Fig. 2). Assessment of ALP activity in
MC3T3-E1 cells suggested that the ALP enzymatic activity increased
from 3.51±0.76 to 22.82±2.05 U/mg on day 7 as the concentration of
irisin increased from 0 to 100 ng/ml, and from 22.82±2.05 to
29.1±1.86 U/mg at 100 mg/ml irisin as the culture time increased
from 7 to 21 days (Fig. 3). The
increase over time appeared more notable at the low irisin
concentrations of 25 and 50 ng/ml. However, the enzymatic activity
of ALP was not significantly changed over the culture period with
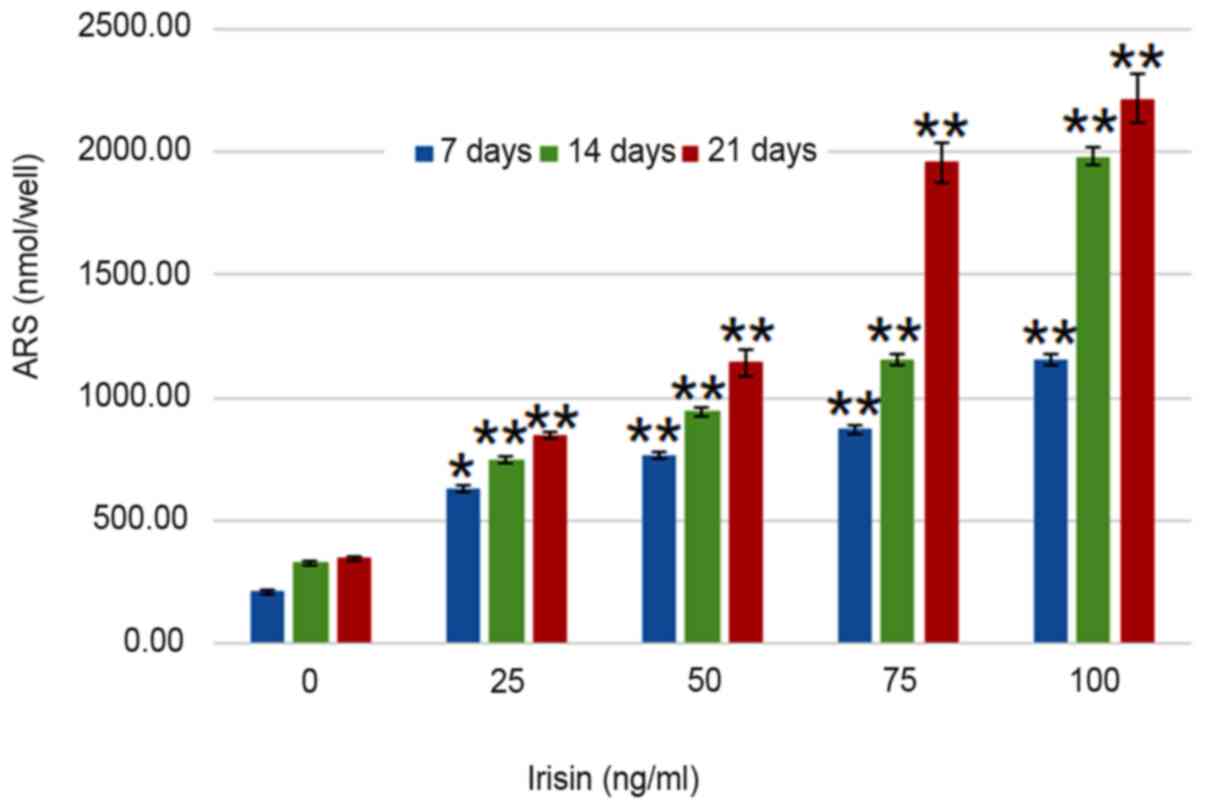
irisin. Analyses suggested that calcium deposits increased in an
irisin concentration- and time-dependent manner (P<0.05;
Fig. 4). On day 7, calcium in
MC3T3-E1 cells increased from 209.30±7.24 nmol/well at 0 mg/ml
irisin to 1,153.65±25.51 nmol/well at 100 mg/ml and this trend was
seen on days 14 and 21, and the amount of ARS increased as the
culture time increased. On the other hand, calcium contents in the
control group only exhibited an insignificant increase over the
experimental period (P>0.05; Fig.
