Introduction
Controlling infection with Mycobacterium
tuberculosis (M.tb.) has become one of the top priorities in
the last few years as tuberculosis (TB) remains a global threat
(1,2). More than 7 million cases were
estimated to be diagnosed worldwide in 2018, from which 85% were
diagnosed with pulmonary TB (3).
Almost 1.5 million deaths were reported in 2018 among both HIV
infected and uninfected individuals (2). These facts increase the need to use
host-directed immunomodulatory therapies among classical
antituberculosis treatment, as susceptibility to infection is
determined by both bacteria and host (1,4).
Autophagy is an essential intracellular homeostatic proteolytic
mechanism which has been recently discussed as an antimicrobial
strategy in pulmonary TB (5,6). The
specific histopathological hallmark of TB is represented by the
granuloma, an immunological environment where infected macrophages
induce secretion of inflammatory cytokines and further recruitment
of neutrophils, monocytes and lymphocytes (7). Macrophages restrain M.tb. by
phago-lysosomal fusion and bacterial degradation to immunogenic
polypeptides, therefore preventing dissemination of the bacilli
(7,8). The question is if macrophages are more
than antigen-presenting cells and immunological barriers (8). Indeed, macrophages possess
bactericidal activity, creating a dynamic connection between innate
immunity and adaptive host-responses (7). Specific M.tb. recognition
membrane-receptors, Toll-like receptors (TLR), lead to cytokines
and antimicrobial effector production (7,8). Even
more, TLR signals involve CYP27B1 stimulation
which catalyses hydroxylation of cholecalciferol (vitamin
D3) into its most active form
1,25-(OH)2-cholecalciferol called calcitriol (8). Following upregulation of T-cells as an
immune adaptive response, the activated vitamin D3 (VD)
form binds to specific vitamin D receptors (VDRs), constitutively
expressed in immune cells such as macrophages, monocytes, and
dendritic cells (5,8,9).
Therefore, the role of VD as an essential endogenous immune
mediator in pulmonary TB and not just as a maintainer of calcium
homeostasis and musculoskeletal health is highlighted (5,9). The
overall amount of evidence reporting the anti-inflammatory and
immunoregulatory action of the active VD forms suggests a function
that might be generally called ‘stress-quenching activity’
(7-9).
VD activates immunological pathways by direct induction of specific
antimicrobial peptides, such as cathelicidin (LL-37) and
β-defensins, in phagocytes and by stimulation of autophagic
processes, assessed through autophagy-related genes, such as
beclin-1 and Atg5 (4,5). Many studies have emphasized the
dose-dependent bactericidal activity against M.tb. of both
VD and LL-37 (4,5,9) and
also the essential role of beclin-1 in phagosome maturation and
cellular apoptosis of macrophages (6,10). On
the other hand, VD deficiency leads to negative lymphoproliferative
response, decreased autophagy, lower regulation of the pathological
response and downregulated bacterial clearance (4,6,7).
M.tb. is further able to multiplicate and persist at a low
metabolic rate in the granuloma, developing into extensive tissue
injury, caseous necrosis and cavitary severe forms (7,8).
In the case of persistent infection, as an attempt
to eliminate infected immune cells, epithelioid cells of the
tuberculous granuloma produce M30 (an epitope of caspase-cleaved
cytokeratin 18) (11,12). M30 is a specific apoptosis
biomarker, obtained after cleavage of various cellular proteins
through caspase activity, that can be measured from plasma through
immunochemical methods (11). The
programmed cellular death of macrophages could reduce the viability
and limit the spread of M.tb, without spilling cellular
affected contents into the tissue, and eventually promote host
defense by stimulating an adaptive immune response through T cells
(10,12). Yet, the mycobacteria are able to
adopt different strategies, yet incompletely known, in order to
avoid apoptosis. One of the most relevant is the preferential
undergoing necrosis of macrophages, which causes acute inflammation
and tissue swelling (13).
In the present study, we assessed serum levels of
VD, VDR, LL-37, beclin-1 and M30 before and after two months of
anti-TB treatment in order to assess the crosstalk between VD axis,
inflammation and host defense mechanisms and to determinate the
outcome of first-line anti-TB pharmacotherapy.
