Introduction
Lumbar degenerative disease (LDD) is a common
disease in adults, often causing lower back pain and leg pain that
frequently requires surgery (1).
Decompression with or without spinal fusion has been thought to be
the ‘gold standard’ for the treatment of LDD (2-4).
To prevent the instability caused by wide decompression,
instrumented spinal fusion is recommended (5-7).
However, rigid fixation and fusion pose inherent problems,
including greater invasiveness, longer surgical time and higher
blood loss, and may cause adjacent segment degeneration (ASD)
(8-11).
To address the limitations and disadvantages
associated with fusion, novel techniques and implants with motion
preservation, such as an interlaminar device, have been designed to
provide segmental stability following decompression (12). A Coflex interlaminar device
(Paradigm Spine) was designed to overcome the adverse effects of
fusion (13). This device focuses
on decelerating the degenerative process through dynamic
stabilisation of the lumbar spine to provide adequate stability for
restoring normal segmental kinematics, which allows for more
physiological load transmission (14,15). A
number of previous studies have suggested that Coflex interspinous
stabilisation is safe and efficacious (16,17).
However, the majority of previous studies have only
investigated the short-term results of Coflex with decompressive
surgery. Whether Coflex interspinous stabilisation reduces the
incidence of ASD compared with fusion requires further study with
long-term follow-up data. Therefore, the purpose of the current
retrospective study was to compare the radiographic and clinical
outcomes between patients who underwent Coflex interspinous
stabilisation and those who underwent decompression and fusion
(PLIF) for single-level LDD and had a minimum follow-up time of 8
years. This was performed in order to investigate whether Coflex
interspinous stabilisation affects the incidence of ASD, and to
determine the risk factors for ASD in each group.
Materials and methods
Study design
The current study is a retrospective, comparative,
single-institute study of two surgical procedures for the treatment
of LDD with instability. The present study was approved by the
Medical Research Ethics Committee of the Guangdong Provincial
People's Hospital. The patients were well informed of the details
of the study and signed an informed consent.
Patient selection
Between June 2007 and February 2011, a total of 82
patients (51 males, 31 females; mean age, 59.70±5.97 years) with
LDD treated with decompression and Coflex interspinous
stabilisation (Coflex group; 39 patients; 23 males, 16 females;
mean age, 58.79±6.46 years) or decompression and PLIF (PLIF group;
43 patients; 28 males, 15 females; mean age, 60.51±5.43 years) in
Guangdong Provincial People's Hospital were retrospectively
reviewed. The inclusion criteria were as follows: Aged 40-70 years
and follow-up duration of at least 8 years; patients who complained
of significant low back pain; radiating leg pain with or without
neurogenic claudication; radiographic confirmation of no segmental
instability at the adjacent segments; stable degenerative
spondylolisthesis up to Meyerding grade II (18), lumbar spinal stenosis or lumbar disc
herniation, or both. The exclusion criteria were as follows:
History of lumbar spine surgery; damage of the vertebral body in
the affected segment (for example, osteoporotic compression
fracture or tumours), degenerative lumbar scoliosis (>25˚), body
mass index (BMI) >40 kg/m2, cauda equina syndrome,
spinal infection or two or more segments requiring treatment.
Operative technique
All procedures were performed under general
anaesthesia, and patients were placed in the prone position. The
surgical segment was located using radiography.
In the Coflex group, a median incision ~4-6 cm long
was performed. Paraspinal muscles were separated subperiosteally,
keeping the supraspinous ligament intact and exposing the bilateral
facet joints to perform bilateral partial laminectomy. Undercutting
facetectomy was performed carefully until freely movable nerve
roots were identified. Microdiscectomy was performed if disk
herniation was present. The supraspinous ligament was cut with a
knife over the lower spinous process and reflected upwards, thereby
separating it from both spinous processes, and the intervening
interspinous ligament was excised to insert an optimal size of the
Coflex spacer. The wings were subsequently tightened with a clamp,
and the supraspinous ligament was re-sutured.
In the PLIF group, an 8-10 cm median incision was
performed to expose the spinous process and both laminae. The
ligamentum flavum and interspinous ligaments were subsequently
removed, and the spinous process and laminae were resected to
expose the entire nerve root and intervertebral space. Adequate
decompression was performed, and an appropriately sized
polyetheretherketone cage filled with an autograft bone was
implanted into the intervertebral space and fixed with a pedicle
screw system.
Clinical evaluation
The Oswestry disability index (ODI), 100 mm visual
analogue scale (VAS)-back pain and VAS-leg pain (6) were compared to assess the clinical
outcomes preoperatively, at 1, 2 and 5 years, and at the final
follow-up at ≥8 years. The ODI recovery rate represented the degree
of normal functioning after surgery and was calculated as follows:
(postoperative ODI score-preoperative ODI score)/preoperative ODI
score x100%. The degree of recovery was as follows: Excellent,
<50% improvement; good, improvement between 25 and 50% in ODI;
fair, change of -25 to +25% in ODI; poor, decrease of >25% in
ODI. A decrease of >20 mm in VAS was considered a significant
improvement. For each patient,, the operative time, amount of blood
loss and post-treatment complications were also compared.
