Introduction
Neuronal loss frequently results in neurological
injury and underlies several neurological diseases (1,2).
Treatments aimed to replace lost neurons have shown significant
success, both in studies and when used clinically (3-5).
Induced pluripotent stem cells (iPSCs), which are reprogrammed
somatic cells, exhibit similar functional abilities to that of
embryonic stem cells (ESCs) with regard to self-renewal ability and
differentiation capacity. They show great promise for the
development of clinical cell-based applications (6-8).
Recently, trials on ESCs or iPSCs derived from neural cells have
been assessed as a treatment for Parkinson's disease and macular
degeneration (9,10). However, the generation of a
sufficient number of functional neural cells from pluripotent stem
cells (PSCs) remains challenging, owing to certain hurdles,
including unclear neural differentiation mechanisms and low
differentiation efficiency (11).
Therefore, an effective method for production of functional neural
cell types is required. In addition, certain obstacles remain, such
as the effects of the external regulatory environment, as well as
the specific molecular mechanisms involved with regard to neural
differentiation of PSCs.
To date, there are three major established regimens
used for differentiation of PSCs into neural precursor cells:
Promoting the direct neural differentiation of PSCs; co-culture of
PSCs with stromal cells, such as MS5 and PA6; or use of a multistep
procedure that includes the formation of embryoid bodies (EBs)
(12). Based on the use of a
suspension culture or the hanging drop method in vitro, the
structure of EBs formed exhibit definitive aspects of early
embryogenesis with lineage specific regions, similar to what is
observed in vivo (7).
Retinoic acid (RA), one of the most significant morphogens, is
required for neural differentiation of mouse ESCs (13). In the present study, the neural
differentiation protocol that was involved in the formation of EBs
was established. Through the combination of RA with N2B27 medium,
cytokines were supplemented to promote neuronal differentiation
(3). Polycomb group proteins (PcG)
are primarily described in relation to their roles in Drosophila,
in which embryonic development is regulated through the repression
of homeotic genes (6,14). Bmi1, a member of the PcG family of
proteins, is required for the maintenance of self-renewing adult
neural stem cells (NSCs) in vivo and in vitro
(15,16). Bmi1 knockout mice experiments showed
that it was essential for postnatal self-renewal of NSCs by
regulating the cell-cycle inhibitors, p16/p19(17). Additionally, short hairpin
RNA-mediated knockdown of Bmi1 revealed that the p21-Rb pathway is
crucial for self-renewal of NSCs during embryonic development
(18). Together, these previous
studies highlight the essential role of Bmi1 in maintaining the
biological function of NSCs. To date, the role of Bmi1 in neuronal
differentiation of PSCs has not been determined, to the best of our
knowledge. In the present study, whether Bmi1 could regulate the
neuronal differentiation of mouse iPSCs via the formation of EBs
was assessed. The aim of the present study was to establish an
ex vivo detection paradigm to explore the neuronal
development capacity of PSCs.
Materials and methods
Cell culture
Mouse embryonic fibroblasts (MEFs) were separated
from the embryos of female mice after 14.5 days of pregnancy. The
specific procedure of deriving MEFs was as follows: The ICR mouse
was sacrificed by cervical spondylolisthesis, and the abdomen was
saturated with 70% ethanol. Sterilized instruments were used to cut
the peritoneal wall and expose the uterine horns, which were
removed and placed in a clean disposable Petri dish in PBS. The
embryos were obtained and minced. The minced tissue was trypsinized
and incubated in DMEM (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 10% of FBS (Gibco; Thermo Fisher Scientific,
Inc.), 1% nonessential amino acids (Invitrogen; Thermo Fisher
Scientific, Inc.), 1% L-glutamine (Invitrogen; Thermo Fisher
Scientific, Inc.) and penicillin/streptomycin (Beijing Solarbio
Science & Technology Co., Ltd.) to grow the MEFs.
293T cells were kindly provided by the Stem Cell
Bank, Chinese Academy of Sciences (Serial no. GNHu17) and cultured
in DMEM high glucose (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS and penicillin/streptomycin. All animal
experiments were performed in accordance with the guidelines
described in the Institutional Animal Care Committee of Zhejiang
Chinese Medical University. The present study was approved by the
Laboratory Animal Management and Welfare Ethical Review Committee
(approval no. ZSLL-2017-181).
Retrovirus production and infection,
and generation of mouse iPSCs
Moloney-based retroviral vectors (pMXs) containing
the human genes encoding c-Myc, Klf4, Sox2 and Oct3/4 (all
established cell-reprogramming factors) (19) were obtained from Addgene, Inc. Each
plasmid was co-transfected into 293T cells with the packaging
plasmids pCMV-GP and pCMV-G (kindly provided by Professor Jing-Kuan
Yee, Department of Diabetes and Metabolic Diseases Research,
Beckman Research Institute, City of Hope National Medical Center)
by co-precipitation of calcium phosphate. Specifically, 15 µg
retroviral vector for Oct3/4, Sox2, Klf4 and c-Myc, 15 µg pCMV-GP
and 4 µg pCMV-G was transfected. After 48 and 72 h,
retrovirus-containing supernatants derived from 293T cultures were
filtered using a 0.45 µm filter (EMD Millipore) and 4 µg/ml
polybrene was added (Sigma-Aldrich; Merck KGaA). Subsequently, the
MEFs were treated with the virus/polybrene mixture twice for 8-10
h, when the cells had reached 60-70% confluence. Next, the media was
replaced with DMEM/F12 (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 15% FBS (Gibco; Thermo Fisher Scientific, Inc.),
1% non-essential amino acids (Invitrogen; Thermo Fisher Scientific,
Inc.), 1% (Invitrogen; Thermo Fisher Scientific, Inc.), 0.1 mM
2-mercaptoethanol (Sigma-Aldrich; Merck KGaA), 1,000 U/ml leukemia
inhibitor factor (Biolead) and penicillin/streptomycin (mouse ESC
medium). Valproic acid (1 mM; Sigma-Aldrich; Merck KGaA) was added
from days 3-8, and Vitamin C (25 µg/ml; Sigma-Aldrich; Merck KGaA)
was added from days 2-12 or 14. After 12-14 days of infection, the
well-defined colonies were screened out and expanded on the
inactivated MEF feeder layers in mouse ESC medium for passaging.
