Introduction
Liver ischemia-reperfusion (IR) injury occurs in
diverse clinical settings, including liver hemorrhage and shock,
surgical resection, transplantation and CA (1,2). Among
these diverse clinical settings, cardiac arrest (CA) is known to
result in high rates of mortality (ranging from 46 to 68%)
(3). Therapeutic hypothermia can
improve the survival rate and the neurological outcomes of post-CA
patients for several years (4,5).
Hypothermia can markedly reduce ischemia-associated tissue damage
in various organs, such as the liver, kidney and heart (6,7). Among
these organs, the liver requires mitochondrial oxidative
phosphorylation for its energy supply; therefore, the liver is a
target of IR injury (8).
During the IR process, hepatocyte injury occurs
through several pathways, such as lipid peroxidation, the release
of reactive oxygen species (ROS), the activation of signal
transduction cascades and the production of diverse inflammatory
mediators (9). An IR event to an
organ causes an acute inflammatory response that results in
important cellular damage and organ dysfunction (10). At an early stage after injury, the
production of pro-inflammatory cytokines is known to be increased
(11,12). Inflammatory responses in the liver
IR injury involve complex interactions between various cellular and
humoral contributors (7). Kupffer
cells, neutrophils and hepatocytes are major cellular players
(8). Pro-inflammatory and
anti-inflammatory cytokines, chemokines and complement proteins are
also involved in the IR-induced liver injury (8). Therefore, it is fundamental to
elucidate the inflammatory process for preventing the liver IR
injury following CA.
The beneficial effects of hypothermia include the
decreased hepatic metabolism, the subsequent reduction in oxygen
requirement and the inflammatory response regulation (13-15).
Pretreatment with mild hypothermia before ischemia can reduce
apoptosis and the production of inflammatory cytokines.
Posttreatment with hypothermia after ischemic events is sufficient
to reduce IR injury by inhibiting inflammatory responses (16). It has been reported that mild
hypothermic treatment (35˚C) for 1-24 h after 60 min of ischemia
enzymatically improved liver function in a rat model of liver
ischemia (17). In addition,
hypothermia or spontaneous cooling for several to 24 h beginning
immediately after CA could improve liver function and reduce death
in rats (17).
To date, it has been reported that hypothermic
therapy can effectively reduce the histopathological damage of
lumbar spinal cord (18,19), hippocampus (20) and cerebellum (21) in the brain of a rat model of
asphyxial CA. However, as aforementioned, few studies have examined
the effects of hypothermia on the liver after CA. In addition, the
therapeutic or protective mechanisms of hypothermic treatment in
the liver after CA are still not well understood. Therefore, the
objective of the present study was to chronically observe the
tissue damage and the alterations in the expression levels of
pro-inflammatory (TNF-α and IL-2) and anti-inflammatory cytokines
(IL-4 and IL-13) in the liver following 5 min of asphyxial CA in
rats. The effects of hypothermic treatment on tissue damage and the
expression levels of pro- and anti-inflammatory cytokines in the
liver after CA were also investigated.
Materials and methods
Experimental animals and groups
Male Sprague-Dawley rats (age, 10 weeks; body
weight, 310-320 g) were obtained from the Experimental Animal
Center of Kangwon National University (Chuncheon, South Korea). The
rats were maintained in pathogen-free conditions under appropriate
temperature (~23˚C), humidity (~60%) and 12-h light/dark cycle with
freely accessible pellet feed and water. All experimental protocols
used in the present study were approved on the basis of ethical
procedures and scientific care proposed by the Institutional Animal
Care and Use Committee of Kangwon National University (approval no.
KW-180124-1; January 2018; Chuncheon, South Korea). Proper animal
handling and care conformed to the guidelines of the current
international laws and policies [NIH Guide for the Care and Use of
Laboratory Animals, The National Academies Press, 8th Edition,
2011(22); AVMA Guidelines for the
Euthanasia of Animals: 2013 Edition (23)]. The number of rats used in the
current study and the suffering caused by the procedures performed
in all experiments were minimized. The body weight and the behavior
of all animals were monitored every other day. Humane endpoints
were determined when the animals exhibited >20% weight loss,
dehydration and loss of ability to ambulate. No animals presented
signs of humane endpoints intended to be euthanized immediately.
Euthanasia involved chemical and physical methods. Cardiac
perfusion was conducted after each animal was profoundly
anesthetized using 60 mg/kg pentobarbital sodium. Death was
confirmed by evaluating vital signs, including heartbeat, pupillary
response and respiratory pattern (lack of cardiac activity for 5
min through cardiac palpation, unresponsiveness to light with
dilated pupils after directing light into the eyes of the animal
and lack of a spontaneous breathing pattern with a shallow and
irregular breathing pattern).
The rats (n=101) used in the current study were
randomly divided into the following groups: i) Normal group (n=5;
data not shown); ii) sham-operated normothermia group (n=5 at each
time point), which was not subjected to CA operation, and the body
temperature was controlled at 37±0.5˚C for 4 h; iii) CA-operated
normothermia group (n=7 at each time point), which was subjected to
CA, and the body temperature was controlled at 37±0.5˚C for 4 h
after ROSC; iv) sham-operated hypothermia group (n=5 at each time
point), which was not subjected to CA operation, and the body
temperature was controlled at 33.0±0.5˚C for 4 h; and v)
CA-operated hypothermia group (n=7 at each time point), which was
subjected to CA, and the body temperature was controlled at
33.0±0.5˚C for 4 h after ROSC. The rats in each group were
sacrificed at 6, 12 h, 1 and 2 days after ROSC.
CA induction and cardiopulmonary
resuscitation (CPR)
CA and CPR were performed according to previously
published protocols (24,25) with minor modifications. Briefly, the
rats were anesthetized with 2-3% isoflurane and mechanically
ventilated to maintain respiration using a rodent ventilator
(Harvard Apparatus). Body temperature was maintained at 37±0.5˚C
during CA surgery. Peripheral oxygen saturation was monitored with
an oxygen saturation probe of pulse oximetry (Nonin Medical Inc.),
which was attached to the left foot. To record the
electrocardiogram, electrocardiographic probes (GE Healthcare) were
placed in the four limbs, and the data were monitored continuously.
Simultaneously, the left femoral artery and right femoral vein were
separately cannulated to monitor the mean arterial pressure (MAP)
and intravenous injections (MLT 1050/D; ADInstruments Ltd.).