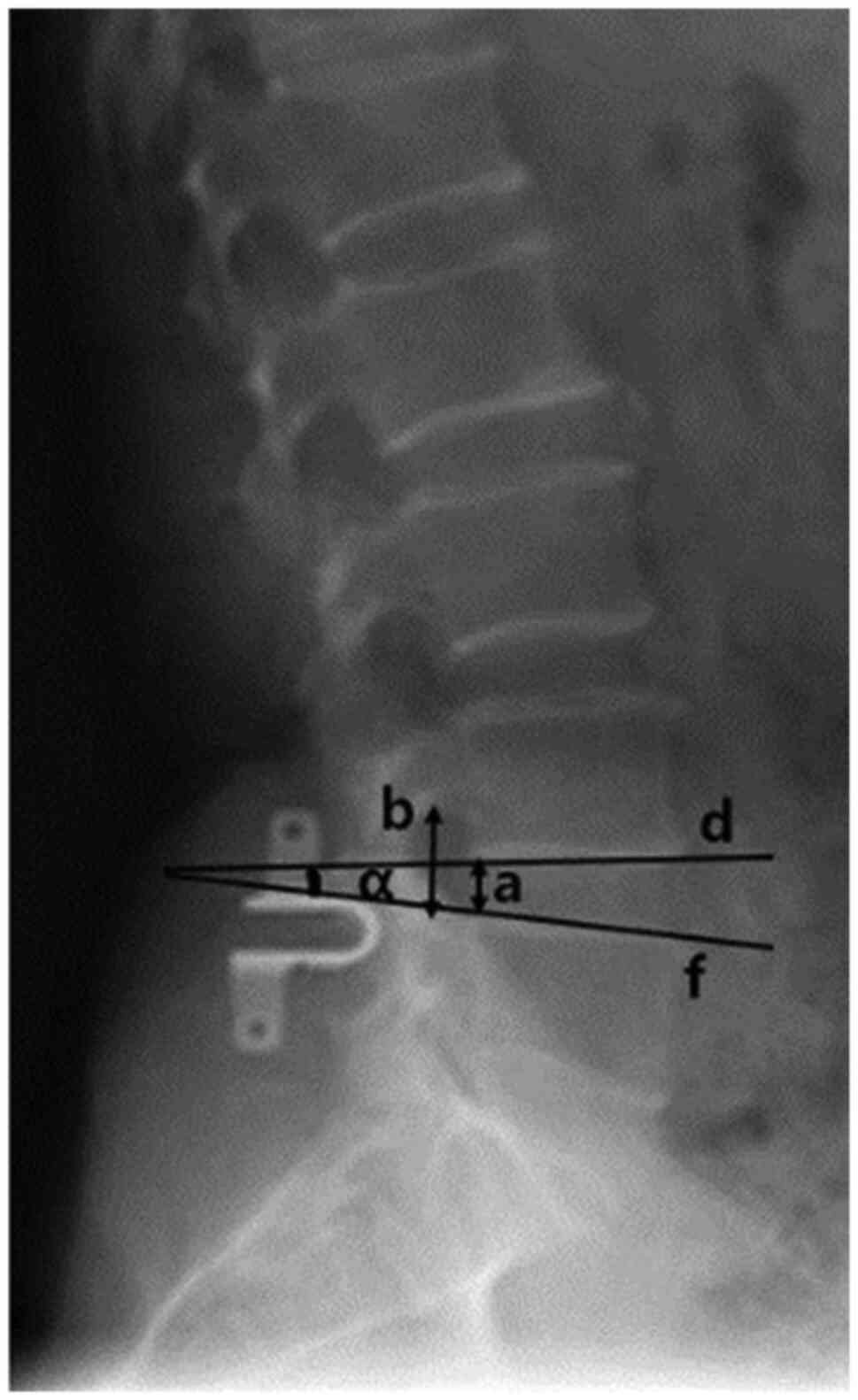
Radiological evaluation
The range of motion (ROM), posterior disk height
(PDH), and foraminal height (FH) of the operated segment and the
upper and lower adjacent segments were measured prior to surgery,
at 1, 3 and 5 years after surgery, and at the final follow-up at ≥8
years (Fig. 1). As all patients
underwent MRI as a routine examination preoperatively and at
follow-up visits, the progression of disk degeneration was
evaluated by the Pfirrmann classification (18). The following radiologic changes
indicated ASD: Disk height reduced to ≥50%, vertebral slip
increased to ≥3 mm on neural lateral radiograph, and angulation of
flexion versus extension >10˚ (19). A total of two experienced surgeons
team evaluated the radiographs, and were also involved in the
treatments of the patients. The examination was repeated twice to
avoid intra-observer variations.
Statistical analysis
Unpaired t-test and χ2 or Fisher's exact
test were used to compare all clinical and radiographic data.
Differences in incidence rates of ASD and complications among the
groups were evaluated using a χ2 test. Multivariable
correlation analysis was used to analyse the risk factors for
developing radiographic ASD. All data are presented as means ±
standard deviation. SPSS version 21.0 (IBM Corp.) was used for all
statistical analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
Demographic and baseline clinical
characteristics
The Coflex group was composed 39 patients of 23 men
and 16 women, with a mean age of 58.79±6.46 years. The follow-up
duration was 104.24±7.23 months. The PLIF group was composed 43
patients of 28 men and 15 women, with a mean age of 60.51±5.43
years. The follow-up duration was 104.16±7.20 months. No
significant difference was identified in age, sex, BMI, follow-up
time, preoperative foraminal height, operated segment ROM, duration
of symptom, PDH, Pfirrmann grade of disk degeneration, VAS scores
for leg and back pain or ODI scores between the two groups (all
P>0.05; Table I). The mean
duration of surgery, amount of blood loss, and duration of hospital
stay were significantly decreased in the Coflex group than in the
fusion group (all P<0.05; Table
I).
 | Table IDemographic data. |
Table I
Demographic data.