The inactivated MEF feeder layers were established via treating the
MEFs for 2 h at 37˚C using mitomycin C (7 µg/ml; Sigma-Aldrich;
Merck KGaA).
Alkaline phosphatase (AP) staining and
immunofluorescence analysis
AP staining was performed using an Alkaline
Phosphatase staining kit (EMD Millipore). For immunofluorescence
staining, cells were cultured in plates, and fixed and
immunostained using established standard protocols (1,20).
After washing with immunostaining wash buffer, the cells were
sealed with immune staining blocking buffer at room temperature for
60 min, and stained with primary antibodies at appropriate
dilutions for 90 min at room temperature. The following antibodies
and dilutions were used: Anti-Oct3/4 (1 µg/ml; cat. no. ab19857;
Abcam), anti-SSEA-1 (5 µg/ml; cat. no. ab16285; Abcam),
anti-α-Fetoprotein (1:50; cat. no. GTX30030; GeneTex), anti-Sox2 (1
µg/ml; cat. no. ab97959; Abcam), anti-Smooth Muscle Actin (SMA;
1:300; cat. no. ab124964; Abcam), anti-βIII Tubulin (1 µg/ml; cat.
no. ab68193; Abcam), anti-Vimentin (1:300; cat. no. ab92547;
Abcam), anti-Nestin (1 µg/ml; cat. no. ab68193; Abcam),
anti-Vimentin (1:300; cat. no. NBP1-02419; Novus Biologicals LLC),
Subsequently, cells were stained with secondary antibodies
(1:1,000; Alexa Fluor 488 labeled Goat anti-Rabbit IgG; cat. no.
ab150077; Abcam or 1:1,000; Alexa Fluor 555 labeled Donkey
anti-Rabbit IgG; cat. no. A-31572; Thermo Fisher Scientific, Inc.)
for 30-60 min at room temperature. To stain the nuclei, 1 µg/ml
DAPI was used for 10 min at room temperature. Cells were imaged at
x100 magnification using an inverted fluorescence microscope (Nikon
Corporation). All of the immune reagents including the Fixative
Solution, Immunol Staining Wash Buffer, Primary Antibody Dilution
Buffer and Secondary Antibody Dilution Buffer were purchased from
Hang Zhou Da Wen Biotechnology Co., Ltd.
Formation of EBs
For formation of EBs, mouse iPSCs were suspended in
the mouse ESC medium without LiF and cultured in 10 mm culture
dishes at 37˚C for 40 min to remove the MEF layers. Then,
2x105 single mouse iPSCs were cultured in 60 mm petri
dishes with mouse ESC medium and without LiF (termed EB medium).
After the first and second day, petri dishes were gently shaken to
prevent the adherence of the cells. After 8 days, EBs had formed
and images were taken at x40 magnification using an inverted light
microscope (Nikon Corporation).
Neural differentiation ability of
mouse iPSCs
First, mouse iPSCs were suspended for 3 days upon
the formation of EBs (days 0-3). Then, EBs were treated with 1 µM
all-trans RA (Sigma-Aldrich; Merck KGaA) for 4 days (days 3-7). EBs
were plated onto 0.1% gelatin-coated dishes in N2B27 medium
supplemented with 10 µg/ml basic fibroblast growth factor (bFGF)
(PeproTech, Inc.),10 µg/ml epidermal growth factor (EGF)
(PeproTech, Inc.) and 1 µM/ml PTC-209 (Selleck-chem) for 6-7 days
(days 7-15). The media was replaced every other day. The N2B27
medium was a 1:1 mixture of DMEM/F12 supplemented with N2
(Invitrogen; Thermo Fisher Scientific, Inc.) and neurobasal media
added, and supplemented with B27 (Invitrogen; Thermo Fisher
Scientific, Inc.). Images were obtained at x100 magnification using
an inverted light microscope (Nikon Corporation).
Flow cytometry analysis
The general flow cytometry analysis protocols were
performed as follows: The cells were centrifuged for 5 min at 400 x
g and 10˚C. Single cell suspensions were obtained in a solution
consisting of PBS supplemented with 2% FBS, and then re-suspended
in 200 µl 4% paraformaldehyde and fixed at room temperature for 10
min. The cells were permeabilized using 100 µl PBS with 0.1% Triton
X-100 (cat. no. ST797; Beyotime Institute of Biotechnology) for 20
min at 4˚C. The primary antibodies were added and cells were
incubated at 4˚C for 30 min, and then subsequently, cells were
treated with the corresponding secondary antibody at 4˚C for 30
min. Next, flow cytometry was performed using a BD Fortessa
(Becton-Dickinson and Company). Analysis of the flow data was
performed using FlowJo version 10 (FlowJo, LLC). The primary
antibodies used were mouse anti-Nestin polyclonal antibody (1:200;
Abcam; cat. no. ab1642) and mouse anti-GFAP polyclonal antibody
(1:200; Abcam; cat. no. ab10062). The secondary antibody used was
an anti-mouse IgG H&L-AlexaFluor 488 (1:1,000; Abcam; cat. no.
ab150105).
