Introduction
Osteoporosis(OP) is a systemic bone metabolic
disorder, which is characterized by decreased bone density and
degeneration of the bone microstructure, resulting in increased
bone brittleness and risk of fractures (1). Bone modeling and remodeling are
dynamic metabolic processes that are mainly regulated by two types
of bone cells: Osteoblasts, which secrete bone matrix and
accelerate calcium deposition; and osteoclasts, which dissolve
mineralized bone matrix (2); the
imbalance between these two processes leads to the development of
OP. Along with an increase in age, the absorption of calcium in the
body decreases with the reduction of osteoblast production, whereas
endocrine and metabolic factors also play a crucial role in
reducing the bioavailability of calcium (3). At present, due to the aging of the
population, the incidence of OP among the elderly is increasing
annually. Research by several patient investigation centers in
China in 2014 indicated that the prevalence of OP in individuals
aged 50-59 years was 15.5%, and its prevalence in individuals aged
80-89 years was 81% (4).
Furthermore, among patients with OP aged between 50 and 89 years,
the spine accounts for 28% of the total morbidity, the femur
accounts for 15%, and both the spine and femur account for 31%
(5). With an increasing incidence
of OP, the social burden has greatly increased.
It has been demonstrated that oxidative stress is
associated with the pathogenesis of OP, and the pathogenesis of OP
is accompanied by an increase in oxidative stress and apoptosis
(6). Accumulating evidence has
indicated that reactive oxygen species (ROS)-induced oxidative
stress increases with aging, which is related to the
pathophysiology of postmenopausal OP (7). An excess of ROS can inhibit osteoblast
differentiation and proliferation, enhance osteoclastic
differentiation, and ultimately lead to greater bone reabsorption
(8). Dietary supplementation with
antioxidants is an effective approach to improving the damage
caused by excessive ROS generation. N-acetyl cysteine (NAC) is one
of the most widely used antioxidants in the context of clinical
studies, animal and cell culture experiments (9). A number of conclusions have been based
on the outcome of experiments using NAC. When treatment with NAC
was found to inhibit a particular cellular process or response, it
was typically concluded that ROS play an active role in this
process. For example, the stimulating effects of gonadectomy on
oxidative stress, osteoblast apoptosis and osteoclastogenesis, and
the loss of bone mass, were shown to be reduced by supplementation
with antioxidants, such as NAC and ascorbate (10,11).
Amongst several other applications, NAC has also been used to
investigate the physiological roles of mitochondrial ROS (12,13).
Additionally, Yamada et al (14) demonstrated that NAC markedly
promoted the differentiation of osteoblastic cells and accelerated
bone regeneration. Calcium ion is an essential structural component
of the skeleton, and oxidative stress is an important mediator of
bone loss (15). The administration
of antioxidants may protect bones from OP and may also help
accelerate the healing of fractured bones.
Under conditions of oxidative stress,
phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) is a vital
signaling pathway that participates in regulating the
proliferation, death and survival of cells, and serves as a
targeted pathway of intervention treatment(16). Studies on the PI3K signaling pathway
have reported that AKT and its related downstream signaling
molecules are key factors for regulating endochondral ossification
(17,18). AKT may affect bone formation and
osteoblast survival by maintaining the class O forkhead box
transcription factors (FOXOs) in the cytoplasm (19). These previous studies have indicated
that the PI3K/AKT signaling pathway is closely associated with the
occurrence of OP.
The present study investigated the use of total
flavonoids of Rhizoma Drynariae (TFRD) and calcium in the treatment
of OP. TFRD are considered to be natural antioxidant agents and
free radical scavengers, and may help prevent bone loss; the intake
of calcium can attenuate bone loss and improve bone mineralization
(20). Therefore, it was
hypothesized that TFRD combined with calcium could improve OP
through relieving oxidative stress. For this purpose, in the
present study, an OP model was established by ovariectomy in female
rats, and the effects of the combined use of TFRD and calcium
carbonate (CaCO3) on bone mineral density (BMD), bone
mineral content (BMC) and oxidative stress were determined. The
findings of the present study may provide a basis for the future
clinical prevention and treatment of OP.
Materials and methods
Experimental animals
A total of 36 female specific pathogen-free (SPF)
Sprague-Dawley rats, aged 3 months and weighing 230±20 g, were
purchased from Chengdu Dashuo Experimental Animal Co., Ltd.
(license no. SYXK 2018-119). The rats were kept in cages with a
controlled environment (temperature 24˚C and humidity 40%) and a
12/12-h light/dark cycle and were provided with free access to food
and water. The present study was approved by the Animal Care Unit
and Use Committee of Chengdu University of TCM (no. 20190078).
Experimental reagents
CaCO3, TFRD (Guangzhou Baozhilin
Pharmacy), NAC (Sigma-Aldrich; Merck KGaA) and kits for superoxide
dismutase (SOD; cat. no. A001-3-2), malondialdehyde (MDA; cat. no.
A003-1-2), ROS (cat. no. E004-1-1) and glutathione peroxidase
(GSH-Px; cat. no. A005-1-2) were purchased from Nanjing Jiancheng
Bioengineering Institute; kits for IL-6 (cat. no. ZC-36404), IL-1β
(cat. no. ZC-M6681), TNF-α (cat. no. ZC-37624) were purchased from
ZCIBIO Technology Co., Ltd.; kits for hematoxylin and eosin
(H&E; cat. no. C0105S) staining were purchased from Beyotime
Institute of Biotechnology; kits for Masson's staining (cat. no.
60532ES58) were purchased from Shanghai Maikun Chemical Co., Ltd.;
kits for protein extraction (cat. no. P1202) and SDS-PAGE gel
preparation (cat. no. PG112) were purchased from Shanghai Epizyme
Biotech; RIPA buffer reagent (cat. no. P0013) and kit for BCA (cat.
no. P0009) were purchased from Beyotime Institute of Biotechnology;
primary antibodies against runt-related transcription factor 2
(RUNX2; cat. no. sc-390351), osteoprotegerin (OPG; cat. no.
sc-390518), osteocalcin (BGP; cat. no. sc-365797), AKT (cat. no.
sc-5298), p-AKT (cat. no. sc-377556), mammalian target of rapamycin
(mTOR; cat. no. sc-517464), p-mTOR (cat. no. sc-293133), PI3K (cat.
no. sc-365290) and p-PI3K (cat. no. sc-1637) were purchased from
Santa Cruz Biotechnology, Inc. Horseradish peroxidase-labeled goat
anti-rabbit secondary antibody (cat. no. #56970) was purchased from
Cell Signaling Technology, Inc. kit for Enhanced chemiluminescence
(ECL; cat. no. KF001) was purchased from Affinity Biosciences.
Experimental instruments
The Dual Energy X-Ray Bone Densitometer (Hologic,
Inc.) continuous-wavelength multifunctional microplate reader
(Tecan Group, Ltd.) and the BA200 Digital trinocular micro-camera
system (McAudi Industry Group) and the 5200 Quantity One gel
imaging software (Tanon Science and Technology Co., Ltd.) were used
in the present study.
Rat model of ovariectomy-induced
OP
A total of 36 senile female SPF rats were adaptively
fed for 1week, and then randomly divided into 6 groups (n=6 per
group) as follows: Non-ovariectomy control group (sham-operated
group), model group (OP group), TFRD group (OP + TFRD group),
CaCO3 group (OP + CaCO3group), TFRD combined
with CaCO3 group (OP + TFRD + CaCO3 group)
and TFRD and CaCO3 combined with NAC group (OP + TFRD +
CaCO3 + NAC group). Following anesthesia with 35 mg/kg
pentobarbital sodium (intraperitoneal), the rats in the
sham-operated group underwent sham surgery to remove 1 g of adipose
tissue around the ovary, and the rats of the other 5 groups
underwent bilateral ovariectomy. At 4 weeks post-surgery (from the
5th week onwards), the rats of the respective groups were
intragastrically given CaCO3 (20 mg/kg/day), TFRD (50
mg/kg/day) or TFRD (50 mg/kg/day) and CaCO3 (20
mg/kg/day). The rats in the OP + TFRD + CaCO3 + NAC
group were intraperitoneally injected with 500 mg/kg/day NAC, and
TFRD (50 mg/kg/day) + CaCO3 (20 mg/kg/day) were
administered via intragastric administration once a day. The rats
in the sham-operated and model (OP) groups were administered 0.9%
normal saline intragastrically once daily for 10 consecutive weeks.