| Variable | Coflex group
(n=39) | PLIF group
(n=43) | P-value |
|---|
| Age (years) | 58.79±6.46 | 60.51±5.43 | 0.161 |
| Sex
(male/female) | | | 0.567 |
|
Male | 23 | 28 | |
|
Female | 16 | 15 | |
| Duration of symptom
(months) | 9.85±2.92 | 10.40±3.37 | 0.175 |
| BMI
(kg/m2) | 23.42±0.84 | 23.58±0.92 | 0.349 |
| Follow-up time
(months) | 104.24±7.23 | 104.16±7.20 | 0.676 |
| Preoperative
posterior disc height (mm) | 6.53±1.05 | 7.01±1.12 | 0.387 |
| Preoperative
foraminal height | 19.67±2.62 | 19.74±2.37 | 0.255 |
| Preoperative
operated segment ROM (˚) | 6.81±2.79 | 7.21±2.76 | 0.516 |
| Preoperative
Pfirrmann grade | | | 0.492 |
|
II | 10 | 14 | |
|
III | 29 | 29 | |
| Preoperative VAS
leg pain score (mm) | 65.38±14.48 | 67.21±14.36 | 0.833 |
| Preoperative VAS
back pain score (mm) | 70.00±12.14 | 67.91±13.72 | 0.089 |
| Preoperative ODI
score | 61.59±9.81 | 62.86±10.23 | 0.754 |
| Operative time
(min) | 54.59±9.93 | 100.49±24.17 | 0.001 |
| Blood loss
(ml) | 81.67±18.58 | 188.37±45.05 | <0.001 |
| Duration of
hospital stay (days) | 5.56±1.33 | 7.95±1.02 | 0.026 |
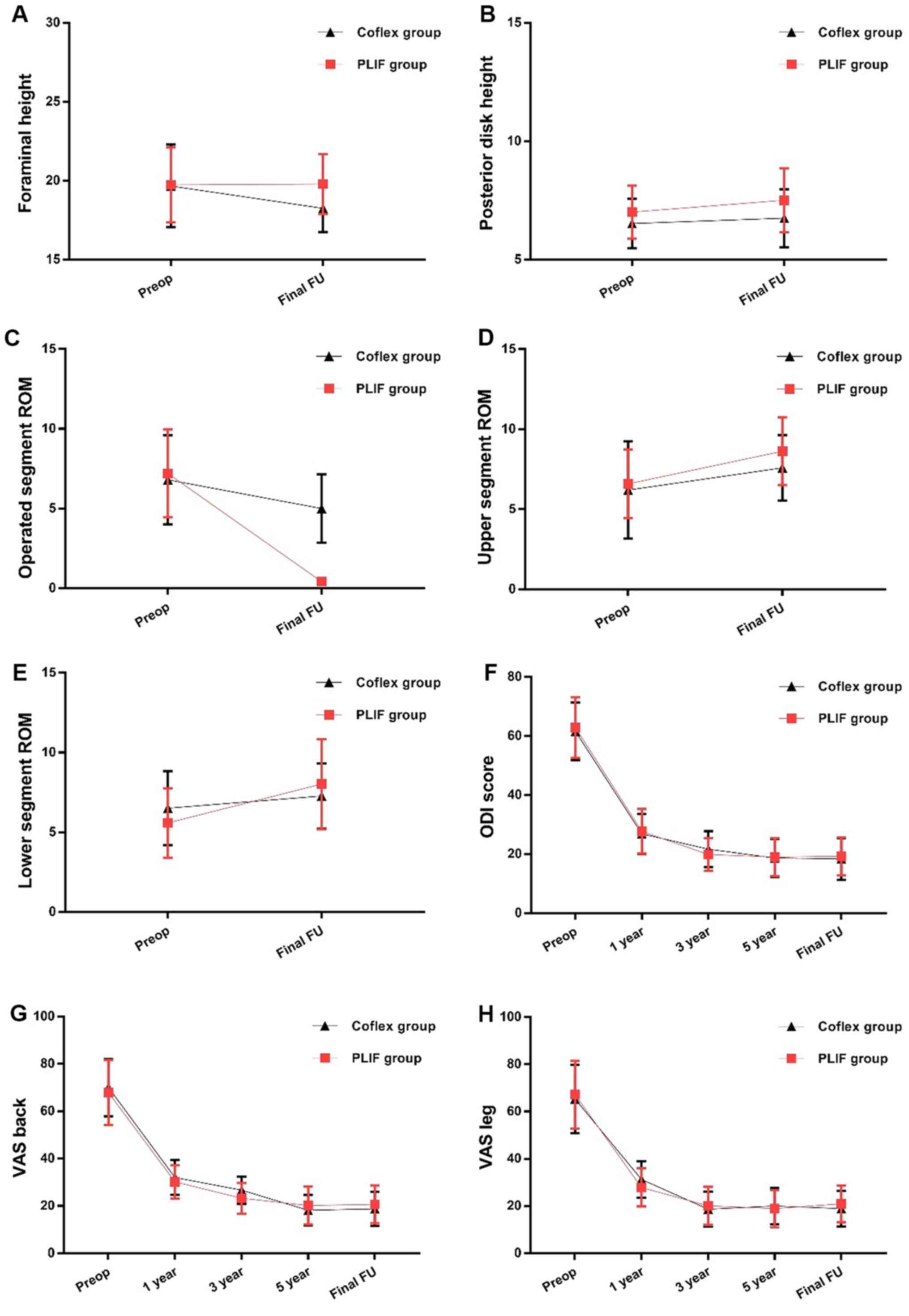
Clinical outcomes
The ODI scores and VAS scores for back and leg pain
markedly improved compared with the baseline scores in both groups
at each follow-up time point (Fig.
2F-H). However, no significant difference was indicated in the
scores between the two groups at each follow-up time point
(Fig. 2F-H). It was demonstrated
that ODI scores at 3 and 5 years postoperatively and at the final
follow-up were markedly lower than the ODI score at 1 year
postoperatively in the Coflex group, whereas no significant changes
were noted at 3 and 5 years postoperatively and at the final
follow-up in the Coflex group (Fig.
2F). In the PLIF group, the ODI scores at 3 and 5 years
postoperatively and at the final follow-up were significantly lower
than the ODI score at 1 year postoperatively, whereas no marked
changes were noted between 3 and 5 years postoperatively and at the
final follow-up (Fig. 2F). At 3 and
5 years postoperatively and at the final follow-up, the VAS scores
for back and leg pain decreased significantly compared with those
at 1 year in the Coflex group (Fig.
