Introduction
Osteoarthritis (OA) is a chronic and progressive
disorder of the joints, characterized by a gradual loss of
articular cartilage and remodeling of the underlying bone. OA of
the knee, the most common type of OA, is one of the fastest growing
causes of disability, and affected >250 million individuals
worldwide (1). Despite its high
prevalence, there is a lack of effective pharmacological
interventions for treatment or management of OA at present and the
most common treatment strategies are pain relief or total knee
replacement (2). Thus far,
pharmaceutical treatment strategies for OA have relied on the use
of analgesics and non-steroidal anti-inflammatory drugs (3). Whilst research into disease-modifying
drugs for OA that may delay disease progression by targeting
various signaling pathways involved in the pathogenesis of OA is
currently underway (2,4), the development of tissue-specific
disease-modifying treatments for knee OA has been plagued with
difficulties (5), primarily due to
a lack of clear understanding of the molecular mechanisms
underlying the initiation and development of this disease.
Knee OA is a multifactorial joint disease that
involves various anatomical structures of the joint, including the
articular cartilage, subchondral bone, synovial membrane, ligaments
and menisci (6,7). A key factor that may contribute to OA
onset and development is the biomechanical characteristics of the
subchondral bone. Indeed, changes in the architecture and
composition of the subchondral bone have been frequently recognized
in animal OA models and patients with OA. These changes include
abnormal bone turnover, sclerosis of the subchondral plate,
osteophyte formation, thinning of the trabecular bone, subchondral
cysts and bone marrow lesions (8,9). These
microstructural changes are highly associated with subchondral bone
stiffening and degeneration of the overlying cartilage (10). Amelioration of the abnormalities in
subchondral bone may not only alleviate joint pain but may also
delay cartilage degeneration (11),
making subchondral bone-targeted treatment a promising approach for
the treatment of OA. However, a comprehensive investigation into
the potential role of subchondral bone changes in OA and the
related underlying molecular mechanisms is still required.
Due to the difficulties in monitoring the initiation
and progression of OA in human patients, animal models with
naturally occurring articular disorders similar to human OA are
instead widely used to investigate the pathogenesis of this disease
(12). The Hartley strain of guinea
pigs can spontaneously develop an OA-related phenotype of the knee
joint. In Hartley guinea pigs, the articular cartilage exhibits
age-dependent degenerative changes, for example, a gradual decrease
in the number of chondrocytes and amount of proteoglycan that
closely resembles what is observed in human OA. In addition,
Hartley guinea pigs share certain OA risk factors with humans, such
as age, sex and body weight (13,14).
All of these advantages make Hartley guinea pigs a suitable animal
model for studying the molecular mechanisms underlying OA
initiation and progression. Previous studies have focused on
altered gene-expression patterns in osteoarthritic articular
cartilage, whereas research concerning the association of
subchondral bone with OA remains limited (15,16).
Thus, determining the molecular mechanisms underlying abnormal
alterations in the subchondral bone in a Hartley guinea pig model
of knee OA may not only increase knowledge of the pathogenesis of
OA, but also highlight potential therapeutic targets for novel OA
disease-modifying drugs.
Liquid chromatography tandem-mass spectrometry
(LC-MS/MS) combined with iTRAQ technology is widely used to
identify new biomarkers and pathways involved in several diseases.
However, to the best of our knowledge, there are no studies
examining the molecular changes in subchondral bone in OA using
large-scale proteomic analysis. To address this gap in the
literature, the objective of the present study was to identify
potentially novel molecular biomarkers associated with the early
pathological process of OA using the established Hartley guinea pig
model of spontaneous knee OA. Differentially expressed proteins
were analyzed and three candidates in subchondral bone tissues
derived from male and female Hartley guinea pigs in the very early
phase of OA were identified by performing iTRAQ-based LC-MS/MS. The
proteomic characterization of osteoarthritic subchondral bone
allowed for a deeper understanding of this disease and may
facilitate future development of nonsurgical treatments for OA.