4).
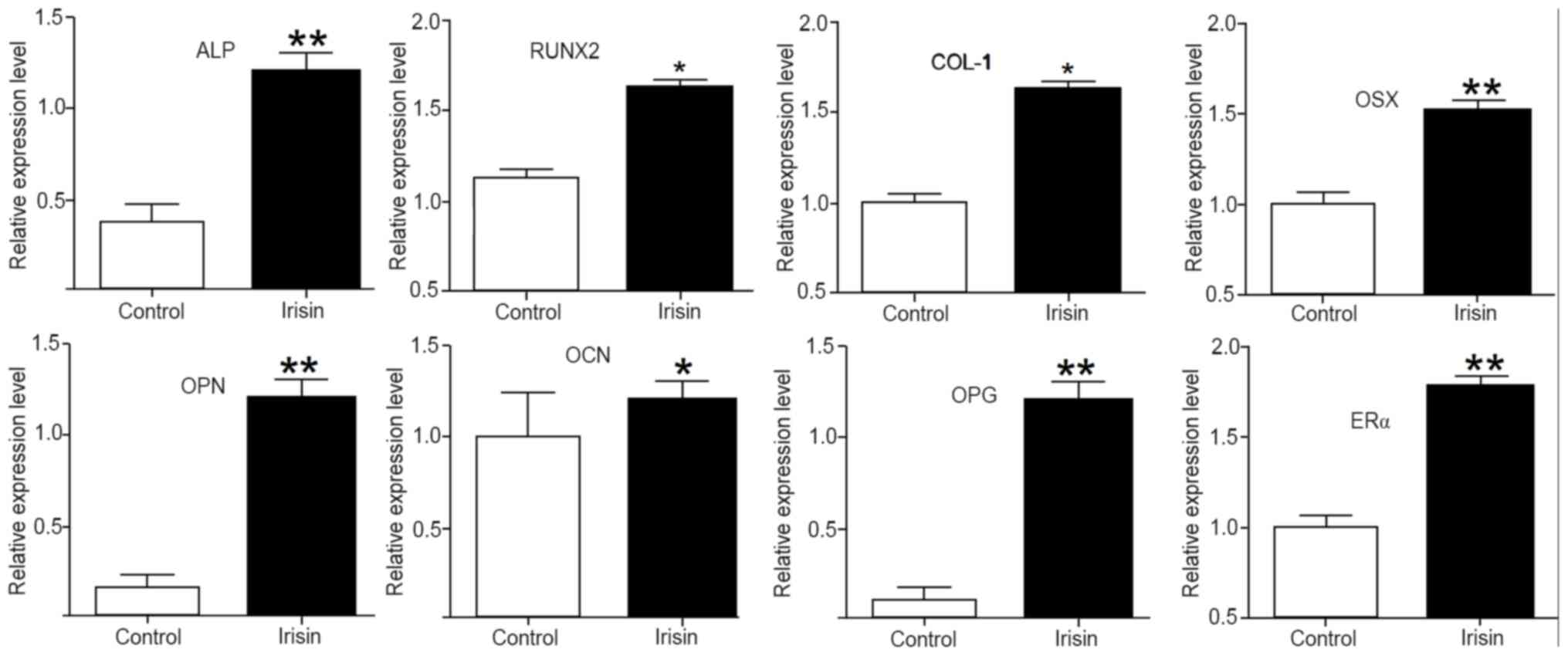
Irisin upregulates the expression of
osteogenic genes and markers
To elucidate the mechanisms underlying the enhanced
osteogenic activity stimulated by irisin, the expression of
osteogenic genes and markers was assessed using RT-qPCR. After
exposure to irisin for 21 days, the mRNA levels of ALP, collagen I
(COL-1), RUNX2, OSX, osteopontin (OPN), osteocalcin (OCN),
osteoprotegerin (OPG) and estrogen receptor α (ERα) were all
significantly increased (P<0.05 or P<0.01) as compared with
those in the control. Among them, the upregulations were more
obvious for ALP, OPN, OPG, Erα and OSX, but less intense for OCN
(Fig. 5).