NAC was injected once every 2 weeks for a total of 5 times. The
dosage of TFRD and CaCO3was selected based on the
research reports of Fang et al (21) and Zhu et al (22).
Sample collection
After treatment for 10 weeks, blood samples were
collected from the abdominal aorta. For blood collection, 35 mg/kg
pentobarbital sodium was injected into the abdominal cavity to
anesthetize the rats. After successful anesthesia, the rats were
transferred to a pre-sterilized super-clean worktable, an incision
was performed along the middle of the abdomen, and then the left
abdominal wall was cut laterally, fully exposing the abdominal
organs, carefully exposing the retroperitoneum and the abdominal
aorta. Then, the needle of a 10-ml syringe was injected through the
blood vessel wall at an angle of 30 degrees with the needle eye
facing down, the direction was then changed to horizontal, and 5-10
ml blood was drawn from each rat. The rats were sacrificed by
exsanguination after blood collection. Next, the hip joint was
exposed by routine peeling with a sterilized scalpel and scissors
under aseptic conditions. The joint capsule was cut open, the round
ligament of the femoral head was severed above the femoral head,
and the bilateral femoral heads were removed. The left femur tissue
of each rat was fixed in 4% paraformaldehyde fixation solution for
24h, and then decalcified with 15% EDTA-2Na for 4 weeks. After
decalcification, the intact femur was embedded in paraffin, cut
into sections(3-5 µm), and the sections were subjected to H&E
and Masson's staining. The left proximal femur was quickly frozen
in liquid nitrogen for western blotting detection. The intact right
femur was stored at 4˚C in tissue fixation solution for follow-up
dual energy X-Ray bone densitometry.
Dual-energy X-Ray bone density
measurement
Dual-energy X-ray bone densitometry uses X-rays to
examine the bones of the body and processes the corresponding data
through a special chip microcomputer system. It can provide
accurate measurement and positioning data, and the detection speed
is high. Therefore, the bone morphometric parameters of the femurs
were determined using a dual-energy X-ray bone densitometer. The
femurs of the rats were resected and the attached cartilage tissue
was removed. The dual-energy X-ray bone densitometer and its
accompanying small animal cascade standard analysis software
(Hologic discovery Wi; Hologic, Inc.) were then immediately used to
determine the BMD (g/cm2) and BMC
(g/cm2).
H&E and Masson's staining
Bone tissue from each group of rats was decalcified
in 15% EDTA-2Na solution. Then cleaned using xylene and embedded in
melted paraffin. The paraffin block was then cut into 3-5-µm
sections using a microtome. Finally, the sections were dewaxed and
at 50˚C subjected to H&E (hematoxylin staining for 10-20 min in
warm water and eosin staining for 3-5 min at 50˚C) and Masson's
trichrome staining (celestine blue staining for 2-3 min, followed
by hematoxylin staining for 2-3 min, Ponceau masson acid fuchsin
staining for 10min and aniline blue staining for 5 min; all
performed at 37˚C). The BA200 Digital trinocular micro-camera
system was then used to capture images of the sections. Bone
structure parameters, including bone volume over tissue volume
(bone volume fraction; BV/TV, %) and trabecular thickness (Tb.Th,
mm) were then analyzed using the VIDAS automated image analysis
system (Zeiss AG).
Detection of serum indices
After treatment for 10 weeks, blood (5-10 ml) was
extracted from the abdominal aorta as mentioned above and
centrifuged at 2,030 x g at 4˚C for 10 min. The serum was separated
and cryopreserved at -80˚C. The serum levels ofIL-6, IL-1β, TNF-α,
SOD, ROS, MDA and GSH-Px were determined by sequential sample
loading according to the instructions of the manufacturer of the
respective kits. The content of IL-6 in each group was measured at
450 nm using a continuous-wavelength multifunctional enzyme
analyzer. The content of IL-1β in each group was determined at 570
nm, the content of TNF-α in each group was measured at 492 nm, the
content of SOD in each group was detected at 550 nm, the content of
MDA in each group was determined at 532 nm, the content of GSH-Px
in each group was detected at 412 nm, and the content of ROS in
each group was determined at 485 nm. Three test repetitions were
performed for each indicator.
Western blot analysis
For the determination of protein expression levels,
total protein was collected using RIPA buffer reagent, protein
concentration was determined using a BCA kit. Then, a total of 40
µg protein were loaded per lane and separated by 10% SDS-PAGE and
transferred onto PVDF membranes. Membranes were then blocked with
5% skimmed milk powder at room temperature for 2 h. The membranes
were blotted with primary antibodies against RUNX2, OPG, BGP PI3K,
p-PI3K, AKT, p-AKT, mTOR and p-mTOR (all, 1:1,000) at 4˚C
overnight; β-actin (1:10,000) was used as a loading control. The
membranes were washed 3 times with 0.1% TBST buffer for 10 min each
time, followed by incubation at room temperature for 2-3 h with the
secondary antibody (1:5,000). The protein bands resulting from
Western blot analysis were visualized by ECL. The A-value of the
target band was analyzed using Quantity One gel imaging software,
and the ratio of the A-value of the target band to the β-actin band
was considered as the relative expression of the target protein;
the blots were performed in triplicate.
Statistical analysis
The data were statistically analyzed using SPSS 20.0
software (IBM Corp.). After verification of a normal or non-normal
distribution by the Shapiro-Wilk test, two-tailed Student's t-test
and one-way ANOVA followed by Tukey's post-hoc test was performed
to analyze the variables of normal distribution. When data were
non-normally distributed, log-transformation was applied. For all
the analyses, P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of TFRD combined with
CaCO3 on the femur microstructure of OP rats
Compared with the sham-operated group, the BMD, BMC,
BV/TV and Tb.Th were markedly decreased in the OP, OP + TFRD, OP +
CaCO3, OP + TFRD + CaCO3 and OP + TFRD +
CaCO3 + NAC groups (P<0.05; Fig. 1A-D); compared with the OP group, the
BMD, BMC, BV/TV and Tb.Th were markedly increased in the OP + TFRD,
OP + CaCO3, OP + TFRD + CaCO3 and OP + TFRD +
CaCO3 + NAC groups (P<0.05; Fig. 1A-D); compared with the OP + TFRD and
OP + CaCO3 groups, BMD, BMC and BV/TV were markedly
elevated in the rats in the OP + TFRD + CaCO3 + NAC
group (P<0.05; Fig. 1A-D). These
results indicated that TFRD, CaCO3 and NAC markedly
ameliorated bone density in OP rats.
