Introduction
Acute kidney injury (AKI) is caused by a variety of
factors and leads to a sharp decline in renal function, which can
affect a variety of organs and systems (1). The overall incidence of AKI is
estimated to be 0.2-0.3%, the incidence of AKI in hospitalized
patients is 10%, the incidence in intensive care units is 22-67%
and the mortality rate is as high as 50-80% (2). In addition, the overall mortality rate
of AKI is 60% in adults and 57-66% in children (3). In the USA, ~300,000 individuals
succumb to AKI each year. Furthermore, AKI seriously affects the
quality of life of patients and increases the economic burden on
society (4). At present, there is a
lack of uniform and effective treatments. Renal replacement therapy
(RRT) is currently the only effective supportive treatment.
However, under the maintenance of RRT, the mortality rate of AKI
remains at 50-60%. Moreover, with the increasing progress of RRT
technology, the aforementioned mortality rate has not changed
significantly, and the medical burden generated by AKI has also
increased (5). AKI has long been
considered a reversible clinical syndrome and previous studies have
demonstrated that, although the clinical symptoms of AKI can be
restored, it can cause permanent damage to kidney tissue (5,6).
Therefore, it is important to establish effective targets for the
treatment of AKI.
Renal tubular injury, inflammation and vascular
dysfunction are the main pathological features of AKI. Acute
tubular necrosis is the most important mechanism of AKI, with
damage and death of tubule cells considered to be major markers of
AKI severity (7). The recovery and
regeneration of tubule cells is considered to be a major marker of
renal injury recovery. Apoptosis is an active process and is a type
of programmed cell death. However, excessive apoptosis can cause
tissue and organ atrophy, resulting in irreversible damage
(8).
Thioredoxin (Trx) is a type of protein molecule with
a catalytically active dithiol site that is widely present in
living organisms. It was first identified as a component of
supernatant from human T lymphocyte virus type 1 transformed T
cells (9). The Trx system protects
proteins and cell membranes from oxidative injury (9). In addition to its antioxidant effects,
it regulates cytokine transcription and apoptosis. In reactive
oxygen species (ROS)-induced cardiomyocyte injury and apoptosis,
Trx clears ROS and upregulates DnaJ heat shock protein family
(Hsp40) member B5 in signal transduction pathways to enhance
mitochondrial function, thereby inhibiting cardiac hypertrophy
(10). Numerous studies have found
that Trx could protect cells from oxidative and inflammatory
injury, while no significant adverse symptoms have been found
(11,12).
Therefore, the present study hypothesized that the
anti-inflammatory and anti-oxidative features of Trx may be useful
in the treatment of AKI. To verify this, C57/BL6 mice were used to
construct an AKI model and were administered Trx. In addition, the
underlying mechanism of Trx was investigated using HK-2 cells.
Materials and methods
Animals and grouping
The present study was approved by the Animal Ethics
Committee of Changzhou Fourth People's Hospital Animal Center. A
total of 80 C57/BL6 male mice (age, 8 weeks; weight, 20±2 g) were
obtained from Changzhou Fourth People's Hospital Animal Center. All
mice were housed in a specific pathogen free laboratory animal room
at a temperature of 22-24°C and a relative humidity of
50-60%. Mice received specific-pathogen-free grade food and
drinking water, ad libitum. An artificial 12 h light/dark
cycle with suitable ventilation was maintained in the animal room.
Mice were divided into three groups as follows: Control group, AKI
group and AKI + Trx (Abcam) group. Mice in the AKI group and the
AKI + Trx group were used to construct the AKI model. Mice in the
AKI + Trx group were administered a daily subcutaneous injection of
recombinant mouse Trx (50 mg/kg) one week prior to the construction
of the AKI model (13). Mice in the
control group were routinely housed in squirrel cages and were
injected with the same quantity of saline.
Surgical procedure for mouse AKI model
construction
Lipopolysaccharide (LPS) was used to induces sepsis
in mice (14). The mice were
anesthetized with pentobarbital sodium at a dose of 40 mg/kg
intraperitoneally. The mice were then administered 10 mg/kg LPS
(Sigma-Aldrich; Merck KGaA) intraperitoneally using a microsyringe.
After the injection, the syringe was gently rotated 90˚ clockwise
and slowly extracted. After the modeling was completed, all of the
mice were returned to the squirrel cage for rearing. After 24 h,
mouse urine was obtained for analysis and the mice were sacrificed
by cervical dislocation (14).
Enzyme linked immunosorbent assay
(ELISA)
The expression levels of relevant indicators in the
serum, urine and cellular supernatant of mice were evaluated by
ELISA. ELISA kits [Jianglai Biotechnology (Jianglaibio) Co., Ltd.
and Hangzhou Multisciences (Lianke) Biotech, Co., Ltd.] were used
to detect the expression of neutrophil gelatinase-associated
lipocalin (NGAL), kidney injury molecule 1 (KIM-1), netrin-1 and
liver-type fatty acid binding protein (L-FABP) in mouse urine. In
addition, the eyeballs of the mice were removed for blood
collection and the serum was obtained by centrifugation (3,000 x g
for 5 min at 4°C). The ELISA kits were used to detect
the expression of serum creatinine [Scr; cat. no. JL20633; Jianglai
Biotechnology (Jianglaibio) Co., Ltd.], blood urea nitrogen [BUN;
cat. no. JL20491; Jianglai Biotechnology (Jianglaibio) Co., Ltd.],
IL-1β [cat. no. 70-EK201B/3-96; Hangzhou Multisciences (Lianke)
Biotech, Co., Ltd.] and TNF-α [cat. no. 70-EK282/3-96; Hangzhou
Multisciences (Lianke) Biotech, Co., Ltd.] in the serum of the
mice. The expression of IL-1β [cat. no. 70-EK101BHS-96; Hangzhou
Multisciences (Lianke) Biotech, Co., Ltd.], IL-6 [cat. no.
70-EK106/2-96; Hangzhou Multisciences (Lianke) Biotech, Co., Ltd.]
and TNF-α [cat. no. 70-EK182HS-96; Hangzhou Multisciences (Lianke)
Biotech, Co., Ltd.] in the cellular supernatant was also detected
by ELISA.
Malondialdehyde (MDA) and superoxide
dismutase (SOD) activity assay
The mouse kidneys were collected and stored at
-80°C. The kidney tissue was ground into powder at
4°C, then dissolved in phosphate buffered saline (PBS).
The level of oxidative stress in mouse kidneys was determined by
measuring the levels of MDA and SOD in solution. MDA and SOD kits
were obtained from Hangzhou Multisciences (Lianke) Biotech, Co.,
Ltd. and were used to detect the levels of MDA and SOD in the
kidneys of mice in accordance with the manufacturer's
instructions.
Hematoxylin and eosin (H&E)
staining
After the AKI models were constructed, the mice were
sacrificed and their kidneys were harvested. After stripping the
renal capsule of the mouse kidney, the kidneys were fixed with 4%
paraformaldehyde at room temperature. After 48 h, the kidneys were
washed with PBS and embedded in paraffin by dehydration. A
microtome was used to slice the paraffin sections at 5 µm. After
the paraffin sections were dried, an H&E kit (Beyotime
Institute of Biotechnology) was used to perform H&E staining.
