Introduction
Sepsis is an excessive systemic inflammatory
reaction caused by infection or trauma and accompanied by organ
dysfunction and respiratory failure (1-3).
Sepsis is one of the main causes of death of critically ill
patients in intensive care units due to the lack of specific
diagnostic markers and broad-spectrum therapies that are restricted
to antibiotics and supportive care (4,5).
Currently, sepsis is a leading cause of morbidity and mortality in
children worldwide (6). Due to the
non-specific clinical characteristics of sepsis, the difficulty in
differentiating sepsis from non-infectious etiologies and the low
positivity rates of blood culture, the early diagnosis and
treatment of children with sepsis is complicated and challenging
(7,8). Thus, it is urgently necessary to
discover novel and accurate biomarkers and therapeutic targets for
sepsis at an earlier stage.
MicroRNAs (miRs/miRNAs) are small, single-stranded
endogenous RNA molecules that bind to target mRNAs to regulate gene
expression and induce translational repression in eukaryotes,
thereby serving vital roles in complicated human diseases (9-11).
There is evidence that aberrant miRNA expression is associated with
various autoimmune and inflammatory diseases, including sepsis
(12). For example, in one study,
miR-15a and miR-16 were reported to be upregulated in neonatal
patients with sepsis, and to suppress lipopolysaccharide
(LPS)-induced toll-like receptor (TLR)-4/interleukin (IL)-1
receptor-associated kinase 1 signaling in vitro, thereby
inhibiting LPS-induced inflammation (13). In another study, it was observed
that miR-375 levels were downregulated and miR-21 levels were
upregulated in patients with sepsis, and a negative correlation was
detected between the expression of these two miRNAs. Furthermore,
the ectopic expression of miR-375 reduced the number of
marrow-derived suppressor cells in a mouse model of sepsis, thus
inhibiting sepsis development (14). Another miRNA, miR-1184, has been
shown to be significantly decreased in the blood of neonates with
sepsis (15). However, the role of
miR-1184 and its underlying mechanism in children with sepsis
remain to be elucidated.
The present study detected the downregulation of
miR-1184 expression in the blood of children with sepsis and
analyzed the effects of miR-1184 on inflammation and apoptosis in
an LPS-induced cell model of sepsis. In addition, the present study
investigated the target gene regulated by miR-1184 in order to
further understand the molecular mechanism of sepsis.
Materials and methods
Clinical specimens
The peripheral blood samples of 30 children (13
female, 17 male) with sepsis and 30 healthy control children (13
female, 17 male) were obtained at the Guiyang Maternal and Child
Health Care Hospital between January 2017 and March 2019. The age
range of control group was 8 to 11 years and the age range of
sepsis group was 8 to 12 years. The length of hospitalization of
control group was 42.9±10.3 h and the length of hospitalization of
sepsis group was 43.9±11.9. Experiments were approved by the Ethics
Committee of Guiyang Maternal and Child Health Care Hospital and
written informed consent was signed by the parents or guardians of
all participants. Inclusion criteria: i) complete clinical data and
ii) consent was obtained from the parents or guardians of the child
and informed consent was signed. Exclusion criteria: i) children
discharged from hospital within 24 h after admission; ii) onset
time exceeds 72 h; iii) children with any other health conditions
complicated with major visceral lesions such as those affecting the
heart, liver and/or kidney; iv) withdrawal from the study.
For the collection of peripheral blood serum, blood
samples were centrifuged at 400 x g for 10 min at 4˚C. The
supernatant was collected immediately and stored at -80˚C prior to
further analysis. For the collection of peripheral blood
mononuclear cells, blood samples with anti-coagulation treatment
(blood was stored in anticoagulant tubes, which contains sodium
heparin) were added to an equal volume of lymphocyte isolation
buffer (Beijing Solarbio Science & Technology Co., Ltd.), and
centrifuged at 800 x g for 18 min at 20˚C. The upper white layer
was slowly transferred into a new tube with 10 ml serum-free
RPMI-1640 medium (Sigma-Aldrich; Merck KGaA), followed by
centrifugation at 600 x g for 15 min at 20˚C. Following removal of
the supernatant, cell precipitates were resuspended in RPMI-1640
medium and centrifuged at 400 x g for 8 min at 20˚C. The collected
cells were mononuclear cells.
Cell culture and treatment
The THP-1 human monocytic cell line was obtained
from The Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences and maintained in RPMI-1640 medium supplemented
with 10% FBS (HyClone; Cytiva), 100 µg/ml streptomycin and 100 U/ml
penicillin at 37˚C in a humidified incubator with 5%
CO2. To establish the in vitro sepsis model,
THP-1 cells were treated with l µg/ml LPS (Sigma-Aldrich; Merck
KGaA) for 24 h to simulate a septic environment at 37˚C (16,17).
Cell Counting Kit 8 (CCK8)
Cell proliferation was assessed using CCK-8 (Dojindo
Molecular Technologies, Inc.) according to the instructions of the
manufacturer. Cells were seeded at a density of 8,000 cells/well in
a plate with 96 wells in complete medium overnight. The cells were
treated accordingly a in 5% CO2 at 37˚C for 72 h. A 10
µl volume of CCK-8 solution was added to each well and the
absorbance at 450 nm in 4 h was measured using a microplate reader
(Thermo Fisher Scientific, Inc.).
Cell transfection
Cells were inoculated in a six-well plate at a
concentration of 1x105/well, and transfection
experiments were conducted after the cells grew to 70-80%
confluence. A miR-1184-overexpression plasmid (miR-1184
double-stranded mimic: 5'-AUUUCCCGCGGUUUCAAACUCUCGGC-3', forward;
5'-UAAACGCGCUUCAACGCCUGCGUUAAA-3', reverse), TRADD overexpression
vector (pcDNA-TRADD) and corresponding negative controls (miR-NC
and pcDNA-NC) were designed and synthesized by Shanghai GenePharma
Co., Ltd. The vectors or negative controls were transfected at a
concentration of 20 nM into THP-1 cells using Lipofectamine™ 2000
(cat. no. 11668027; Invitrogen; Thermo Fisher Scientific, Inc.) in
accordance with the manufacturer's instructions. Follow-up
experiments were conducted 48 h after transfection.