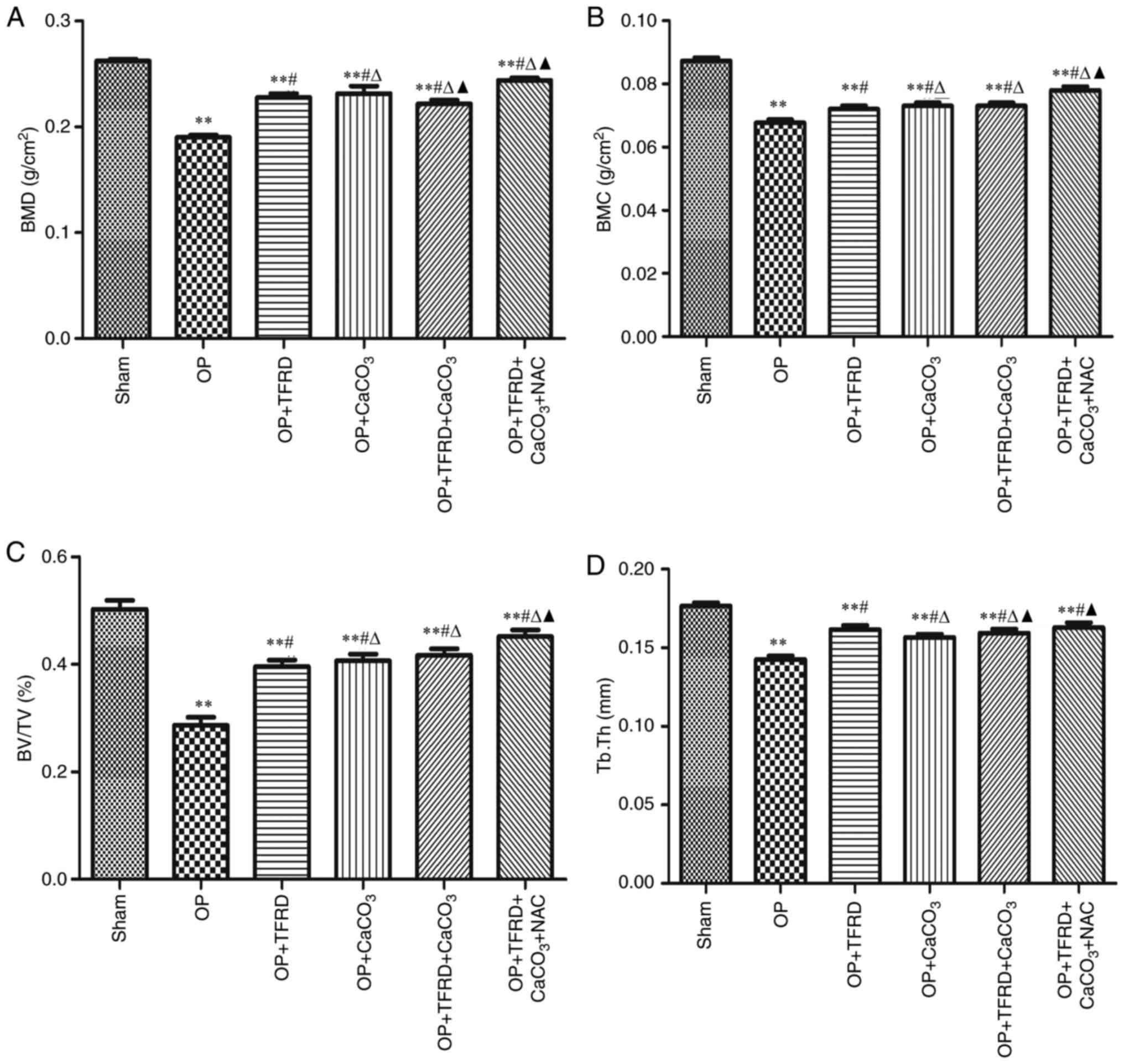 | Figure 1Comparisons of femur microstructure
between the rats in the different groups. (A) BMD; (B) BMC; (C)
BV/TV; and (D) Tb.Th. The data are expressed as mean ± SD. Compared
with the sham-operated group, **P<0.01; compared with
the OP group, #P<0.05; compared with the OP + TFRD
group, rP<0.05; compared with the OP +
CaCO3 group, pP<0.05. OP, osteoporosis;
TFRD, total flavonoids of Rhizoma Drynariae; CaCO3,
calcium carbonate; NAC, N-acetylcysteine; BMD, bone mineral
density; BMC, bone mineral content; BV/TV, bone volume fraction;
Tb.Th, trabecular thickness. |
Effects of TFRD combined with
CaCO3 on the pathological changes of bone in OP
rats
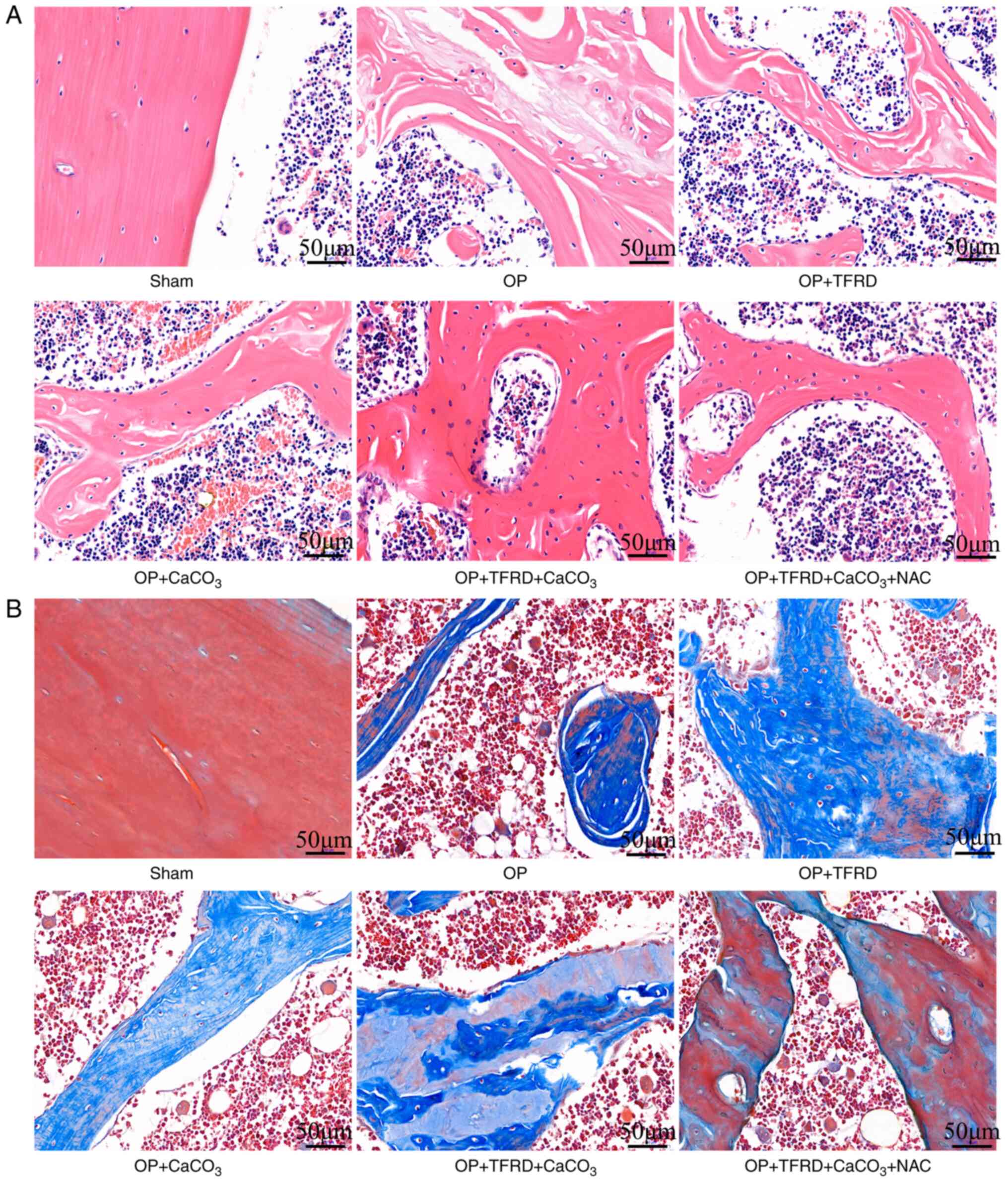
Compared with the sham-operated group, in the OP
model rats, the osteolysis of the femur, bone trabeculae were
disconnected, and there was a large number of bone marrow cells in
the bone marrow cavity; blood cells, macrophages, adipocytes or
mesenchymal cells at different developmental stages were also
observed. Compared with the OP, OP + TFRD and OP + CaCO3
groups, the structure of the bone tissue was clear, complete and
continuous, and the bone trabeculae exhibited a uniform thickness
in the OP + TFRD + CaCO3 + NAC group (Fig. 2A). Compared with the sham-operated
group, in the OP model rats, osteogenic tissue fractures were
observed in some regions, the trabecular bone was markedly reduced,
and the trabeculae were fine and evidently disconnected. Compared
with the OP, OP + TFRD and OP + CaCO3 groups, the
structure of the bone tissue was complete and clear, and a small
number of small cracks were observed in some bone trabeculae in the
OP + TFRD + CaCO3 + NAC group (Fig. 2B). These results indicated that
TFRD, CaCO3 and NAC supplementation promoted the
pathological improvement of bone tissue in OP rats.