The kidney injury was evaluated according to the method of Paller
et al (15). An optical
microscope (Leica Microsystems, Inc.) was used to record the
results. Ten diseased tubules were randomly selected from the field
of view at high magnification (x200) and the kidney tissue was
scored by two members (JW and WZ). Marked dilatation of renal
tubules or flattening of cells was scored as 1 point; damage to the
brush border was scored as 1 point; cell shedding was scored as 2
points; the renal tubular cells were scored as 2 points;
irregularly shaped or fragmented cells are scored as 1 point.
Cell culture and treatment
HK-2 cells (Procell Life Science & Technology
Co., Ltd.) were cultured in Dulbecco's modified Eagle's medium
(HyClone) containing 10% fetal bovine serum (HyClone) and placed in
an incubator at 37˚C and 5% CO2 until further
processing. After the cell growth density reached 50-60%, the cells
were stimulated with LPS or Trx. LPS was used to stimulate HK-2
cells to induce AKI at the cellular level.
Western blot analysis
When the HK-2 cell growth density reached ≥90%, the
cells were passaged into 6-well plates and stimulated with Trx or
LPS. After the treatment was complete, the cells were lysed with
protein lysate and the protein concentration was detected using a
bicinchoninic acid (BCA) kit (Beyotime Institute of Biotechnology).
The Murine kidney samples were also lysed using RIPA lysis buffer
(Beyotime Institute of Biotechnology) to produce the protein
lysate, the concentration of which was determined using a BCA kit
(Beyotime Institute of Biotechnology). Protein (20 µg) was added to
each well of the electrophoresis gel and the different components
of the protein were separated by 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis. The protein was then
transferred to a polyvinylidene fluoride (PVDF) membrane (EMD
Millipore), which was blocked with 5% skimmed milk-PBST for 1 h at
room temperature. The PVDF membrane was incubated at 4˚C overnight
with the following primary antibodies: IL-1β (1:3,000; cat. no.
31202S; CST Biological Reagents Co., Ltd.), IL-6 (1:3,000; cat. no.
ab9324; Abcam), IL-8 (1:3,000; cat. no. ab154390; Abcam), TNF-α
(1:3,000; cat. no. ab183218; Abcam), caspase-3 (1:3,000; cat. no.
ab184787; Abcam), caspase-9 (1:2,000; cat. no. ab202068; Abcam),
p65 (1:5,000; cat. no. ab32536; Abcam), IKKα (1:4,000; cat. no.
ab32041; Abcam), IκBα (1:4,000; cat. no. ab32518; Abcam) and
β-actin (1:5,000; cat. no. ab8226; Abcam). The next day the PVDF
membrane was washed with PBST and incubated at room temperature for
2 h using goat anti-rabbit secondary antibody (1:5,000; cat. no.
ab6721; Abcam). Finally, an electrochemiluminescence kit
(Sigma-Aldrich; Merck KGaA) was used to detect the protein
quantity. ImageJ Software 1.52 (National Institutes of Health) was
used for gray value analysis.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA from the HK-2 cells
and the RNA concentration was detected using a spectrophotometer
(260 nm). The reverse transcription reaction system was prepared on
ice to avoid the degradation of RNA. After reverse transcription
was completed, an SYBR Green kit (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to amplify cDNA with different primers
(Table I). The expression of
endogenous GAPDH served as the control and the 2-ΔΔCq
method was used to statistically analyze the relative expression
levels of the target genes (16).
 | Table IReverse transcription-quantitative
PCR primers. |
Table I
Reverse transcription-quantitative
PCR primers.
| | Sequence
(5'-3') |
|---|
| Primer Human | Sense | Anti-sense |
|---|
|
SOD1 |
GGTGAACCAGTTGTGTTGTC |
CCGTCCTTTCCAGCAGTC |
|
SOD2 |
CAGACCTGCCTTACGACTATGG |
CTCGGTGGCGTTGAGATTGTT |
|
GPX1 |
ATCATATGTGTGCTGCTCGGCTAGC |
TACTCGAGGGCACAGCTGGGCCCTTGAG |
|
GPX3 |
AGAGCCGGGGACAAGAGAA |
ATTTGCCAGCATACTGCTTGA |
|
Catalase |
CGTACTGATCTATGCGCTCTACA |
GCTACTGAGCCACACTGAGCACGA |
|
Caspase-3 |
CAGAATCATAAGCCCCTGGA |
TCTGCGAGTCAGGCATTTG |
|
Caspase-9 |
TTCTTGAGCAACACCCTC |
CGCATACACTGTCTACCT |
|
Bax |
CAGTTGAAGTTGCCATCAGC |
CAGTTGAAGTTACCATCAGC |
|
Bcl-2 |
GACTGAGTACCTGAACCGGCATC |
CTGAGCAGCGTCTTCAGAGACA |
|
p65 |
ACTGCCGGGATGGCTACTAT |
TCTGGATTCGCTGGCTAATGG |
|
IKKα |
GTCAGGACCGTGTTCTCAAGG |
GCTTCTTTGATGTTACTGAGGGC |
|
IκBα |
GGATCTAGCAGCTACGTACG |
TTAGGACCTGACGTAACACG |
|
GAPDH |
ACAACTTTGGTATCGTGGAAGG |
GCCATCACGCCACAGTTTC |
| Mouse | | |
|
p65 |
CCAGGGTGTGTCCATGTCT |
GTGTGGGAGCTGGGTCA |
|
IKKα |
GTGGCCCTCAGTAACATCA |
AAGAGAGCGGGCAGAAC |
|
IκBα |
CGATGAATGGTGCGACAG |
GCCCAGGAGGAAATCCA |
|
GAPDH |
GCAGTGGCAAAGTGGAG |
GCCGTGAGTGGAGTCATAC |
Cell counting kit-8 (CCK-8) assay
HK-2 cells were cultured in 96-well plates. After
the cell growth density reached 60%, 0, 10, 30, 50 and 100 ng/ml of
Trx was administered to stimulate the HK-2 cells. CCK-8 reagent (10
µl; Dojindo Molecular Technologies, Inc.) was added to each well of
the 96-well plate and the cells were returned to the incubator for
an additional 2 h. A microplate reader was used to measure the
absorbance of each well.
Immunocytofluorescence (IF)
staining
HK-2 cells were cultured in 24-well plates. When the
cell growth density reached 60%, the cells were treated as
aforementioned. The 12-well plate was removed and the medium was
discarded. After washing with PBS, cells were fixed with 4%
paraformaldehyde for 15 min at room temperature. The cells were
soaked for 20 min using 0.2% Triton-PBS. After washing cells with
PBS, the cells were incubated with 10% goat serum-PBS for 30 min at
room temperature. The goat serum was discarded and the cells were
incubated with the following primary antibody dilution: IL-6
(1:300; cat. no. ab9324; Abcam), IL-8 (1:200; cat. no. ab154390;
Abcam), SOD1 (1:500; cat. no. ab51254; Abcam), SOD2 (1:800; cat.
no. ab110300; Abcam), Bax (1:400; cat. no. ab53154; Abcam), Bcl-2,
(1:400; cat. no. ab218123; Abcam) and p65 (1:200; cat. no. ab32536;
Abcam) at 4˚C overnight. After washing the cells with PBS the next
day, the cells were incubated for 1 h using fluorescent secondary
antibody, goat anti-rabbit-FITC (1:500; cat. no. ab150077; Abcam).