Patients and methods
Study design
A total of 30 active newly diagnosed pulmonary TB
patients from ‘Victor Babeș’ Clinical Hospital of Infectious
Diseases and Pneumophthisiology Craiova and Pneumology Hospital
Leamna, Dolj County, diagnosed through both laboratory methods
(sputum-smear positivity, gene Xpert) and radiologic examinations,
were recruited for the prospective study, after obtaining written
informed consent. Patients were included in our study if they had
not received more than one week of anti-TB treatment. Patients with
extra-pulmonary TB and co-morbidities such as HIV, diabetes,
hypertension, hepatic or renal disorders, cancer, thyroid and
parathyroid gland dysfunctionalities were excluded, after
examination of patient history and baseline laboratory
investigations. Patients treated with immunosuppressants,
corticosteroids, thiazide diuretics, other drugs that may interfere
with VD levels (some anticonvulsants, theophylline) and pregnant
women were also excluded from the study. We also excluded those who
were administered supplements based on VD.
For 17 patients it was possible to quantify all the
parameters at the inclusion of the study (T0) and after they
received for two months (T2) a different regimen of first-line
anti-TB drugs (isoniazid, rifampicin, pyrazinamide, ethambutol). It
is important to underline that none of the patients received VD
supplements or any other medication that could interfere with VD
plasmatic levels from T0 to T2.
Sample collection and handling
At every visit, three samples of blood were
obtained, using heparinized vacutainers, with EDTA and without
anticoagulant, and further centrifuged at 1,000 x g, within 30 min
of collection. Plasma/serum separated were aliquoted (minimum 500
µl) and stored at -80˚C until biomarker analysis.
Laboratory assessments
Assessment of the serum 25-(OH)-D level, the
recommended analyte for evaluation of VD status, was performed at
the Clinical Laboratory of the Clinical Emergency Hospital of
Craiova on the platform Roche Cobas e601 by
electrochemiluminescence immunoassay using Roche Cobas Vitamin D
total reagent (Roche Diagnostics GmbH). VDR, M30, LL-37 and
beclin-1 were assayed at the Biochemistry Laboratory of the
University of Medicine and Pharmacy of Craiova using commercially
available kits based on enzyme immunoassays. Cusabio Human Vitamin
D Receptor ELISA kit (CUSABIO) was used for VDR dosage following
the steps recommended by the manufacturer. M30 Apoptosense ELISA
(PEVIVA) kit (TECOmedical AG), a one-step solid-phase in
vitro immunologic assay, was used for dosage of serum M30 as it
measures levels of soluble caspase-cleaved K18 fragments. Both
ELISA assays for LL-37 (Human LL-37 ELISA kit, Elabscience
Biotechnology) and beclin-1 (Human Beclin-1 ELISA kit, Cloud-Clone
Corp.), with excellent sensitivity and specificity, were carried
out according to the manufacturer's protocols. All enzyme
immunoassays were performed in duplicate using a complete StatFax
ELISA line (orbital shaker, washing system, plate reader) provided
by Awareness Technology Inc.
Statistical analysis
In the statistical analysis, nonparametric data
(small sample size) are presented as the median ± interquartile
range (IQR). Qualitative variables (sex, environment, culture) are
presented as percentages. The nonparametric Wilcoxon matched-pairs
signed rank test was used to evaluate the significant differences
between variables at T0 (before anti-TB treatment) and T2 (after
two months of anti-TB treatment). Patients were divided into two
groups according to the results obtained at culture analysis
(positive or negative) after two months of treatment. The two
groups were compared using the nonparametric Mann-Whitney U test.
Differences were considered statistically significant at the 5%
level (two-tailed). The strength of quantitative relationship
between different clinical characteristics of the patients was
measured with Spearman coefficients and heatmap. Data were analyzed
using GraphPad Prism 8.4.3 software (GraphPad Software, LLC).
Results
We recruited 30 patients but only 17 were analyzed
during the period of this study. Their mean age ± standard
deviation (SD) was 48.76±8.83 (range: 32-61 years) and weight was
53.88±7.77 (range: 39-64 kg). Demographic characteristics are
presented in Table I and
biochemical characteristics are presented in Table II.
 | Table IDemographic characteristics of the TB
patients (N=17) included in this study. |
Table I
Demographic characteristics of the TB
patients (N=17) included in this study.
| Characteristics | Data |
|---|
| Age, median
(IQR) | 51 (43-55) |
| Weight, median
(IQR) | 58 (47.5-59.5) |
| Sex, n (%) | |
|
Female | 3 (17.7) |
|
Male | 14 (82.3) |
| Environment, n
(%) | |
|
Urban | 4 (23.5) |
|
Rural | 13 (76.5) |
 | Table IIBiochemical characteristics of the TB
patients (N=17) included in this study. |
Table II
Biochemical characteristics of the TB
patients (N=17) included in this study.