Discussion
In vitro osteogenic differentiation may be
detected and assessed in several ways, including measuring ALP
enzymatic activity, extracellular matrix mineralization and levels
of osteogenesis related genes, such as OSX, RUNX2, BMP-2, OPN,
COL-1, OCN, OPG and ALP (26).
Several of these genes are considered as osteogenic markers, which
have important roles during early osteoblastic differentiation and
late mineralization (27) and were
chosen to measure the osteogenic induction ability of irisin in the
present study. The results indicated that irisin increased the
enzymatic activity of ALP and calcium deposits, and promoted the
differentiation of MC3T3-E1 cells into osteoblasts and the
formation of mineralized nodules in cultured MC3T3-E1 cells. At the
molecular level, it upregulated the expression of
osteogenesis-related genes and markers. Furthermore, at the
concentrations used, it did not have a significant negative impact
on the viability of MC3T3-E1 cells. Therefore, the peptide may be
further explored as a potential candidate for OP therapy.
Osteogenesis is a complex biological process where a
number of genes are involved in shaping the direction of
differentiation. It has been demonstrated that early/late
osteoblastic proliferation and differentiation may be estimated by
assessing the expression of COL-I, ALP, OCN and OPN, although these
markers have not been demonstrated to indicate the differentiation
of MC3T3-E1 cells towards the osteogenic lineage (27). ALP is associated with a perosseous
cellular metabolism and to the elaboration of a bone matrix that is
chemically calcifiable (28). The
activity of ALP increases during osteogenic differentiation in
various cell types, such as mesenchymal stem cells (29). In the present study, MC3T3-E1 cells
exposed to irisin had significantly higher ALP activity than the
control cells, suggesting that irisin increases ALP activity,
likely via upregulating the expression of the ALP gene, as
indicated by RT-qPCR analysis.
RUNX2 was significantly upregulated in the cells
grown in irisin-supplemented medium. As a major transcription
factor of the bone, RUNX2 is essential for the differentiation of
pluripotent mesenchymal cells to osteoblasts and also controls the
proliferation, differentiation and maintenance of these cells
(30). It is regarded as an early
specific biomarker for osteogenic differentiation of osteoblasts.
However, since its expression was assessed 21 days after the cells
were exposed to irisin in the present study, further study is
required to demonstrate its role in early osteogenic
differentiation.
Collagens are the major components of bone matrix
and other connective tissues (31).
A previous study indicated that insulin-like growth factor I and
transforming growth factor-β1 promote osteoblast differentiation
via upregulating COL-1, which, on the other hand, inhibits the
production of osteoblasts in vitro (32). In the present study, increased COL-1
expression was observed. It would be worthwhile investigating
whether such an increase is able to reduce the proliferation of
osteoblasts simultaneously. Furthermore, the biological action of
collagen is impacted by the interactions with other bone materials,
such as glycosaminoglycan and minerals. When crosslinked with
chitosan and hydroxyapatite, it may form a matrix that enhances
bone growth and reduces bone resorption (33). Therefore, it is likely that COL-1
expression has a role in the later stage of osteogenesis.
OSX, also known as transcription factor Sp7, is a
member of the Sp family of zinc-finger transcription factors. Along
with RUNX2, it functions as a major player to drive the
differentiation of mesenchymal precursor cells into osteoblasts and
eventually osteocytes (34). A
recent study indicated that OSX may be activated by long non-coding
RNA MALAT1 to promote osteogenic differentiation of human BM-MSCs
(35). This is consistent with the
present results.
As a multifunctional protein, OPN has a crucial role
in bone remodeling and biomineralization. It has been indicated to
have a regulatory effect on hydroxyapatite crystal growth and may
activate the signaling pathways mediated by various osteogenic
genes, including OPN, bone sialoprotein, OSX and OCN (36). OCN, also known as bone
γ-carboxyglutamic acid-containing protein, is specifically
synthesized and secreted by osteoblasts in the non-proliferative
phase. Generally, OCN is accumulated after the peak period of bone
mineralization and is regulated by RUNX2. In the present study, OCN
was relatively less upregulated as compared with the other
osteogenic genes assessed. This may be attributed to the timing of
the assay, since this gene may be more active after bone
mineralization.