The cells were incubated for 15 min with
4',6-diamidino-2-phenylindole at room temperature. After washing
the cells with PBS, the outcome of staining was observed and
recorded using a fluorescence microscope.
Flow cytometry
ROS levels and apoptosis rates were detected in HK-2
cells by flow cytometry. After cell growth density reached 60%, the
cells were treated as aforementioned. We then used the DCFH-DA kit
(Invitrogen; Thermo Fisher Scientific, Inc.) and the Annexin
V-FITC/PI kit (Invitrogen; Thermo Fisher Scientific, Inc.) to
determine ROS levels and apoptosis rates in HK-2 cells according to
the manufacturer's instructions. Flow cytometry (Thermo Fisher
Scientific, Inc.; cat. no. A28997) was used to detect apoptosis
with Attune NxT Software 3.2.1 (Thermo Fisher Scientific,
Inc.).
Statistical analysis
All experiments were repeated more than three times.
SPSS V21.0 (IBM Corp.) was used for statistical analysis and
GraphPad Prism 7.0 (GraphPad Software, Inc.) was used to construct
and present the data. Comparisons between multiple groups were
performed using One-way ANOVA followed by the least significant
difference post hoc test. Kruskal-Wallis test followed by Dunn's
test was were used for the histological scoring. P<0.05 was
considered to indicate a statistically significant difference.
Results
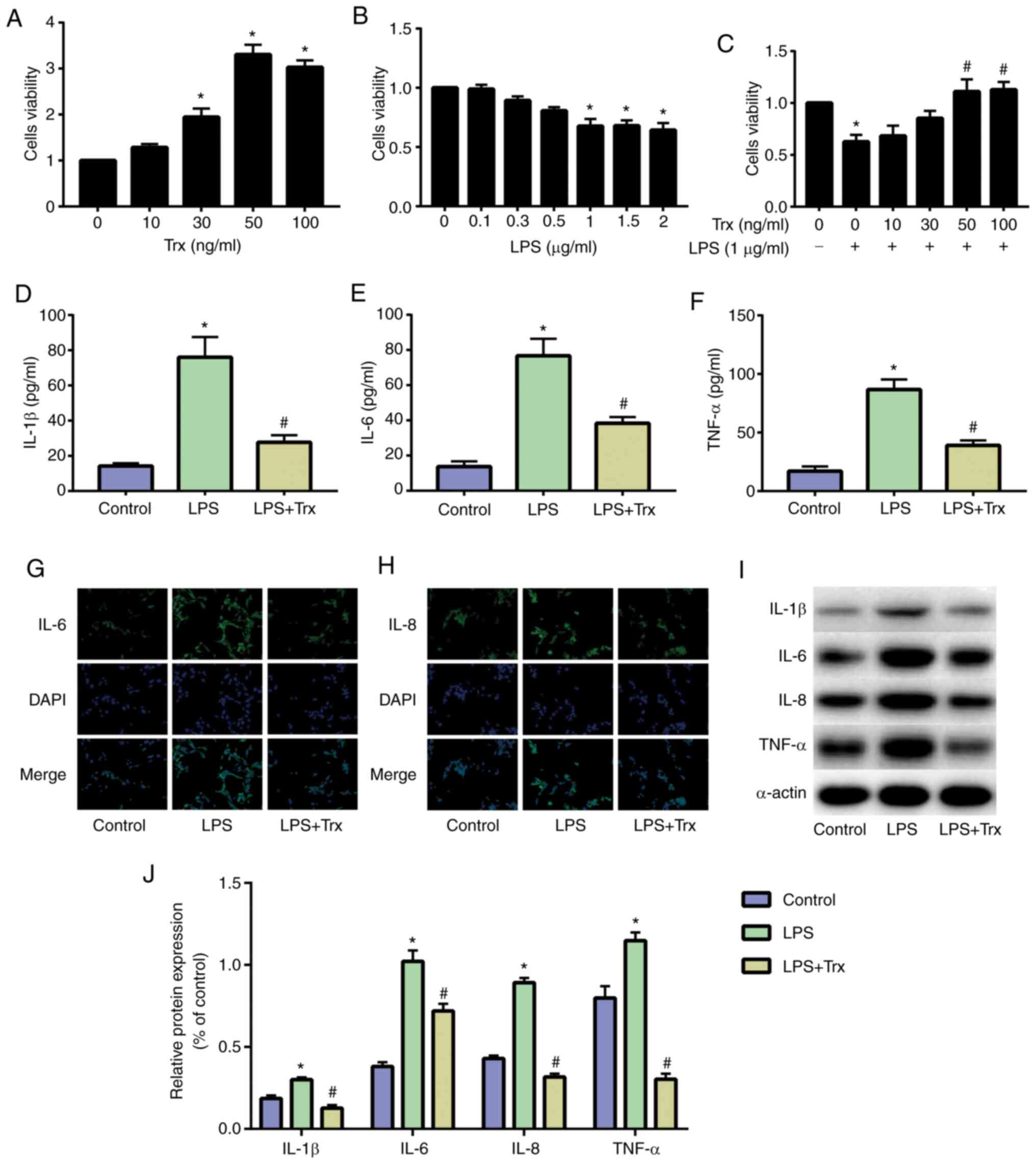
Trx reduces LPS-induced inflammation
levels in HK-2 cells
HK-2 cells were cultured and stimulated with Trx. A
CCK-8 assay was used to determine the optimal concentration of Trx
for HK-2 cells. Results indicated that 50 ng/ml Trx achieved
optimal cell viability (Fig. 1A).
The HK-2 cells were then stimulated with different concentrations
of LPS and the results demonstrated that 1 µg/ml LPS significantly
reduced the activity of HK-2 cells (Fig. 1B). When stimulating HK-2 cells with
both LPS (1 µg/ml) and Trx, 50 ng/ml Trx significantly increased
the activity of HK-2 cells (Fig.
1C). The results of ELISA showed that inflammatory factors
(IL-1β, IL-6 and TNF-α) were significantly increased in HK-2 cells
of the LPS group and Trx could reduce the expression of
inflammatory factors (Fig. 1D-F).
The results of IF staining also indicated that Trx reduced IL-6
(Fig. 1G) and IL-8 (Fig. 1H). The results of western blot
analysis were similar to those of ELISA and IF staining (Fig. 1I and J).
Trx reduces LPS-induced oxidative
stress levels in HK-2 cells
Oxidative stress is a notable feature in the
development of AKI. SOD is an important antioxidant enzyme in the
body. The results of IF staining showed that the expression of SOD1
and SOD2 in the LPS group was significantly lower than that in the
control group, indicating that LPS reduced the anti-oxidant
capacity of HK-2 cells. Following stimulation of HK-2 cells with
Trx, the anti-oxidant capacity of HK-2 cells increased
significantly (Fig. 2A and B). In addition, the results of RT-qPCR
showed that Trx increases the expression of SOD1, SOD2, glutathione
peroxidases (GP)X1, GPX3 and catalase (CAT; Fig. 2C-G). GPX1/3 is an important
peroxidase enzyme that is widely expressed in various organisms and
CAT is a key enzyme that catalyzes the breakdown of hydrogen
peroxide. The results of flow cytometry indicated that Trx reduces
ROS levels in HK-2 cells (Fig. 2H).