A total of 2 mg/kg vecuronium bromide (Gensia Sicor
Pharmaceuticals, Inc.) was intravenously administered after a 5-min
stabilization period, anesthesia was stopped, and mechanical
ventilation was halted. At this point, MAP was <25 mmHg, and
subsequent pulseless electric activity and asystolic rhythm were
used to define CA (26). CA was
confirmed at 3-4 min after the injection of vecuronium bromide and
was performed for 5 min. CPR was initiated by intravenously
administering 0.005 mg/kg epinephrine and 1 mEq/kg sodium
bicarbonate. Mechanical ventilation with 100% oxygen and mechanical
chest compression were provided at a rate of 300/min until MAP
reached 60 mm Hg and electrocardiographic activity was observed. If
ROSC was not detected, half the amount of epinephrine was
administered during a 1-min CPR; however, the rats subjected to a
third CPR were excluded from the present study. In the normothermic
groups, body temperature was maintained at 37±0.5˚C after the CA
surgery.
Hypothermic treatment
According to published protocols (21,27),
hypothermic treatment was performed after ROSC. In brief,
hypothermic treatment was provided by cooling the body surface with
isopropyl alcohol wipes, ice packs, an electrical fan and a cooling
blanket. Body temperature in the hypothermia groups was maintained
at 33±0.5˚C for 4 h by rectal temperature sensor monitoring. The
rats were re-warmed to 37±0.5˚C for 30 min using a warming blanket
and a hot pad.
Tissue preparation
According to our previous study (28), the rats were anesthetized by
intraperitoneal administration of sodium pentobarbital (60 mg/kg;
JW Pharmaceutical Corp.) (29), and
the vital signs of the animals were examined to ensure that the
animals were profoundly anesthetized. After anesthesia, the rats
were transcardially perfused with 0.1 M PBS (pH 7.4), followed by
perfusion with 4% paraformaldehyde solution (diluted in 0.1 M PBS,
pH 7.4). Their livers were isolated and post-fixed with the same
fixative for 24 h at room temperature. Subsequently, the liver
tissues were cut, embedded in paraffin and sectioned into 6-µm
thickness. Finally, the sections were mounted on gelatin-coated
microscopy slides.
H&E staining
CA-induced pathological alterations in the livers of
each group were examined by H&E staining according to our
previous study (28). In brief, the
paraffin sections were deparaffinized in xylene and rehydrated in a
descending ethanol gradient and washed briefly in distilled water.
Next, the sections were stained with hematoxylin solution for 7 min
followed by eosin solution for 5 min at room temperature and
dehydrated by immersion in a descending ethanol gradient. Finally,
they were mounted with Canada balsam (Kanto Chemical Co.,
Inc.).
CA-induced damage and the hypothermic effect on the
CA-induced damage were analyzed under AxioM1 light microscope
(magnification, x20; Carl Zeiss AG,) equipped with a digital camera
(Axiocam; Carl Zeiss AG) connected to a PC monitor.
Immunohistochemistry
Immunohistochemistry was carried out to examine
alterations in the expression of proinflammatory (TNF-α and IL-2)
and anti-inflammatory cytokines (IL-4 and IL-13). According to our
previous study (30), the paraffin
sections were deparaffinized in xylene and rehydrated using a
descending ethanol gradient and washed briefly in distilled water.
Thereafter, endogenous peroxidase activity was blocked with 0.3%
hydrogen peroxide (H2O2) for 20 min at room
temperature. Antigen retrieval was performed for 5 min at 95˚C in
sodium citrate buffer. Unspecific proteins were blocked using 5%
normal donkey serum (Vector Laboratories Inc.) for 30 min at room
temperature. Next, the sections were incubated with solutions of
the following primary antibodies overnight at 4˚C: Rabbit
anti-TNF-α (Cat. no. ab66579, diluted 1:500; Abcam), mouse
anti-IL-2 (Cat. no. sc-133118, diluted 1:200; Santa Cruz
Biotechnology, Inc.), mouse anti-IL-4 (Cat. no. sc-53084, diluted
1:200; Santa Cruz Biotechnology, Inc.) and mouse anti-IL-13 (Cat.
no. sc-393365, diluted 1:200; Santa Cruz Biotechnology, Inc.). The
sections were subsequently incubated with the secondary antibody
solution (1:250, Vector Laboratories Inc.), biotinylated goat-anti
rabbit (Cat. no. BA-1000) or horse anti-mouse IgG (Cat. no.
BA-2000) for 60 min at room temperature and developed using
VECTASTAIN ABC kit, which contains the avidin/biotin-based
peroxidase system (Vector Laboratories Inc.). Finally, the signal
was visualized with 3,3'-diaminobenzidine, and the sections were
dehydrated and mounted with Canada balsam.
Quantitative analyses of TNF-α, IL-2, IL-4 and IL-13
immunoreactivity in each group were performed according to our
previous study (28). Briefly,
images of TNF-α, IL-2, IL-4 and IL-13-immunoreactive structures
were captured using AxioM1 light microscope (magnification, 20x)
equipped with a digital camera connected to a PC monitor. The
density of each immunoreactive structure in the liver tissue
section was evaluated as relative optical density (ROD) using
ImageJ v1.59 software (National Institutes of Health). The results
are presented as a ratio of ROD (%) vs. the sham-operated
group.
Statistical analysis
All statistical analyses were performed with
GraphPad InStat (v3.05; GraphPad Software, Inc.) and data are
presented as the mean ± SD for physiological variables and mean ±
SEM for ROD. Differences between the groups were assessed using
one-way ANOVA followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Physiological variables and survival
rate
Before CA operation, all the animals in the
CA-operated normothermia and hypothermia groups exhibited normal
physiological values of body weight, temperature, heart rate and
MAP (Table I). In addition, at 2
days after ROSC, the survival rate was 14.3% in the CA-operated
normothermia group (the survival rate immediately after ROSC was
85.7%), while it was 42.9% in the CA-operated hypothermia groups
(the survival rate immediately after ROSC was 100%).
 | Table IPhysiological variables in the
CA-operated normothermia and hypothermia groups. |
Table I
Physiological variables in the
CA-operated normothermia and hypothermia groups.