Materials and methods
Animal handling and gross
examination
Male (n=66) and female (n=66) Hartley guinea pigs
(age, 1 month old; mean weight, 328 g for female animals, 374 g for
male animals) were obtained from Beijing Vital River Laboratory
Animal Technology Co., Ltd.. Animals were randomly assigned to
groups of six and housed together in plastic cages, measuring
90x60x15 cm with a stainless-steel grid lid, and maintained at a
temperature of 25˚C, a relative humidity of 55±10% and a 12 h
light/dark cycle. The animals were provided with ad libitum
access to water and standard rodent chow. All animal experiments
were approved by the Beijing Jishuitan Hospital Animal Care and Use
Committee and were performed in accordance with the institutional
guidelines for care and use of animals.
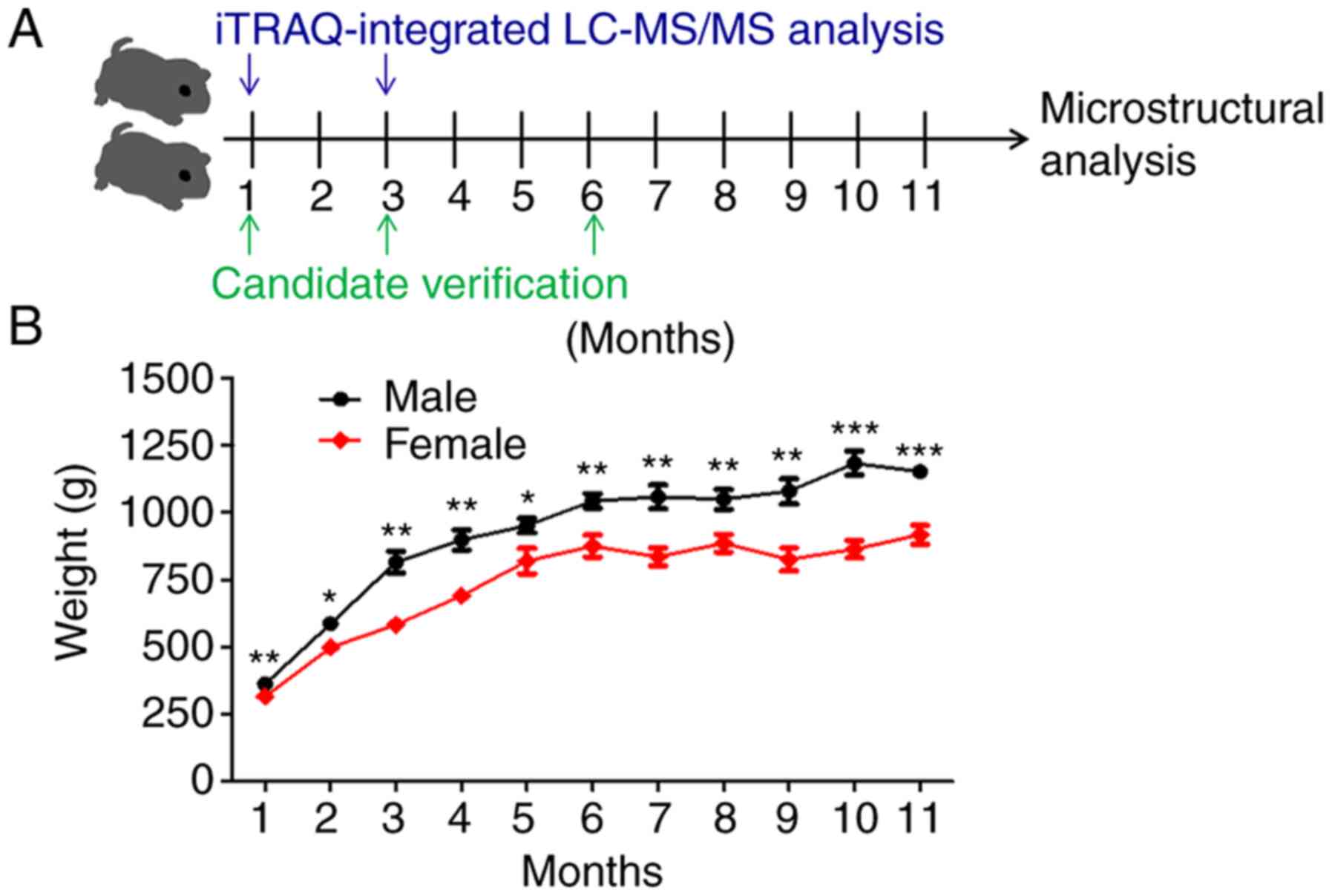
The general design of the study is shown in Fig. 1A. Animals were randomly selected for
sacrifice at 1-month intervals for 11 months, 6 animals at a time.