Reverse transcription-quantitative PCR
(RT-qPCR)
Cells were inoculated in a 96-well plate at a
concentration of 1x103/well. The cells were treated with
or without LPS accordingly and then total RNA was extracted from
THP-1 cells and blood specimens using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The extracted RNA was reverse
transcribed into cDNA using the PrimeScript™ RT reagent kit (Takara
Bio, Inc.) at about 65˚C for 10 min. cDNA amplification was
performed via qPCR using the SYBR Premix Ex Taq™ II kit (Takara
Bio, Inc.) on an ABI PRISM 7900 Real-Time system (Applied
Biosystems; Thermo Fisher Scientific, Inc). Amplification
conditions were as follows: 95˚C for 10 min, followed by 40 cycles
of 95˚C for 10 sec and 60˚C for 60 sec. GAPDH and U6 served as the
internal controls. The primer sequences were as follows, IL-6
forward, 5'-GGCCCTTGCTTTCTCTTCG-3' and reverse,
5'-ATAATAAAGTTTTGATTATGT-3'; TNF-α forward,
5'-CAGCCTCTTCTCCTTCCTGA-3' and reverse,
5'-GGAAGACCCCTCCCAGATAGA-3'; IL-1β forward,
5'-GGCGAATTCCTTCATTGCCCAGGTTTC-3' and reverse,
5'-GGCGAATTCCTTCATTGCCCAGGTTTC-3'; GAPDH forward,
5'-TGGGTGTGAACCACGAGAA-3' and reverse, 5'-GGCATGGACTGTGGTCATGA-3';
U6 forward 5'-CTCGCTTCGGCAGCACA-3' and reverse,
5'-ACGCTTCACGAATTTGC-3'. The relative expression levels of target
genes were calculated using the 2-ΔΔCq method (18). Agarose gel electrophoresis (1%) was
used to detect the PCR products. In brief, 8 µl PCR products and 2
µl sample loading buffer were mixed for sample loading and a DNA
Marker of appropriate size was used for electrophoresis separation
with ethidium bromide staining. The results were analyzed by
ImageQuant TL (version 7.0, GE Healthcare; Cytiva).
Cell apoptosis analysis
The cells were grown at a density of
1x105 in a six-well plate. Flow cytometry was performed
to detect apoptosis of the cells using an FITC Annexin V/PI
Apoptosis Detection kit I (Guangzhou RiboBio Co., Ltd.). Cells with
or without prior LPS treatment were transfected with miR-1184 mimic
or miR-NC, alone or in combination with pcDNA-NC or pcDNA-TRADD for
48 h. Subsequently, cells were collected, resuspended and stained
with Annexin V/PI reagent according to the manufacturer's
instructions. FlowJo software (version 7.6.1; FlowJo LLC.) was used
to analyze cells in the early and late stages of apoptosis. Each
experiment was repeated three times.
TargetScan analysis
The binding sites between miR-1184 and TRADD were
predicted using TargetScan bioinformatics software (version 7.2;
http://www.targetscan.org/vert_72/).
Luciferase reporter assay
An association between miR-1184 and TRADD was
predicted using TargetScan software. A luciferase reporter assay
was then performed to verify the putative binding sites. The DNA
sequence of the TRADD 3'-untranslated region (3'-UTR) containing
wild-type or mutant target sites for miR-1184 was subcloned and
inserted into a pGL3-Control Vector (Promega Corporation) to create
wild-type TRADD (TRADD-WT) or mutated-TRADD (TRADD-MUT) reporter
vectors, respectively. Subsequently, THP-1 cells were
co-transfected with a luciferase reporter vector and either
miR-1184 mimic or miR-NC for 48 h using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.). Relative
luciferase activities were detected using a Dual-Luciferase
Reporter Assay kit (Promega Corporation), which was compared with
Renilla luciferase activity according to the manufacturer's
instructions.
Western blot analysis
Cells were inoculated in a 96-well plate at a
concentration of 1x103/well. The cells were treated with
LPS, as previously described, and then total protein was extracted
from the cells using RIPA buffer (Auragene; Hunan Aijia
Biotechnology Co., Ltd.). Protein quantities were determined using
a BCA protein assay kit (Bio-Rad Laboratories, Inc.). Samples
containing 40 µg protein/lane were separated by 10% SDS-PAGE and
transferred onto PVDF membranes (EMD Millipore). Following blocking
with 5% non-fat milk for 1 h at room temperature, the membranes
were incubated with primary antibodies targeting TRADD (1:1,000;
cat. no. ab110644; Abcam), p65 (1:1,000; cat. no. ab16502; Abcam),
phosphorylated (p)-p65 (1:1,000; cat. no. ab183559; Abcam) and
GAPDH (1:1,000; cat. no. ab9485; Abcam) overnight at 4˚C. Following
washing with 0.1% PBS-Tween-20 four times, the membranes were
incubated with horseradish peroxidase-conjugated secondary antibody
(1:5,000; ab150113; Abcam) for 1 h at room temperature. Finally,
the bands were visualized using an ECL detection system (Beyotime
Institute of Biotechnology) and Image J software (version 146;
National Institutes of Health) was used to analyze the fold-changes
of protein levels.