| | Body weight
(g) | Temperature
(˚C) | Heart rate
(beats/min) | MAP (mmHg) |
|---|
| CA +
normothermia | 303.3±3.9 | 36.4±0.2 | 348.8±4.2 | 117.0±1.1 |
| CA +
hypothermia | 301.6±1.4 | 36.5±0.1 | 346.5±1.7 | 114.2±1.9 |
H&E staining
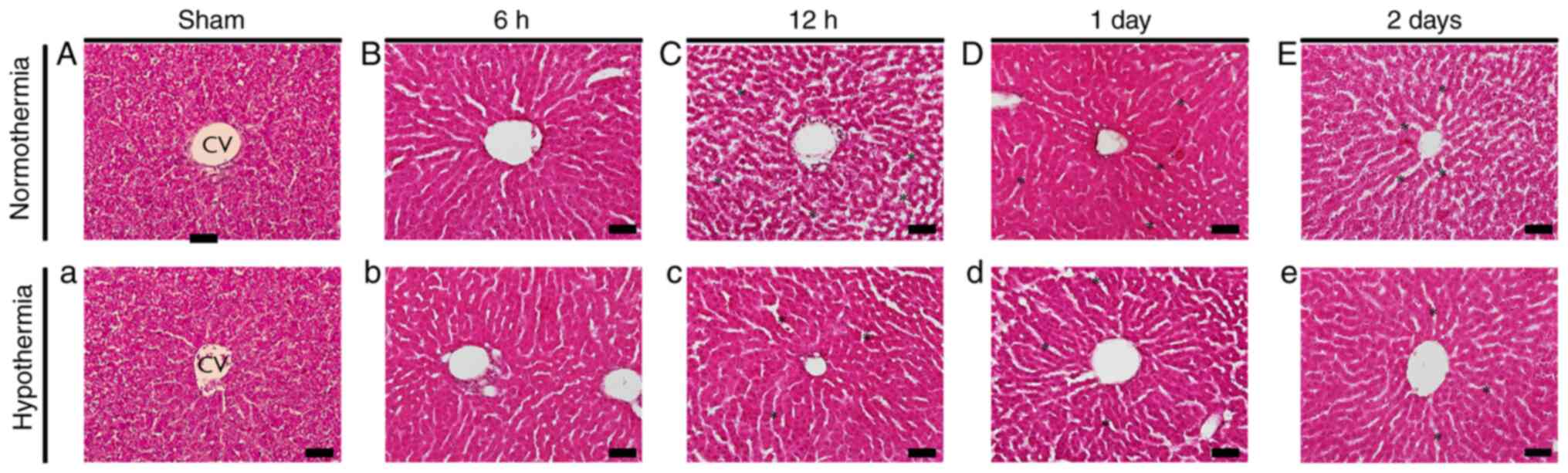
Normal rat liver histology was observed in the
sham-operated normothermia and hypothermia groups (Fig. 1A and a). In the CA-operated normothermia group,
hepatocyte swelling was detected at 6 h after CA (Fig. 1B). From 12 h after CA, sinusoidal
dilatation, vacuolization and infiltration of inflammatory cells in
the interstitial tissue were observed (Fig. 1C-E). On the other hand, in the
CA-operated hypothermia group, structural alterations, such as
those in the CA-operated normothermia group, were less profound
compared with those in the CA-operated normothermia group (Fig. 1b-e).
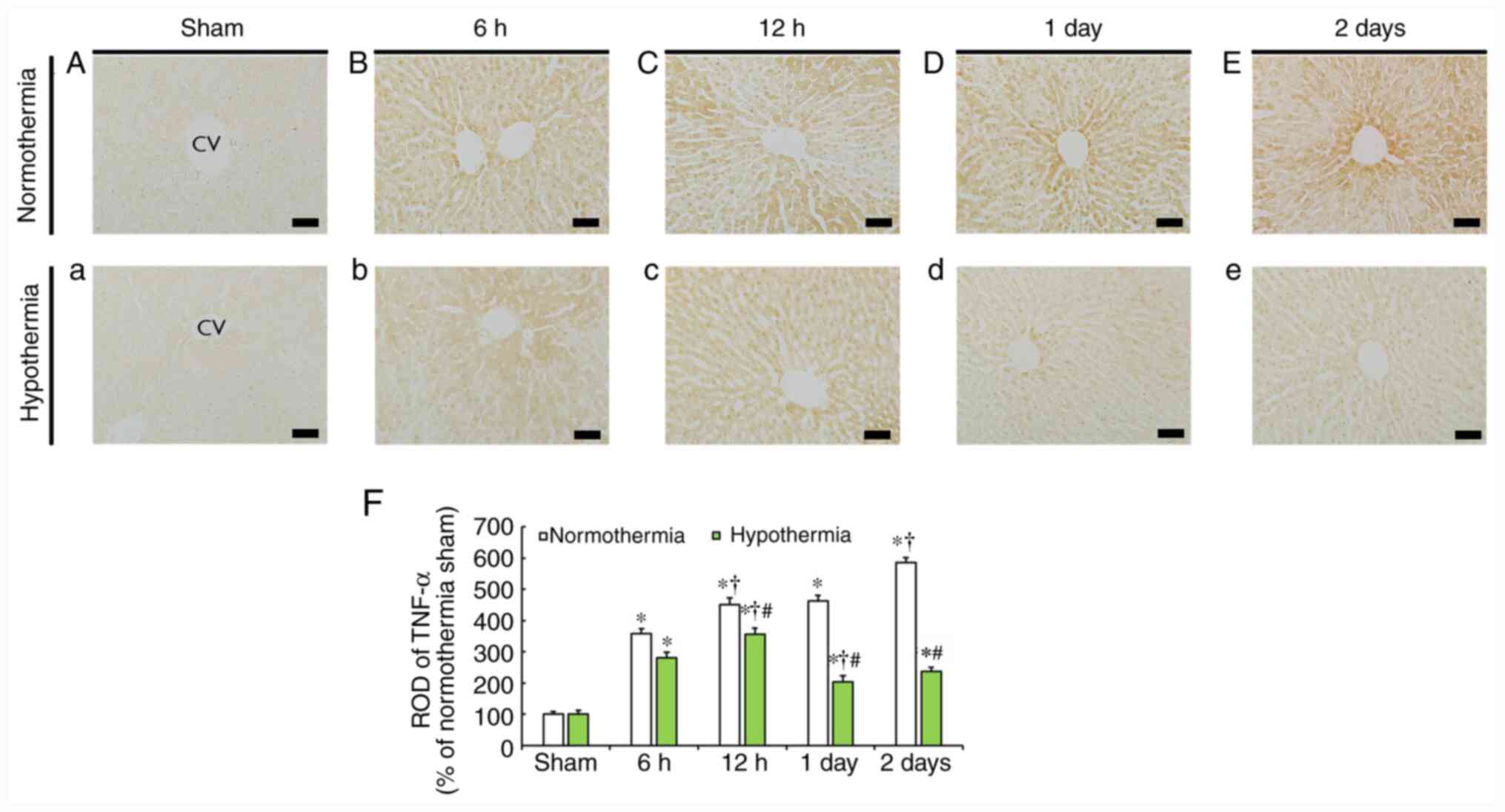
TNF-α immunoreactivity
In the sham-operated normothermia and hypothermia
groups, weak TNF-α immunoreactivity was detected in the rat liver
(Fig. 2A and a), and this finding did not differ from
that in the normal group (data not shown).