2G and H), whereas no
significant changes at 3 and 5 years postoperatively and at the
final follow-up was observed in the Coflex group (Fig. 2G and H). In the PLIF group, the VAS scores for
back and leg pain at 3 and 5 years postoperatively and at the final
follow-up decreased markedly compared with those at 1 year
postoperatively (Fig. 2G and
H), whereas no significant changes
at 3 and 5 years postoperatively and at the final follow-up were
observed (Fig. 2G and H). The recovery ratio in ODI score and
improvement in VAS scores for leg and back pain exhibited good
results in both groups at the final follow-up, and no differences
were indicated in the scores at baseline and in the last follow-up
between the two groups (Table
II).
 | Table IIClinical and radiographic outcomes at
the final follow-up. |
Table II
Clinical and radiographic outcomes at
the final follow-up.
| Variable | Coflex group
(n=39) | PLIF group
(n=43) | P-value |
|---|
| ODI recovery ratio
(%) | | | 0.797 |
|
>50 | 35 (89.74) | 37 (86.05) | |
|
25-50 | 4 (10.26) | 6 (13.95) | |
|
-25-25 | 0 | 0 | |
|
<-25 | 0 | 0 | |
| ODI score | 18.44±7.05 | 19.30±6.40 | 0.674 |
| VAS score of leg
pain (%) | | | |
|
>20 mm
decrease | 37 (94.87) | 40 (93.02) | |
|
≤20 mm
decrease | 2 (5.13) | 3 (6.98) | |
| VAS score of leg
pain | 18.97±7.54 | 20.93±7.81 | 0.729 |
| VAS score of back
pain (%) | | | 0.512 |
|
>20 mm
decrease | 37 (94.87) | 38 (88.37) | |
|
≤20 mm
decrease | 2 (5.13) | 5 (11.63) | |
| VAS score of back
pain | 18.79±7.18 | 20.70±7.99 | 0.336 |
| Posterior disk
height (mm) | 6.75±1.22 | 7.51±1.35 | 0.301 |
| Foraminal height
(mm) | 18.25±1.49 | 19.79±1.91 | 0.060 |
| ROM (˚) | | | |
|
Operated
segment | 5.01±2.15 | 0.42±0.26 | <0.001 |
|
Upper
adjacent segment | 7.60±2.24 | 8.63±2.11 | 0.035 |
|
>10˚
(%) | 1 (2.56) | 7 (16.28) | 0.037 |
|
Lower
adjacent segment | 7.28±2.05 | 8.03±2.82 | 0.176 |
|
>10˚
(%) | 0 | 2 (4.65) | 0.495 |
Radiographic outcomes
The FH at baseline and at the final follow-up were
not markedly different between the two groups (Table II; Fig.
2A), and no marked difference was identified between the scores
at baseline and at the final follow-up (Fig. 2A). At baseline, no marked difference
was demonstrated in the PDH between the two groups (Fig. 2B). In both two groups, the PDH at
the final follow-up had no difference than that at baseline
(Fig. 2B). Additionally, no marked
difference was observed in the PDH between the two groups at the
final follow-up (Table II;
Fig. 2B).
No marked difference was indicated in the ROM of the
operated segment at baseline between the two groups (P=0.516;
Table I; Fig. 2); however, the ROM decreased
compared with the baseline after surgery in both groups (Fig. 2C). Moreover, the ROM of the operated
segment in the Coflex group was markedly higher compared with the
PLIF group at the final follow-up (Fig.
2C; Table II). At baseline, no
marked difference was found in the ROM of the upper adjacent
segment in both groups (Fig. 2D).
The ROM of the upper adjacent segment markedly increased from
baseline to the final follow-up in both groups (Fig. 2D). However, the ROM of the upper
adjacent segment was markedly higher in the PLIF group than in the
Coflex group at the final follow-up (Fig. 2D; Table
II). The ROM of the lower adjacent segment also demonstrated no
marked difference at baseline in both groups (Fig. 2E). However, in the PLIF group,
values markedly increased from baseline to the final follow-up
(Fig. 2E), whereas no marked
changes was noted in the Coflex group (Fig. 2E). At the final follow-up, no marked
difference in ROM of the lower adjacent segment was indicated
between the two groups (Fig.
2E).
Complications
In the PLIF group, post-op, one patient had a
transient neurological deficit, one patient had a hematoma, and two
patients had dural tears. On the contrary, in the Coflex group, one
patient had a transient neurological deficit and three patients had
anterior thigh pain. However, none of the perioperative
complications in either group were persistent. As for long-term
complications, in the PLIF group, 14 patients were diagnosed with
ASD. Of these patients, four were severely symptomatic and
underwent a subsequent operation. In the Coflex group, five
patients were diagnosed with ASD, while three of these patients
were severely symptomatic and underwent re-surgery. A total of two
patients underwent recurrence disc herniation of the operation
segment in the Coflex group. At the last follow-up, six patients in
the Coflex group developed bone resorption of the spinous processes
and looseness of internal fixation. All of them had undergone PLIF
reoperation. No significant difference in complication and
resorption rates were indicated between the two groups (Table III).
 | Table IIIComplication and resorption rates in
the Coflex and PLIF groups. |
Table III
Complication and resorption rates in
the Coflex and PLIF groups.