| Biochemical
parameters | T0 median (IQR) | T2 median (IQR) | P-value |
|---|
| VDR | 169
(124.5-290.3) | 242.8
(149.5-340.9) | 0.1439 |
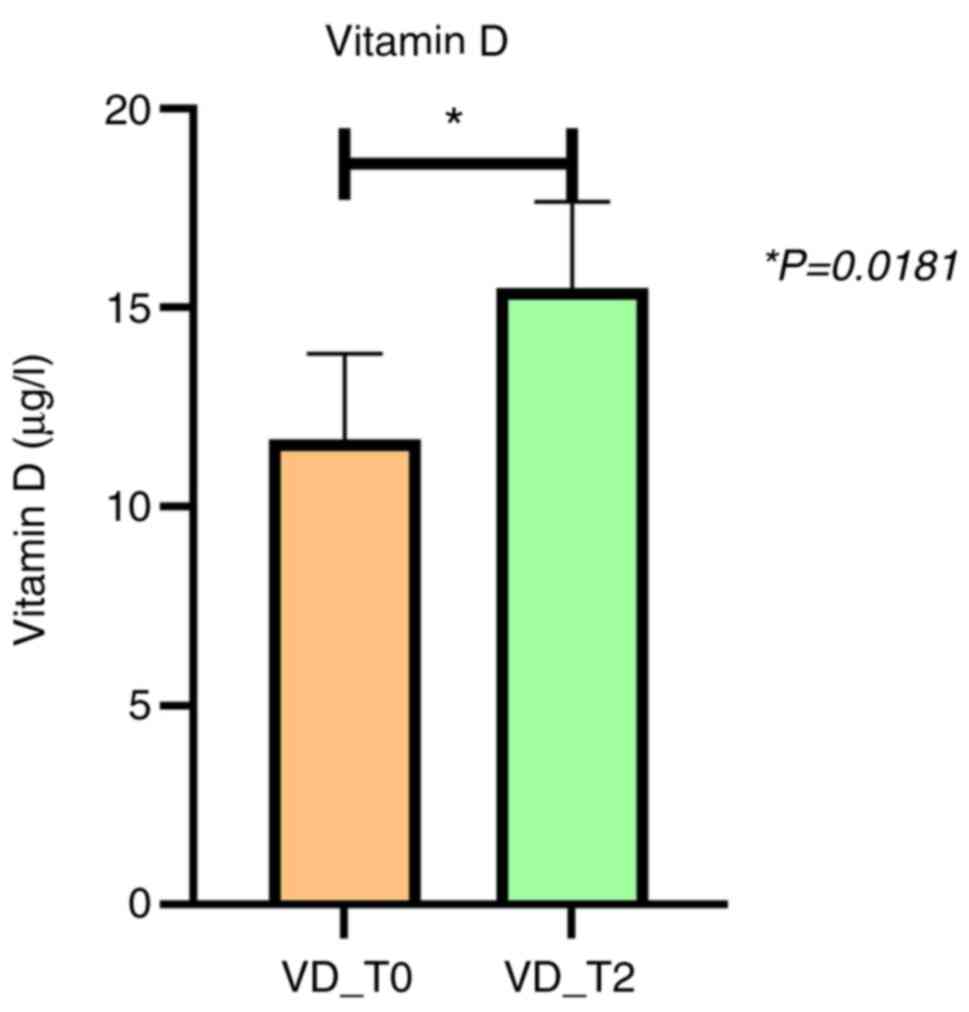
| VD | 9.1 (5.5-16) | 14.4 (8-22.6) | 0.0181a |
| LL-37 | 25.6
(13.75-76.25) | 26.1
(12.9-51.88) | 0.6322 |
| Beclin-1 | 0.21
(0.145-0.25) | 0.13
(0.08-0.30) | 0.3755 |
| M30 | 202.2
(113.1-289.7) | 151.3
(133.2-264.4) | 0.8603 |
Serum VD levels were significantly different between
the two periods of time (before and after two months of anti-TB
treatment) being lower at T0 than at T2 (Fig. 1). After two months of first-line
anti-TB treatment, patients were divided into two different groups
according to the results obtained after sputum-culture analysis; 6
patients were still positive and had bacillary load at T2 and 11
patients were negative at T2. In patients with positive
sputum-culture, the serum levels of VD and VDR varied differently
(3 patients presented lower VDR levels at T2, while VD levels
increased, 2 patients presented higher VDR levels at T2, while VD
levels decreased and only 1 patient was identified with higher
values for both VD and VDR).