The body weights of all animals were recorded before sacrifice.
Articular cartilage was collected immediately after guinea pigs
were sacrificed by cervical dislocation under anesthesia using 3%
sodium pentobarbital (30 mg/kg body weight) intraperitoneally.
After sacrifice the knee-joints were disarticulated, the femurs and
tibias were separated, the articular cartilage of the femur and the
tibia were cleaned with 0.9% normal saline and the gross visual
appearance of the articular cartilage was recorded using a Canon
EOS 40D digital camera with a 50-mm lens (Canon Inc.).
Microstructural analysis of
subchondral bone
The microarchitecture of whole knee-joint specimens
was analyzed using a SkyScan 1172 micro-computed tomography (CT)
system (SkyScan; Bruker Corporation). The microfocus X-ray source
was set at 86 kV and 116 µA, with a pixel size of 12 µm. After
scanning, 3D reconstructions with a voxel size of 12 µm were
acquired using NRecon version 1.6.6.15 (SkyScan; Bruker
Corporation). For analysis of the subchondral trabecular bone, a
cuboid of trabecular bone measuring 2.05x2.05x0.6 mm3
was selected as the volume of interest (VOI). After VOI selection,
the images were binarized using the following gray-level thresholds
in Hounsfield units: 105-252 for ages 1-2 months; 120-252 for ages
3-6 months; 125-252 for age 7 months; and 130-252 for ages 8-11
months. The binarized images were used to calculate morphological
parameters using CTAn version 1.14.4 (SkyScan; Bruker Corporation).
The bone volume fraction (BV/TV as a percentage), trabecular
thickness (Tb. Th, mm), trabecular number (Tb. N, 1/mm), structure
model index (SMI), trabecular bone pattern factor (Tb. Pf, 1/mm),
degree of anisotropy (DA) and trabecular separation (Tb. Sp, mm)
were calculated. Additionally, the bone mineral density (BMD,
g/cm3) was calibrated using the attenuation coefficients
of two hydroxyapatite phantom materials with known mineral
densities of 0.25 and 0.75 g/cm3.
Protein digestion and iTRAQ
labeling
Protein digestion was performed using the
filter-aided sample-preparation procedure described by Wisniewski
et al (17). The mixture of
peptides generated was labeled with 4-plex iTRAQ reagents,
according to the manufacturer's protocol (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Briefly, 200 µg total protein for
each tibial subchondral bone sample was incorporated into a 30 µl
solution containing 4% sodium dodecyl sulfate, 100 mM
dithiothreitol, and 100 mM Tris-HCl (pH 8.0). After heating at 95˚C
for 5 min and then cooling to room temperature, each sample was
filtered using an ultrafiltration filter (pore size, 10 kDa;
Sartorius AG) and treated with 200 µl UT buffer (8 M urea and 150
mM Tris-HCl at pH 8.0). Subsequently, 100 µl 50 mM iodoacetamide in
UT buffer was added to block reduced cysteines, and the samples
were incubated at room temperature for 20 min in the dark. The
filters were washed three times with 100 µl UT buffer and then
twice with 100 µl dissolution buffer (50 mM triethylammonium
bicarbonate at pH 8.5). Finally, the protein suspensions were
digested overnight with 40 µl trypsin (Promega Corporation) buffer
(2 µg trypsin in 40 µl dissolution buffer) at 37˚C. The generated
peptide concentrations were estimated using UV light spectral
density at 280 nm. For labeling, each iTRAQ reagent was dissolved
in 70 µl ethanol and added to the respective mixture of peptides.