| Complication | Coflex group (n
=39) | PLIF group (n
=43) | P-value |
|---|
| Current (%) | 4 (10.26) | 4 (9.30) | 0.999 |
| Long-term (%) | 13 (33.33) | 14 (32.56) | 0.818 |
| Reoperation
(%) | 9 (23.08) | 4 (9.30) | 0.130 |
Risk factor analysis of ASD
A total of 5 patients (12.82%) in the Coflex group
and 14 patients (32.56 %) in the PLIF group exhibited ASD at the
final follow-up (P=0.040; Table
IV). More upper adjacent segment (15/19) deteriorations than
lower adjacent segment (4/19) deteriorations were observed in these
patients. The proportion of patients who underwent reoperation for
ASD was not significantly different between the groups (P=0.794;
Table IV). The Pfirrmann grades of
both upper and lower adjacent segments were significantly different
between the two groups (P<0.001 and P=0.020, respectively;
Table IV). The results of the
logistic regression analysis demonstrated that the surgical method
and ROM were significant risk factors of ASD (P=0.031 and P=0.021,
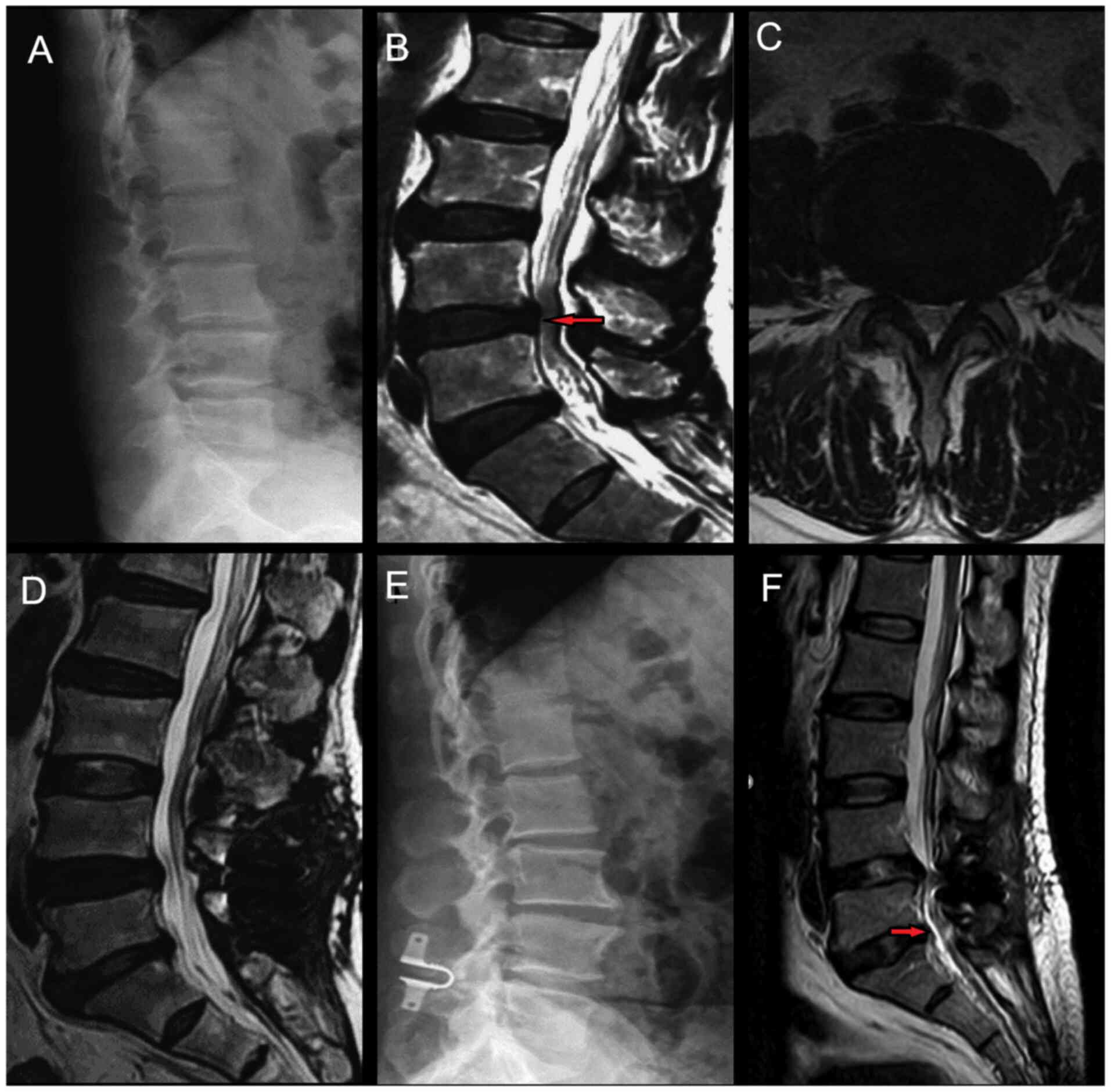
respectively; Table V). Fig. 3 presents a patient who underwent
Coflex interspinous stabilisation had ASD in the lower adjacent
segment.
 | Table IVPatients with ASD and Pfirrmann grade
in the Coflex and PLIF groups at the final follow-up. |
Table IV
Patients with ASD and Pfirrmann grade
in the Coflex and PLIF groups at the final follow-up.
| Variable | Coflex group
(n=39) | PLIF group
(n=43) | P-value |
|---|
| Number of ASD
(%) | 5 (12.82) | 14 (32.56) | 0.040 |
| Upper adjacent
segment | 4 | 11 | |
| Lower adjacent
segment | 1 | 3 | |
| Disc height reduced
≥50% | 3 | 6 | |
| Vertebral slip ≥4
mm | 2 | 4 | |
| ROM >10˚ | 1 | 9 | |
| Reoperation for ASD
(%) | 3 (7.69) | 4 (9.30) | 0.794 |
| Upper adjacent
segment Pfirrmann grade | | | <0.001 |
|
≤III | 23 | 9 | |
|
≥IV | 16 | 34 | |
| Lower adjacent
segment Pfirrmann grade | | | 0.020 |
|
≤III | 32 | 28 | |
|
≥IV | 7 | 15 | |
 | Table VRisk factors for developing
radiographic ASD. |
Table V
Risk factors for developing
radiographic ASD.
| Variable | No ASD (n=63) | ASD (n=19) | P-value |
|---|
| Surgical
method | | | 0.031 |
|
Coflex | 34 | 5 | |
|
Fusion | 29 | 14 | |
| Age (years) | | | 0.929 |
|
<55 | 12 | 3 | |
|
≥55 | 51 | 16 | |
| Sex | | | 0.302 |
|
Male | 39 | 12 | |
|
Female | 24 | 7 | |
| BMI
(kg/m2) | | | 0.807 |
|
≥25 | 57 | 18 | |
|
<25 | 6 | 1 | |
| Perioperative ROM
(˚) | | | 0.021 |
|
≤10˚ | 61 | 16 | |
|
>10˚ | 2 | 3 | |
| Perioperative
Pfirrmann grade | | | 0.442 |
|
II | 22 | 2 | |
|
III | 41 | 17 | |
Discussion
In the current study, the clinical outcomes between
Coflex interspinous stabilisation and fusion surgery alone were
compared. The results demonstrated that Coflex interspinous
stabilisation was able to reduce the incidence of ASD. The
occurrence rate of ASD in the Coflex group (12.82%) was
significantly lower compared with the fusion group (32.56%), and
the Pfirrmann grade of both the upper and lower adjacent segments
in the Coflex group was significantly lower than that in the fusion
group at the final follow-up. Moreover, the results of the logistic
regression analysis revealed that the surgical method and
perioperative ROM were significant risk factors of ASD. These
results revealed that Coflex interspinous stabilisation is more
advantageous in decreasing the incidence of ASD than fusion
surgery.