Statistical analysis
SPSS version 20.0 (IBM Corp.) and GraphPad Prism 6.0
(GraphPad Software, Inc.) were used to analyze the data. All
results are presented as the mean ± SD from at least three
independent experiments. The differences between and among groups
were evaluated using Student's t-test or one-way ANOVA followed by
Tukey's post hoc test, respectively. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-1184 expression is downregulated
in clinical samples from children with sepsis
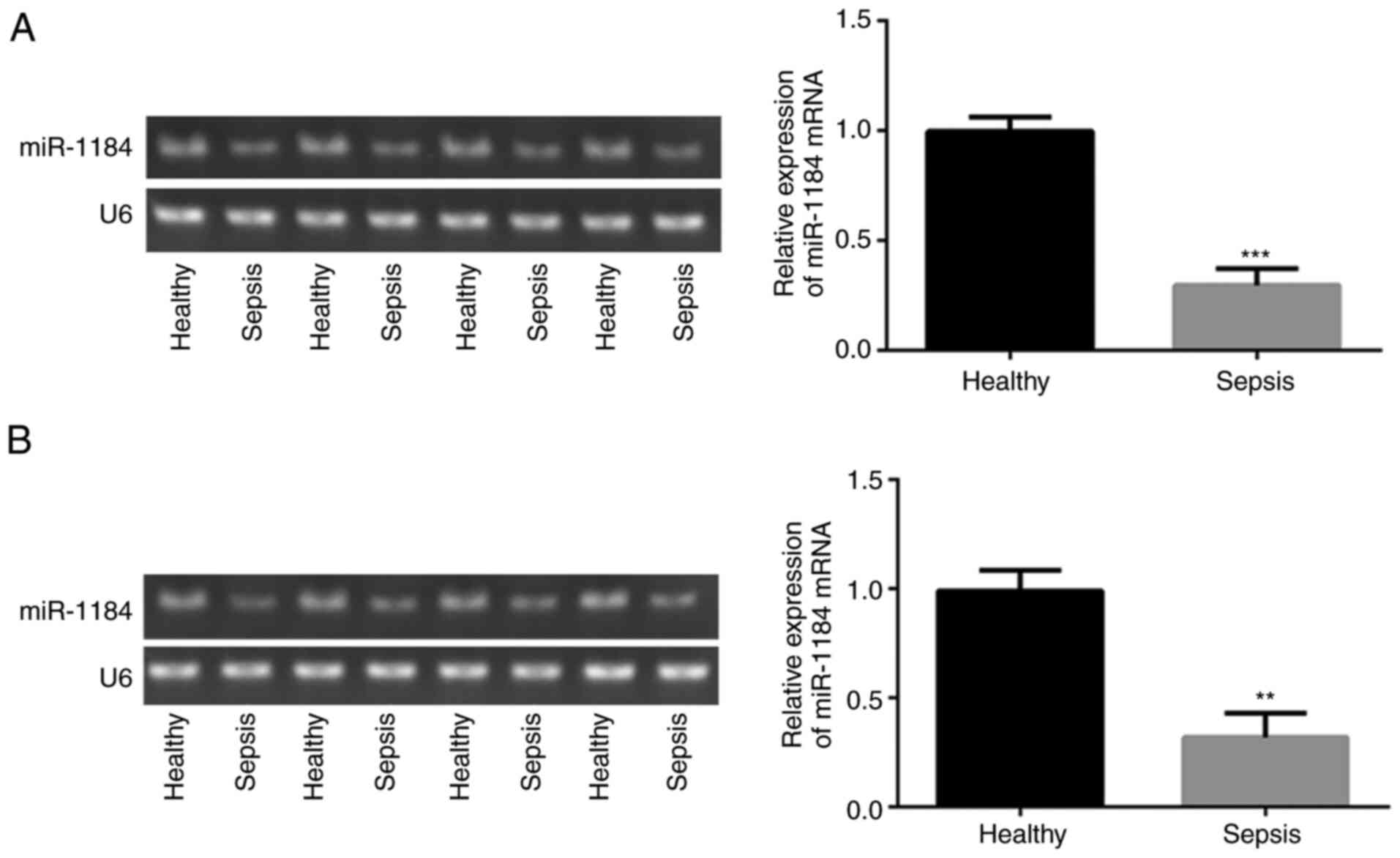
To assess the expression levels of miR-1184 in the
blood of children with sepsis, RT-qPCR was performed. As shown in
Fig. 1A, compared with healthy
controls, miR-1184 expression was significantly decreased in the
blood mononuclear cells of patients with sepsis. Similar results
were observed for the detection of miR-1184 expression in the serum
(Fig. 1B). The data indicate that
miR-1184 may be associated with the occurrence of sepsis in
children.
Levels of miR-1184 and inflammatory
factors are increased and decreased, respectively, in THP-1 cells
under LPS treatment
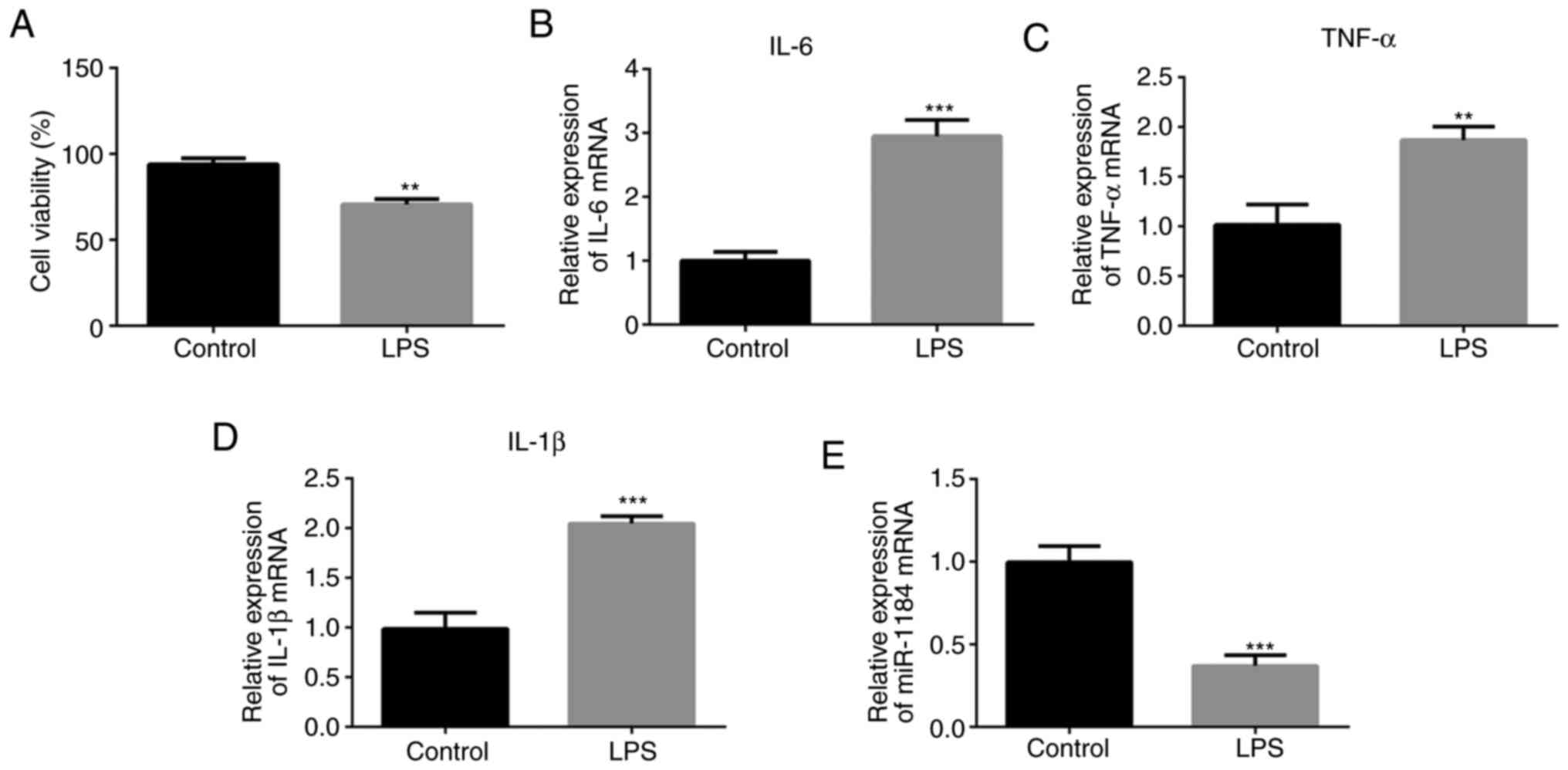
To assess whether the in vitro model of
sepsis was successfully established, a CCK-8 assay was conducted to
detect the cell viability. LPS was shown to inhibit cell viability
compared with that of the control group without LPS stimulation
(Fig. 2A). The levels of