The peptides obtained from the tibial subchondral bone samples from
the guinea pigs were named as follows: 1-month-old male guinea pigs
(1MM)-1, -2 and -3; 1-month-old female guinea pigs (1MF)-1, -2 and
-3; 3-month-old male guinea pigs (3MM)-1, -2 and -3; and
3-month-old female guinea pigs (3MF)-1, -2 and -3. Tibial
subchondral bone samples from the 1MM, 1MF, 3MM and 3MF groups were
labeled with isobaric iTRAQ tags of masses 114, 115, 116 and 117
Da, respectively, and were multiplexed and vacuum dried.
Peptide fractionation using strong
cationic-exchange chromatography
The iTRAQ-labeled peptides were fractionated using
strong cationic-exchange (SCX) chromatography performed on the AKTA
Purifier system (GE Healthcare). The dried peptide mixture was
reconstituted and acidified with 2 ml buffer A consisting of 10 mM
KH2PO4 in 25% (v/v) of acetonitrile (pH 2.7),
and then subjected to SCX fractionation on a PolySULFOETHYL column
(4.6x100 mm, 5 µm, 200 Å; PolyLC Inc.). The peptides were eluted at
a flow rate of 1 ml/min with a gradient of 0-10% buffer B (buffer A
with 500 mM KCl added) for 2 min, 10-20% buffer B for 25 min,
20-45% buffer B for 5 min and 50-100% buffer B for 5 min. The
elution was monitored using the absorbance at 214 nm and fractions
were collected every min. Finally, ~30 collected fractions were
combined into 10 pools and desalted on C18 Cartridges
[Empore™ SPE Cartridges C18 (standard density), bed
inner diameter 7 mm, volume 3 ml; Sigma-Aldrich; Merck KGaA]. Each
SCX salt step fraction was concentrated by vacuum centrifugation
and then reconstituted in 40 µl 0.1% (v/v) acetic acid. All samples
were stored at -80˚C until required for LC-MS/MS analysis.
LC-MS/MS analysis
Experiments were performed on a Q Exactive mass
spectrometer coupled with an Easy nLC (Proxeon Biosystems; Thermo
Fisher Scientific, Inc.). For each fraction, 5 µg peptide mixture
was subjected to nano-LC-MS/MS analysis. The peptide mixture was
loaded onto a reverse phase trap column (Thermo Fisher Scientific,
Inc.; Acclaim PepMap100, 100 µm x 2 cm, nanoViper C18) connected to
a C18-reversed phase analytical column (Thermo Fisher Scientific,
Inc.; Easy Column; 10-cm long, 75-µm inner diameter, 3-µm resin) in
buffer C (0.1% formic acid) and separated with a linear gradient of
buffer D (buffer C with 84% acetonitrile added) at a flow rate of
300 nl/min controlled using IntelliFlow technology over 120 min. MS
data were acquired using a data-dependent top 10 method that
dynamically selected the most abundant precursor ions from the
survey scan (300-1,800 m/z) for higher energy collisional
dissociation (HCD) fragmentation. The target value was determined
based on predictive automatic gain control. The dynamic exclusion
duration was 40 sec. Survey scans were acquired at a resolution of
70,000 at m/z 200, and the resolution for HCD spectra was set to
17,500 at m/z 200; the isolation width was 2 m/z. The normalized
collision energy was 30 eV, and the underfill ratio was defined as
0.1% on Q Exactive.
Sequence database examination and data
analysis
MS/MS spectra were searched using the MASCOT engine
(Matrix Science; version 2.2) embedded into Proteome Discoverer
version 1.4 (Thermo Fisher Scientific, Inc.) against the Uniprot
Cavia porcellus database (20,415 sequences, downloaded on
October 10th, 2015) and a decoy database. For protein
identification, the following parameters were used: Peptide mass
tolerance, 20 ppm; fragment mass tolerance, 0.1 Da; enzyme,
trypsin; maximum missed cleavages, 2; fixed modifications,
carbamidomethyl (C), iTRAQ4plex (K), and iTRAQ4plex (N-term);
variable modification, oxidation (M); peptide false discovery rate
(FDR)≤0.01.