Effects of TFRD combined with
CaCO3 on the expression of bone formation-related genes
in OP rats
Compared with the sham-operated group, the protein
expression of RUNX2was significantly decreased in rats in the OP
and OP + CaCO3 groups (P<0.01 and P<0.05,
respectively; Fig. 3A); the
expression of OPG in the rats in the OP, OP + TFRD and OP +
CaCO3 groups was significantly decreased (P<0.01,
P<0.05 and P<0.01, respectively; Fig. 3A); and the expression of BGP in the
rats in the OP group was markedly decreased (P<0.01; Fig. 3A). Compared with the OP group, the
protein expression of RUNX2 and BGP were markedly increased in the
rats in the OP + TFRD + CaCO3 and OP + TFRD +
CaCO3 + NAC groups (P<0.05, P<0.05, P<0.05 and
P<0.01, respectively; Fig. 3A);
the protein expression of OPG was significantly increased in rats
of the OP + TFRD, OP + TFRD + CaCO3 and OP + TFRD +
CaCO3 + NAC groups (P<0.05, P<0.05 and P<0.01;
Fig. 3A). These results suggested
that TFRD, CaCO3 and NAC supplementation increased the
expression levels of bone formation-related genes in OP rats.
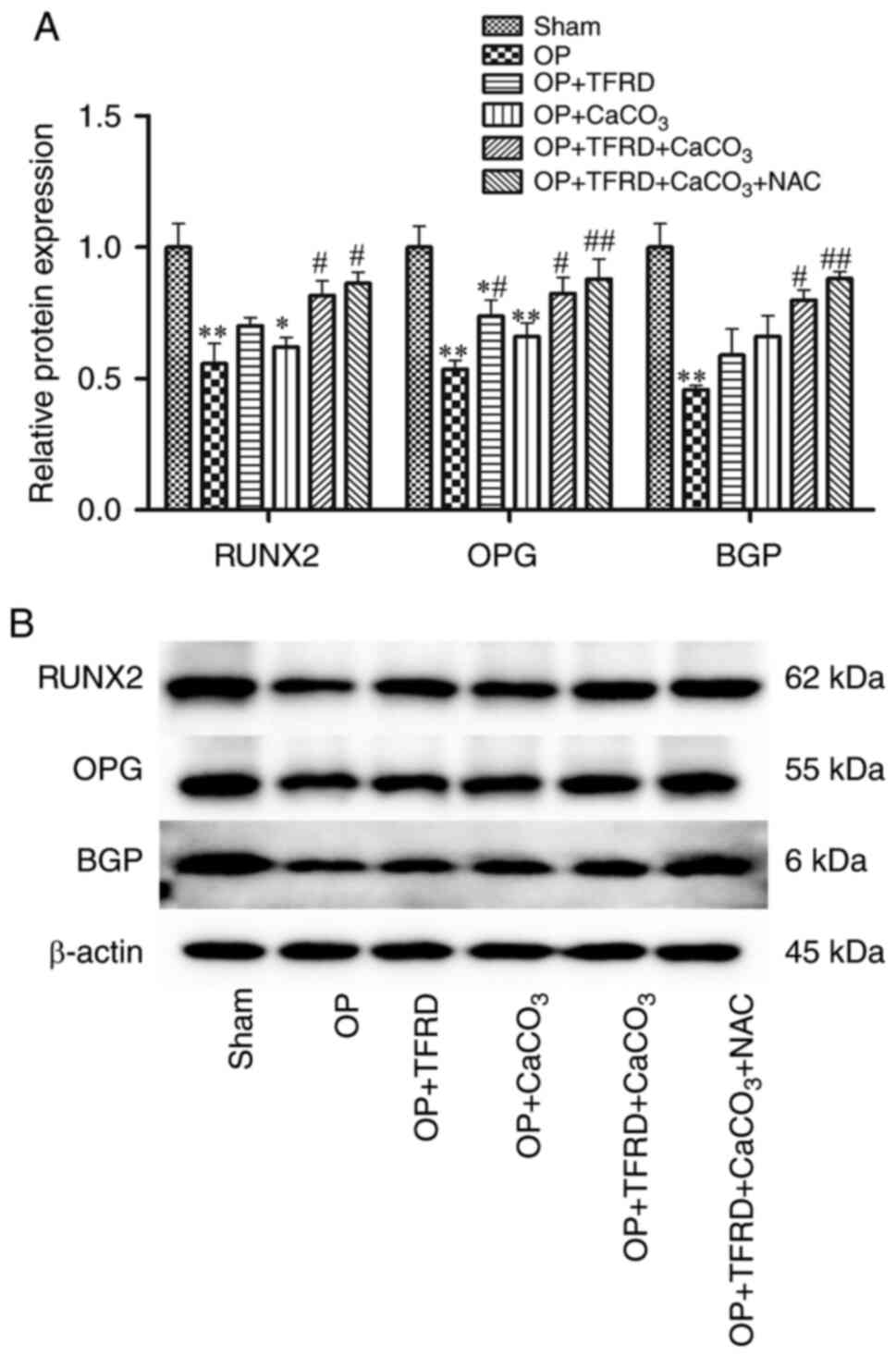 | Figure 3Western blot analysis of the
expression of bone formation-related proteins in OP rats. (A)
Relative expression of RUNX2, OPG and BGP; (B) protein band density
was calculated as a ratio relative to β-actin protein levels. The
data are expressed as the mean ± SD. Compared with the
sham-operated group, *P<0.05 and
**P<0.01; compared with the OP group,
#P<0.05 and ##P<0.01; RUNX2,
Runt-related transcription factor 2; OPG, osteoprotegerin; BGP,
osteocalcin; OP, osteoporosis; TFRD, total flavonoids of Rhizoma
Drynariae; CaCO3, calcium carbonate; NAC, N-acetyl
cysteine. |
Effects of TFRD combined with
CaCO3 on inflammation and oxidative stress in OP
rats
Compared with the sham-operated group, the levels of
IL-6 and IL-1β were significantly increased in rats of the OP, OP +
TFRD and OP + CaCO3 groups (all P<0.05; Fig. 4A and B), the levels of TNF-α, ROS and MDA were
significantly increased in rats of the OP and OP + TFRD groups (all
P<0.05; Fig. 4C-E), whereas SOD
and GSH-Px activity was significantly decreased in rats of the OP,
OP + TFRD, OP + CaCO3 and OP + TFRD + CaCO3
groups (all P<0.05; Fig. 4F and
G). Compared with the OP group, the
levels of IL-6 and TNF-α were significantly decreased in rats of
the OP + TFRD + CaCO3and OP + TFRD + CaCO3 +
NAC groups, the levels of IL-1β, ROS and MDA were markedly
decreased in rats of the OP + TFRD + CaCO3 + NAC group,
and SOD and GSH-Px activity was significantly increased in rats of
the OP + TFRD + CaCO3 + NAC group (all P<0.05;
Fig. 4A-G). Compared with the OP +
TFRD group, the levels of ROS and MDA were markedly decreased in
rats of the OP + TFRD + CaCO3 + NAC group (P<0.05;
Fig. 4D-E). These results revealed
that OP increased the levels of oxidative stress-related markers in
the serum, whereas TFRD, CaCO3 and NAC supplementation
reduce oxidative stress in OP rats.