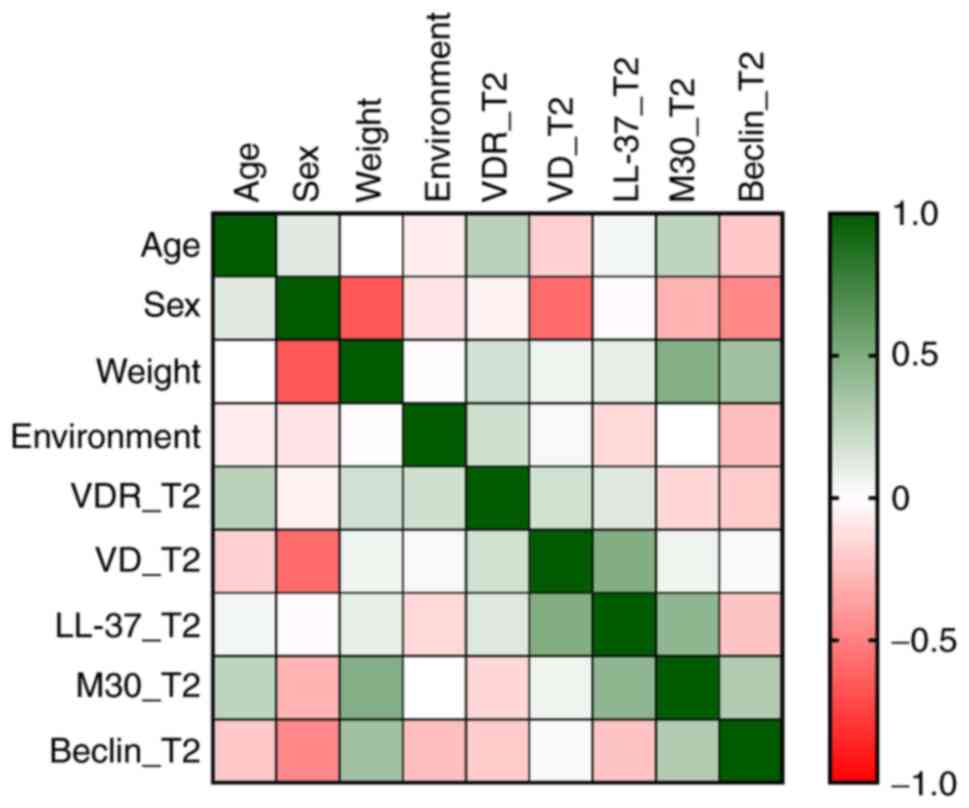
A negative moderate correlation was found between
sex and VD level (rho=-0.58, P=0.032), VD decreasing more for
female patients (Table III). No
significant correlations were found between serum levels of the
biomarkers (Fig. 2).
 | Table IIICorrelations between biochemical and
demographic characteristics of the TB patients (Spearman
coefficients with correlation matrix). |
Table III
Correlations between biochemical and
demographic characteristics of the TB patients (Spearman
coefficients with correlation matrix).
| | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|
| Age (1) | 1.00 | | | | | | | | |
| Sex (2) | 0.13 | 1.00 | | | | | | | |
| Weight (3) | 0.00 | -0.67b | 0.00 | | 1 | | | | |
| Environment
(4) | -0.07 | -0.11 | -0.01 | 1.00 | | | | | |
| VDR_T2(5) | 0.28 | -0.05 | 0.17 | 0.19 | 1.00 | | | | |
| VD_T2(6) | -0.19 | -0.58a | 0.06 | 0.03 | 0.18 | 1.00 | | | |
| LL-37_T2(7) | 0.05 | -0.02 | 0.10 | -0.16 | 0.13 | 0.49 | 1.00 | | |
| M30_T2(8) | 0.25 | -0.30 | 0.48 | 0.00 | -0.16 | 0.07 | 0.44 | 1.00 | |
|
Beclin-1_T2(9) | -0.22 | -0.47 | 0.37 | -0.25 | -0.21 | 0.03 | -0.23 | 0.30 | 1.00 |
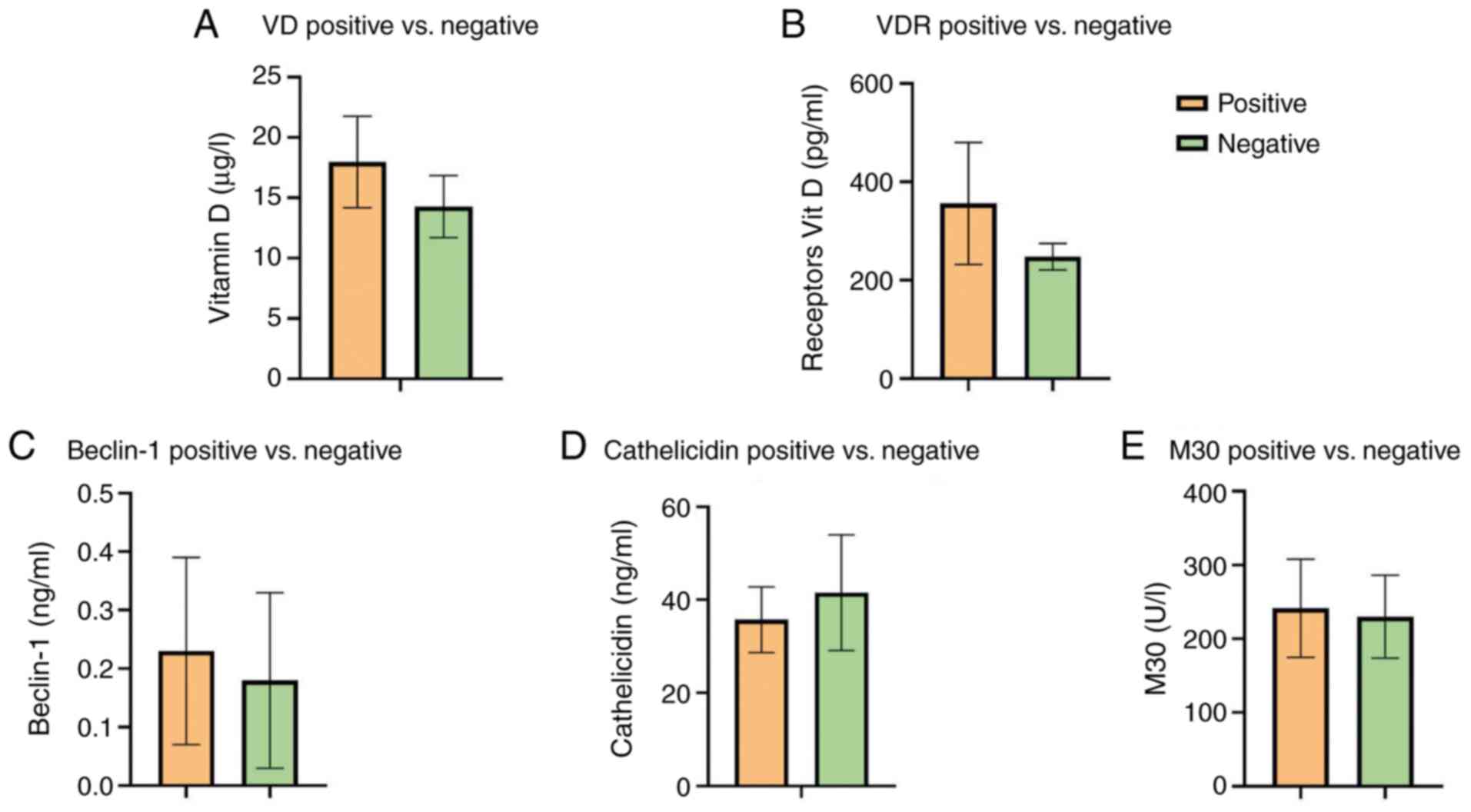
No significant differences in VD, VDR, LL-37,
beclin-1 or M30 were noted among the TB-positive culture and
TB-negative culture group after two months of treatment (Table IV). The results revealed decreased
values in negative patients compared with positive TB patients,
except for LL-37, but the differences were not significant
(Fig. 3).
 | Table IVBiomarkers after two months of
anti-TB treatment. |
Table IV
Biomarkers after two months of
anti-TB treatment.