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from untransfected MEFs, as
well as MEFs transfected with retroviral particles expressing
Oct3/4, SOX-2, c-Myc and Klf4 using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. cDNA was synthesized from the RNA using
the PrimeScript™ RT reagent kit (Takara Bio, Inc.) according to the
manufacturer's protocol. PCR was performed using 2X
TSINGKE® MasterMix (Bejing TsingKe Biotech Co., Ltd.) in
a 20 µl reaction mixture containing specific primers. PCR
amplification reaction was conducted as follows: 5 min at 94˚C,
followed by 40 cycles of 30 sec at 94˚C, 30 sec at 55˚C, and 1 min
at 72˚C, Then 8 min at 72˚C. Nat1 was used as a loading control
between the control MEFs and the MEFs 48-72 h after the
transduction with the four retroviruses. The sequences of the
primers used for PCR are listed in Table I.
 | Table ISequences of the primers used for
PCR. |
Table I
Sequences of the primers used for
PCR.
| Gene | Sequence, 5'-3' |
|---|
| Oct3/4 | |
|
Forward |
CCCCAGGGCCCCATTTTGGTACC |
|
Reverse |
CCCTTTTTCTGGAGACTAAATAAA |
| SOX2 | |
|
Forward |
GGCACCCCTGGCATGGCTCTTGGCTC |
|
Reverse |
CCCTTTTTCTGGAGACTAAATAAA |
| c-Myc | |
|
Forward |
CAACAACCGAAAATGCACCAGCCCCAG |
|
Reverse |
CCCTTTTTCTGGAGACTAAATAAA |
| Klf4 | |
|
Forward |
ACGATCGTGGCCCCGGAAAAGGACC |
|
Reverse |
CCCTTTTTCTGGAGACTAAATAAA |
| Nat1 | |
|
Forward |
ATTCTTCGTTGTCAAGCCGCCAAAGTGGAG |
|
Reverse |
AGTTGTTTGCTGCGGAGTTGTCATCTCGTC |
For qPCR, total RNA was extracted from iPSCs and MEF
and cDNA was synthesized from the RNA as described above. qPCR was
performed using SYBR Premix Ex Taq™ (Takara Bio, Inc.) in a 10 µl
reaction mixture containing 0.4 µl specific primers. Each sample
was run in triplicate, and expression was normalized to the
endogenous reference (GAPDH). All the amplifications were performed
on a LightCycler 480 system (Roche Diagnostics), PCR amplification
reaction was conducted as follows: 10 min at 95˚C, followed by 40
cycles of 15 sec at 95˚C and 1 min at 60˚C. and fold expression
relative to the reference gene was calculated using the comparative
method 2-ΔΔCq method (21). The sequences of the primers used for
qPCR are listed in Table II.
 | Table IISequences of the primers used for
quantitative PCR. |
Table II
Sequences of the primers used for
quantitative PCR.
| Gene | Sequence,
5'-3' |
|---|
| Oct3/4 | |
|
Forward |
AGAGGATCACCTTGGGGTACA |
|
Reverse |
CGAAGCGACAGATGGTGGTC |
| SOX2 | |
|
Forward |
GCGGAGTGGAAACTTTTGTCC |
|
Reverse |
CGGGAAGCGTGTACTTATCCTT |
| c-Myc | |
|
Forward |
CCGCTCAAGTTGCTCGAAAAG |
|
Reverse |
TCTCCTTGTAAGACATTGCTGAC |
| Nanog | |
|
Forward |
TCTTCCTGGTCCCCACAGTTT |
|
Reverse |
GCAAGAATAGTTCTCGGGATGAA |
| Klf4 | |
|
Forward |
CCAGACCAGATGCAGTCACA |
|
Reverse |
GCAGGTGTGCCTTGAGATGA |
| GAPDH | |
|
Forward |
AGGTCGGTGTGAACGGATTTG |
|
Reverse |
TGTAGACCATGTAGTTGAGGTCA |
Western blot analysis
Following induction of neural differentiation by a
range of cytokines and RA, iPSC-derived cells were washed with cold
PBS and lysed with RIPA lysis buffer (Boster Biological Technology)
supplemented with a protease inhibitor cocktail (Thermo Fisher
Scientific, Inc.) and phosphatase inhibitor tablets (Roche
Diagnostics). The protein concentration of the supernatant was
measured using BCA reagents. Equivalent amounts of protein lysates
(~25 µg/lane) were loaded on a 10% SDS-gel, resolved using SDS-PAGE
and transferred to a nitrocellulose membrane (Pall Life Sciences),
which was then blocked using 5% skimmed milk in TBST (150 mM NaCl,
0.1% Tween-20, 25 mM Tris-HCl, pH 7.6) at room temperature for 2 h.
The membranes were subsequently incubated with primary antibodies
overnight at 4˚C and washed with TBST the following day. After
incubating with the secondary IRDye 680 goat anti-mouse antibody
(1:5,000; Abcam; cat. no. ab216776) for 2 h at room temperature,
signals were visualized using an Odyssey Infrared Imaging system
(LI-COR Biosciences). The primary antibodies used were rabbit
anti-Bmi1 (1:5,000; Abcam; cat. no. ab38295) and mouse anti-β-actin
(1:5,000; Sigma-Aldrich; Merck KGaA; cat. no. A5441).
Statistical analysis
Data are presented as the mean ± the standard error
of the mean of three independent repeats. Comparisons between two
groups were performed using a Student's t-test (for two groups) or
a one-way ANOVA followed by a Student-Newman-Keuls post-hoc test
for multiple groups. P<0.05 was considered to indicate a
statistically significant difference. The data were analyzed using
GraphPad Prism version 5 (GraphPad Software, Inc.).