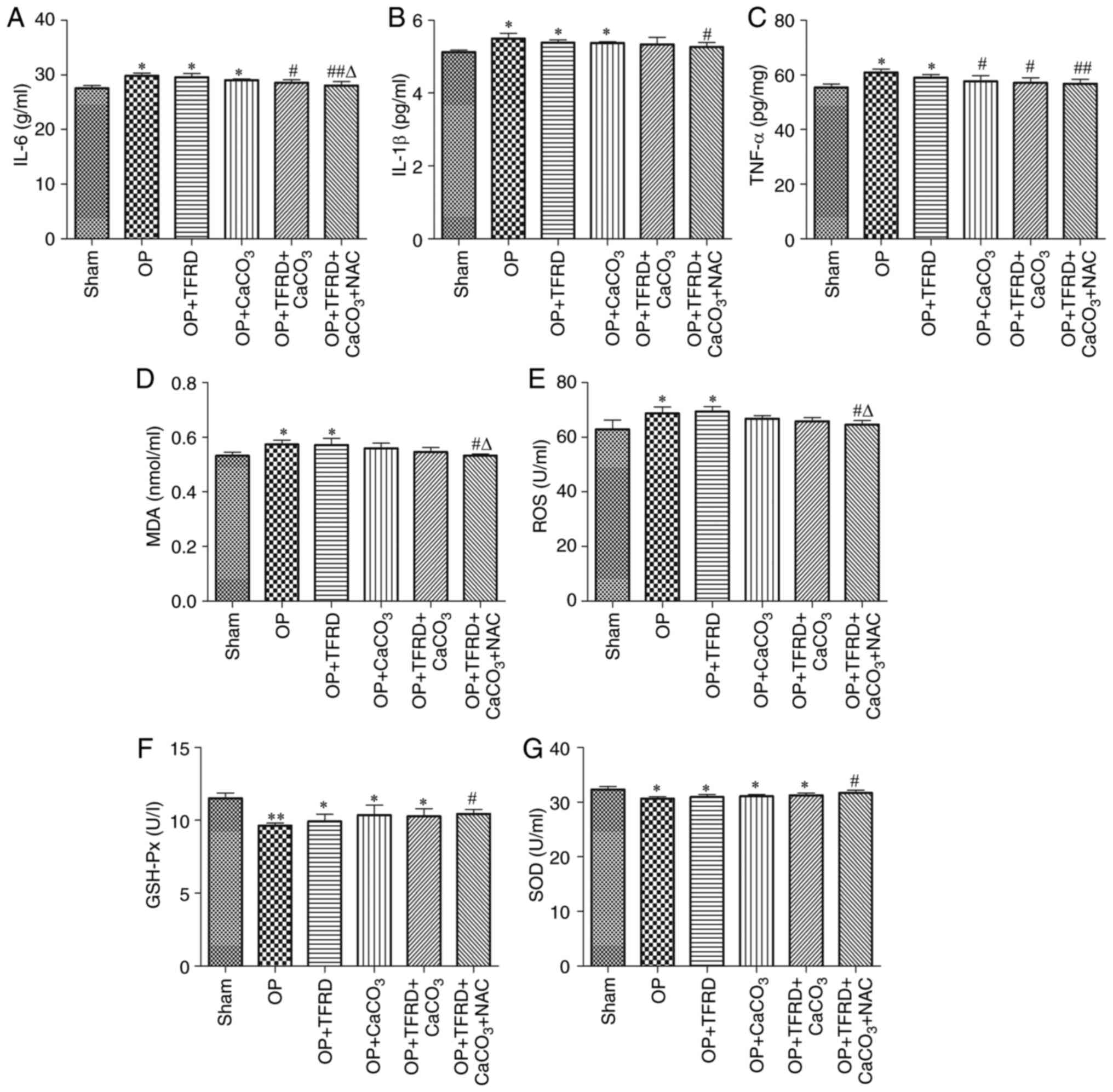 | Figure 4Analysis of inflammatory and
oxidative stress-related factors.Serum levels of (A) IL-6; (B)
IL-1β; and (C) TNF-α. (D)MDA content. Activity of (E) ROS; (F)
GSH-Px; and (G) SOD. The data are expressed as the mean ± SD.
Compared with the sham-operated group, *P<0.05 and
**P<0.01; compared with the OP group,
#P<0.05 and ##P<0.01; compared with the
OP + TFRD group, rP<0.05.MDA, malonaldehyde; ROS,
reactive oxygen species; GSH-Px, glutathione peroxidase; SOD,
superoxide dismutase; OP, osteoporosis; TFRD, total flavonoids of
Rhizoma Drynariae; CaCO3, calcium carbonate; NAC,
N-acetyl cysteine. |
Effects of TFRD combined with
CaCO3 on the oxidative stress pathway in OP rats
Compared with the sham-operated group, the contents
of AKT, p-AKT, mTOR, p-mTOR and PI3K exhibited no significant
differences in the other groups (P>0.05; Fig. 5A). The content of p-PI3K was
significantly increased in rats of the OP group (P<0.01;
Fig. 5A). Compared with the OP
group, the content of p-PI3K in the OP + TFRD + CaCO3
and OP + TFRD + CaCO3 + NAC groups was significantly
decreased (all P<0.01; Fig. 5A).
Compared with the sham-operated group, the p-AKT/AKT ratio was
markedly increased in OP rats (P<0.01), the p-mTOR/mTOR ratio
was significantly increased in the OP, OP + TFRD and OP +
CaCO3 groups (P<0.01, P<0.05 and P<0.01,
respectively), and the p-PI3K/PI3K ratio was markedly increased in
rats of the OP, OP + TFRD, OP + CaCO3 and OP + TFRD +
CaCO3 groups (P<0.01, P<0.01, P<0.05 and
P<0.05, respectively; Fig. 5B).
Compared with the OP group, the p-AKT/AKT ratio was significantly
decreased in rats of the OP + TFRD + CaCO3 + NAC group
(P<0.01; Fig. 5B), and the
p-mTOR/mTOR and p-PI3K/PI3K ratios were markedly decreased in rats
of the OP + TFRD + CaCO3 and OP + TFRD +
CaCO3 + NAC groups (P<0.01 and P<0.05,
respectively; Fig. 5B). Compared
with the OP + TFRD group, the of p-mTOR/mTOR ratio was
significantly decreased in the OP + TFRD + CaCO3 + NAC
group (P<0.05; Fig. 5B).
Compared with the OP + CaCO3group, the p-PI3K/PI3Kratio
was significantly decreased in rats of the OP + TFRD +
CaCO3 + NAC group (P<0.05; Fig. 5B). These results indicated that OP
increased the levels of phosphorylated proteins in the oxidative
stress pathway, while TFRD, CaCO3 and NAC
supplementation reduced the level of phosphorylated proteins in the
oxidative stress pathway in OP rats.