representative inflammatory mediators were then detected using
RT-qPCR. As presented in Fig. 2B-D,
the expression levels of IL-6, TNF-α and IL-1β were significantly
increased in the LPS-induced THP-1 cells compared with cells
without LPS stimulation. In addition, miR-1184 expression was
significantly decreased in the LPS-induced cells (Fig. 2E), which is consistent with the
results shown in Fig. 1.
miR-1184 overexpression inhibits the
production of inflammatory cytokines and apoptosis induced by
LPS
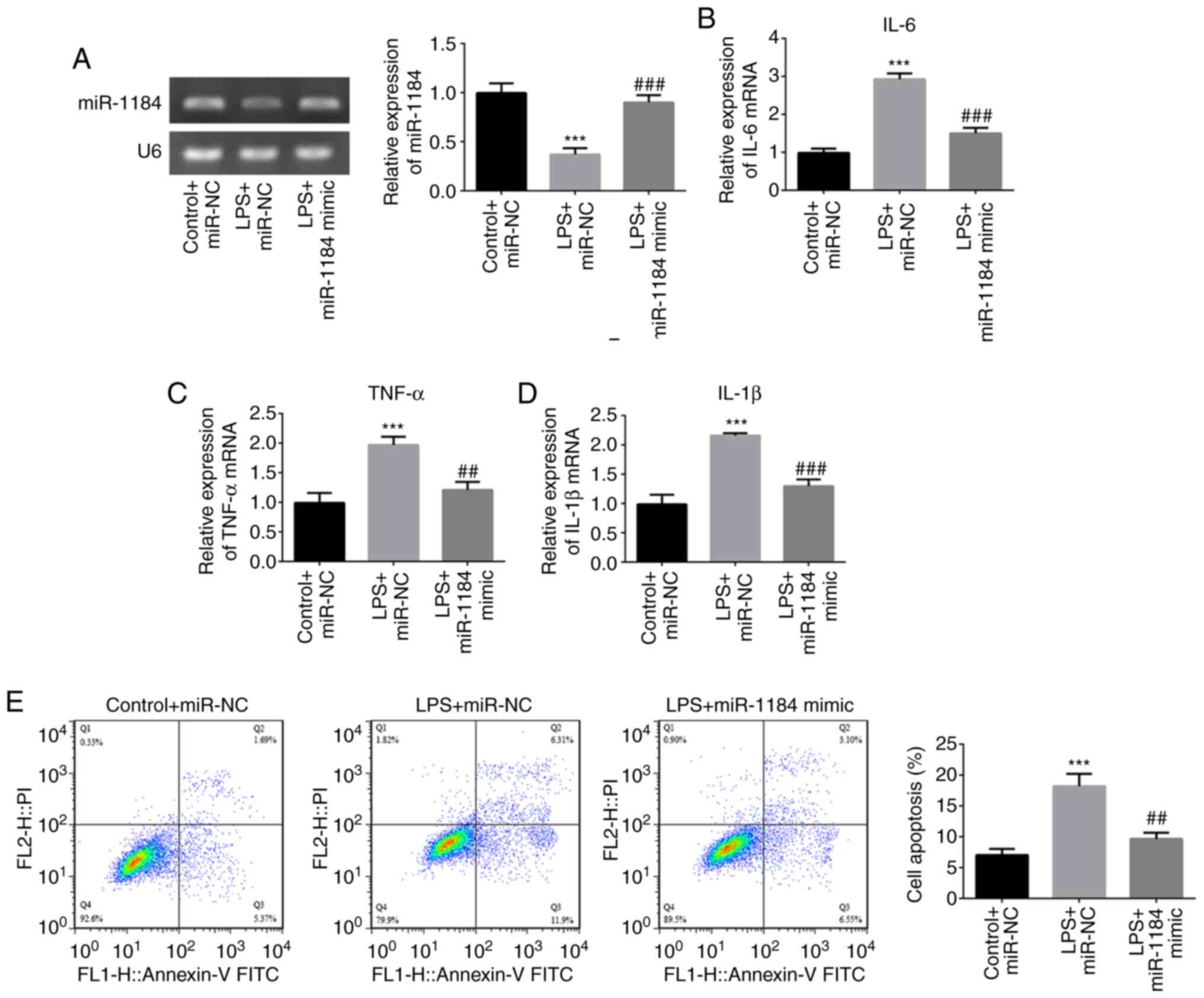
To investigate the role of miR-1184 in LPS-induced
THP-1 cells, miR-1184 was overexpressed by transfection with
miR-1184 mimic. RT-qPCR results showed that miR-1184 expression was
significantly increased following transfection with miR-1184 mimic
compared with miR-NC under conditions of LPS stimulation (Fig. 3A). The LPS-induced levels of IL-6,
TNF-α and IL-1β were decreased by transfection with miR-1184 mimic
compared with those in the negative control (LPS + miR-NC) group
(Fig. 3B-D). Furthermore, cell
apoptosis rates in the LPS + miR-NC group were significantly
increased compared with those in the control + miR-NC group, while
the miR-1184 mimic decreased the LPS-induced cell apoptosis
(Fig. 3E). These results indicate
that the overexpression of miR-1184 plays a suppressive role in the
LPS-induced inflammation and apoptosis of THP-1 cells.