These results demonstrate that Trx reduces oxidative stress levels
in HK-2 cells.
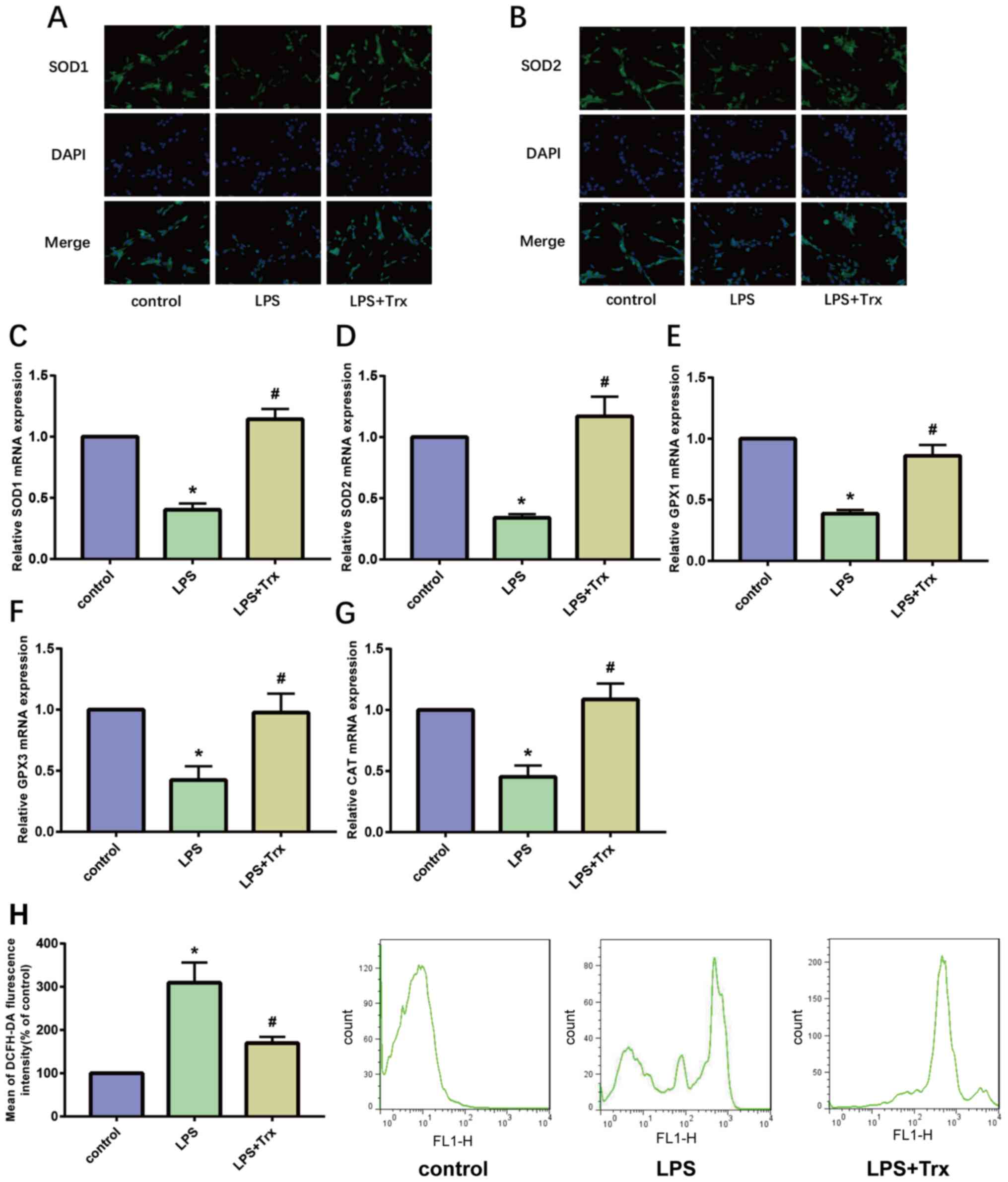 | Figure 2Trx reduces LPS-induced oxidative
stress levels in HK-2 cells. (A and B) Immunocytofluorescence
staining of SOD1 and SOD2 (magnification, x400). (C-G) Reverse
transcription-quantitative PCR of SOD1, SOD2, GPX1, GPX3 and CAT.
(H) ROS level in HK-2 cells. *P<0.05 vs. the control
group; #P<0.05 vs. the LPS group. Data are presented
as the mean ± SD. Trx, thioredoxin; LPS, lipopolysaccharide; SOD,
superoxide dismutase; GP, glutathione peroxidase; CAT, catalase;
ROS, reactive oxygen species. |
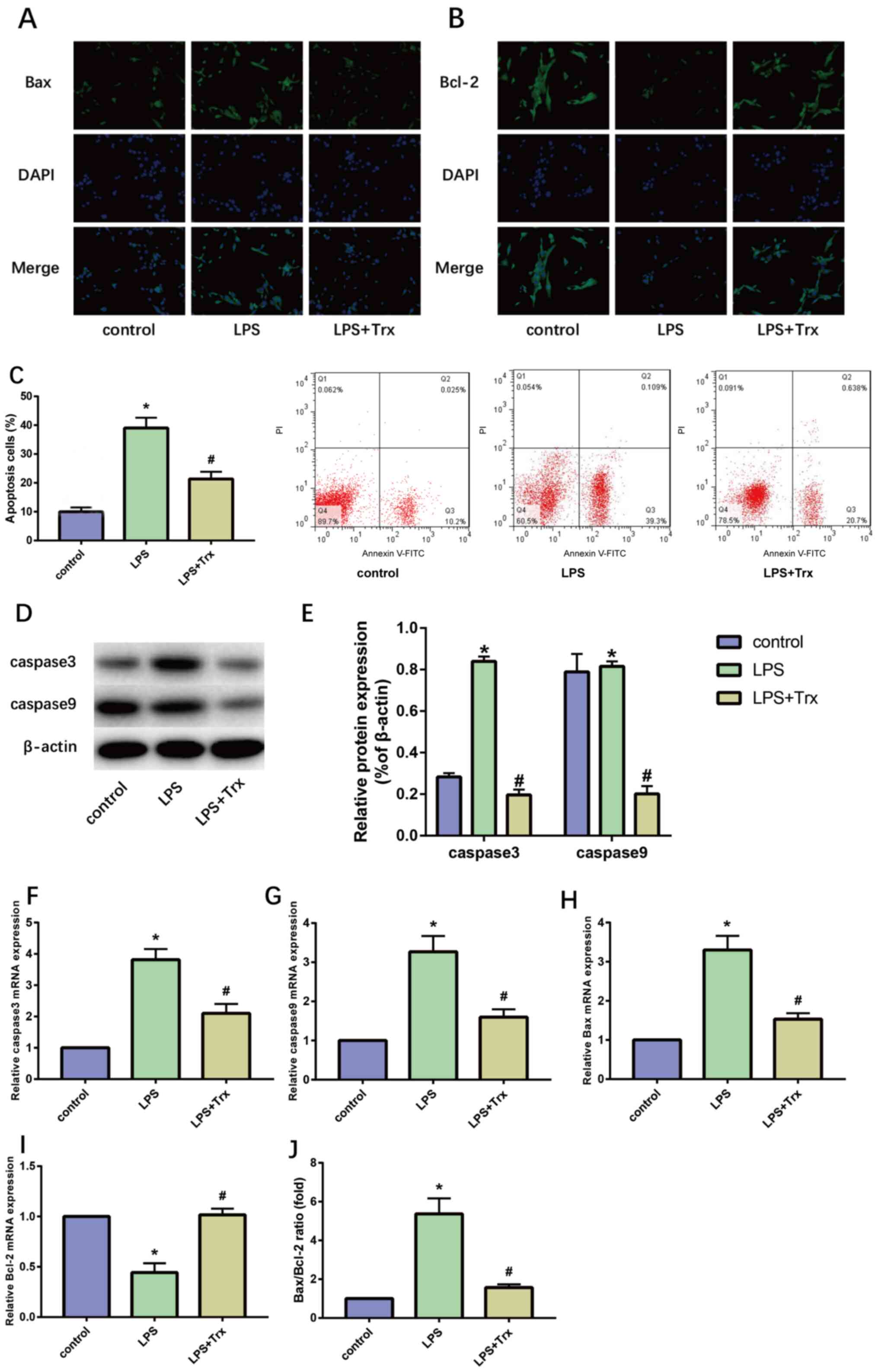
Trx reduces LPS-induced apoptosis in
HK-2 cells
The pathological process of AKI is accompanied by
the apoptosis of a large number of renal tubular epithelial cells,
therefore, the effect of Trx on the apoptosis of HK-2 cells was
detected. The results of IF staining indicated that Trx
significantly reduced the expression of Bax in HK-2 cells and
increased the expression of Bcl-2 (Fig.