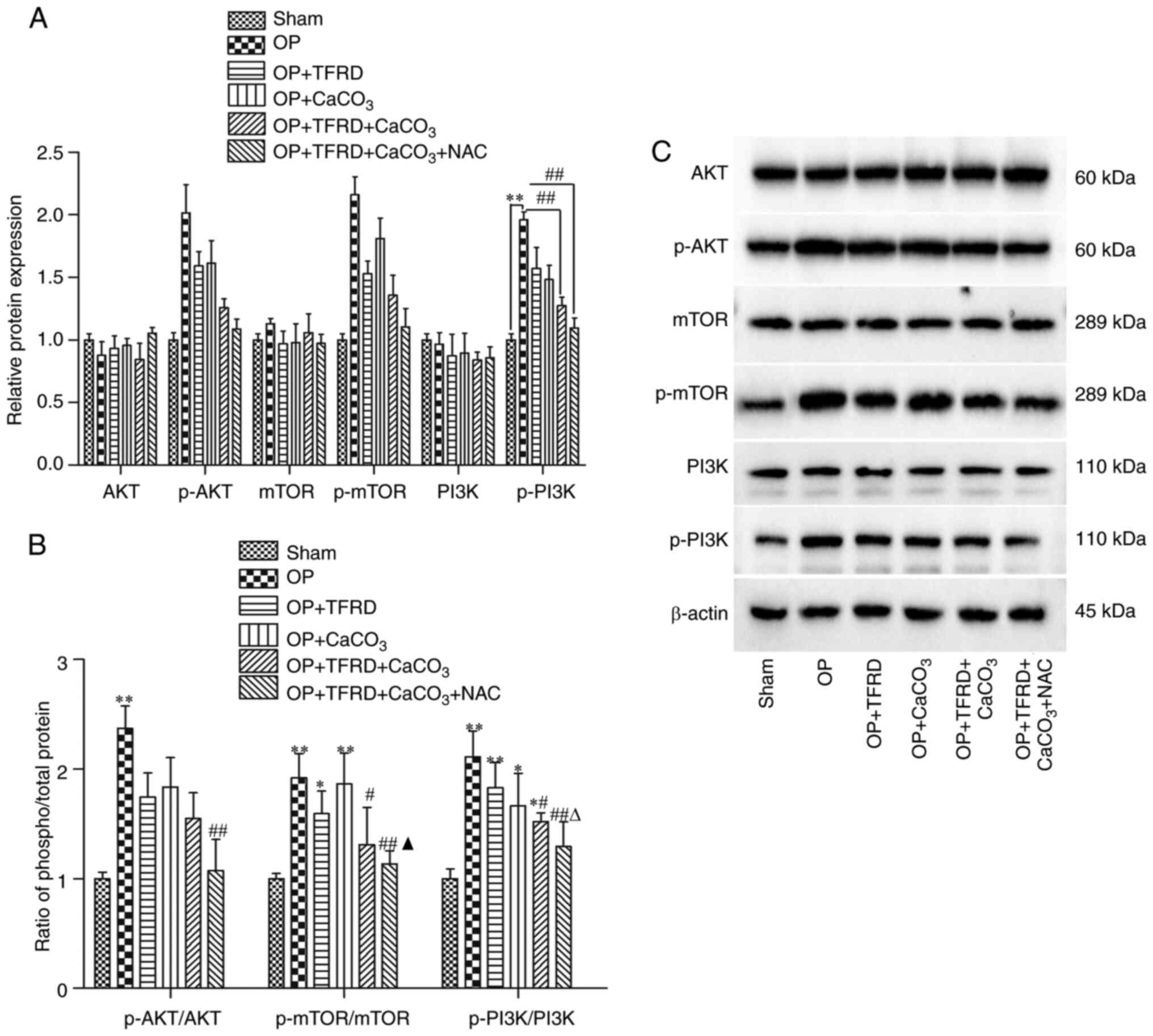 | Figure 5Western blot analysis of oxidative
stress pathway-related factors in OP rats. (A) Relative expression
of AKT, p-AKT, mTOR, p-mTOR, PI3K and p-PI3K; (B) p-AKT/AKT,
p-mTOR/mTORandp-PI3K/PI3K ratio; (C) Protein band density was
calculated as a ratio relative to β-actin protein levels. The data
are expressed as the mean ± SD. Compared with the sham-operated
group, *P<0.05 and **P<0.01; compared
with the OP group, #P<0.05 and
##P<0.01; compared with the OP + TFRD group,
rP<0.05; compared with the OP + CaCO3
group, pP<0.05. OP, osteoporosis; TFRD, total
flavonoids of Rhizoma Drynariae; CaCO3, calcium
carbonate; NAC, N-acetyl cysteine. |
Discussion
The present study reported that TFRD combined with
calcium inhibited OP by reducing ROS generation. The results
revealed that the supplementation of TFRD, calcium and NAC
significantly increased antioxidant capacity, increased BMD and
reduced bone mineral loss in rats with OP. These findings confirmed
that TFRD combined with calcium inhibited the occurrence of OP by
suppressing ROS generation.
OP is mainly characterized by degenerative changes
in bone tissue microstructure, osteopenia and increased bone
fragility, thus greatly increasing the risk of fractures (23). Calcium is an important mineral of
the body, and the main pathological change of OP is bone mineral
loss (15). The decrease of the
calcium content leads to an increase in ROS level; by contrast, the
addition of antioxidants can significantly restore calcium
absorption (24). Therefore,
calcium deficiency is an important cause of OP, and calcium
supplementation is the most effective and safe method for the
prevention and treatment of OP (25). Appropriate calcium supplementation
can enhance bone calcium levels and increase bone density, thereby
significantly ameliorating bone formation (26). An adequate intake of calcium and
vitamin D can prevent bone loss in postmenopausal women and
maintain the bone density of the femoral neck and lumbar vertebrae,
thus reducing the incidence of fractures (27). The OP rat model is a widely used
animal model in the study of OP (28). In the present study, an OP rat model
was constructed to evaluate the effects of TFRD combined with
calcium on OP. The rats with OP exhibited a severely decreased BMD,
BMC, BV/TV and Tb.Th. Treatment with TFRD combined with
CaCO3 significantly increased bone density and reduced
bone mineral loss in OP rats. In addition, the BMD, BMC and BV/TV
were normalized in rats with OP following treatment with NAC. These
results suggest that NAC enhances the preventive effects of TFRD
combined with CaCO3 on bone loss in OP rats, and this
recovery may be mainly achieved through passive calcium
absorption.
Accumulating evidence indicates that increased ROS
generation and decreased antioxidant defense mechanisms lead to
bone loss in OP (29,30). The present study sought to determine
whether OP is associated with increased oxidative stress. It has
recently been demonstrated that oxidative stress is associated with
decreased levels and activity of antioxidant enzymes in mouse
vertebral tissue, which leads to decreased osteoblast-mediated bone
formation and increased osteoclast-mediated bone resorption
(31). The results revealed that
the level of ROS and MDA content were significantly increased, and
the activity of SOD and the expression of GSH-Px were significantly
decreased in rats with OP. TFRD combined with CaCO3
increased the activities of SOD and GSH-Px, and reduced the
production of ROS and MDA in rats with OP, thus exerting
antioxidant effects. TFRD and CaCO3 in combination
exerted more prominent effects compared with either treatment
alone. Inflammatory factors (such as ILs) are released in patients
with OP and it has been demonstrated that they are negatively
associated with BMD (32). IL-6 is
a multicellular cytokine, and its level changes may reflect the
severity of the inflammatory response; under stress conditions,
such as infection or tissue injury, the level of serum IL-6
increases (33). IL-1β and TNF-α
can jointly promote the production of other cytokines, such as
IL-11 and macrophage colony-stimulating factor, thus promoting the
imbalance of bone metabolic coupling (34). In the present study, the results
revealed that the expression of IL-6, IL-1β and TNF-α in OP rats
was significantly increased, and the levels of IL-6, IL-1β and
TNF-α were significantly decreased following treatment with TFRD,
CaCO3 and NAC. These results suggested that TFRD,
CaCO3 and NAC treatment improved inflammatory factor
levels in rats with OP, and this improvement was at least partly
accompanied by changes in the oxidative stress level and
inflammatory response.