In the CA-operated normothermia group, the level of
TNF-α immunoreactivity progressively increased in hepatocytes from
6 h to 2 days after CA in a time-dependent manner (Fig. 2B-E), and the mean ROD of TNF-α
immunoreactivity was indicated to be 358.9, 450.4, 462.9 and 586.2%
at 6, 12 h, 1 and 2 days post-CA, respectively, compared with that
in the sham-operated normothermia group (Fig. 2F).
TNF-α immunoreactivity in the CA-operated
hypothermia group also increased from 6 to 12 h after CA (Fig. 2b and c), but ROD was significantly lower (280.7
and 355.2%, respectively) compared with that at the corresponding
time points of the CA-operated normothermia group (Fig. 2F). Subsequently, TNF-α
immunoreactivity was markedly decreased (Fig. 2d and e), with ROD being 203.7% at 1 day and
248.2% at 2 days, respectively, compared with that at corresponding
time of the CA-operated normothermia groups (Fig. 2F).
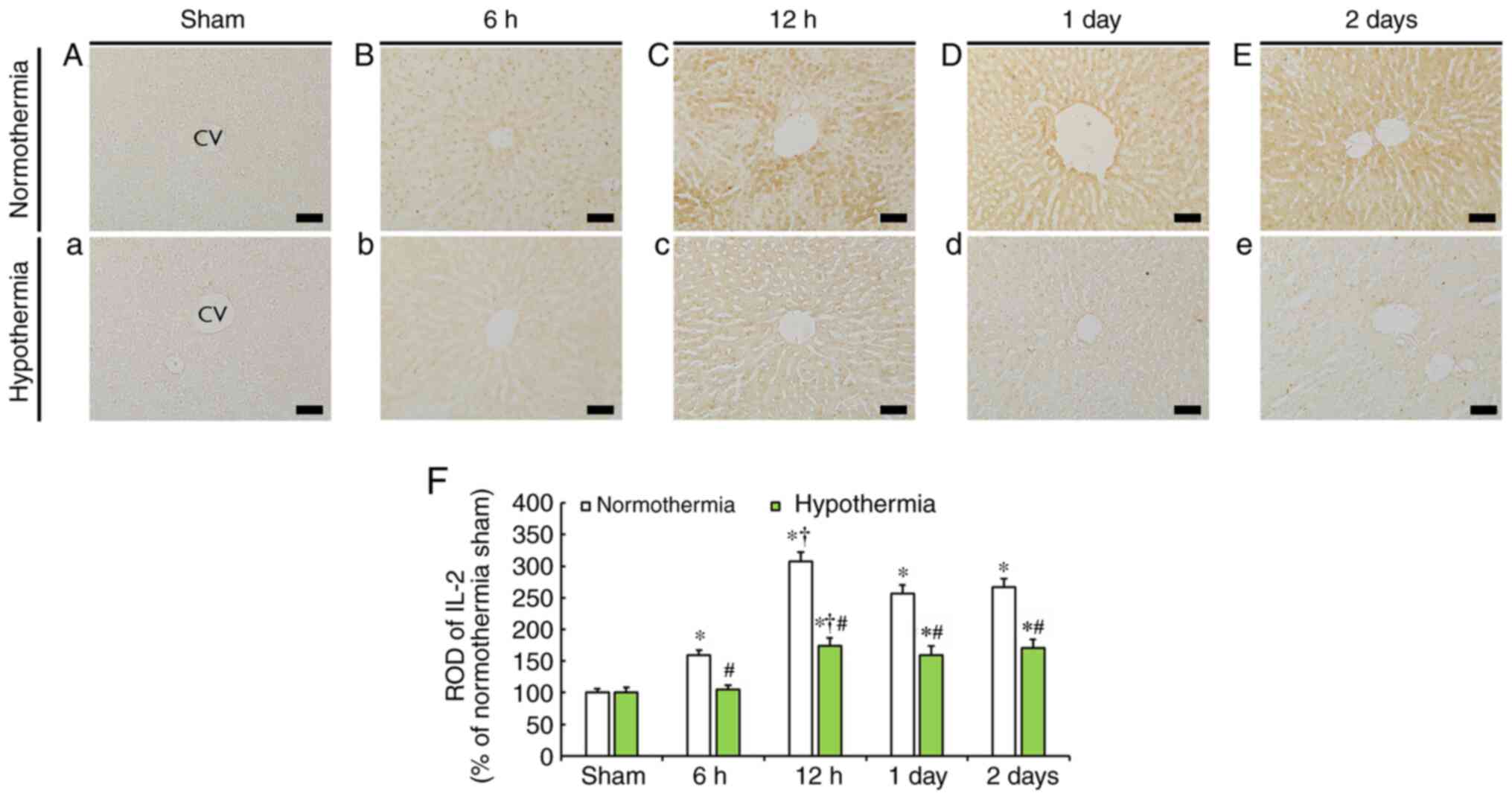
IL-2 immunoreactivity
Weak IL-2 immunoreactivity was observed in the liver
of the sham-operated normothermia and hypothermia groups (Fig. 3A and a).
IL-2 immunoreactivity in the liver of the
CA-operated normothermia group was significantly increased at 6 h
(159.7% of the sham-operated normothermia group) and reach the
highest value at 12 h (307.3% of the sham-operated normothermia
group) after CA (Fig. 3B, C and F).
At the following time points, the increased IL-2 immunoreactivity
was reduced, but was still higher than that in the sham-operated
normothermia group (Fig. 3D and
E), with ROD being 256.9 and 267.1%
of the sham-operated normothermia group, respectively, at 1 and 2
days post-CA (Fig. 3F).
In the CA-operated hypothermia group, IL-2
immunoreactivity at 6 h post-CA was similar to that in the
sham-operated hypothermia group (Fig.
3b). In the subsequent time points, IL-2 immunoreactivity was
increased compared with that of the sham-operated hypothermia group
(Fig. 3c-e); however, ROD at 12 h,
1 and 2 days post-CA was significantly lower (174.2, 159.6 and
170.9%, respectively) compared with that at the corresponding time
points of the CA-operated normothermia group (Fig. 3F).