The PLIF is often considered to be the ‘gold
standard’ treatment for LDD with instability following failure of
conservative treatment (2).
However, increased trauma, increased amount of blood loss and a
high incidence of ASD after fusion surgery are receiving increased
attention (20,21). To reduce the incidence of serious
complications that are associated with spinal fusion,
motion-preserving devices, such as dynamic interspinous spacer
devices, were developed (10,22).
In the 1990s, Jacques Samani first invented the
interspinous U-shaped fixture and demonstrated good results during
clinical application (15).
Furthermore, in 2005, Paradigm Spine improved the dynamic
stabilisation device and named it Coflex, which can provide
stability for the operated segment for unloading the facet joint
and for maintaining the direct decompression effect (23). A level I study that was approved by
the Food and Drug Administration directly assessed the outcome of
Coflex interspinous stabilisation and demonstrated that Coflex
interspinous stabilisation is an effective and sustainable
treatment option for LDD and is not an inevitable precursor to
fusion (16). A biomechanical study
conducted by Kong et al (17) revealed that Coflex interspinous
stabilisation with fusion could stabilise the transition segment
and restrict flexion and extension of that segment. A study
performed by Richter et al (24) involving a large sample of nearly
14,000 patients who underwent Coflex interspinous stabilisation
demonstrated that the Coflex interlaminar device is a safe and
viable option in the selection of instrumentation for spinal
stabilisation. However, few convincing long-term follow-up clinical
studies have compared the effectiveness and safety between Coflex
interspinous stabilisation and fusion only in the treatment of
LDD.
The results of the current study revealed that
Coflex is able to provide comparable clinical outcomes to PLIF in
the treatment of LDD with instability. All patients achieved
significant improvement in ODI and VAS scores for leg and back pain
compared with the baseline. Furthermore, Coflex interspinous
stabilisation has been indicated to present a number advantages
over PLIF, such as shorter operative time, decreased amount of
blood loss and shorter duration of hospital stay, due to the
minimally invasive nature of Coflex implantation. Furthermore,
Coflex interspinous stabilisation requires less laminar resection
and does not necessitate interbody fusion. The exposure of the
transverse process and facet joints to reduce damage to the
paraspinal muscles was not necessary during Coflex implantation.
These results are similar to those of a previous study (19). A randomised controlled trial
demonstrated that Coflex interspinous stabilisation can provide
comparable clinical outcomes with lumbar fusion surgery in the
treatment of LDD after a 5-year follow-up (21). Furthermore, a previous study has
also demonstrated the advantages in terms of perioperative
outcomes, such as shorter length of hospital stay, decreased amount
of blood loss and shorter operative time, over fusion (10). In a retrospective study by Yuan
et al (21), no significant
difference was indicated between VAS and ODI improvements during a
minimum 5-year follow-up between the Coflex and PLIF groups. Errico
et al (25) also conducted a
retrospective study of 127 patients and revealed that Coflex
implantation markedly relieved back and leg pain for at least 5
years. In the current study, similar results were observed,
indicating that patients who underwent Coflex interspinous
stabilisation achieved significantly improved clinical outcomes
after a minimum 8-year follow-up, which were comparable with those
with PLIF. These findings indicated that both groups had similar
and satisfactory clinical outcomes.
The results of the present study demonstrated that
the FH at baseline and at the final follow-up was not significantly
different between the two groups. The PDH in both groups was
maintained and with no significant difference compared with the
baseline at the final follow-up. A previous study revealed that
Coflex interspinous stabilisation significantly increased the FH
and PDH at the implanted segment in the immediate postoperative
period or at the 1-year follow-up (21). However, in the current study, the FH
and PDH were decreased and close to the baseline value at the final
follow-up. The results suggested that the FH and PDH were well
preserved after a minimum 8-year follow-up in the Coflex group,
which were comparable with the findings in the PLIF group.
Reduction of ROM at the implanted segment has been frequently
reported in previous studies (20,21,26).
Davis et al (26)
demonstrated that the ROM at the implanted segment decreased
significantly at 24 months postoperatively. Yuan et al
(21) demonstrated that the ROM at
the adjacent segments in the Coflex group had no significant
changes, whereas the ROM at the superior adjacent segment in the
PLIF group was significantly increased. Furthermore, the ROM of the
implanted segment in the Coflex group was significantly improved
compared with the PLIF group at the final follow-up. The results of
the aforementioned study were similar to the results of the current
study, demonstrated that ROM decreased significantly at the
implanted segment in both groups, whereas greater preservation of
motion was observed in the Coflex group. However, the present study
indicated that the ROM in the adjacent segment was significantly
increased at the final follow-up in the PLIF group. These
postoperative ROM changes are considered to be an advantage of
Coflex interspinous stabilisation compared with fusion, as a higher
degree of ROM changes at the adjacent segments may lead to ASD.
ASD has been a significant problem in clinical
practice after spinal fusion. Alentado et al (20) reported that reoperation accounts for
9% of patients who developed ASD after undergoing fusion surgery.
Previous studies have confirmed that the degeneration of
intervertebral disk nucleus cells was an intrinsic factor in the
development of ASD (27,28), whereas the increased disk pressure
was an important stimulative factor for accelerating the
development of ASD (29). A
biomechanical study indicated that increased stress and ROM of
adjacent segments were the most important factors causing and
accelerating the development of ASD (23). Although previous studies have
investigated the risk factors for ASD progression (19,30-32),
the precise pathogenesis of ASD remains uncertain. Additionally,
the logistic regression analysis demonstrated that the surgical
method was a significant risk factor of ASD. The results of the
current study revealed that Coflex interspinous stabilisation
decreases the incidence of ASD compared with fusion surgery. The
results also indicated that the preoperative ROM of the implanted
segment was another significant risk factor of ASD. This was caused
by the need to compensate for the hypermobility of the adjacent
segment after fusion. Furthermore, a larger preoperative ROM of the
implanted segment indicated that adjacent segments need to
compensate more. Previous biomechanical and clinical studies have
explained the compensatory loading transfer (33) and increased ROM (34-36)
at adjacent levels after rigid fixation.