3A and B). The results of flow
cytometry also indicated that Trx reduced the apoptosis rate in
HK-2 cells (Fig. 3C). Western blot
analysis (Fig. 3D and E) and RT-qPCR (Fig. 3F-I) showed that the expression of
Bax, caspase-3 and caspase-9 in LPS-induced HK-2 cells was
significantly increased while the expression of Bcl-2 was
decreased. Treatment with Trx attenuated the effects of LPS. The
mRNA expression levels of Bax and Bcl-2 were compared and Trx
reduced the Bax/Bcl-2 ratio (Fig.
3J).
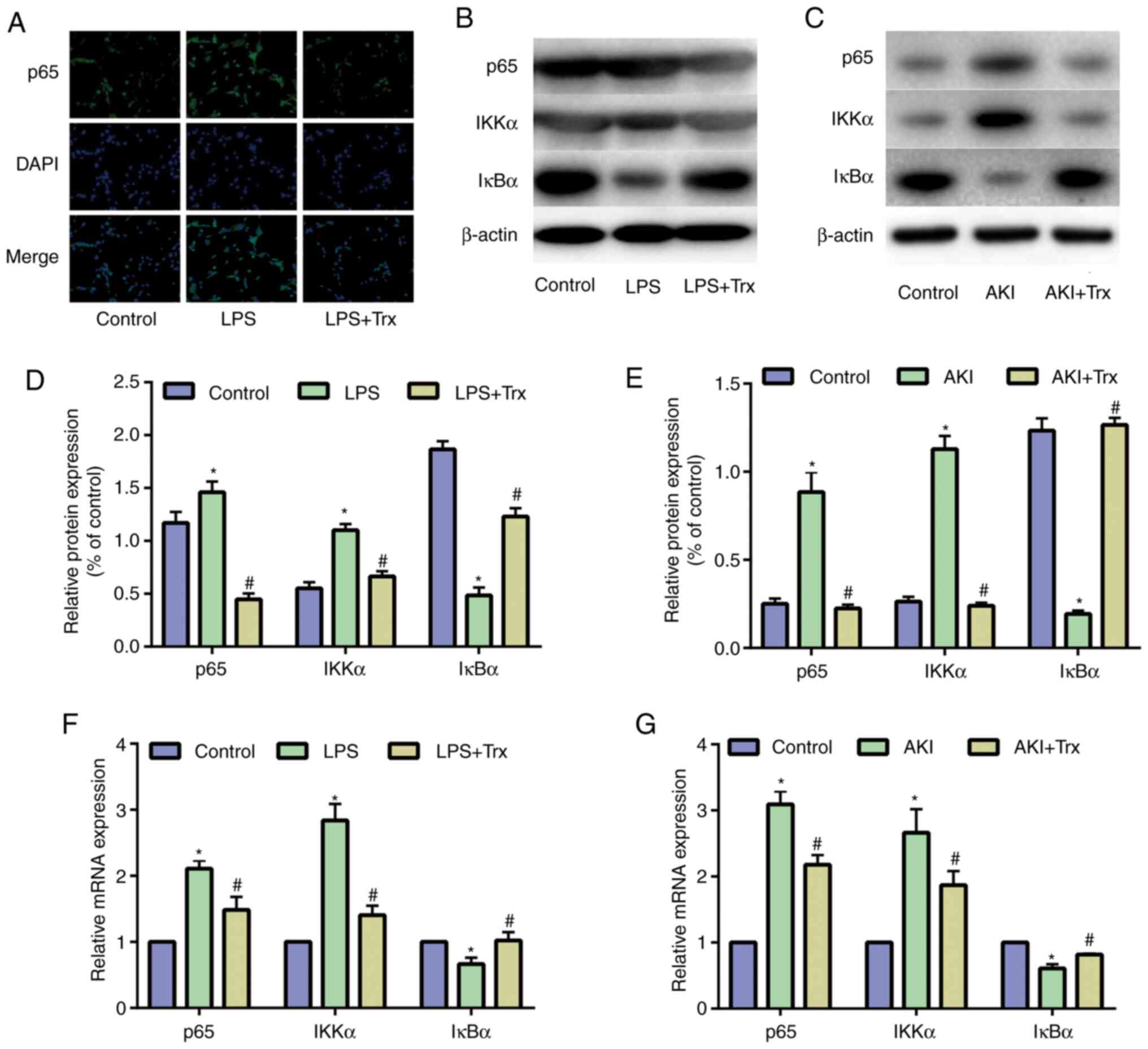
Trx reduces activity of the NF-κB
signaling pathway
NF-κB is widespread in glomerular epithelial cells,
renal tubular epithelial cells and mesangial cells, and is involved
in the production and regulation of cytokines, such as TNF-α, IL-1β
and interferon in the pathogenesis of glomerulonephritis, renal
toxicity damage and renal disease (17). To evaluate the underlying mechanism
by which Trx attenuated AKI, the activity of the NF-κB signaling
pathway was examined in HK-2 cells. P65, IKKα and IκBα are the key
signaling molecules in the NF-κB signaling pathway. The results of
IF staining indicated that Trx reduces the expression of p65
(Fig. 4A). The western blot
analysis (Fig. 4B) demonstrated
that the expression of p65 and IKKα in the LPS group was
significantly higher than that in the control group, while the
expression of IκBα was decreased. Treatment with Trx attenuated the
effect of LPS. Similar results have also been shown in vivo
(Fig. 4C). Quantitative analysis of
protein expression was performed (Fig.
4D and E). The results of
RT-qPCR indicated that Trx reduced the mRNA expression of p65 and
IKKα, and promoted the mRNA expression of IκBα in vitro and
in vivo (Fig. 4F and
G).