The RANK/RANKL/OPG axis has an important role in
osteoclast regulation and bone homeostasis. Knockout of OPG, the
soluble decoy receptor for RANKL, was indicated to generate
profound osteoporosis (37). In
addition, OPG reduces osteoclast resorption on mineralized collagen
scaffolds (38). Therefore,
increased OPG expression changes the balance of
osteogenic-osteoclastic functions and results in osteogenic
differentiation, probably via increasing the activity of the
Wnt/β-catenin pathway (39).
ERα and ERβ have been indicated to influence
osteogenic differentiation of bone marrow mesenchymal stem cells
(BM-MSCs). For instance, simvastatin-induced osteogenesis of
BM-MSCs is mediated via an ERα-dependent pathway (40). They are involved in bone-cell
responses to mechanical stress that stimulate osteoblast
proliferation and bone formation (41). As a mechanism of the
isopsoralen-induced anti-osteoporotic effects, ERα promotes
osteoblast differentiation and mineralization, increases calcium
nodule levels and ALP activity and upregulates osteoblast markers
(42). On other hand, ER inhibitor
is able to significantly reduce the expression of the RUNX2, OSX
and OPN genes and block osteogenic differentiation (43).
Taken together, the present results indicated that
osteogenic activity stimulated by irisin is accompanied with
upregulated expression of osteogenesis-related genes. However, the
temporal and spatial patterns of expression require further
investigation to elucidate the role and contributions of these
genes in irisin-induced osteogenic differentiation. In addition, it
is not clear the mechanism by which irisin acts to regulate the
expression of these genes, although irisin is able to activate
P38/ERK MAP kinase signaling cascades, leading to increased
osteogenic activity (17).
Furthermore, further in vitro and in vivo studies
using different cells, animal models and human subjects are
required to validate the present results and to develop irisin into
a potential therapeutic agent for OP.
In conclusion, the present study demonstrated that
irisin induces the differentiation of calvaria-derived
pre-osteoblast cells into osteoblasts and the formation of
mineralized nodules in vitro. This activity appears to be
dose-dependent and is mediated by the activation of a number of
osteogenesis-associated genes. Further studies are required to
investigate the role of irisin in osteogenesis in vivo and
determine its potential as a therapeutic for OP.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY, KY and DZ designed the study. JY, KY, DZ, DL, JY
and LT collected the data and performed the analysis. JY, KY and DZ
drafted the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
No authors listed. Osteoporosis
prevention, diagnosis, and therapy. NIH Consens Statement. 17:1–45.
2000.
|
|
2
|
Ensrud KE and Crandall CJ: Osteoporosis.
Ann Intern Med. 168:306–307. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tu KN, Lie JD, Wan CKV, Cameron M, Austel
AG, Nguyen JK, Van K and Hyun D: Osteoporosis: a review of
treatment options. P T. 43:92–104. 2018.PubMed/NCBI
|
|
4
|
Ogdie A, Nowell WB, Applegate E, Gavigan
K, Venkatachalam S, de la Cruz M, Flood E, Schwartz EJ, Romero B
and Hur P: Patient perspectives on the pathway to psoriatic
arthritis diagnosis: Results from a web-based survey of patients in
the United States. BMC Rheumatol. 4(2)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Crandall CJ, Newberry SJ, Diamant A, Lim
YW, Gellad WF, Booth MJ, Motala A and Shekelle PG: Comparative
effectiveness of pharmacologic treatments to prevent fractures: An
updated systematic review. Ann Intern Med. 161:711–723.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Cawthon PM, Fullman RL, Marshall L, Mackey
DC, Fink HA, Cauley JA, Cummings SR, Orwoll ES and Ensrud KE:
Osteoporotic Fractures in Men (MrOS) Research Group. Physical
performance and risk of hip fractures in older men. J Bone Miner
Res. 23:1037–1044. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Prestwood KM, Pilbeam CC and Raisz LG:
Treatment of osteoporosis. Annu Rev Med. 46:249–256.
1995.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cappola AR and Shoback DM: Osteoporosis
therapy in postmenopausal women with high risk of fracture. JAMA.