Results
Generation and characterization of
mouse iPSCs derived from MEFs
Before reprogramming, the transfection efficiency of
retroviruses was evaluated. Retroviruses were generated by
transfection of 80% confluent 293T cells with the control plasmid
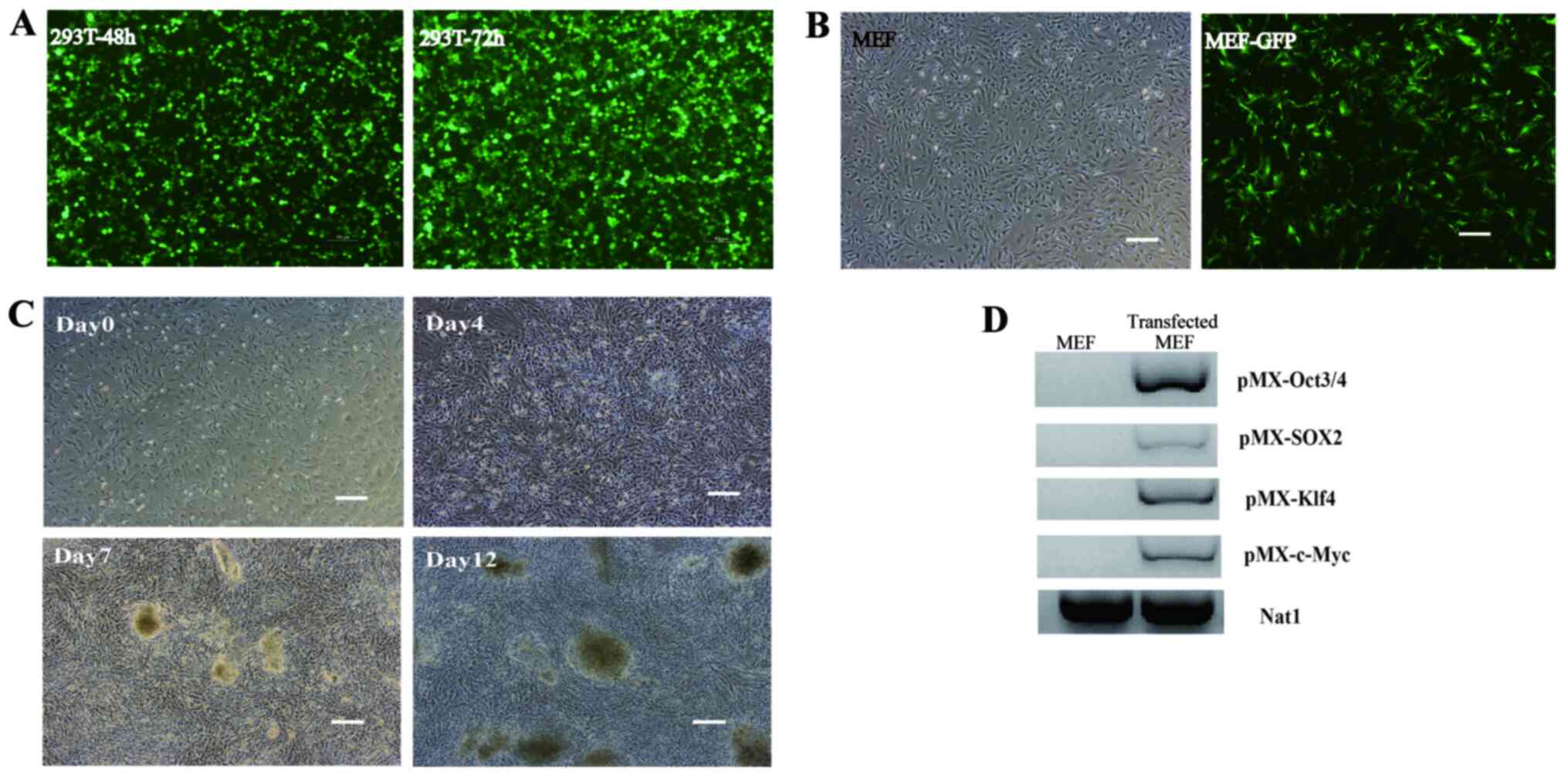
pMXs-GFP (Fig. 1A). Retroviruses
were harvested after 48 and 72 h post-transfection to infect the
MEFs. The green signal from GFP in MEFs was visible following
infection (Fig. 1B), which
suggested that the cells had been successfully transfected, and
that the retroviruses could be used to reprogram the MEFs into
iPSCs. MEFs from ICR mice at passage 3 were used to reprogram iPSCs
by retrovirally expressing Klf4, c-Myc,Sox2 and Oct4. Colonies were
visualized 7 days after transduction, and numerous large colonies
were observed on day 12 (Fig. 1C).
PCR analysis showed that MEFs expressed pMX-Oct3/4, pMX-Sox2,
pMX-c-Myc and pMX-Klf4 genes 48-72 h after transfection with the
respective retrovirus particles (Fig.
1D). The large colonies were screened out and passaged for
culture in mouse ESC medium (Fig.
2A). Through AP staining and immunofluorescence staining
analysis, the mouse iPSC colonies were shown to be strongly
positive for AP. iPSCs exhibited positive staining for Oct3/4 and
SSEA-1 (Fig. 2B-D). The mouse iPSCs
were capable of achieving in-vitro differentiation to form
EBs that were positive for α-smooth muscle actin (mesoderm marker),
α-fetoprotein/(endoderm marker), and βIII tubulin (ectoderm marker)
as shown by immunostaining (Fig. 2E
and F). The endogenous pluripotency
factors, including Oct3/4, Sox2, c-Myc, Klf4 and Nanog were
expressed in the reprogrammed iPSCs (Fig. 2G). These data confirmed that the
reprogrammed mouse iPSCs had similar pluripotency properties to
that of mouse ESCs, and that mouse iPSCs had been generated through
the reprogramming of MEFs.