Exogenous Trx attenuates LPS-induced
mouse AKI
Trx was subcutaneously injected into mice to verify
the effect of Trx on AKI in mice. The results of H&E staining
revealed that some tubular epithelial cells in the AKI group
disappeared and inflammatory cells infiltrated the stroma. The
tubular epithelial cells in the Trx-treated mice were almost
intact, and the renal histopathology was significantly improved
when compared with that of the AKI group (Fig. 5A and B). MDA and SOD are important indicators of
oxidative stress. The expression levels of MDA and SOD in mouse
kidney tissue samples were detected by ELISA. It was found that Trx
effectively increased the expression of SOD and decreased the
expression of MDA, indicating that Trx reduced the level of
oxidative stress in mouse kidney tissues (Fig. 5C and D). In addition, the expression of AKI
biomarkers, NGAL, KIM-1, netrin-1 and L-FABP, in the urine of mice
in the AKI group was significantly higher than that in the control
group, while the expression of AKI biomarkers in the urine of mice
in the AKI + Trx group was significantly reduced (Fig. 5E-H). The expression levels of Scr,
BUN, IL-1β and TNF-α in the serum of LPS-induced mice were higher
than that of the control group. Treatment with Trx decreased their
expression (Fig. 5I-L).
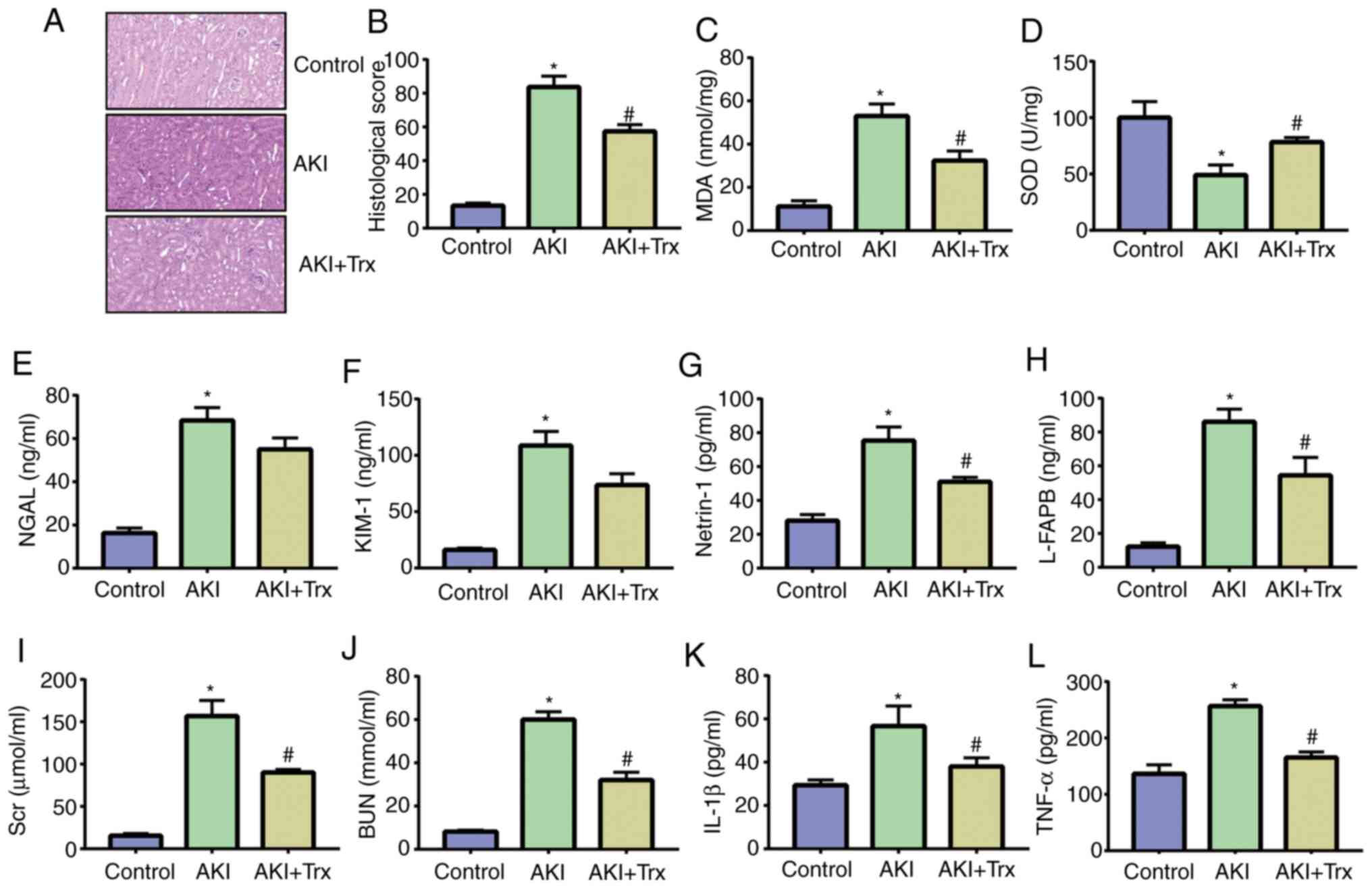 | Figure 5Exogenous Trx attenuates LPS-induced
mouse AKI. (A and B) H&E staining and histological scoring of
mouse kidney samples (magnification, x100). (C) MDA and (D) SOD
activity in mouse kidney samples. (E-H) ELISA of NGAL, KIM-1,
netrin-1 and L-FABP. (I-L) ELISA of Scr, BUN, IL-1β and TNF-α.
*P<0.05 vs. the control group; #P<0.05
vs. the LPS group. Data are presented as the mean ± SD. Trx,
thioredoxin; LPS, lipopolysaccharide; AKI, acute kidney injury;
MDA, malondialdehyde; SOD, superoxide dismutase; NGAL, neutrophil
gelatinase-associated lipocalin; KIM-1, kidney injury molecule 1;
L-FABP, liver-type fatty acid binding protein; Scr, serum
creatinine; BUN, blood urea nitrogen. |
Discussion
After AKI, tubular epithelial cells swell, necrosis
and shedding are observed, and the kidneys exhibit localized
ischemia, hypoxia and oxidative stress cascades, accompanied by
inflammatory cell infiltration. These complex pathophysiological
processes initiate the self-repairing function of the kidneys, but
the kidneys have limited ability to regenerate compared to organs
with abundant blood supply and powerful functions, such as the
heart and liver. When injury is severely beyond the kidney load,
the kidney cannot complete self-repair and the remaining renal
tubular epithelial cells lose cell polarity, showing continuous and
uncontrolled proliferation of abnormalities (18). These paracrine products of renal
tubular epithelial cells also interfere with the interaction
between normal renal tubular epithelial cells, perivascular
capillary endothelial cells and pericytes, resulting in epithelial
to mesenchymal transformation, endothelial to mesenchymal
transformation and pericytes to myofibroblast transformation
(19). Myofibroblasts continue to
proliferate and activate, secreting a large quantity of collagen
fibers deposited in the renal parenchyma, gradually developing into
renal interstitial fibrosis and progressing to end-stage renal
disease (19). Renal tubular
epithelial cell injury is the initiating factor for the progression
of AKI to chronic kidney disease (20). Therefore, interventions for renal
tubular epithelial cells will effectively promote kidney repair and
regeneration.