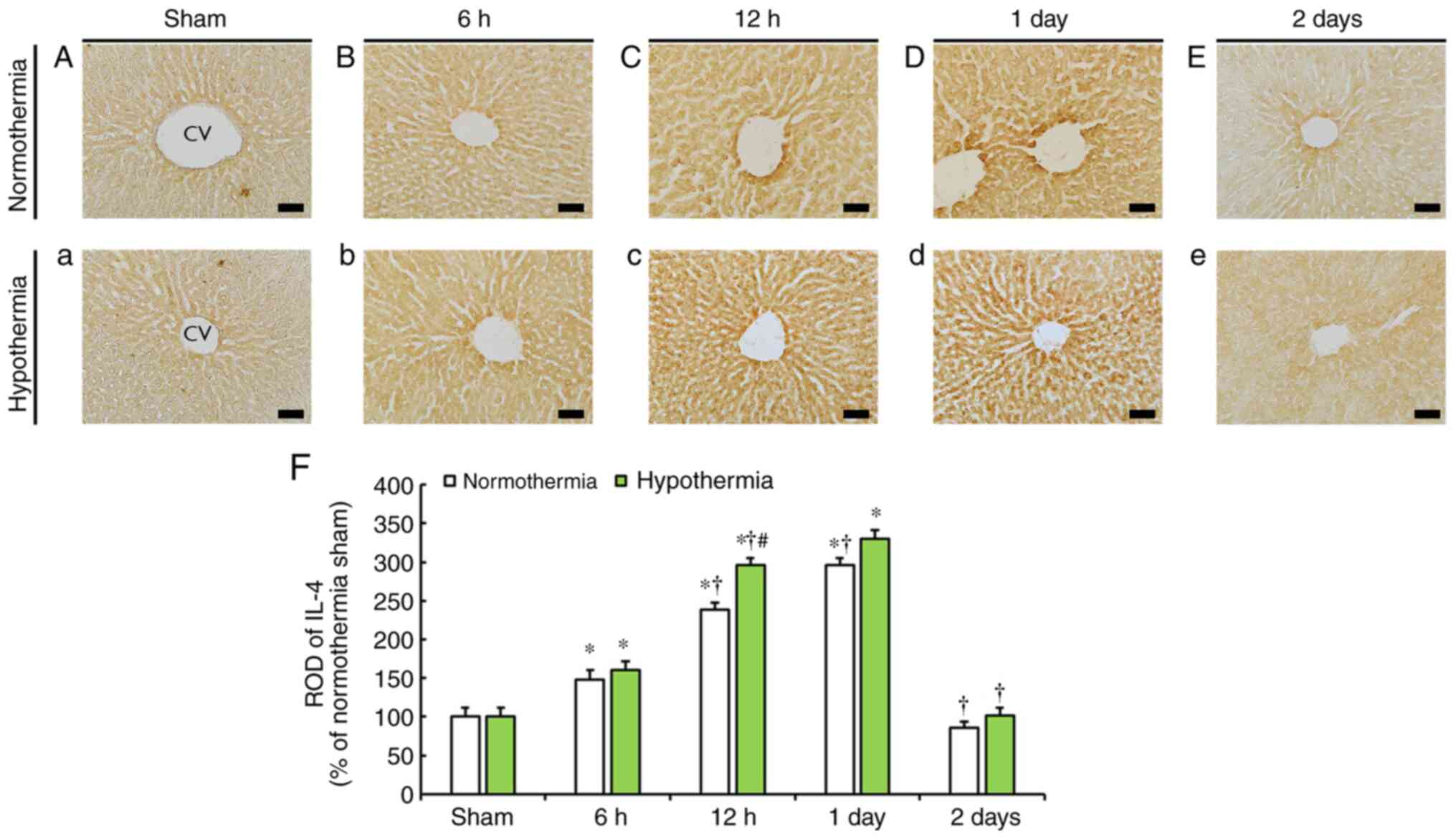
IL-4 immunoreactivity
IL-4 immunoreactivity was generally evident in the
liver, and the level of immunoreactivity was mainly observed around
the central veins in both sham-operated normothermia and
hypothermia groups (Fig. 4A and
a).
In the CA-operated normothermia group, IL-4
immunoreactivity gradually increased from 6 h after CA and reached
the highest level at 1 day after CA (Fig. 4B-D), with ROD at 6, 12 h and 1 day
post-CA being 148.1, 239.0 and 296.6% of that in the sham-operated
normothermia group (Fig. 4F). At 2
days after CA, IL-4 immunoreactivity in the liver was notably
decreased (Fig. 4E and F; 85.6% of that in the sham-operated
normothermia group).
In the CA-operated hypothermia group, the pattern of
IL-4 immunoreactivity was similar to that in the CA-operated
normothermia group (Fig. 4b-e);
however, IL-4 immunoreactivity in this group was higher by 12.7% at
6 h, 56.8% at 12 h, 33.8% at 1 day and 16.4% at 2 days post-CA
compared with that at the corresponding time points of the
CA-operated normothermia group (Fig.
4F).