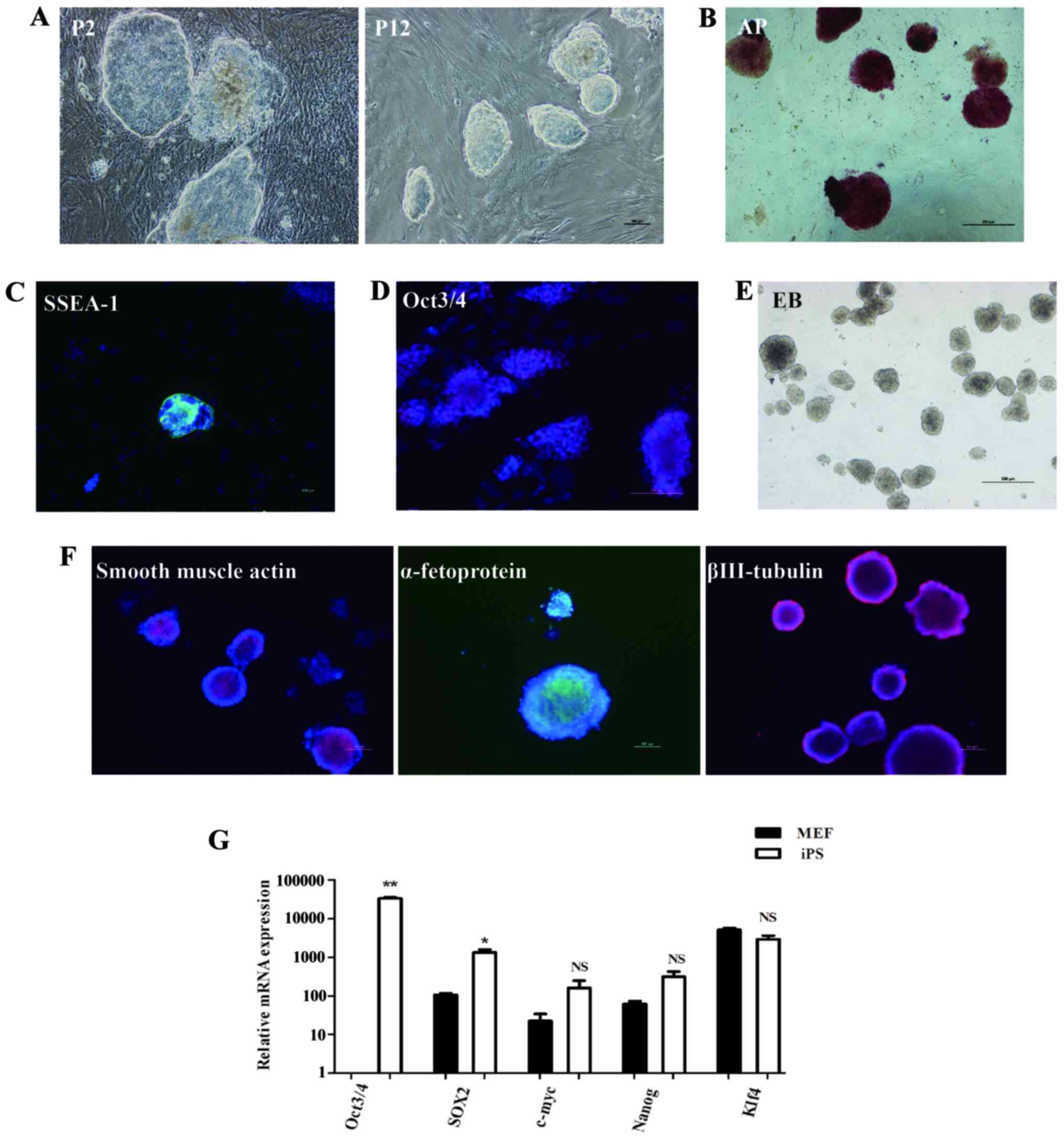 | Figure 2Characteristics of the reprogrammed
mouse iPSCs derived from MEFs. (A) Representative bright-field
microscopy showing the morphology of the mouse iPSCs after 2 and 12
passages. (B) Characterization of mouse iPSCs was performed by AP
staining. Reprogrammed iPSCs were immunostained for detection of
(C) SSEA-1 or (D) Oct3/4 expression. Scale bars, 100 µm. (E) In
vitro EB formation. Scale bar, 500 µm. (F) Immunostaining
confirming the differentiated three germ layers of reprogrammed
iPSCs in vitro. Scale bar, 100 µm. (G) mRNA expression of
pluripotency factors, including Oct3/4, Sox2, c-Myc, Klf4 and Nanog
in the reprogrammed iPSCs and 2 days cultured MEFs of passage 2.
*P<0.05, **P<0.01. MEF, Mouse embryonic
fibroblast; iPSC, induced pluripotent stem cell; AP, alkaline
phosphatase; EB, embryoid body. |
In-vitro neural differentiation of
mouse iPSCs
After confirming the pluripotency of mouse iPSCs,
they were differentiated into neuronal cells, with the aim of
establishing neural stem cells (NSCs). iPSCs were first induced to
form EBs, and then EBs were induced after 7 days to further form
neural cells in N2B27 medium containing EGF (10 µg/ml) and bFGF (10