Gene ontology (GO) annotation and
Kyoto encyclopedia of genes and genomes (KEGG) pathway
analysis
GO analysis was performed to annotate the identified
differentially expressed proteins. Firstly, the protein sequences
of the differentially expressed proteins identified were retrieved
in batches from the UniProtKB database (https://www.uniprot.org/) in the FASTA format. Then,
the functional GO annotations and classifications of all the
differentially expressed proteins were applied using the Blast2GO
program. Biological pathway enrichment analysis was performed based
on KEGG pathway analysis. The FASTA sequences of all the
differentially expressed proteins were compared against the online
KEGG database (http://www.genome.ad.jp/kegg/) and mapped to the
pathways included in KEGG. The corresponding KEGG pathways were
subsequently extracted.
Western blot analysis
At sacrifice, samples of the right tibial
subchondral bone were collected for western blot analysis. For
coronin 1A (CORO1A), S100 calcium-binding protein A8 (S100A8) and
TCIRG1 protein expression studies, 100 µg total protein from tibial
subchondral bone was resolved using a 4-12% SDS-PAGE gel. After
transfer to PVDF membranes, the membranes were blocked for 1 h at
room temperature in 1X PBS containing 0.15% Tween-20 (1X PBST) and
5% non-fat dry milk, and subsequently immunoblotted with the
indicated primary antibodies in 1X PBST with 5% non-fat dry milk at
4˚C overnight. The primary antibodies included anti-CORO1A (Aviva
Systems Biology, Corp; cat. no. ARP38899_T100; 1:400), anti-S100A8
(Abcam; cat. no. ab92331; 1:1,000) and anti-TCIRG1 (Santa Cruz
Biotechnology, Inc.; cat. no. sc-162300; 1:500). Signals were
visualized using enhanced chemiluminescence reagent (Thermo Fisher
Scientific, Inc.) and horseradish peroxidase HRP-conjugated goat
anti-rabbit IgG (Abcam; cat. no. ab97051; 1:5,000), HRP-conjugated
rabbit anti-mouse IgG (Abcam; cat. no. ab6728; 1:5,000) and
HRP-conjugated rabbit anti-goat IgG (Abcam; cat. no. ab6741;
1:5,000) secondary antibodies for 2 h at room temperature. β-actin
(Abcam; cat. no. ab6276; 1:1,000) was used as the internal
control.
Statistical analysis
SAS version 9.2 (SAS Institute, Inc.) was used for
statistical analyses for between-group differences. GraphPad Prism
version 8.0 (GraphPad Software, Inc.) was used for statistical
analyses of differences between sexes. Differences between ages in
BMD, BV/TV, Tb. Th, Tb. N, SMI, Tb. Pf, DA and Tb. Sp values of the
subchondral trabecular bone were analyzed using a non-parametric
Kruskal-Wallis test, and a pair-wise comparison among 11 ages was
performed for each parameter based upon Dunn's post hoc test. Sex
differences in BMD, BV/TV, Tb. Th, Tb. N, SMI, Tb. Pf, DA and Tb.
Sp values were analyzed using a Student's t-test or non-parametric
Mann Whitney U test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Gross morphological observation of
spontaneous degeneration of articular cartilage
After an initial sharp increase from 1 to 5 months,
the weight of all guinea pigs remained relatively stable over time.