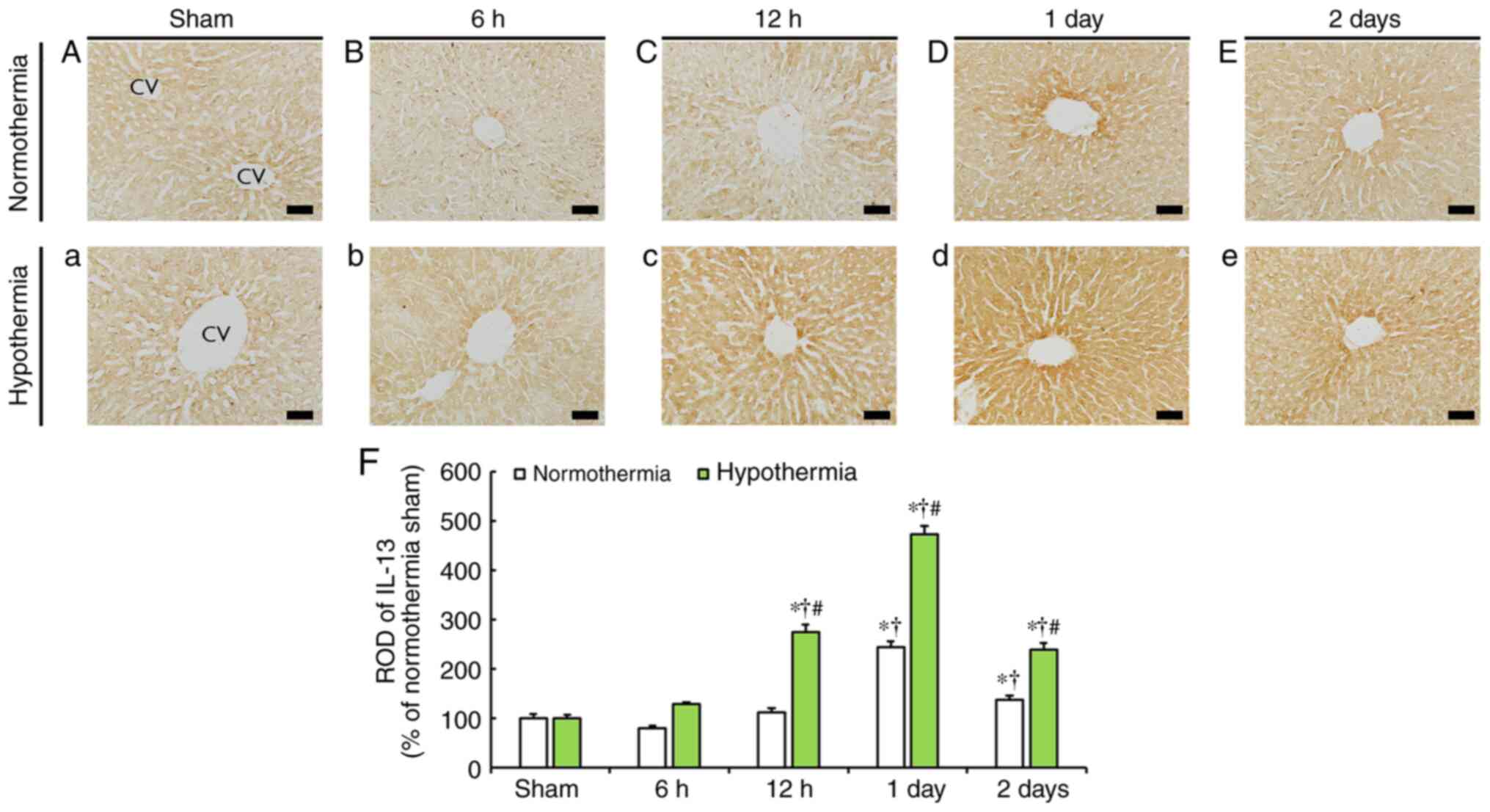
IL-13 immunoreactivity
IL-13 immunoreactivity was evident in the liver of
the sham-operated normothermia and hypothermia groups and was
mainly observed around the central veins (Fig. 5A and a).
IL-13 immunoreactivity in the CA-operated
normothermia group was slightly decreased at 6 h post-CA (Fig. 5B) and recovered at 12 h post-CA
(Fig. 5C and F). In the subsequent time points, IL-13
immunoreactivity was significantly increased by 244.8% at 1 day and
by 37.5% at 2 days post-CA (Fig.
5D-F) compared with that in the sham-operated normothermia
group.
In the CA-operated hypothermia group, IL-13
immunoreactivity was slightly increased at 6 h post-CA (127.9% of
the sham-operated hypothermia group) and significantly increased
(274.6% of the sham-operated hypothermia group) at 12 h post-CA and
reached the highest level (472.3% of the sham-operated hypothermia
group) at 1 day post-CA (Fig. 5b,
c, d and F).
At 2 days post-CA, IL-13 immunoreactivity was decreased compared
with the previous time point (Fig.
5e), but ROD was 238.7% of that in the sham-operated
hypothermia group (Fig. 5F).
Discussion
In the present study, the histopathological
alterations and expression levels of pro- and anti-inflammatory
cytokines in the liver following 5 min of asphyxial CA were
examined. Hypothermic treatment for 4 h after CA was indicated to
attenuate the histopathological damage, reduce the expression
levels of pro-inflammatory cytokines and enhance the expression
levels of anti-inflammatory cytokines compared with normothermic
treatment.
In CA-induced normothermic livers, histopathological
damage, including sinusoidal dilatation and vacuolization, became
more apparent with time after CA. However, hypothermic treatment
for 4 h after CA markedly attenuated these structural alterations
in the liver. In accordance with the results of the current study,
Behrends et al (16) have
reported that hypothermic treatment protected against IR-induced
liver injury. Taken together, these results indicated that the
transient block of blood supply to the liver can evoke structural
alterations in the liver. However, hypothermia after CA could
decrease hepatocellular damage, therefore hypothermia exhibited
protective effects against hepatic IR damage.
It has been reported that IL-2 administration in
mice enhanced the activity of phagocyting Kupffer cells in the
liver, causing leukocyte-endothelial adhesion and impeding
sinusoidal microcirculation (31).
These changes were indicated to induce hypoxic deterioration near
central venules by inhibiting the hepatic sinusoidal blood flow
(31). In addition, it has been
reported that IL-2 can induce secretion of TNF-α from hepatic
Kuepfer cells, monocytes and macrophages (31,32),
suggesting that treatment with IL-2 can biologically activate
TNF-α. TNF-α is a central pro-inflammatory cytokine produced from
Kupffer cells in the liver or neutrophils recruited to the liver
(33). It is known to be a primary
inflammatory mediator in IR liver injury in rats (2). TNF-α has been indicated to induce the
production of chemokines, ROS and adhesion molecules that are
capable of causing direct cellular damage to the liver (8). TNF-α can also interact with TNF-α
receptor 1 and activate signal transduction pathways of hepatocyte
death (2). In the present study, 5
min of CA in the CA-operated normothermia group evoked a
significant increase in TNF-α and IL-2 expression levels in the
liver at 6 and 12 h after CA. At 1 and 2 days after CA, IL-2
expression was slightly decreased compared with the those at 12 h
after CA, whereas TNF-α expression was continuously increased in
the liver, indicating that liver damage at 2 days post-CA under
normothermia may be severe.
Hypothermic treatment for 4 h after CA significantly
reduced the CA-induced increase in IL-2 and TNF-α expression
levels, indicating a remarkable reduction in TNF-α expression at 1
and 2 days after CA compared with the normothermia group. These
findings indicated that hypothermia resulted in the inhibition of
the inflammatory response, which is typically observed following
hepatic IR injury (8). It has been
reported that inhibition of TNF-α production or its neutralization
with a TNF-α antibody can decrease the number of neutrophils
infiltrating into the liver after IR and rescue hepatic IR injury
(33-35).
In addition, it has been reported that administration of anti-TNF-α
antibody improved the survival rate after treatment with a high
dose of IL-2 in mice (31). Based
on the results of the aforementioned studies and the current
findings, it is likely that CA can induce an early increase in IL-2
and TNF-α expression levels in the liver under normothermia, while
hypothermic treatment may be sufficient to reduce the IL-2 and
TNF-α expression levels at later time points.