| Biomarkers Mean (±
SD) Median (IQR) | TB-positive culture
group (n=6) | TB-negative-culture
group (n=11) | P-value |
|---|
| VD | 17.96 (±9.29) | 14.27 (±8.12) | 0.4938 |
| | 14.40
(11.75-25.94) | 11.7
(6.51-23.18) | |
| VDR | 356 (±304.2) | 247.7 (±85.67) | 0.4923 |
| | 236
(130.1-608.1) | 248.5
(163.5-332.6) | |
| LL-37 | 35.73 (±17.29) | 41.55 (±39.33) | 0.8131 |
| | 42
(17.73-49.03) | 21.8
(10.98-91.65) | |
| Beclin-1 | 0.23 (±0.16) | 0.18 (±0.15) | 0.7112 |
| | 0.20
(0.07-0.39) | 0.10
(0.09-0.23) | |
| M30 | 241.4 (±162.9) | 229.9 (±177.6) | 0.7925 |
| | 187.9
(130-339.9) | 151.3
(123.9-319.2) | |
Discussion
All the enrolled patients included in the present
study were diagnosed with primary active pulmonary tuberculosis
(TB) through acid-fast bacilli microscopic identification and
radiologic chest examinations at T0 and administration of
first-line specific anti-TB treatment (isoniazid, rifampicin,
pyrazinamide, ethambutol) was initiated at a weight-dependent
dosage. Pyridoxine (vitamin B6) was the only supplement
administered during the therapy.
The risk of developing infectious pulmonary
diseases, including TB, is related to malnutrition, tobacco and
alcohol consumption, and low socioeconomic status (14). In line with this, all the included
patients presented at the hospitals were active smokers or alcohol
consumers, with malnutrition and 76.5% came from rural environment.
Moreover, our analysis showed vitamin D3 (VD) deficiency
before anti-TB treatment (T0) as a feature of all patients with
pulmonary TB included in our study, suggesting impaired
antimycobacterial innate immunity mechanisms, in congruence with
other studies (14-16)
which report that the VD axis plays an essential role in innate
defense against intracellular microorganisms.
It is important to emphasize that both VD and the
vitaminD receptor (VDR) increased after two months of first-line
anti-TB pharmacotherapy without administering other drugs
interfering with VD serum levels or nutritive supplements.
Increased serum VD levels as a treatment outcome support the
antimycobacterial effects of anti-TB treatment and a higher sputum
conversion rate (14).
Macrophages recognize mycobacteria through specific
receptors, Toll-like receptors (TLRs) and further enhance VDR
expression (15,16). VDR promotes cathelicidin (LL-37)
synthesis and activity, leading to bacterial membrane
disintegration (15). In our study,
81.81% of the patients (9 patients) with negative sputum-culture at
T2 presented proportionally increased serum levels for both VD and
VDR, while only 18.18% (2 patients who initially presented higher
bacillary loads at T0) were identified with lower levels of VDR and
higher levels of VD. Decreased levels of VDR may be due to
downregulation of its expression. In addition, VDR gene
polymorphisms may influence VDR activity and downstream VD-mediated
effects (15-18).
Lack of VDR could suggest that serum VD are not sufficient to
promote innate immune responses (15-18).
Further studies regarding VD supplementation in pulmonary TB
patients may be useful in establishing the importance of this
liposoluble vitamin in immunity and antimycobacterial processes, as
available clinical trials have given inconclusive results to date
(18).
VD-mediated innate immunity and autophagy have been
shown to provide protection against infection with Mycobacterium
tuberculosis (M.tb) (14-17). After VD binding to VDR through VD
response elements and induction of oxidative stress burst, LL-37
expression is increased and autophagy is upregulated through
phagosomal maturation (14,18). Elimination of M.tb. is
dependent on both LL-37 and beclin-1, as higher levels determine
better intracellular bacterial killing (1,6).
Several studies have pointed out that not only susceptibility to
infection, but also impaired expression of LL-37 has been
associated with VD deficiency, as this antimicrobial peptide is
highly dependent on VD concentration (18-20).
As Ayelign et al emphasize, lower VD levels are connected
with lower antimicrobial LL-37 influence and delayed autophagy
mechanisms (16).
The present study reported decreased values of
LL-37, beclin-1 and M30 at T2 in culture-negative patients compared
with culture-positive patients. Culture-positive individuals
presented activated immunological pathways and signaling as an
attempt to maintain intracellular homeostasis and restriction of
M.tb. growth (6). VD
actively increases LL-37 expression and promotes pathogen clearance
by recruiting T cells and enhancing immunomodulatory activities,
whereas the mycobacteria downregulate it and delay disruption of
physical microbial membrane integrity through TLR signaling
(8,17,18,21).