Direct interaction between miR-1184
and TRADD
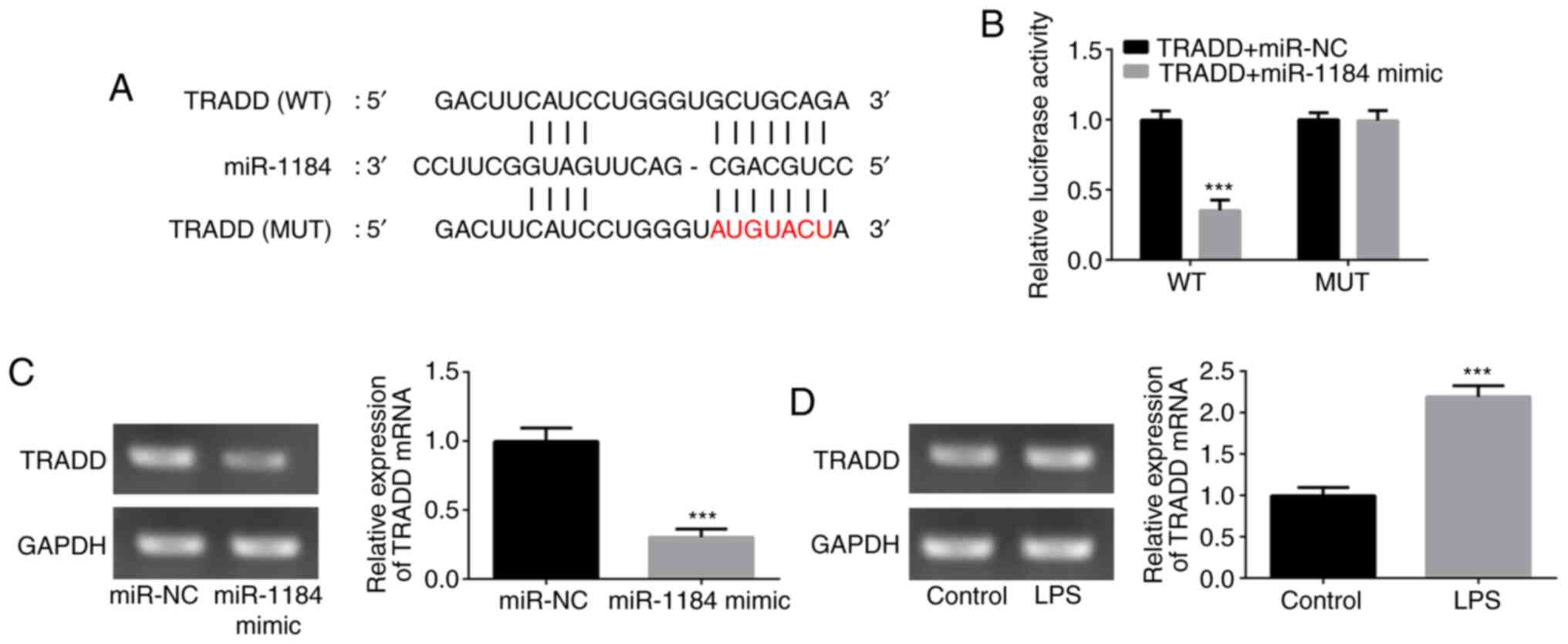
To verify the relationship between miR-1184 and
TRADD, wild-type and mutant TRADD 3'-UTR sequences containing
predicted miR-1184 binding sites were transfected into THP-1 cells
(Fig. 4A). The results from the
dual-luciferase reporter assay revealed that the luciferase
activity significantly declined following co-transfection with
TRADD (WT) and miR-1184 mimic, whereas no obvious effect of
miR-1184 mimic was observed on the mutant TRADD 3'-UTR (Fig. 4B). Additionally, the mRNA expression
level of TRADD was reduced by the upregulation of miR-1184
(Fig. 4C). Furthermore, LPS
stimulation significantly induced TRADD expression in THP-1 cells
(Fig. 4D). These results suggest
that TRADD is a direct target of miR-1184.