The present study revealed that IL-4
immunoreactivity in the CA-operated normothermia group was
significantly increased from 6 h post-CA. It reached its highest
level at 1 day post-CA and was markedly reduced at 2 days post-CA.
IL-4 immunoreactivity in the CA-operated hypothermia group was
altered in a similar pattern as in the normothermia group. However,
the ROD level was significantly higher in the CA-operated
hypothermia group than that in the normothermia group. In addition,
IL-13 immunoreactivity in both groups was altered similarly to IL-4
immunoreactivity, with the ROD level in the hypothermia group being
higher than that in the normothermia group. This finding suggested
that anti-inflammatory cytokines may reduce the expression levels
of pro-inflammatory cytokines. A previous study has demonstrated
that IL-4 reduced the expression levels of pro-inflammatory
cytokines, including TNF-α and IL-1, in hepatocytes following IR
and it protected liver tissues against hepatic IR injury (36). In addition, IL-13 has been indicated
to reduce liver damage following hepatic IR injury and lead to
liver regeneration (37), and it
was suggested that this protective role of IL-13 was mediated by
the downregulation of the pro-inflammatory cytokine IL-2.
Furthermore, it has been reported that the protective effect
against hepatic IR injury was associated with STAT6 activation by
IL-4 and IL-13, thereby suppressing NF-κB-dependent
pro-inflammatory mediators (9,38).
Taken together, the current results indicated that IL-4 may reduce
TNF-α expression from 6 h post-CA and IL-13 may decrease IL-2 at
later time points after CA. In addition, the results of the present
study suggested that hypothermia significantly increased the
anti-inflammatory effect following IR injury compared with
normothermia.
In summary, the present study revealed that CA
significantly increased the expression levels of TNF-α and IL-2
from early time points post-CA and induced ischemic damage in the
liver despite the increase in the expression levels of IL-4 and
IL-13 in the ischemic liver under normothermia. However,
hypothermic treatment after CA significantly increased IL-4 and
IL-13 expression levels in the liver, which may reduce the
expression levels of TNF-α and IL-2 and ameliorate CA-induced liver
damage. These results indicated that hypothermic treatment after CA
resulted in reduced hepatocellular damage, which was closely
associated with decreased expression levels of pro-inflammatory
cytokines and increased expression levels of anti-inflammatory
cytokines in the liver after CA.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National
Research Foundation of Korea funded by the Korean government
(Ministry of Science, ICT and Future Planning; grant no.
2017R1C1B5075773) and by Basic Science Research Program through the
National Research Foundation of Korea funded by the Ministry of
Education, Science and Technology (grant no. 2012R1A1A2001404).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YP, JHA, JunHC and MHW were responsible for the
experimental design, data acquisition, data analysis and manuscript
writing. JeongHC, HJT, TKL and BK performed the experiments and
data analysis. JCL, JHP, MCS and TGO performed data analysis and
made critical comments on the entire process of the study. All
authors have read and approved the final version of manuscript.
JunHC and MHW confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
All experimental protocols used in the present study
were approved on the basis of ethical procedures and scientific
care proposed by the Institutional Animal Care and Use Committee of
Kangwon National University (approval no. KW-180124-1; January
2018; Chuncheon, South Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kato A, Okaya T and Lentsch AB: Endogenous
IL-13 protects hepatocytes and vascular endothelial cells during
ischemia/reperfusion injury. Hepatology. 37:304–312.
2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shuh M, Bohorquez H, Loss GE Jr and Cohen
AJ: Tumor necrosis factor-α: Life and death of hepatocytes during
liver ischemia/reperfusion injury. Ochsner J. 13:119–130.
2013.PubMed/NCBI
|
|
3
|
Carr BG, Kahn JM, Merchant RM, Kramer AA
and Neumar RW: Inter-hospital variability in post-cardiac arrest
mortality. Resuscitation. 80:30–34. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kida K, Shirozu K, Yu B, Mandeville JB,
Bloch KD and Ichinose F: Beneficial effects of nitric oxide on
outcomes after cardiac arrest and cardiopulmonary resuscitation in
hypothermia-treated mice. Anesthesiology. 120:880–889.
2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Miao YF, Wu H, Yang SF, Dai J, Qiu YM, Tao
ZY and Zhang XH: 5'-adenosine monophosphate-induced hypothermia
attenuates brain ischemia/reperfusion injury in a rat model by
inhibiting the inflammatory response. Mediators Inflamm.
2015(520745)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Niemann CU, Feiner J, Swain S, Bunting S,
Friedman M, Crutchfield M, Broglio K, Hirose R, Roberts JP and
Malinoski D: Therapeutic hypothermia in deceased organ donors and
kidney-graft function. N Engl J Med. 373:405–414. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Niemann CU, Xu F, Choi S, Behrends M, Park
Y, Hirose R and Maher JJ: Short passive cooling protects rats
during hepatectomy by inducing heat shock proteins and limiting the
induction of pro-inflammatory cytokines. J Surg Res. 158:43–52.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Abu-Amara M, Yang SY, Tapuria N, Fuller B,
Davidson B and Seifalian A: Liver ischemia/reperfusion injury:
Processes in inflammatory networks-a review. Liver Transpl.
16:1016–1032. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–1401.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Lentsch AB, Kato A, Yoshidome H, McMasters
KM and Edwards MJ: Inflammatory mechanisms and therapeutic
strategies for warm hepatic ischemia/reperfusion injury.
Hepatology. 32:169–173. 2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jaeschke H, Bautista AP, Spolarics Z and
Spitzer JJ: Superoxide generation by Kupffer cells and priming of
neutrophils during reperfusion after hepatic ischemia. Free Radic
Res Commun. 15:277–284. 1991.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jaeschke H, Farhood A and Smith C:
Neutrophils contribute to ischemia/reperfusion injury in rat liver
in vivo. FASEB J. 4:3355–3359. 1990.PubMed/NCBI
|
|
13
|
Black PR, van Devanter S and Cohn LH:
Effects of hypothermia on systemic and organ system metabolism and
function. J Surg Res. 20:49–63. 1976.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xiao Q, Ye Q, Wang W, Xiao J, Fu B, Xia Z,
Zhang X, Liu Z and Zeng X: Mild hypothermia pretreatment protects
against liver ischemia reperfusion injury via the PI3K/AKT/FOXO3a
pathway. Mol Med Rep. 16:7520–7526. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Abdo EE, Figueira ER, Rocha-Filho JA,
Chaib E, D'Albuquerque LA and Bacchella T: Preliminary results of
topical hepatic hypothermia in a model of liver
ischemia/reperfusion injury in rats. Arq Gastroenterol. 54:246–249.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Behrends M, Hirose R, Serkova NJ, Coatney
JL, Bedolli M, Yardi J, Park YH and Niemann CU: Mild hypothermia
reduces the inflammatory response and hepatic ischemia/reperfusion
injury in rats. Liver Int. 26:734–741. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Eum HA, Cha YN and Lee SM: Necrosis and
apoptosis: Sequence of liver damage following reperfusion after 60
min ischemia in rats. Biochem Biophys Res Commun. 358:500–505.