316:715–716. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Park E, Kim J, Kim MC, Yeo S, Kim J, Park
S, Jo M, Choi CW, Jin HS, Lee SW, et al: Anti-osteoporotic effects
of kukoamine B isolated from Lycii radicis cortex extract on
osteoblast and osteoclast cells and ovariectomized osteoporosis
model mice. Int J Mol Sci. 20(20)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li TM, Huang HC, Su CM, Ho TY, Wu CM, Chen
WC, Fong YC and Tang CH: Cistanche deserticola extract increases
bone formation in osteoblasts. J Pharm Pharmacol. 64:897–907.
2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang J, Zhang W, Dai J, Wang X and Shen
SG: Overexpression of Dlx2 enhances osteogenic differentiation of
BMSCs and MC3T3-E1 cells via direct upregulation of Osteocalcin and
Alp. Int J Oral Sci. 11(12)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sasa K, Yoshimura K, Yamada A, Suzuki D,
Miyamoto Y, Imai H, Nagayama K, Maki K, Yamamoto M and Kamijo R:
Monocarboxylate transporter-1 promotes osteoblast differentiation
via suppression of p53, a negative regulator of osteoblast
differentiation. Sci Rep. 8(10579)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Boström P, Wu J, Jedrychowski MP, Korde A,
Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, et al: A
PGC1-α-dependent myokine that drives brown-fat-like development of
white fat and thermogenesis. Nature. 481:463–468. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hofmann T, Elbelt U and Stengel A: Irisin
as a muscle-derived hormone stimulating thermogenesis--a critical
update. Peptides. 54:89–100. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Huh JY, Dincer F, Mesfum E and Mantzoros
CS: Irisin stimulates muscle growth-related genes and regulates
adipocyte differentiation and metabolism in humans. Int J Obes.
38:1538–1544. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wu LF, Zhu DC, Tang CH, Ge B, Shi J, Wang
BH, Lu YH, He P, Wang WY, Lu SQ, et al: Association of plasma
irisin with bone mineral density in a large chinese population
using an extreme sampling design. Calcif Tissue Int. 103:246–251.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Qiao X, Nie Y, Ma Y, Chen Y, Cheng R, Yin
W, Hu Y, Xu W and Xu L: Irisin promotes osteoblast proliferation
and differentiation via activating the MAP kinase signaling
pathways. Sci Rep. 6(18732)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Colaianni G, Cuscito C, Mongelli T,
Oranger A, Mori G, Brunetti G, Colucci S, Cinti S and Grano M:
Irisin enhances osteoblast differentiation in vitro. Int J
Endocrinol. 2014(902186)2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pullisaar H, Colaianni G, Lian AM,
Vandevska-Radunovic V, Grano M and Reseland JE: Irisin promotes
growth, migration and matrix formation in human periodontal
ligament cells. Arch Oral Biol. 111(104635)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vitro differentiation and calcification in a new
clonal osteogenic cell line derived from newborn mouse calvaria. J
Cell Biol. 96:191–198. 1983.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nagao M, Tanabe N, Manaka S, Naito M,
Sekino J, Takayama T, Kawato T, Torigoe G, Kato S, Tsukune N, et
al: LIPUS suppressed LPS-induced IL-1α through the inhibition of
NF-κB nuclear translocation via AT1-PLCβ pathway in MC3T3-E1 cells.
J Cell Physiol. 232:3337–3346. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cheleschi S, Giordano N, Volpi N, Tenti S,
Gallo I, Di Meglio M, Giannotti S and Fioravanti A: A complex
relationship between visfatin and resistin and microrna: an in
vitro study on human chondrocyte cultures. Int J Mol Sci.
19(19)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sun J, Zhao J, Bao X, Wang Q and Yang X:
Alkaline phosphatase assay based on the chromogenic interaction of
diethanolamine with 4-aminophenol. Anal Chem. 90:6339–6345.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Palmieri V, Barba M, Di Pietro L, Conti C,
De Spirito M, Lattanzi W and Papi M: Graphene oxide induced
osteogenesis quantification by in-situ 2D-fluorescence
spectroscopy. Int J Mol Sci. 19(19)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Krause U, Seckinger A and Gregory CA:
Assays of osteogenic differentiation by cultured human mesenchymal
stem cells. Methods Mol Biol. 698:215–230. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ching HS, Luddin N, Rahman IA and Ponnuraj
KT: Expression of odontogenic and osteogenic markers in DPSCs and
SHED: a review. Curr Stem Cell Res Ther. 12:71–79. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Siffert RS: The role of alkaline
phosphatase in osteogenesis. J Exp Med. 93:415–426. 1951.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Birmingham E, Niebur GL, McHugh PE, Shaw
G, Barry FP and McNamara LM: Osteogenic differentiation of
mesenchymal stem cells is regulated by osteocyte and osteoblast
cells in a simplified bone niche. Eur Cell Mater. 23:13–27.