Numerous clinical studies have shown that elevated
levels of Trx in plasma or serum can be used as clinical parameters
for inflammatory diseases (21,22).
Trx can be secreted into saliva and respond to the severity of the
disease through the level of salivary Trx. Trx also regulates
macrophage migration inhibitory factor (MIF) to exert
anti-inflammatory effects (23).
MIF has attracted widespread attention as a deteriorating factor in
inflammatory diseases (23). A gene
fusion protein, human serum albumin (HSA)-Trx (consisting of HSA
and Trx) has a long half-life and good biological activity. HSA-Trx
has a certain effect in the treatment of oxidative stress-related
diseases. HSA-Trx also inhibits the production of inflammatory
cytokines by inhibiting MIF to exert an anti-inflammatory effect.
In a previous study, transgenic mice overexpressing Trx suppressed
allergies and inflammation in an allergic contact dermatitis (ACD)
model. Following sensitization with
1-fluoro-2,4-dinitrofluorobenzene, no difference in the
antigen-specific proliferation of lymph node cells was observed in
the Trx transgenic mice and control mice (24). Therefore, overexpression of Trx did
not affect the primary immune response during ACD, and neutrophil
infiltration was reduced. In addition, skin inflammation was
inhibited. These findings indicated that Trx could reduce
inflammation during the induction phase of ACD and its
anti-inflammatory mechanism was different to other
anti-inflammatory agents. The protective effect of exogenous Trx
was also confirmed in a mouse model of irritant contact dermatitis
(ICD) (25). Recombinant human Trx
can inhibit the production and release of pro-inflammatory
mediators at the site of inflammation and inhibit ICD (25). Furthermore, IL-1β produced by
inflammatory cells stimulates renal epithelial cells to produce
downstream inflammatory cytokines, TNF-α and IL-6(26). Similarly, the inhibitory effect of
Trx on inflammatory cytokines (IL-1β and TNF-α) was observed in the
serum of AKI mice in the present study.
MDA is a product of lipid peroxidation and its
content reflects the level of oxygen free radicals and the speed of
lipid oxidation in the body (27,28).
MDA in the serum of AKI mice was also found to be inhibited by Trx
in the present study. SOD is a free radical scavenging factor in
vivo and its activity reflects the ability of cells to scavenge
free radicals and resist lipid peroxidation. SOD plays a key role
in preventing the formation of peroxides, anti-oxidative stress
injury and anti-renal tubular injury (29). The present study also identified the
augmenting effect of Trx on SOD in AKI mice. The ratio of
anti-apoptotic molecule Bcl2 to pro-apoptotic molecule Bax reflects
the level of apoptosis. An increase in Bax and a decrease in Bcl2
lead to increases in the caspase family, thereby contributing to
cell apoptosis (30). In the
present study, Trx treatment significantly reduced LPS-induced AKI
in mice, predominantly manifesting as decreased levels of
inflammation and oxidative stress. Trx not only reduced Scr and BUN
in the mouse serum, but also decreased the expression of
AKI-related molecules in urine samples. In addition, Trx reduced
inflammatory factors, oxidative stress levels and apoptosis levels
in HK-2 cells, demonstrating significant anti-inflammatory,
anti-oxidative and anti-apoptosis effects, which are beneficial for
relieving AKI.
The NF-κB signaling pathway is a typical
inflammatory signaling pathway, which is largely based on the
pivotal position of NF-κB in the expression of inflammatory genes
(22). NF-κB could increase the
expression of cytokines, chemokines, adhesion factors, matrix
metalloproteinases, cyclooxygenase 2 and nitric oxide synthase, and
NF-κB itself is activated and sustained in numerous inflammatory
diseases (31). Inflammatory bowel
disease, glomerulonephritis and septic shock have been identified
as inflammatory diseases closely associated with NF-κB (31). Studies have shown that NF-κB can be
activated by endotoxin to enter the nucleus and regulate the gene
expression of various inflammatory mediators, particularly the
increase of TNF-α and IL-1β, which further acts on macrophages and
other inflammatory mediators and cytokines (32,33).
These cytokines further activate NF-κB, thus forming a positive
feedback cascade amplification effect, producing an excessive
inflammatory response and causing extensive tissue and cell injury
(33). Inflammation associated
cytokine storms during the course of sepsis tends to damage the
mitochondria in cells. Damaged mitochondria produce a large
quantity of ROS and antioxidant enzymes cannot completely eliminate
the excess ROS. Inflammation associated cytokine storms and
oxidative stress lead to various types of cell damage, including
apoptosis and even death (34).
The NF-κB signaling pathway and its downstream
inflammatory cascade play an important role in LPS-induced AKI. In
previous studies, the expression of pro-inflammatory cytokines,
toll-like receptor 4 and phosphorylated p65 in renal tissue were
all increased in AKI animal models induced by different stimuli.
Blocking or inhibiting the expression of these molecules with drugs
prevented the subsequent inflammatory response and promoted kidney
tissue repair (35-37).
Therefore, it is critical for the treatment of these diseases to
inhibit the NF-κB signaling pathway. In the present study,
LPS-induced NF-κB signaling pathway activity in HK-2 cells was
significantly increased, indicating that the NF-κB signaling
pathway was involved in the development of LPS-induced AKI. The
activity of the NF-κB signaling pathway was decreased in
Trx-stimulated HK-2 cells, indicating that Trx can inhibit the
NF-κB signaling pathway in HK-2 cells. Therefore, the role of Trx
in improving AKI may be related to inhibition of the NF-κB
signaling pathway. However, the present study did not conduct Trx
dose-dependency experiments, and further investigations are
required to establish the optimal treatment modality and mechanism
of action of Trx.
The present study identified that Trx reduced
LPS-induced inflammation, oxidative stress and apoptosis levels in
HK-2 cells. In addition, Trx reduced the activity of the NF-κB
signaling pathway in HK-2 cells. The protective effect of Trx on
HK-2 cells was also verified in vivo. Trx effectively
alleviated LPS-induced mouse AKI, reduced inflammatory factors in
mouse serum and reduced MDA levels in kidney tissue samples. To
conclude, Trx treatment reduced inflammation, levels of oxidative
stress and apoptosis in HK-2 cells by inhibiting the NF-κB
signaling pathway, thereby alleviating LPS-induced mouse AKI.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JW designed the study and performed the experiments.
WZ and GL established the animal models. WZ and GL collected the
data and JW, WZ and GL analyzed the data. JW prepared the
manuscript. All authors read and approved the final manuscript. JW
and WZ confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
This study was approved by the Animal Ethics
Committee of Changzhou Fourth People's Hospital Animal Center.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang YM, Han RL, Song SG, Yuan XP and Ren
XS: Inhibition of PARP overactivation protects acute kidney injury
of septic shock. Eur Rev Med Pharmacol Sci. 22:6049–6056.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pakula AM and Skinner RA: Acute kidney
injury in the critically Ill patient: A current review of the
literature. J Intensive Care Med. 31:319–324. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wald R, Kitchlu A, Harel Z and Silver SA:
Care of the acute kidney injury survivor. Nephron. 137:306–309.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Skube SJ, Katz SA, Chipman JG and
Tignanelli CJ: Acute kidney injury and sepsis. Surg Infect
(Larchmt). 19:216–224. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rahman M, Shad F and Smith MC: Acute
kidney injury: A guide to diagnosis and management. Am Fam
Physician. 86:631–639. 2012.PubMed/NCBI
|
|
6
|
Rydén L, Hertzberg D, Sartipy U and
Holzmann M: Acute kidney injury is a common and serious condition.