Research on cell culture has confirmed that inhibition of
M.tb. development was sustained by both LL-37 upregulation
and VD supplementation (18).
M.tb. adopts different strategies in order to
evade immune macrophagic mechanisms, by restraining phago-lysosomal
fusion, increasing secretion of pro-inflammatory biomarkers and
generating necrosis instead of apoptosis (15,18).
Necrosis determines irreversible membrane and unbeneficial
host-cellular destruction, while apoptosis restores normal cellular
functionality as it attenuates accumulation of misfolded peptides
in endoplasmic reticulum, therefore limiting TB progression
(10). Caseous granuloma formation,
tissue remodelling and cavitation result as a consequence of
M.tb.-induced necrosis (10). On the other hand, macrophages and
dendritic cells are responsible for apoptotic body degradation,
enhancing both innate and adaptive responses (10). Along with LL-37 and beclin-1, M30
could also be a useful clinical marker for disease assessment and
prognosis (11), as it contributes
to visualize structural cellular changes and to understand
different pathophysiological mechanisms involved in TB.
Our present study presented several limitations. The
small number of included patients was due to the pandemic period of
COVID-19, as access to hospitals from our town was restricted. We
assessed biomarkers only after two months of anti-TB therapy but a
longer period would have been useful to examine the influence of
treatment on immune mechanisms such as autophagy and apoptosis.
Therefore, we intend to perform further studies with more clinical
cases in the future. In the present study, we recruited only
patients who had not received VD supplements or drugs interfering
with VD metabolic activity, but we did not monitor food intake,
sunlight exposure or genetic polymorphisms of VDR, which can also
affect the risk of TB activation and progression (14). With regard to the demographics, most
of our patients showed a male predominance and this may be a
potential bias.
Nevertheless, one of the major strengths of our
study is represented by the analysis of M30 involvement in
pulmonary TB, as the first so far to the best of our knowledge. The
unique cohort of subjects, without comorbidities and only pulmonary
infection with mycobacteria, strongly indicates the value of our
correlations.
All in all, both autophagy and apoptosis biomarkers
as well as the VD axis and immunological response variations in
pulmonary TB should not be neglected in further studies, as they
may be useful in controlling this highly infectious public
threat.
Acknowledgements
We are thankful for the technical support provided
by chemist Loredana Colhon from the Department of Biochemistry,
University of Medicine and Pharmacy of Craiova, Romania, and to all
the patients who gave consent for participating in the present
study.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ADM, SS, MB and ATS were involved in the literature
research and wrote the manuscript. ATS supported the statistical
analysis and reviewed the results. CGP, ADM and SS conceived,
planned and followed the execution of the experiments. CGP and AMB
administered the 25-OH-D dosage and contributed to the manuscript
revision. FMN, RC and MM collected and analyzed the patient
samples. All authors contributed to the manuscript revision, read
and approved the final version.
Ethics approval and consent to
participate
The study was approved by The Ethics Committees of
The University of Medicine and Pharmacy of Craiova, Romania (Nr.
5/17.01.2019). All patients included in the study provided informed
consent for data publication.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
This study is part of the Ph.D. thesis of
Andreea-Daniela Meca from the University of Medicine and Pharmacy
of Craiova, Romania.
References
|
1
|
Rekha RS, Mily A, Sultana T, Haq A, Ahmed
S, Mostafa Kamal SM, van Schadewjik A, Hiemstra PS, Gudmundsson GH,
Agerberth B and Raqib R: Immune responses in the treatment of
drug-sensitive pulmonary tuberculosis with phenylbutyrate and
vitamin D3 as host directed therapy. BMC Infect Dis.