Although other complications such as postoperative
nausea, vomiting, nerve root injury and internal fixation system
fractures were also reported in previous studies, no significant
differences were indicated in the results of these studies
(10,21,25).
The incidence of complications in the current study is consistent
with reports in the previous literature (10,21,25).
Comfortable and strict indication is important when choosing Coflex
interspinous stabilisation. Additionally, the Coflex interlaminar
device should be placed deep enough between the interlaminar spaces
because insufficient implantation depth would increase stress on
the spinous process. This may cause spinous process bone
resorption, device fixation loosening and possibly a spinous
process fracture.
The current study was retrospective without
randomisation in patient selection, which may generate bias.
Although the cost of the treatment is not free in China, it was not
considered to be a factor in the current study, which may be a
limitation. Additionally, the mean duration of follow-up was
slightly longer than 8 years, and an extended follow-up study is
currently being conducted. The sample size was also relatively
small, although it exceeded the size for detecting a statistical
difference in clinical outcomes. A randomised controlled trial with
a large sample size is warranted in the future to increase the
reliability of the clinical results.
The present study demonstrated that decompression
and Coflex interspinous stabilisation can achieve satisfactory and
comparable long-term clinical outcomes to PLIF in the treatment of
LDD. Coflex stabilisation can preserve the mobility of the operated
segment with lesser amount of blood loss, shorter operative
duration and shorter length of hospital stay, compared with PLIF.
Furthermore, Coflex stabilisation can significantly decrease the
incidence of ASD compared with PLIF. The results of the current
study suggested that Coflex stabilisation is an acceptable
alternative to PLIF for the treatment of LDD.
Acknowledgements
The authors would like to acknowledge Mr Hongwu Mao
and Miss Weiran Ye (Pinghu Hospital Affiliated to Shenzhen
University, Shenzhen, China) for their assistance in data
collection. Dr Yong Xiao (Department of Neurosurgery, University
Medicine Greifswald, Germany) assisted in the development of the
radiographic data collection and measurement methods. The authors
would also like to thank Dr Sascha Marx (Department of
Neurosurgery, University Medicine Greifswald, Germany) for language
modification.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC designed the experiments. XZ and ZC collected and
interpreted the data. HoY and HuY collected the follow-up data. XZ,
JZ and HuY collected and analyzed the data, and wrote the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the Guangdong Provincial
People's Hospital approved the study protocol. Signed informed
consent was obtained from all patients included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Du Bois M, Szpalski M and Donceel P:
Patients at risk for long-term sick leave because of low back pain.
Spine J. 9:350–359. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Brantigan JW, Neidre A and Toohey JS: The
Lumbar I/F Cage for posterior lumbar interbody fusion with the
variable screw placement system: 10-year results of a Food and Drug
Administration clinical trial. Spine J. 4:681–688. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Harrop JS, Youssef JA, Maltenfort M,
Vorwald P, Jabbour P, Bono CM, Goldfarb N, Vaccaro AR and Hilibrand
AS: Lumbar adjacent segment degeneration and disease after
arthrodesis and total disc arthroplasty. Spine (Phila Pa 1976).
33:1701–1707. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lai PL, Chen LH, Niu CC, Fu TS and Chen
WJ: Relation between laminectomy and development of adjacent
segment instability after lumbar fusion with pedicle fixation.
Spine (Phila Pa 1976). 29:2527–2532. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lin B, Yu H, Chen Z, Huang Z and Zhang W:
Comparison of the PEEK cage and an autologous cage made from the
lumbar spinous process and laminae in posterior lumbar interbody
fusion. BMC Musculoskelet Disord. 17(374)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pearson A, Blood E, Lurie J, Abdu W,
Sengupta D, Frymoyer JW and Weinstein J: Predominant leg pain is
associated with better surgical outcomes in degenerative
spondylolisthesis and spinal stenosis: Results from the Spine
Patient Outcomes Research Trial (SPORT). Spine (Phila Pa 1976).
36:219–229. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kleinstück FS, Grob D, Lattig F, Bartanusz
V, Porchet F, Jeszenszky D, O'Riordan D and Mannion AF: The
influence of preoperative back pain on the outcome of lumbar
decompression surgery. Spine (Phila Pa 1976). 34:1198–1203.
2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Imagama S, Kawakami N, Matsubara Y, Tsuji
T, Ohara T, Katayama Y, Ishiguro N and Kanemura T: Radiographic
adjacent segment degeneration at 5 years after L4/5 posterior
lumbar interbody fusion with pedicle screw instrumentation:
Evaluation by computed tomography and annual screening with
magnetic resonance imaging. Clin Spine Surg. 29:E442–E451.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Volkheimer D, Malakoutian M, Oxland TR and
Wilke HJ: Limitations of current in vitro test protocols for
investigation of instrumented adjacent segment biomechanics:
Critical analysis of the literature. Eur Spine J. 24:1882–1892.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Musacchio MJ, Lauryssen C, Davis RJ, Bae
HW, Peloza JH, Guyer RD, Zigler JE, Ohnmeiss DD and Leary S:
Evaluation of decompression and interlaminar stabilization compared
with decompression and fusion for the treatment of lumbar spinal
stenosis: 5-year follow-up of a prospective, randomized, controlled
trial. Int J Spine Surg. 10(6)2016.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Hilibrand AS and Robbins M: Adjacent
segment degeneration and adjacent segment disease: The consequences
of spinal fusion? Spine J. 4 (Suppl 6):190S–194S. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Trautwein FT, Lowery GL, Wharton ND, Hipp
JA and Chomiak RJ: Determination of the in vivo posterior loading
environment of the Coflex interlaminar-interspinous implant. Spine
J. 10:244–251. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gala RJ, Russo GS and Whang PG:
Interspinous implants to treat spinal stenosis. Curr Rev
Musculoskelet Med. 10:182–188. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kaner T, Sasani M, Oktenoglu T and Ozer
AF: Dynamic stabilization of the spine: A new classification
system. Turk Neurosurg. 20:205–215. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sangiorgio SN, Sheikh H, Borkowski SL,
Khoo L, Warren CR and Ebramzadeh E: Comparison of three posterior
dynamic stabilization devices. Spine (Phila Pa 1976).