The clinical significance is great and probably underestimated.
Lakartidningen. 113(DXD3)2016.PubMed/NCBI(In Swedish).
|
|
7
|
Kampaktsis PN, Feldman DN and Charitakis
K: Strategies to avoid TAVI-related acute kidney injury. Curr Pharm
Des. 22:1950–1958. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang J, Li X, Han X, Liu R and Fang J:
Targeting the thioredoxin system for cancer therapy. Trends
Pharmacol Sci. 38:794–808. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Léveillard T and Aït-Ali N: Cell signaling
with extracellular thioredoxin and thioredoxin-like proteins:
Insight into their mechanisms of action. Oxid Med Cell Longev.
2017(8475125)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mattoo RU, Farina Henriquez Cuendet A,
Subanna S, Finka A, Priya S, Sharma SK and Goloubinoff P: Synergism
between a foldase and an unfoldase: Reciprocal dependence between
the thioredoxin-like activity of DnaJ and the polypeptide-unfolding
activity of DnaK. Front Mol Biosci. 1(7)2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jia J, Zeng X, Xu G and Wang Z: The
potential roles of redox enzymes in Alzheimer's disease: Focus on
thioredoxin. ASN Neuro. 13(1759091421994351)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yin Z, Ren H, Liu L, Chen W, Gan C, Jiao H
and Fan J: Thioredoxin protects skin flaps from
ischemia-reperfusion Injury: A novel prognostic and therapeutic
target. Plast Reconstr Surg. 137:511–521. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ueda S, Nakamura T, Yamada A, Teratani A,
Matsui N, Furukawa S, Hoshino Y, Narita M, Yodoi J and Nakamura H:
Recombinant human thioredoxin suppresses lipopolysaccharide-induced
bronchoalveolar neutrophil infiltration in rat. Life Sci.
79:1170–1177. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shen J, Cui ZK, Yao F, Li K, Zhang Y, Chen
Z, Zhou Y, Xu S, Zhang Y, Jiang W, et al: TSC1 deletion in
fibroblasts alleviates lipopolysaccharide-induced acute kidney
injury. Clin Sci (Lond). 132:2087–2101. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Paller MS, Hoidal JR and Ferris TF: Oxygen
free radicals in ischemic acute renal failure in the rat. Clin
Invest. 74:1156–1164. 1984.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sanz AB, Sanchez-Niño MD, Ramos AM, Moreno
JA, Santamaria B, Ruiz-Ortega M, Egido J and Ortiz A: NF-kappaB in
renal inflammation. J Am Soc Nephrol. 21:1254–1262. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Peerapornratana S, Manrique-Caballero CL,
Gómez H and Kellum JA: Acute kidney injury from sepsis: Current
concepts, epidemiology, pathophysiology, prevention and treatment.
Kidney Int. 96:1083–1099. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fragasso T, Ricci Z and Goldstein SL:
Pediatric acute kidney injury. Contrib Nephrol. 193:113–126.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Abdelraheem MB: Acute kidney injury in
low- and middle-income countries: Investigations, management and
prevention. Paediatr Int Child Health. 37:269–272. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen G, An N, Ye W, Huang S, Chen Y, Hu Z,
Shen E, Zhu J, Gong W, Tong G, et al: bFGF alleviates
diabetes-associated endothelial impairment by downregulating
inflammation via S-nitrosylation pathway. Redox Biol.
41(101904)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhou J and Chng WJ: Roles of thioredoxin
binding protein (TXNIP) in oxidative stress, apoptosis and cancer.
Mitochondrion. 13:163–169. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Benhar M: Application of a
thioredoxin-trapping mutant for analysis of the cellular
nitrosoproteome. Methods Enzymol. 585:285–294. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ouyang Y, Peng Y, Li J, Holmgren A and Lu
J: Modulation of thiol-dependent redox system by metal ions via
thioredoxin and glutaredoxin systems. Metallomics. 10:218–228.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mata-Pérez C and Spoel SH:
Thioredoxin-mediated redox signalling in plant immunity. Plant Sci.
279:27–33. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lv Y, Bing Q, Lv Z, Xue J, Li S, Han B,
Yang Q, Wang X and Zhang Z: Imidacloprid-induced liver fibrosis in
quails via activation of the TGF-β1/Smad pathway. Sci Total
Environ. 705(135915)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu B, Yu H, Baiyun R, Lu J, Li S, Bing Q,
Zhang X and Zhang Z: Protective effects of dietary luteolin against
mercuric chloride-induced lung injury in mice: Involvement of
AKT/Nrf2 and NF-κB pathways. Food Chem Toxicol. 113:296–302.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang Q, Han B, Xue J, Lv Y, Li S, Liu Y,
Wu P, Wang X and Zhang Z: Hexavalent chromium induces mitochondrial
dynamics disorder in rat liver by inhibiting AMPK/PGC-1α signaling
pathway. Environ Pollut. 265(114855)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lv Y, Jiang H, Li S, Han B, Liu Y, Yang D,
Li J, Yang Q, Wu P and Zhang Z: Sulforaphane prevents
chromium-induced lung injury in rats via activation of the
Akt/GSK-3β/Fyn pathway. Environ Pollut. 259(113812)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li S, Baiyun R, Lv Z, Li J, Han D, Zhao W,
Yu L, Deng N, Liu Z and Zhang Z: Exploring the kidney hazard of
exposure to mercuric chloride in mice: Disorder of mitochondrial
dynamics induces oxidative stress and results in apoptosis.
Chemosphere. 234:822–829. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen J and Chen LF: Methods to detect
NF-κB acetylation and methylation. Methods Mol Biol. 1280:395–409.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Neale PA, Leusch FDL and Escher BI: What
is driving the NF-κB response in environmental water extracts?
Chemosphere. 210:645–652. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1(a001651)2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Turkmen K: Inflammation, oxidative stress,
apoptosis, and autophagy in diabetes mellitus and diabetic kidney
disease: The four horsemen of the apocalypse. Int Urol Nephrol.
49:837–844. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lee JW, Kim SC, Ko YS, Lee HY, Cho E, Kim
MG, Jo SK, Cho WY and Kim HK: Renoprotective effect of paricalcitol
via a modulation of the TLR4-NF-κB pathway in
ischemia/reperfusion-induced acute kidney injury. Biochem Biophys
Res Commun. 444:121–127. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nair AR, Masson GS, Ebenezer PJ, Del Piero
F and Francis J: Role of TLR4 in lipopolysaccharide-induced acute
kidney injury: Protection by blueberry. Free Radic Biol Med.
71:16–25. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Korrapati MC, Shaner BE and Schnellmann
RG: Recovery from glycerol-induced acute kidney injury is
accelerated by suramin. J Pharmacol Exp Ther. 341:126–136.
2012.PubMed/NCBI View Article : Google Scholar
|