The increased accumulation of ROS can significantly
decrease the expression of the osteogenic marker proteins RUNX2,
BGP and OPG (35). RUNX2is an early
marker of osteoblast differentiation, whereas the Wnt pathway and
BMP play an osteogenic role by stimulating the expression of
RUNX2(36). Furthermore, high
expression of RUNX2can activate OPN and regulate BGP, while BGP can
maintain the normal mineralization rate of bone (37). The regulatory mechanism of
osteogenesis and bone resorption was demonstrated by Dufresne et
al (38); the process of bone
remodeling mainly depends on the expression of OPG and receptor
activator of NF-κΒ ligand (RANKL) in osteoblasts; OPG blocks the
binding of RANKL to RANK and regulates bone resorption by
inhibiting osteoclast differentiation (39). The present study demonstrated that
the protein expression of RUNX2, OPG and BGP decreased
significantly in rats of the OP group. However, the protein
expression of RUNX2, OPG and BGP in the OP + TFRD +
CaCO3 and OP + TFRD + CaCO3 + NAC groups was
significantly increased. Adding TFRD to CaCO3 following
ovariectomy increased RUNX2 expression, while CaCO3
alone following ovariectomy did not increase RUNX2 expression,
which may be due to the fact that TFRD can initiate the
differentiation of rat bone marrow mesenchymal cells into
osteoblasts and regulate the expression of osteogenic factor mRNA
in the Wnt/β-catenin signaling pathway. In addition, TFRD can also
promote the maturation of osteoblasts, thereby stimulating the
expression of RUNX2. These results indicated that TFRD,
CaCO3 and NAC supplementation inhibited osteoclast
differentiation and prevented the occurrence of OP.
The PI3K/AKT signaling pathway regulates the
function of osteoblasts and osteoclasts by affecting their
formation, differentiation, proliferation and apoptosis (40). It has been reported that the
PI3K/AKT signaling pathway maintains the dynamic balance of bone
tissue, not only under normal conditions, but also under
pathological conditions (17). It
has been demonstrated that RUNX2 can enhance the activity of the
PI3K/AKT signaling pathway by upregulating the protein levels of
PI3KP85 subunit, p110 β subunit and AKT (41). At the same time, PI3K/AKT can
significantly promote the DNA binding ofRUNX2 and RUNX2-dependent
transcription (42). When AKT2 is
deficient, the gene and protein expression of RUNX2is low, and the
AKT pathway can promote the gene expression of the RUNX2(43). The feedback regulation between
PI3K/AKT and RUNX2 enhances the activity of RUNX2, thus promoting
osteogenic differentiations44). Therefore, the PI3K/AKT pathway is
crucial for bone growth and development. In the present study, the
results revealed that the PI3K, AKT and mTOR levels in the OP model
group exhibited no significant differences from those in the sham
operation group; however, the p-PI3K, p-AKT and p-mTOR levels
increased significantly, while the p-PI3K, p-AKT, and p-mTOR levels
decreased significantly following treatment with TFRD,
CaCO3 and NAC. These results suggested that TFRD,
CaCO3 and NAC supplementation reduced the level of
phosphorylated proteins of the oxidative stress pathway in OP
rats.
In conclusion, the present study demonstrated that
rats with OP exhibited a severely decreased BMD, bone mineral loss,
increased oxidative stress and osteogenic marker protein levels;
however, treatment with exogenous TFRD and CaCO3
significantly improved the antioxidant defense, increased BMD and
reduced bone mineral loss, thereby preventing or improving OP. The
mechanisms of action may be related to the antioxidant properties
of these agents.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81403405),
the Sichuan Provincial Department of Health (grant no. CKY2014006),
the Sichuan Science and Technology Project (grant no. 2019YJ0612)
and the Education Department of Sichuan Province (grant no.
14ZB0085).
Availability of materials and data
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PM and YH conceived and designed the current study.
PM, XM, JS, LH and ZZ performed the experiments. PM, XM and JS
collected and analyzed the data. PM and YH drafted the manuscript.
PM, YH and ZZ revised the manuscript. PM and YH confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
Unit and Use Committee of Chengdu University of TCM (approval
no.20190078).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Raisz LG: Pathogenesis of osteoporosis:
Concepts, conflicts, and prospects. J Clin Invest. 115:3318–3325.
2005.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Cano A, Chedraui P, Goulis DG, Lopes P,
Mishra G, Mueck A, Senturk LM, Simoncini T, Stevenson JC, Stute P,
et al: Calcium in the prevention of postmenopausal osteoporosis:
EMAS clinical guide. Maturitas. 107:7–12. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bonaccorsi G, Piva I, Greco P and
Cervellati C: Oxidative stress as a possible pathogenic cofactor of
post-menopausal osteoporosis: Existing evidence in support of the
axis oestrogen deficiency-redox imbalance-bone loss. Indian J Med
Res. 147:341–351. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zeng Q, Li N, Wang Q, Feng J, Sun D, Zhang
Q, Huang J, Wen Q, Hu R, Wang L, et al: The Prevalence of
Osteoporosis in China, a Nationwide, Multicenter DXA Survey. J Bone
Miner Res. 34:1789–1797. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Omsland TK and Magnus JH: Forecasting the
burden of future postmenopausal hip fractures. Osteoporos Int.
25:2493–2496. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Manolagas SC: From estrogen-centric to
aging and oxidative stress: A revised perspective of the
pathogenesis of osteoporosis. Endocr Rev. 31:266–300.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang J, Wang G, Gong L, Sun G, Shi B, Bao
H and Duan Y: Isopsoralen regulates PPAR γ/WNT to inhibit oxidative
stress in osteoporosis. Mol Med Rep. 17:1125–1131. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
An J, Yang H, Zhang Q, Liu C, Zhao J,
Zhang L and Chen B: Natural products for treatment of osteoporosis:
The effects and mechanisms on promoting osteoblast-mediated bone
formation. Life Sci. 147:46–58. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shieh P, Jan CR and Liang WZ: The
protective effects of the antioxidant N-acetylcysteine (NAC)
against oxidative stress-associated apoptosis evoked by the
organophosphorus insecticide malathion in normal human astrocytes.
Toxicology. 417:1–14. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen L, Wang G, Wang Q, Liu Q, Sun Q and
Chen L: N-acetylcysteine prevents orchiectomy-induced osteoporosis
by inhibiting oxidative stress and osteocyte senescence. Am J
Transl Res. 11:4337–4347. 2019.PubMed/NCBI
|
|
11
|
Raffaele M, Barbagallo I, Licari M, Carota
G, Sferrazzo G, Spampinato M, Sorrenti V and Vanella L:
N-Acetylcysteine (NAC) ameliorates lipid-related metabolic
dysfunction in bone marrow stromal cells-derived adipocytes. Evid
Based Complement Alternat Med. 2018(5310961)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Matera MG, Calzetta L and Cazzola M:
Oxidation pathway and exacerbations in COPD: The role of NAC.
Expert Rev Respir Med. 10:89–97. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dludla PV, Dias SC, Obonye N, Johnson R,
Louw J and Nkambule BB: A Systematic Review on the Protective
Effect of N-Acetyl Cysteine Against Diabetes-Associated
Cardiovascular Complications. American journal of cardiovascular
drugs: drugs, devices, and other interventions. 18:283–298.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yamada M, Tsukimura N, Ikeda T, Sugita Y,
Att W, Kojima N, Kubo K, Ueno T, Sakurai K and Ogawa T: N-acetyl
cysteine as an osteogenesis-enhancing molecule for bone
regeneration. Biomaterials. 34:6147–6156. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sheweita SA and Khoshhal KI: Calcium
metabolism and oxidative stress in bone fractures: Role of
antioxidants. Curr Drug Metab. 8:519–525. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhou RP, Lin SJ, Wan WB, Zuo HL, Yao FF,
Ruan HB, Xu J, Song W, Zhou YC, Wen SY, et al: Chlorogenic Acid
Prevents Osteoporosis by Shp2/PI3K/Akt Pathway in Ovariectomized
Rats. PLoS One. 11(e0166751)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xi JC, Zang HY, Guo LX, Xue HB, Liu XD,
Bai YB and Ma YZ: The PI3K/AKT cell signaling pathway is involved
in regulation of osteoporosis. J Recept Signal Transduct Res.