18(303)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Geneva, World Health Organization: WHO
Global Tuberculosis Report. Geneva World Health Organization,
pp1-261, 2019. https://www.who.int/tb/publications/global_report/en/.
|
|
3
|
WHO Regional Office for Europe, European
Centre for Disease Prevention and Control. Tuberculosis
Surveillance and Monitoring in Europe 2018-2016 Data. World Health
Organization, pp181-182, 2018. https://www.ecdc.europa.eu/en/publications-data/tuberculosis-surveillance-and-monitoring-europe-2018.
|
|
4
|
Ashenafi S, Mazurek J, Rehn A, Lemma B,
Aderaye G, Bekele A, Assefa G, Chanyalew M, Aseffa A, Andersson J,
et al: Vitamin D3 status and the association with human
cathelicidin expression in patients with different clinical forms
of active tuberculosis. Nutrients. 10(721)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jo EK: Autophagy as an innate defense
against mycobacteria. Pathog Dis. 67:108–118. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Paik S, Kim JK, Chung C and Jo EK:
Autophagy: A new strategy for host-directed therapy of
tuberculosis. Virulence. 10:448–459. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Balcells ME, Yokobori N, Hong BY, Corbett
J and Cervantes J: The lung microbiome, vitamin D, and the
tuberculous granuloma: A balance triangle. Microb Pathog.
131:158–163. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Torres-Juarez F, Cardenas-Vargas A,
Montoya-Rosales A, González-Curiel I, Garcia-Hernandez MH,
Enciso-Morenzo JA, Hancock RE and Rivas-Santiago B: LL-37
immunomodulatory activity during Mycobacterium tuberculosis
infection in macrophages. Infect Immun. 83:4495–4503.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gupta S, Winglee K, Gallo R and Bishai WR:
Bacterial subversion of cAMP signalling inhibits cathelicidin
expression, which is required for innate resistance to
Mycobacterium tuberculosis. J Pathol. 242:52–61.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lam A, Prabhu R, Gross CM, Riesenberg LA,
Singh V and Addarwal S: Role of apoptosis and autophagy in
tuberculosis. Am J Physiol Lung Cell Mol Physiol. 313:L218–L229.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lee KS, Chung JY, Jung YJ, Chung WY, Park
JH, Sheen SS, Lee KB and Park KJ: The significance of
caspase-cleaved cytokeratin 18 in pleural effusion. Tuberc Respir
Dis (Seoul). 76:15–22. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mohareer K, Asalla S and Banerjee S: Cell
death at the cross roads of host-pathogen interaction in
Mycobacterium tuberculosis infection. Tuberculosis (Edinb).
113:99–121. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Elliott TO, Owolabi O, Donkor S, Kampmann
B, Hill PC, Ottenhoff TH, Haks MC, Kaufmann SH, Maertzdorf J and
Sutherland JS: Dysregulation of apoptosis is a risk factor for
Tuberculosis disease progression. J Infect Dis. 212:1469–1479.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang M, Kong W, He B, Li Z, Song H, Shi P
and Wang J: Vitamin D and the promoter methylation of its metabolic
pathway genes in association with the risk and prognosis of
tuberculosis. Clin Epigenetics. 10(118)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chung C, Silwal P, Kim I, Modlin RL and Jo
EK: Vitamin D-cathelicidin axis: At the crossroads between
protective immunity and pathological inflammation during infection.
Immune Netw. 20(e12)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ayelign B, Workneh M, Molla MD and Dessie
G: Role of vitamin-D supplementation in TB/HIV co-infected
patients. Infect Drug Resist. 13:111–118. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Alford MA, Baquir B, Santana FL, Haney EF
and Hancock RE: Cathelicidin host defense peptides and inflammatory
signaling: Striking a balance. Front Microbiol.
11(1902)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rode AKO, Kongsbak M, Hansen MM, Lopez DV,
Levring TB, Woetmann A, Ødum N, Bonefeld CM and Geisler C: Vitamin
D counteracts Mycobacterium tuberculosis-induced
cathelicidin downregulation in dendritic cells and allows Th1
differentiation and IFNγ secretion. Front Immunol.
8(656)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xia J, Shi L, Zhao L and Xu F: Impact of
vitamin D supplementation on the outcome of tuberculosis treatment:
A systematic review and meta-analysis of randomized controlled
trials. Chin Med J (Engl). 127:3127–3134. 2014.PubMed/NCBI
|
|
20
|
Talat N, Perry S, Parsonnet J, Dawood G
and Hussain R: Vitamin D deficiency and tuberculosis progression.
Emerg Infect Dis. 16:853–855. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rajamanickam A, Munisankar S, Dolla CK and
Babu S: Diminished systemic and mycobacterial antigen specific
anti-microbial peptide responses in low body mass index-latent
tuberculosis co-morbidity. Front Cell Infect Microbiol.
10(165)2020.PubMed/NCBI View Article : Google Scholar
|