miR-1184 attenuates inflammatory
responses and apoptosis of THP-1 cells induced by LPS by targeting
TRADD
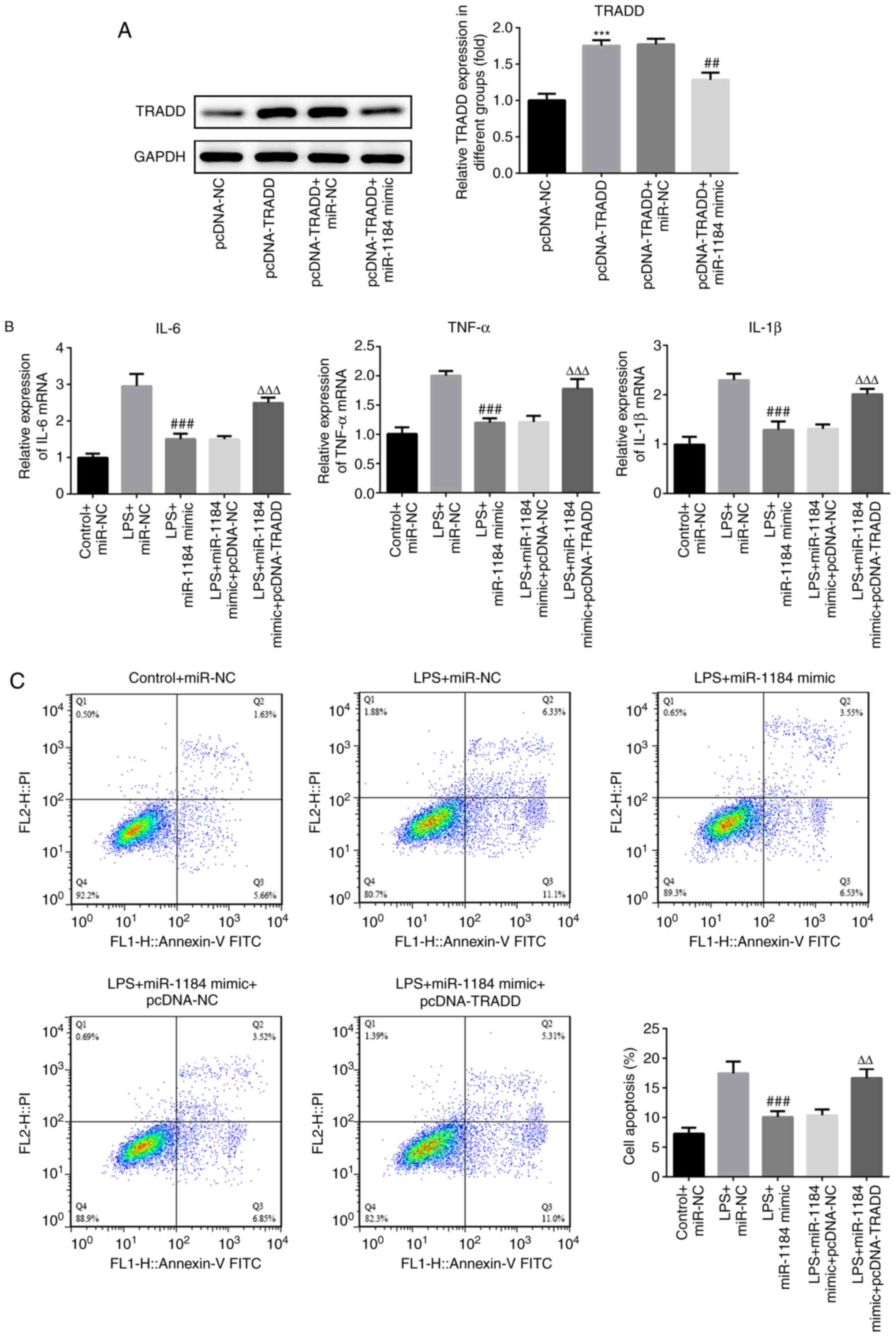
To assess the role of TRADD in sepsis, TRADD was
overexpressed in THP-1 cells. Western blotting results showed that
the protein levels of TRADD were significantly increased following
transfection with TRADD overexpression vector, and the increased
levels were reduced by co-transfection with miR-1184 mimic
(Fig. 5A). The effects of TRADD
overexpression on the levels of inflammatory factors were then
measured. As shown in Fig. 5B,
compared with LPS-induced cells transfected with miR-1184 mimic,
the expression levels of IL-6, TNF-α and IL-1β were significantly
increased in cells following co-transfection with miR-1184 mimic
and pcDNA-TRADD. In addition, the overexpression of TRADD
attenuated the suppression of cell apoptosis induced by the
miR-1184 mimic (Fig. 5C). The
results indicate that TRADD reverses the effects of miR-1184 on the
inflammatory response and apoptosis of LPS-induced THP-1 cells.
miR-1184/TRADD exerts biological
functions in LPS-induced THP-1 cells via regulation of NF-κB
signaling
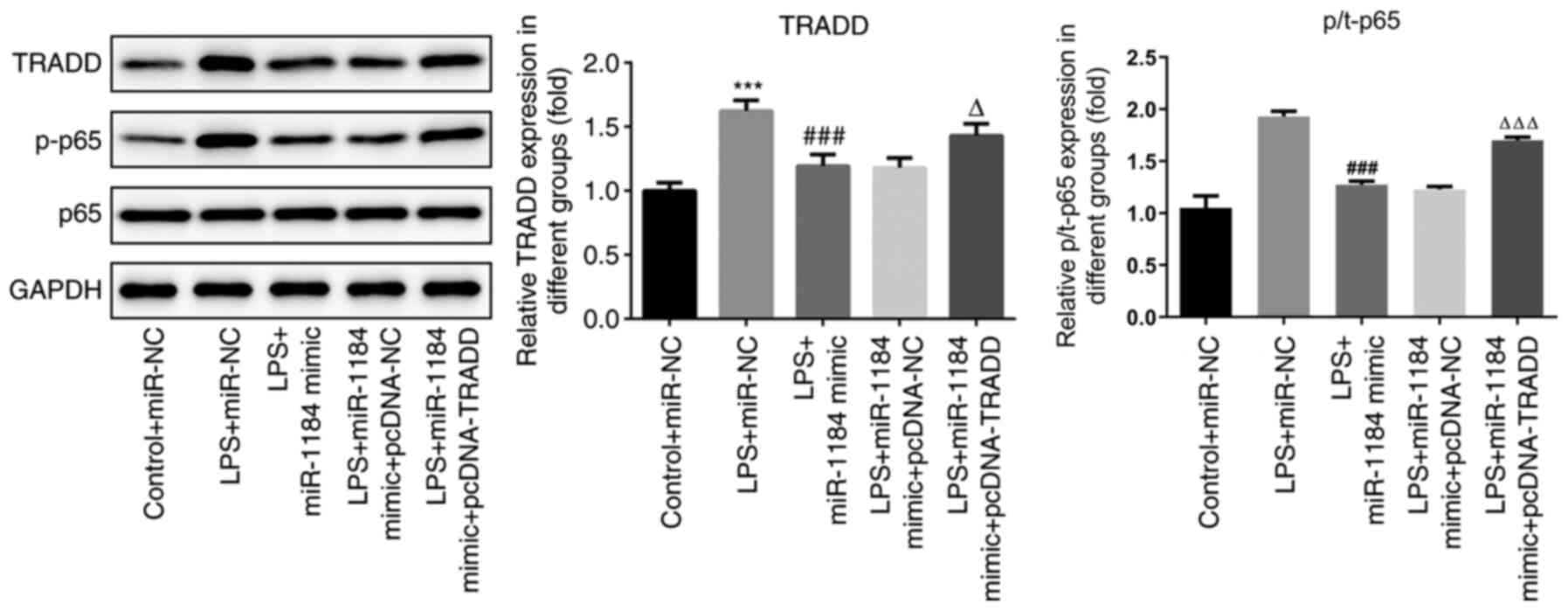
To further study the underlying mechanism of
miR-1184 in sepsis, the levels of proteins associated with the
NF-κB signaling pathway were detected. As shown in Fig. 6, the protein levels of TRADD and
p-p65 were significantly increased by LPS stimulation. miR-1184
mimic repressed the LPS-induced protein expression of TRADD and
p-p65, while TRADD overexpression reversed these effects. The data
indicate that miR-1184 and TRADD exert biological functions in
LPS-induced THP-1 cells via the regulation of NF-κB signaling.