Compared with the female guinea pigs, male animals showed a
significantly faster growth rate until the age of 11 months
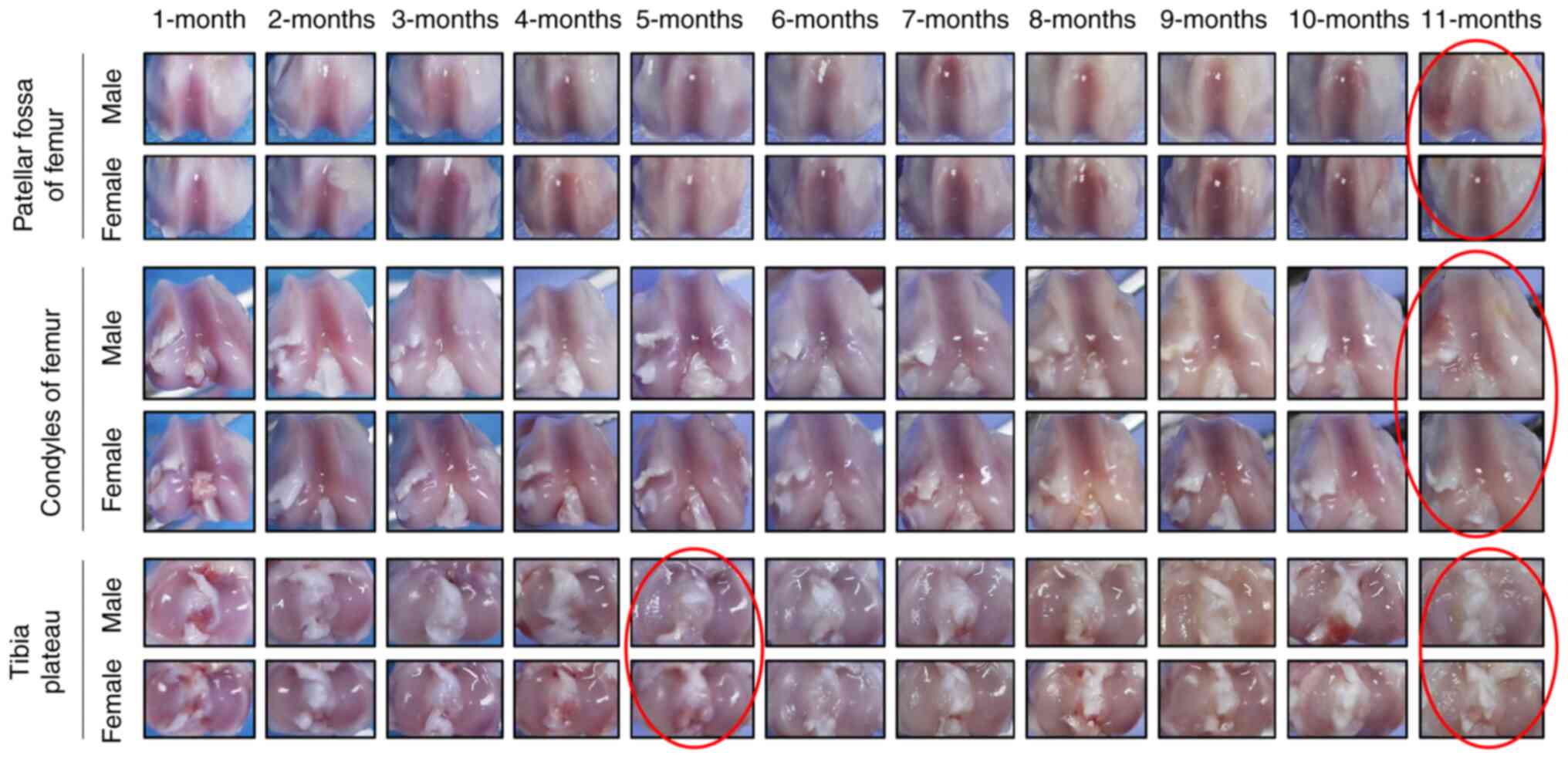
(Figs. 1B and S1). Macroscopic articular cartilage
lesions initially appeared in the region of the medial tibial
plateau not covered by the meniscus in both male and female Hartley
guinea pigs at the age of 5 and 11 months (red circle; Fig. 2). The degeneration of the articular
cartilage worsened with age, starting at 5 months. By the age of 11
months, the degenerative cartilage lesions were noticeable and had
invaded all aspects of the medial and lateral compartments of the
tibial plateau in addition to the condyles and patellar fossa of
the femur. Moreover, spontaneous cartilage degeneration in the knee
joints tended to be more serious in male Hartley guinea pigs
compared with female Hartley guinea pigs.