µg/ml). After another 7 days of adherent inducement, rosette-like
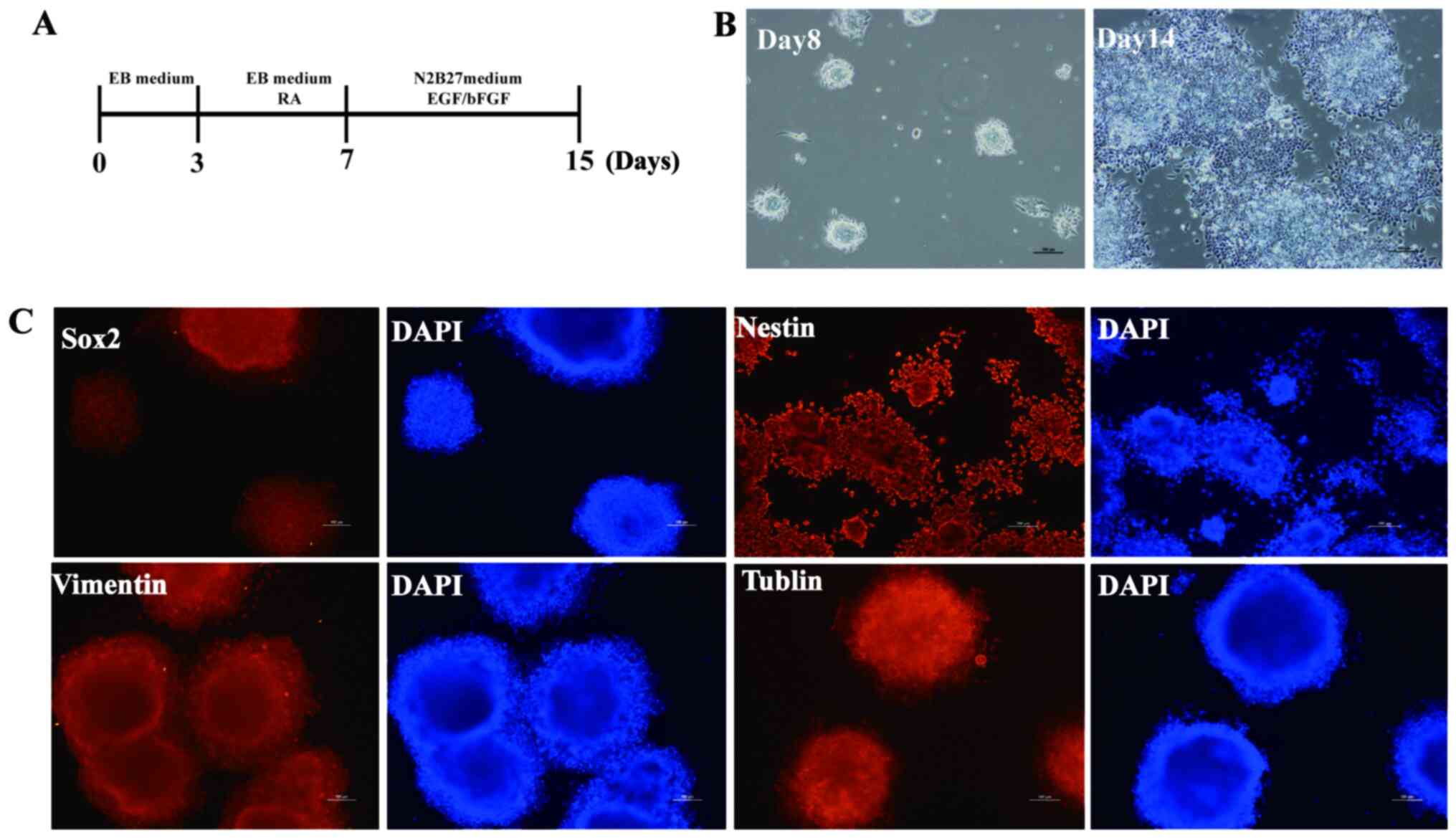
structures were observed (Fig. 3A
and B). Immunofluorescence analysis
was performed to detect expression of neural proteins. Nestin,
Vimentin and Glast were expressed in these cells, suggesting that
neural cells were successfully established from mouse iPSCs
(Fig. 3C).
Bmi1 participates in the neural
differentiation of mouse iPSCs
During the neural differentiation of mouse iPSCs, it
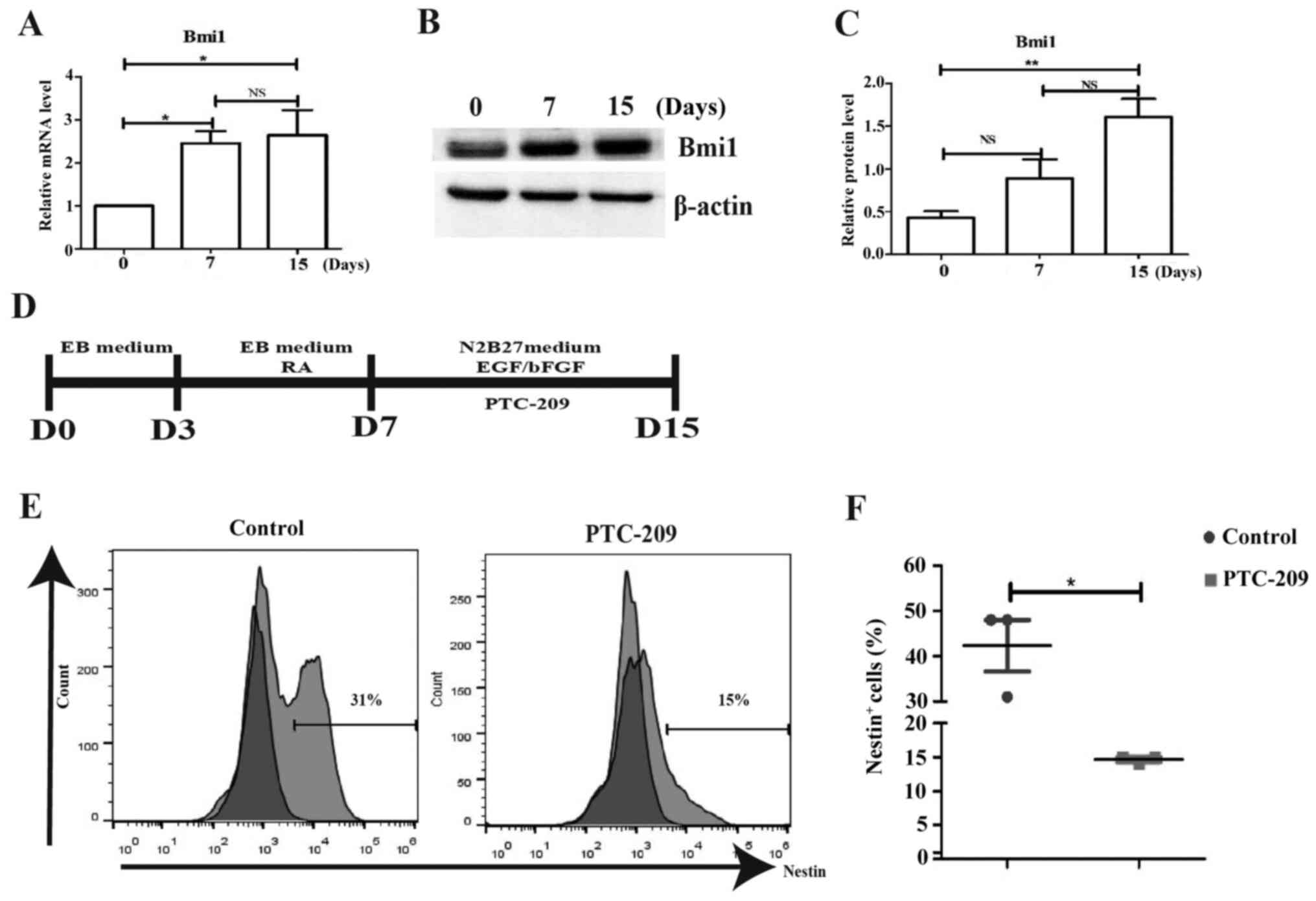
was found that Bmi1 gene expression was increased, suggesting that
it may participate in the regulation of neuronal cells (Fig. 4A). Meanwhile, Bmi1 protein levels
showed a similar trend to that of its mRNA expression levels
(Fig. 4B and C). In order to confirm this hypothesis, a
small molecule inhibitor of Bmi1, 1 µM/ml PTC-209 was used to
inhibit Bmi1 gene expression for 6-7 days (days 7-15) (Fig. 4D). Using flow cytometry analysis, it
was shown that Nestin protein expression was decreased on day 14
compared with the control (Fig. 4E
and F), suggesting that Bmi1 gene
expression was required for neuronal differentiation of mouse
iPSCs. However, the specific mechanisms by which Bmi1 participates
in this process requires further study.