Discussion
Sepsis is a blood infection mainly of bacterial,
viral or fungal (yeast) origin that leads to substantial morbidity
and mortality worldwide (19-21).
The present study compared the expression of miR-1184 between
children with sepsis and healthy children and found that miR-1184
expression was significantly decreased in the mononuclear cells and
serum from the blood of the children with sepsis. A previous study
revealed that the expression of numerous miRNAs were altered in
neonatal patients with sepsis compared with uninfected neonates,
among which miR-1184 was reported to be significantly reduced in
the blood samples of the neonates with sepsis (15). To further understand the role of
miR-1184 in childhood sepsis, an in vitro sepsis model was
established in the present study by the LPS treatment of THP-1
human monocytic leukemia cells. THP-1 cells were treated with l
µg/ml LPS for 24 h to simulate a septic environment (16,17).
Following construction of the in vitro sepsis model, the
expression levels of IL-6, TNF-α and IL-1β were detected. miR-1184
expression in THP-1 cells was shown to decline under LPS
stimulation. Following the overexpression of miR-1184, the
production of the pro-inflammatory cytokines IL-6, TNF-α and IL-1β
was inhibited and cell apoptosis decreased in LPS-induced cells. In
the present study, a binding site between miR-1184 and TRADD was
predicted using bioinformatics analysis, and the predicted binding
between miR-1184 and TRADD was verified through a dual-luciferase
reporter assay. Following the overexpression of miR-1184, the
expression of TRADD in the THP-1 cells significantly decreased,
indicating that miR-1184 can target and regulate TRADD. In
addition, the overexpression of TRADD was found to attenuate the
inhibitory effect of miR-1184 overexpression on apoptosis.
Therefore, it may be concluded that miR-1184 directly regulates
cell proliferation and other functions by interacting with TRADD.
Moreover, western blotting results indicated that the NF-κB
signaling pathway was inactivated in THP-1 cells after transfection
with miR-1184 mimic.
miRNAs are small noncoding single-stranded RNA
molecules that modulate gene expression and are involved in
cellular physiological activities, including proliferation,
invasion, metabolism and apoptosis (22-24).
Alterations in miRNA expression play a crucial role in the
regulation of the immune response (25). For example, Wu et al
(26) reported that miR-23b
expression was downregulated in LPS-induced vascular endothelial
cells and miR-23b mimic inhibited the expression of inflammatory
factors during sepsis. miR-1184 has been found to have biological
functions in several diseases. For instance, Yang et al
(27) revealed that miR-1184 was
involved in the regulatory effect of circular RNA VANGL1 silencing
on the progression of bladder cancer. In addition, changes in
miR-1184 expression have been reported in breast cancer and
prostatic benign hyperplasia (28,29).
In the present study, miR-1184 expression was observed to be
reduced in the mononuclear cells and serum isolated from the blood
samples of children with sepsis, as well as in LPS-induced THP-1
cells. Furthermore, overexpression of miR-1184 decreased the levels
of the LPS-stimulated inflammatory cytokines TNF-α, IL-1β and IL-6,
and inhibited LPS-induced apoptosis in THP-1 cells. These results
indicate that miR-1184 plays an inhibitory role in the
pathophysiology of sepsis.
Studies have demonstrated that miRNAs exert
biological effects by binding to specific mRNA molecules and
degrading mRNA (30). The present
study showed that TRADD is a direct target of miR-1184. In
addition, the overexpression of miR-1184 reduced TRADD expression,
while the expression of TRADD was elevated by LPS stimulation.
TRADD is a multifunctional protein that is important in TNF
receptor 1 (TNFR1) signaling and other signaling pathways involved
in immune responses (31). TRADD
serves a role in the TNF-α-induced pro-inflammatory response by
interacting with TNFR1 and regulates the TLR signaling pathway by
participating in the formation of complexes with TLR4 under LPS
induction (31). Consistent with
previous studies, the present results showed that TRADD promoted
LPS-induced inflammatory cytokine production and cell apoptosis as
it attenuated the inhibitory effects of miR-1184 overexpression on
inflammatory responses and apoptosis in sepsis.
A previous study demonstrated that the
phosphorylation level of p65 in TRADD-deficient cells was reduced
following LPS treatment (32).
TRADD serves a vital role in TIR domain-containing adapter-inducing
interferon-β (TRIF) signaling by participating in TRIF-dependent
NF-κB activation and regulating the production of inflammatory
cytokines (33). Thus, we
hypothesized that miR-1184 may modulate NF-κB signaling by directly
targeting TRADD. The present study observed that the
phosphorylation of p65 was upregulated upon LPS stimulation.
miR-1184 overexpression suppressed p65 phosphorylation and TRADD
attenuated the effects of miR-1184. These findings suggest that
NF-κB activation may be associated with the regulatory role of
miR-1184 on TRADD expression.
In summary, the present study suggests that miR-1184
may exert a therapeutic role in sepsis by repressing inflammatory
cytokine production and cell apoptosis. Bioinformatics analysis and
mechanistic investigations revealed that miR-1184 bound to the
3'-UTR of TRADD and mediated NF-κB signaling in an in vitro
model of sepsis. These findings may contribute to the understanding
of the pathogenesis of sepsis and the development of efficient
therapies for children with sepsis.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by Investigation and Analysis
of Children with Sepsis in Guizhou Province (grant no.
20161001).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PL and JC designed the study, and drafted and
revised the manuscript. RT searched the literature. HW analyzed the
data. PL, RT, HW and XD performed the experiments. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Experiments were approved by the Ethics Committee of
Guiyang Maternal and Child Health Care Hospital and written
informed consent was signed by the parents or guardians of all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vincent JL, Opal SM, Marshall JC and
Tracey KJ: Sepsis definitions: Time for change. Lancet.