2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ducy P, Zhang R, Geoffroy V, Ridall AL and
Karsenty G: Osf2/Cbfa1: A transcriptional activator of osteoblast
differentiation. Cell. 89:747–754. 1997.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Stadlinger B, Pilling E, Mai R, Bierbaum
S, Berhardt R, Scharnweber D and Eckelt U: Effect of biological
implant surface coatings on bone formation, applying collagen,
proteoglycans, glycosaminoglycans and growth factors. J Mater Sci
Mater Med. 19:1043–1049. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Schmidmaier G, Wildemann B, Lübberstedt M,
Haas NP and Raschke M: IGF-I and TGF-beta 1 incorporated in a
poly(D,L-lactide) implant coating stimulates osteoblast
differentiation and collagen-1 production but reduces osteoblast
proliferation in cell culture. J Biomed Mater Res B Appl Biomater.
65:157–162. 2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Elango J, Saravanakumar K, Rahman SU,
Henrotin Y, Regenstein JM, Wu W and Bao B: Chitosan-collagen 3D
matrix mimics trabecular bone and regulates RANKL-mediated
paracrine cues of differentiated osteoblast and mesenchymal stem
cells for bone marrow macrophage-derived osteoclastogenesis.
Biomolecules. 9(9)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hekmatnejad B, Gauthier C and St-Arnaud R:
Control of Fiat (factor inhibiting ATF4-mediated transcription)
expression by Sp family transcription factors in osteoblasts. J
Cell Biochem. 114:1863–1870. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gao Y, Xiao F, Wang C, Wang C, Cui P,
Zhang X and Chen X: Long noncoding RNA MALAT1 promotes osterix
expression to regulate osteogenic differentiation by targeting
miRNA-143 in human bone marrow-derived mesenchymal stem cells. J
Cell Biochem. 119:6986–6996. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Singh A, Gill G, Kaur H, Amhmed M and
Jakhu H: Role of osteopontin in bone remodeling and orthodontic
tooth movement: A review. Prog Orthod. 19(18)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Simonet WS, Lacey DL, Dunstan CR, Kelley
M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et
al: Osteoprotegerin: A novel secreted protein involved in the
regulation of bone density. Cell. 89:309–319. 1997.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ren X, Zhou Q, Foulad D, Tiffany AS, Dewey
MJ, Bischoff D, Miller TA, Reid RR, He TC, Yamaguchi DT, et al:
Osteoprotegerin reduces osteoclast resorption activity without
affecting osteogenesis on nanoparticulate mineralized collagen
scaffolds. Sci Adv. 5(eaaw4991)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gao X, Zheng J, Tu S, Cai B, Zeng R and
Xiang L: Role of osteoprotegerin in the regulation of dental
epithelial mesenchymal signaling during tooth development. Mol Med
Rep. 20:3035–3042. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chuang SC, Chen CH, Fu YC, Tai IC, Li CJ,
Chang LF, Ho ML and Chang JK: Estrogen receptor mediates
simvastatin-stimulated osteogenic effects in bone marrow
mesenchymal stem cells. Biochem Pharmacol. 98:453–464.
2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Galea GL, Price JS and Lanyon LE: Estrogen
receptors' roles in the control of mechanically adaptive bone
(re)modeling. Bonekey Rep. 2(413)2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ge L, Cui Y, Cheng K and Han J:
Isopsoralen Enhanced Osteogenesis by Targeting AhR/ERα. Molecules.
23(23)2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Abdallah BM, Ditzel N, Mahmood A, Isa A,
Traustadottir GA, Schilling AF, Ruiz-Hidalgo MJ, Laborda J, Amling
M and Kassem M: DLK1 is a novel regulator of bone mass that
mediates estrogen deficiency-induced bone loss in mice. J Bone
Miner Res. 26:1457–1471. 2011.PubMed/NCBI View Article : Google Scholar
|