2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lee JC, Tae HJ, Cho JH, Kim IS, Lee TK,
Park CW, Park YE, Ahn JH, Park JH, Yan BC, et al: Therapeutic
hypothermia attenuates paraplegia and neuronal damage in the lumbar
spinal cord in a rat model of asphyxial cardiac arrest. J Therm
Biol. 83:1–7. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ahn JH, Lee TK, Kim B, Lee JC, Tae HJ, Cho
JH, Park Y, Shin MC, Ohk TG, Park CW, et al: Therapeutic
hypothermia improves hind limb motor outcome and attenuates
oxidative stress and neuronal damage in the lumbar spinal cord
following cardiac arrest. Antioxidants (Basel).
9(38)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kim YS, Cho JH, Shin MC, Park Y, Park CW,
Tae HJ, Cho JH, Kim IS, Lee TK, Park YE, et al: Effects of regional
body temperature variation during asphyxial cardiac arrest on
mortality and brain damage in a rat model. J Therm Biol.
87(102466)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cho JH, Tae HJ, Kim IS, Song M, Kim H, Lee
TK, Kim YM, Ryoo S, Kim DW, Lee CH, et al: Melatonin alleviates
asphyxial cardiac arrest-induced cerebellar Purkinje cell death by
attenuation of oxidative stress. Exp Neurol.
320(112983)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
National Research Council (US): Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press, Washington, DC, 2010.
|
|
23
|
Leary S, Underwood W, Anthony R, Corey D,
Grandin T, Greenacre C, Gwaltney-Brant S, McCrackin MA, Meyer R,
Miller D, et al: AVMA guidelines for the euthanasia of animals:
2013 edition. Journal, pp1-102, 2013.
|
|
24
|
Han F, Boller M, Guo W, Merchant RM, Lampe
JW, Smith TM and Becker LB: A rodent model of emergency
cardiopulmonary bypass resuscitation with different temperatures
after asphyxial cardiac arrest. Resuscitation. 81:93–99.
2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Drabek T, Foley LM, Janata A, Stezoski J,
Hitchens TK, Manole MD and Kochanek PM: Global and regional
differences in cerebral blood flow after asphyxial versus
ventricular fibrillation cardiac arrest in rats using ASL-MRI.
Resuscitation. 85:964–971. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hu T, Wang J, Wang S, Li J, Chen B, Zuo F,
Zhang L, Huang Y and Li Y: Effects of the duration of
postresuscitation hyperoxic ventilation on neurological outcome and
survival in an asphyxial cardiac arrest rat model. Sci Rep.
9(16500)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jia X, Koenig MA, Shin HC, Zhen G, Pardo
CA, Hanley DF, Thakor NV and Geocadin RG: Improving neurological
outcomes post-cardiac arrest in a rat model: Immediate hypothermia
and quantitative EEG monitoring. Resuscitation. 76:431–442.
2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lee CH, Park JH, Cho JH, Kim IH, Ahn JH,
Lee JC, Chen BH, Shin BN, Tae HJ, Bae EJ, et al: Effect of oenanthe
javanica extract on antioxidant enzyme in the rat liver. Chin Med J
(Engl). 128:1649–1654. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Carpenter JW: Exotic Animal Formulary. 4th
edition. Elsevier Health Sciences, p744, 2012.
|
|
30
|
Park JH, Park O, Cho JH, Chen BH, Kim IH,
Ahn JH, Lee JC, Yan BC, Yoo KY, Lee CH, et al: Anti-inflammatory
effect of tanshinone I in neuroprotection against cerebral
ischemia-reperfusion injury in the gerbil hippocampus. Neurochem
Res. 39:1300–1312. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nakagawa K, Miller FN, Sims DE, Lentsch
AB, Miyazaki M and Edwards MJ: Mechanisms of interleukin-2-induced
hepatic toxicity. Cancer Res. 56:507–510. 1996.PubMed/NCBI
|
|
32
|
Strieter RM, Remick DG, Lynch JP III,
Spengler RN and Kunkel SL: Interleukin-2-induced tumor necrosis
factor-alpha (TNF-alpha) gene expression in human alveolar
macrophages and blood monocytes. Am Rev Respir Dis. 139:335–342.
1989.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li J, Ke W, Zhou Q, Wu Y, Luo H, Zhou H,
Yang B, Guo Y, Zheng Q and Zhang Y: Tumour necrosis factor-α
promotes liver ischaemia-reperfusion injury through the PGC-1α/Mfn2
pathway. J Cell Mol Med. 18:1863–1873. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Colletti LM, Remick DG, Burtch GD, Kunkel
SL, Strieter RM and Campbell DA Jr: Role of tumor necrosis
factor-alpha in the pathophysiologic alterations after hepatic
ischemia/reperfusion injury in the rat. J Clin Invest.
85:1936–1943. 1990.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yang YL, Li JP, Xu XP, Dou KF, Yue SQ and
Li KZ: Protective effects of tumor necrosis factor alpha antibody
and ulinastatin on liver ischemic reperfusion in rats. World J
Gastroenterol. 10:3161–3164. 2004.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kato A, Yoshidome H, Edwards MJ and
Lentsch AB: Reduced hepatic ischemia/reperfusion injury by IL-4:
Potential anti-inflammatory role of STAT6. Inflamm Res. 49:275–279.
2000.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cannistrà M, Ruggiero M, Zullo A, Gallelli
G, Serafini S, Maria M, Naso A, Grande R, Serra R and Nardo B:
Hepatic ischemia reperfusion injury: A systematic review of
literature and the role of current drugs and biomarkers. Int J
Surg. 33 (Suppl 1):S57–S70. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Girotra S, Chan PS and Bradley SM:
Post-resuscitation care following out-of-hospital and in-hospital
cardiac arrest. Heart. 101:1943–1949. 2015.PubMed/NCBI View Article : Google Scholar
|