381:774–775. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Angus DC, Linde-Zwirble WT, Lidicker J,
Clermont G, Carcillo J and Pinsky MR: Epidemiology of severe sepsis
in the United States: Analysis of incidence, outcome, and
associated costs of care. Crit Care Med. 29:1303–1310.
2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Skrupky LP, Kerby PW and Hotchkiss RS:
Advances in the management of sepsis and the understanding of key
immunologic defects. Anesthesiology. 115:1349–1362. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zilberberg MD, Shorr AF, Micek ST,
Vazquez-Guillamet C and Kollef MH: Multi-drug resistance,
inappropriate initial antibiotic therapy and mortality in
Gram-negative severe sepsis and septic shock: A retrospective
cohort study. Crit Care. 18(596)2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Martin GS, Mannino DM and Moss M: The
effect of age on the development and outcome of adult sepsis. Crit
Care Med. 34:15–21. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cecconi M, Evans L, Levy M and Rhodes A:
Sepsis and septic shock. Lancet. 392:75–87. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Plunkett A and Tong J: Sepsis in children.
BMJ. 350(h3017)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Martinot A, Leclerc F, Cremer R, Leteurtre
S, Fourier C and Hue V: Sepsis in neonates and children:
Definitions, epidemiology, and outcome. Pediatr Emerg Care.
13:277–281. 1997.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ward LD and Kellis M: HaploReg v4:
Systematic mining of putative causal variants, cell types,
regulators and target genes for human complex traits and disease.
Nucleic Acids Res. 44:D877–D881. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tufekci KU, Oner MG, Meuwissen RL and Genc
S: The role of microRNAs in human diseases. Methods Mol Biol.
1107:33–50. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang X, Wang X, Liu X, Wang X, Xu J, Hou
S, Zhang X and Ding Y: miR-15a/16 are upreuglated in the serum of
neonatal sepsis patients and inhibit the LPS-induced inflammatory
pathway. Int J Clin Exp Med. 8:5683–5690. 2015.PubMed/NCBI
|
|
14
|
Sheng B, Zhao L, Zang X, Zhen J and Chen
W: miR-375 ameliorates sepsis by downregulating miR-21 level via
inhibiting JAK2-STAT3 signaling. Biomed Pharmacother. 86:254–261.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen J, Jiang S, Cao Y and Yang Y: Altered
miRNAs expression profiles and modulation of immune response genes
and proteins during neonatal sepsis. J Clin Immunol. 34:340–348.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cheng Q, Tang L and Wang Y: Regulatory
role of miRNA-26a in neonatal sepsis. Exp Ther Med. 16:4836–4842.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
How CK, Hou SK, Shih HC, Huang MS, Chiou
SH, Lee CH and Juan CC: Expression profile of MicroRNAs in
gram-negative bacterial sepsis. Shock. 43:121–127. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Muller MC, Meijers JC, Vroom MB and
Juffermans NP: Utility of thromboelastography and/or
thromboelastometry in adults with sepsis: A systematic review. Crit
Care. 18(R30)2014.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Hermans G and Van den Berghe G: Clinical
review: Intensive care unit acquired weakness. Crit Care.
19(274)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pavlakou P, Liakopoulos V, Eleftheriadis
T, Mitsis M and Dounousi E: Oxidative stress and acute kidney
injury in critical illness: Pathophysiologic
mechanisms-biomarkers-interventions, and future perspectives. Oxid
Med Cell Longev. 2017(6193694)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lenkala D, LaCroix B, Gamazon ER, Geeleher
P, Im HK and Huang RS: The impact of microRNA expression on
cellular proliferation. Hum Genet. 133:931–938. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dvinge H, Git A, Graf S, Salmon-Divon M,
Curtis C, Sottoriva A, Zhao Y, Hirst M, Armisen J, Miska EA, et al:
The shaping and functional consequences of the microRNA landscape
in breast cancer. Nature. 497:378–382. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Melton C, Judson RL and Blelloch R:
Opposing microRNA families regulate self-renewal in mouse embryonic
stem cells. Nature. 463:621–626. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tsitsiou E and Lindsay MA: microRNAs and
the immune response. Curr Opin Pharmacol. 9:514–520.
2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wu M, Gu JT, Yi B, Tang ZZ and Tao GC:
microRNA-23b regulates the expression of inflammatory factors in
vascular endothelial cells during sepsis. Exp Ther Med.
9:1125–1132. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yang D, Qian H, Fang Z, Xu A, Zhao S, Liu
B and Li D: Silencing circular RNA VANGL1 inhibits progression of
bladder cancer by regulating miR-1184/IGFBP2 axis. Cancer Med.
9:700–710. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Danza K, De Summa S, Pinto R, Pilato B,
Palumbo O, Carella M, Popescu O, Digennaro M, Lacalamita R and
Tommasi S: TGFbeta and miRNA regulation in familial and sporadic
breast cancer. Oncotarget. 8:50715–50723. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Knyazev EN, Fomicheva KA, Mikhailenko DS,
Nyushko KM, Samatov TR, Ya Alekseev B and Yu Shkurnikov M: Plasma
levels of hsa-miR-619-5p and hsa-miR-1184 differ in prostatic
benign hyperplasia and cancer. Bull Exp Biol Med. 161:108–111.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Smillie CL, Sirey T and Ponting CP:
Complexities of post-transcriptional regulation and the modeling of
ceRNA crosstalk. Crit Rev Biochem Mol Biol. 53:231–245.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen NJ, Chio IIC, Lin WJ, Duncan G, Chau
H, Katz D, Huang HL, Pike KA, Hao Z, Su YW, et al: Beyond tumor
necrosis factor receptor: TRADD signaling in toll-like receptors.
Proc Natl Acad Sci USA. 105:12429–12434. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wilson NS, Dixit V and Ashkenazi A: Death
receptor signal transducers: Nodes of coordination in immune
signaling networks. Nat Immunol. 10:348–355. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Shukla K, Sharma AK, Ward A, Will R,
Hielscher T, Balwierz A, Breunig C, Münstermann E, König R,
Keklikoglou I and Wiemann S: MicroRNA-30c-2-3p negatively regulates
NF-kB signaling and cell cycle progression through downregulation
of TRADD and CCNE1 in breast cancer. Mol Oncol. 9:1106–1119.
2015.PubMed/NCBI View Article : Google Scholar
|