Discussion
iPSC technology allows for differentiation of
pluripotent cells into almost any type of neuronal cell type,
including, but not limited to, NSCs, neurons, astrocytes, microglia
and oligodendrocytes. This prevents the need for the use of ESCs,
with which there are additional ethical concerns and the potential
for immunological rejection (22,23).
Previously, numerous strategies for the regeneration of neural
cells from PSCs have been assessed (24,25).
In the present study, mouse iPSCs were established from MEFs
obtained from ICR mice by introducing four Yamanaka factors, Klf4,
c-Myc, Sox2 and Oct3/4(19).
According to previous studies, a protocol for differentiation of
mouse iPSCs into neural cell lineages was established, providing a
platform for studying neural regulatory mechanisms in vitro,
whilst also laying down a theoretical foundation for iPSC-based
disease modeling and drug screening (1,26,27).
In the present study, the procedure used to
differentiate mouse iPSCs into neural cell lineages was dependent
on the sequential induction at the right time intervals through the
use of growth factors and small molecular compounds that serve a
role in embryonic neural development in vivo and in
vitro (3,4,28,29).
RA, EGF and bFGF were used to promote the genesis of neural cells
from mouse iPSCs. Previous studies have confirmed that RA promotes
neural differentiation of PSCs in EB culture, in which 0.5 µM RA
may have induced the generation of large numbers of neurons through
the suppression of endogenous Wnt-dependent nodal signaling in a
non-cell-autonomous manner (28,30).
EGF and bFGF are required for proliferation of neural progenitor
cells (29). Nestin, Vimentin,
Tubulin and Sox2 expression are characteristic of multipotent NSCs,
and were expressed in the neural differentiated cells in the
present study.
Bmi1 is required to maintain the pool of adult stem
cells, such as NSCs and HSCs (17,18,31).
In the present study, Bmi1 exhibited a positive regulatory role in
the neural differentiation of mouse iPSCs, suggesting that
overexpression of this gene may improve differentiation from mouse
iPSCs to neural stem cells, and the subsequent NSC-derived cells.
When the small molecule Bmi1 inhibitor PTC-209 was used to inhibit
the neural differentiation of iPSCs, Nestin protein expression was
downregulated. The dependence on Bmi1 for stem cell maintenance has
been illustrated, where Bmi1 suppresses the lnk4a/ARF cell cycle
inhibitory proteins (p16 and p19), whose activities are increased
with postnatal time and age in culture, and are further upregulated
in Bmi1 knockout mice when compared with wild-type mice (14). Bmi1 can regulate the neural
differentiation of iPSCs by lnk4a/ARF cell cycle inhibitory
proteins as well as the downstream signaling pathways. Thus, the
specific mechanism by which Bmi1 regulates these processes may be
worthy of further study.
In conclusion, the present study is the first to
show that Bmi1 positively regulates neural differentiation of mouse
iPSCs. The neural differentiation of iPSCs may provide a novel
platform for studying neuronal development, tissue repair,
regenerative medicine and disease modeling, and may also be used as
a useful tool for individualized assessment of novel therapeutic
compounds.
Acknowledgements
The authors would like to thank Ms. Yanwei Li
(Department of Core Facilities, Zhejiang University School of
Medicine) for providing technical support concerning the present
study.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 31570994).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QY and WS conceived and designed the experiments. WS
and DL performed the experiments. WS, LZ, LL and DL were
responsible for data analysis and interpretation. WS wrote the
manuscript. LL and QY confirm the authenticity of all the raw data.
All authors reviewed and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were performed in accordance
with the guidelines described in the Institutional Animal Care
Committee of Zhejiang Chinese Medical University. The present study
was approved by the Laboratory Animal Management and Welfare
Ethical Review Committee (approval no. ZSLL-2017-181).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ford E, Pearlman J, Ruan T, Manion J,
Waller M, Neely GG and Caron L: Human pluripotent stem cells-based
therapies for neurodegenerative diseases: Current status and
challenges. Cells. 9(2517)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kolagar TA, Farzaneh M, Nikkar N and
Khoshnam SE: Human pluripotent stem cells in neurodegenerative
diseases: Potentials, advances and limitations. Curr Stem Cell Res
Ther. 15:102–110. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yao XL, Liu Q, Ye CH, Li ZP, Lu XL, Li PL,
Li XB and Li WQ: Neuronal differentiation potential of mouse
induced pluripotent stem cells. Neuroreport. 22:689–695.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Aharonowiz M, Einstein O, Fainstein N,
Lassmann H, Reubinoff B and Ben-Hur T: Neuroprotective effect of
transplanted human embryonic stem cell-derived neural precursors in
an animal model of multiple sclerosis. PLoS One.