36:E1251–E1258. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bae HW, Lauryssen C, Maislin G, Leary S
and Musacchio MJ Jr: Therapeutic sustainability and durability of
coflex interlaminar stabilization after decompression for lumbar
spinal stenosis: A four year assessment. Int J Spine Surg.
9(15)2015.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Kong C, Lu S, Hai Y and Zang L:
Biomechanical effect of interspinous dynamic stabilization adjacent
to single-level fusion on range of motion of the transition segment
and the adjacent segment. Clin Biomech (Bristol, Avon). 30:355–359.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pfirrmann CW, Metzdorf A, Zanetti M,
Hodler J and Boos N: Magnetic resonance classification of lumbar
intervertebral disc degeneration. Spine (Phila Pa 1976).
26:1873–1878. 2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen XL, Guan L, Liu YZ, Yang JC, Wang WL
and Hai Y: Interspinous dynamic stabilization adjacent to fusion
versus double-segment fusion for treatment of lumbar degenerative
disease with a minimum follow-up of three years. Int Orthop.
40:1275–1283. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Alentado VJ, Lubelski D, Healy AT, Orr RD,
Steinmetz MP, Benzel EC and Mroz TE: Predisposing characteristics
of adjacent segment disease after lumbar fusion. Spine (Phila Pa
1976). 41:1167–1172. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yuan W, Su QJ, Liu T, Yang JC, Kang N,
Guan L and Hai Y: Evaluation of Coflex interspinous stabilization
following decompression compared with decompression and posterior
lumbar interbody fusion for the treatment of lumbar degenerative
disease: A minimum 5-year follow-up study. J Clin Neurosci.
35:24–29. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lawrence BD, Wang J, Arnold PM, Hermsmeyer
J, Norvell DC and Brodke DS: Predicting the risk of adjacent
segment pathology after lumbar fusion: A systematic review. Spine
(Phila Pa 1976). 37 (Suppl 22):S123–S132. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tsai KJ, Murakami H, Lowery GL and Hutton
WC: A biomechanical evaluation of an interspinous device (Coflex)
used to stabilize the lumbar spine. J Surg Orthop Adv. 15:167–172.
2006.PubMed/NCBI
|
|
24
|
Richter A, Schütz C, Hauck M and Halm H:
Does an interspinous device (Coflex) improve the outcome of
decompressive surgery in lumbar spinal stenosis? One-year follow up
of a prospective case control study of 60 patients. Eur Spine J.
19:283–289. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Errico TJ, Kamerlink JR, Quirno M, Samani
J and Chomiak RJ: Survivorship of coflex interlaminar-interspinous
implant. SAS J. 3:59–67. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Davis R, Auerbach JD, Bae H and Errico TJ:
Can low-grade spondylolisthesis be effectively treated by either
coflex interlaminar stabilization or laminectomy and posterior
spinal fusion? Two-year clinical and radiographic results from the
randomized, prospective, multicenter US investigational device
exemption trial: Clinical article. J Neurosurg Spine. 19:174–184.
2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Soukane DM, Shirazi-Adl A and Urban JP:
Computation of coupled diffusion of oxygen, glucose and lactic acid
in an intervertebral disc. J Biomech. 40:2645–2654. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Galbusera F, van Rijsbergen M, Ito K,
Huyghe JM, Brayda-Bruno M and Wilke HJ: Ageing and degenerative
changes of the intervertebral disc and their impact on spinal
flexibility. Eur Spine J. 23 (Suppl 3):S324–S332. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Adams MA and Dolan P: Intervertebral disc
degeneration: Evidence for two distinct phenotypes. J Anat.
221:497–506. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mannion AF, Leivseth G, Brox JI, Fritzell
P, Hägg O and Fairbank JC: ISSLS Prize winner: Long-term follow-up
suggests spinal fusion is associated with increased adjacent
segment disc degeneration but without influence on clinical
outcome: results of a combined follow-up from 4 randomized
controlled trials. Spine (Phila Pa 1976). 39:1373–1383.
2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yang JY, Lee JK and Song HS: The impact of
adjacent segment degeneration on the clinical outcome after lumbar
spinal fusion. Spine (Phila Pa 1976). 33:503–507. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhu Z, Liu C, Wang K, Zhou J, Wang J, Zhu
Y and Liu H: Topping-off technique prevents aggravation of
degeneration of adjacent segment fusion revealed by retrospective
and finite element biomechanical analysis. J Orthop Surg Res.
10(10)2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Perez-Orribo L, Zucherman JF, Hsu KY,
Reyes PM, Rodriguez-Martinez NG and Crawford NR: Biomechanics of a
posterior lumbar motion stabilizing device: In vitro comparison to
intact and fused conditions. Spine (Phila Pa 1976). 41:E55–E63.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ghiselli G, Wang JC, Bhatia NN, Hsu WK and
Dawson EG: Adjacent segment degeneration in the lumbar spine. J
Bone Joint Surg Am. 86:1497–1503. 2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kumar MN, Baklanov A and Chopin D:
Correlation between sagittal plane changes and adjacent segment
degeneration following lumbar spine fusion. Eur Spine J.
10:314–319. 2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dong Y, Zheng X, Gu H, Liang G, Zhuang J,
Liang CX, Liu B and Chang Y: Is the interspinous device (Coflex)
outdated in the treatment of lumbar spinal stenosis? A seven-year
follow-up. Spine Res. 4(2)2018.
|