35:640–645. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yuan FL, Xu RS, Jiang DL, He XL, Su Q, Jin
C and Li X: Leonurine hydrochloride inhibits osteoclastogenesis and
prevents osteoporosis associated with estrogen deficiency by
inhibiting the NF-κB and PI3K/Akt signaling pathways. Bone.
75:128–137. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang H, Zhao W, Tian QJ, Xin L, Cui M and
Li YK: Effect of lncRNA AK023948 on rats with postmenopausal
osteoporosis via PI3K/AKT signaling pathway. Eur Rev Med Pharmacol
Sci. 24:2181–2188. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Song S, Gao Z, Lei X, Niu Y, Zhang Y, Li
C, Lu Y, Wang Z and Shang P: Total flavonoids of Drynariae
Rhizoma prevent bone loss induced by Hindlimb unloading in
rats. Molecules. 22(22)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fang J, Yang L, Shen JZ, et al: Effects of
total flavonoids of Rhizoma Drynariae on glutamate signal
Glu, mGluR5 and EAAT1 in bone of ovariectomized rats. Chin J
Biochem Drugs. 34:10–12,16. 2014.
|
|
22
|
Zhu F, Liu Z and Ren Y: Mechanism of
melatonin combined with calcium carbonate on improving osteoporosis
in aged rats. Exp Ther Med. 16:192–196. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ensrud KE and Crandall CJ: Osteoporosis.
Ann Intern Med. 167:ITC17–ITC32. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bidwell JP, Alvarez MB, Hood M Jr and
Childress P: Functional impairment of bone formation in the
pathogenesis of osteoporosis: The bone marrow regenerative
competence. Curr Osteoporos Rep. 11:117–125. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Weaver CM, Alexander DD, Boushey CJ,
Dawson-Hughes B, Lappe JM, LeBoff MS, Liu S, Looker AC, Wallace TC
and Wang DD: Calcium plus vitamin D supplementation and risk of
fractures: an updated meta-analysis from the National Osteoporosis
Foundation. Osteoporos Int. 27:367–376. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shuid AN, Mohamad S, Mohamed N, Fadzilah
FM, Mokhtar SA, Abdullah S, Othman F, Suhaimi F, Muhammad N and
Soelaiman IN: Effects of calcium supplements on fracture healing in
a rat osteoporotic model. Orthop Res. 28:1651–1656. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Paschalis EP, Gamsjaeger S, Hassler N,
Fahrleitner-Pammer A, Dobnig H, Stepan JJ, Pavo I, Eriksen EF and
Klaushofer K: Vitamin D and calcium supplementation for three years
in postmenopausal osteoporosis significantly alters bone mineral
and organic matrix quality. Bone. 95:41–46. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang F, Xie J, Wang G, Zhang G and Yang
H: Anti-osteoporosis activity of Sanguinarine in preosteoblast
MC3T3-E1 cells and an ovariectomized rat model. J Cell Physiol.
233:4626–4633. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cervellati C, Bonaccorsi G, Cremonini E,
Bergamini CM, Patella A, Castaldini C, Ferrazzini S, Capatti A,
Picarelli V, Pansini FS, et al: Bone mass density selectively
correlates with serum markers of oxidative damage in
post-menopausal women. Clin Chem Lab Med. 51:333–338.
2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cervellati C and Bergamini CM: Oxidative
damage and the pathogenesis of menopause related disturbances and
diseases. Clin Chem Lab Med. 54:739–753. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wu X, Li J, Zhang H, Wang H, Yin G and
Miao D: Pyrroloquinoline quinone prevents testosterone
deficiency-induced osteoporosis by stimulating osteoblastic bone
formation and inhibiting osteoclastic bone resorption. Am J Transl
Res. 9:1230–1242. 2017.PubMed/NCBI
|
|
32
|
Orchard T, Yildiz V, Steck SE, Hébert JR,
Ma Y, Cauley JA, Li W, Mossavar-Rahmani Y, Johnson KC, Sattari M,
et al: Dietary inflammatory index, bone mineral density, and risk
of fracture in postmenopausal women: Results from the Women's
Health Initiative. J Bone Miner Res. 32:1136–1146. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen B and Li HZ: Association of IL-6
174G/C (rs1800795) and 572C/G (rs1800796) polymorphisms with risk
of osteoporosis: A meta-analysis. BMC Musculoskelet Disord.
21(330)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Al-Daghri NM, Aziz I, Yakout S, Aljohani
NJ, Al-Saleh Y, Amer OE, Sheshah E, Younis GZ and Al-Badr FBM:
Inflammation as a contributing factor among postmenopausal Saudi
women with osteoporosis. Medicine (Baltimore).
96(e5780)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang Q, Li Y and Zhang Y, Ma L, Lin L,
Meng J, Jiang L, Wang L, Zhou P and Zhang Y: lncRNA MEG3 inhibited
osteogenic differentiation of bone marrow mesenchymal stem cells
from postmenopausal osteoporosis by targeting miR-133a-3p. Biomed
Pharmacother. 89:1178–1186. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhu W, He X, Hua Y, Li Q, Wang J and Gan
X: The E3 ubiquitin ligase WWP2 facilitates RUNX2 protein
transactivation in a mono-ubiquitination manner during osteogenic
differentiation. J Biol Chem. 292:11178–11188. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhu XB, Lin WJ, Lv C, Wang L, Huang ZX,
Yang SW and Chen X: MicroRNA-539 promotes osteoblast proliferation
and differentiation and osteoclast apoptosis through the
AXNA-dependent Wnt signaling pathway in osteoporotic rats. J Cell
Biochem. 119:8346–8358. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Dufresne SS, Dumont NA, Bouchard P,
Lavergne É, Penninger JM and Frenette J: Osteoprotegerin protects
against muscular dystrophy. Am J Pathol. 185:920–926.
2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wolski H, Drews K, Bogacz A, Kamiński A,
Barlik M, Bartkowiak-Wieczorek J, Klejewski A, Ożarowski M,
Majchrzycki M and Seremak-Mrozikiewicz A: The RANKL/RANK/OPG signal
trail: Significance of genetic polymorphisms in the etiology of
postmenopausal osteoporosis. Ginekol Pol. 87:347–352.
2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Mukherjee A and Rotwein P: Selective
signaling by Akt1 controls osteoblast differentiation and
osteoblast-mediated osteoclast development. Mol Cell Biol.
32:490–500. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ouyang N, Zhang P, Fu R, Shen G, Jiang L
and Fang B: Mechanical strain promotes osteogenic differentiation
of bone mesenchymal stem cells from ovariectomized rats via the
phosphoinositide 3 kinase/Akt signaling pathway. Mol Med Rep.
17:1855–1862. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kita K, Kimura T, Nakamura N, Yoshikawa H
and Nakano T: PI3K/Akt signaling as a key regulatory pathway for
chondrocyte terminal differentiation. Genes to cells: Devoted to
molecular & cellular mechanisms. 13:839–850. 2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li M, Luo R, Yang W, Zhou Z and Li C:
miR-363-3p is activated by MYB and regulates osteoporosis
pathogenesis via PTEN/PI3K/AKT signaling pathway. In Vitro Cell Dev
Biol Anim. 55:376–386. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhang Y, Cao X, Li P, Fan Y, Zhang L, Li W
and Liu Y: PSMC6 promotes osteoblast apoptosis through inhibiting
PI3K/AKT signaling pathway activation in ovariectomy-induced
osteoporosis mouse model. J Cell Physiol. 235:5511–5524.
2020.PubMed/NCBI View Article : Google Scholar
|