3(e3145)2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ben-Hur T, Idelson M, Khaner H, Pera M,
Reinhartz E, Itzik A and Reubinoff BE: Transplantation of human
embryonic stem cell-derived neural progenitors improves behavioral
deficit in Parkinsonian rats. Stem Cells. 22:1246–1255.
2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lee TI, Jenner RG, Boyer LA, Guenther MG,
Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, Isono K,
et al: Control of developmental regulators by Polycomb in human
embryonic stem cells. Cell. 125:301–313. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Du ZW and Zhang SC: Neural differentiation
from embryonic stem cells: Which way? Stem Cells Dev. 13:372–381.
2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Salewski RP, Buttigieg J, Mitchell RA, van
der Kooy D, Nagy A and Fehlings MG: The generation of definitive
neural stem cells from PiggyBac transposon-induced pluripotent stem
cells can be enhanced by induction of the NOTCH signaling pathway.
Stem Cells Dev. 22:383–396. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Souied E, Pulido J and Staurenghi G:
Autologous induced stem-cell-derived retinal cells for macular
degeneration. N Engl J Med. 377(792)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cyranoski D: Trials of embryonic stem
cells to launch in China. Nature. 546:15–16. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
White N and Sakiyama-Elbert SE: Derivation
of specific neural populations from pluripotent cells for
understanding and treatment of spinal cord injury. Dev Dyn.
248:78–87. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Malgrange B, Borgs L, Grobarczyk B,
Purnelle A, Ernst P, Moonen G and Nguyen L: Using human pluripotent
stem cells to untangle neurodegenerative disease mechanisms. Cell
Mol Life Sci. 68:635–649. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Okada Y, Shimazaki T, Sobue G and Okano H:
Retinoic-acid-concentration-dependent acquisition of neural cell
identity during in vitro differentiation of mouse embryonic stem
cells. Dev Biol. 275:124–142. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ding X, Lin Q, Ensenat-Waser R, Rose-John
S and Zenke M: Polycomb group protein Bmi1 promotes hematopoietic
cell development from embryonic stem cells. Stem Cells Dev.
21:121–132. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
He S, Iwashita T, Buchstaller J, Molofsky
AV, Thomas D and Morrison SJ: Bmi-1 over-expression in neural
stem/progenitor cells increases proliferation and neurogenesis in
culture but has little effect on these functions in vivo. Dev Biol.
328:257–272. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yadirgi G, Leinster V, Acquati S, Bhagat
H, Shakhova O and Marino S: Conditional activation of Bmi1
expression regulates self-renewal, apoptosis, and differentiation
of neural stem/progenitor cells in vitro and in vivo. Stem Cells.
29:700–712. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Molofsky AV, He S, Bydon M, Morrison SJ
and Pardal R: Bmi-1 promotes neural stem cell self-renewal and
neural development but not mouse growth and survival by repressing
the p16Ink4a and p19Arf senescence pathways. Genes Dev.
19:1432–1437. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fasano CA, Dimos JT, Ivanova NB, Lowry N,
Lemischka IR and Temple S: shRNA knockdown of Bmi-1 reveals a
critical role for p21-Rb pathway in NSC self-renewal during
development. Cell Stem Cell. 1:87–99. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li L, Chao J and Shi Y: Modeling
neurological diseases using iPSC-derived neural cells: iPSC
modeling of neurological diseases. Cell Tissue Res. 371:143–151.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Deng J, Zhang Y, Xie Y, Zhang L and Tang
P: Cell transplantation for spinal cord injury: Tumorigenicity of
induced pluripotent stem cell-derived neural stem/progenitor cells.
Stem Cells Int. 2018(5653787)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Czepiel M, Balasubramaniyan V, Schaafsma
W, Stancic M, Mikkers H, Huisman C, Boddeke E and Copray S:
Differentiation of induced pluripotent stem cells into functional
oligodendrocytes. Glia. 59:882–892. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Onorati M, Camnasio S, Binetti M, Jung CB,
Moretti A and Cattaneo E: Neuropotent self-renewing neural stem
(NS) cells derived from mouse induced pluripotent stem (iPS) cells.
Mol Cell Neurosci. 43:287–295. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yan Y, Shin S, Jha BS, Liu Q, Sheng J, Li
F, Zhan M, Davis J, Bharti K, Zeng X, et al: Efficient and rapid
derivation of primitive neural stem cells and generation of brain
subtype neurons from human pluripotent stem cells. Stem Cells
Transl Med. 2:862–870. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
D'Aiuto L, Zhi Y, Kumar Das D, Wilcox MR,
Johnson JW, McClain L, MacDonald ML, Di Maio R, Schurdak ME, Piazza
P, et al: Large-scale generation of human iPSC-derived neural stem
cells/early neural progenitor cells and their neuronal
differentiation. Organogenesis. 10:365–377. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tonge PD and Andrews PW: Retinoic acid
directs neuronal differentiation of human pluripotent stem cell
lines in a non-cell-autonomous manner. Differentiation. 80:20–30.
2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Reynolds BA, Tetzlaff W and Weiss S: A
multipotent EGF-responsive striatal embryonic progenitor cell
produces neurons and astrocytes. J Neurosci. 12:4565–4574.
1992.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Engberg N, Kahn M, Petersen DR, Hansson M
and Serup P: Retinoic acid synthesis promotes development of neural
progenitors from mouse embryonic stem cells by suppressing
endogenous, Wnt-dependent nodal signaling. Stem Cells.
28:1498–1509. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Park IK, Qian D, Kiel M, Becker MW,
Pihalja M, Weissman IL, Morrison SJ and Clarke MF: Bmi-1 is
required for maintenance of adult self-renewing haematopoietic stem
cells. Nature. 1423:302–305. 2003.PubMed/NCBI View Article : Google Scholar
|