Micro-CT analysis of subchondral
trabecular bone
To further characterize the microarchitecture of the
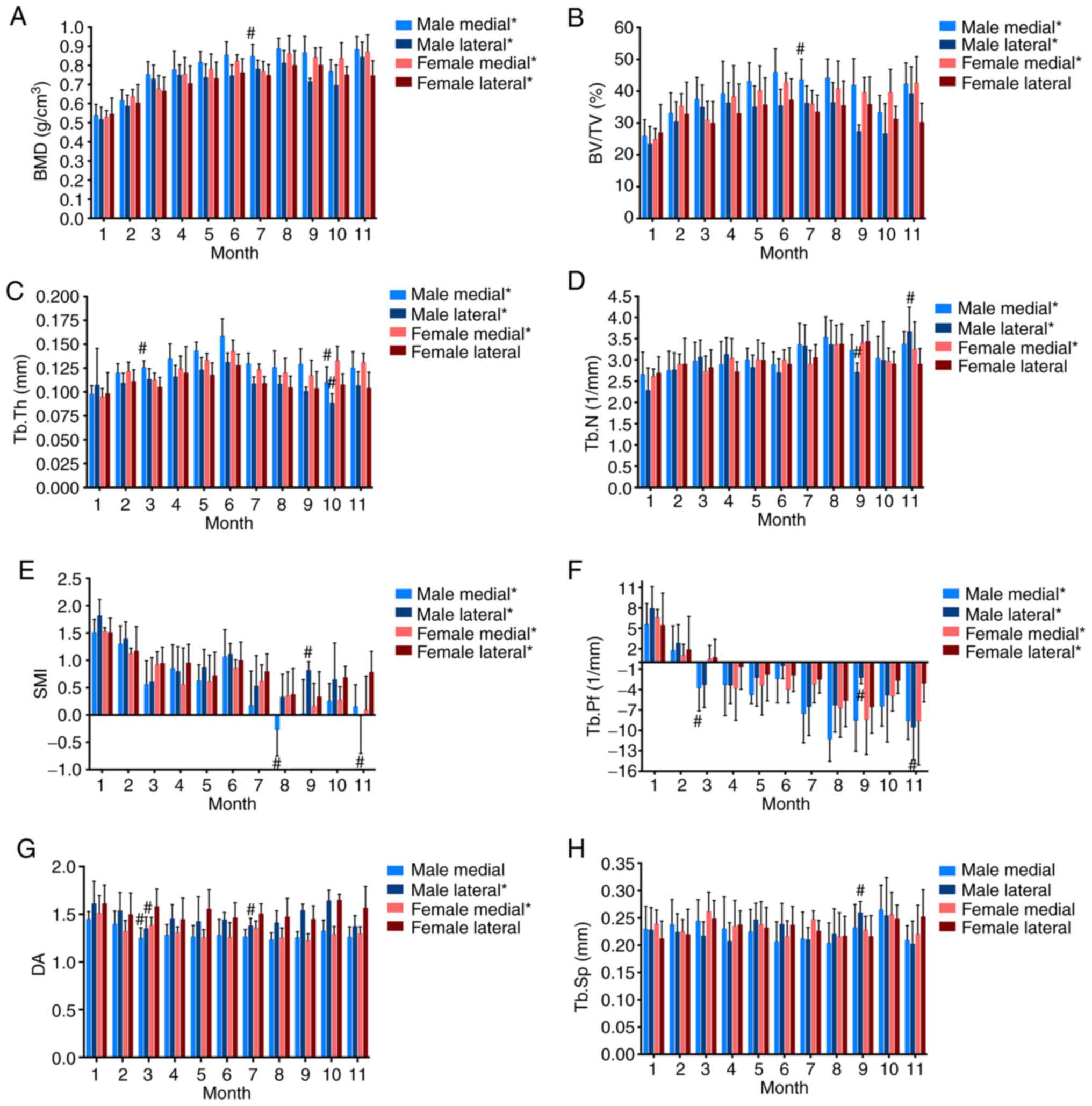
subchondral trabecular bone in the guinea pigs, Micro-CT analysis
for both sexes was performed (Fig.
3). The BMD of the medial tibial trabecular bone showed an
age-related increase in both male and female guinea pigs from 1 to
6 months of age (Fig. 3A). After 6
months, BMD values exhibited slight fluctuations; however, the
differences between ages were not significant (Fig. 3A). A similar tendency was observed
for the lateral tibial trabecular bone in female guinea pigs.
However, the BMD of the lateral tibial trabecular bone in male
animals showed a gradual upward trend from ages 1 to 4 months,
followed by temporary fluctuations. The BV/TV increased over time
on both the medial and lateral sides in the female animals, peaking
at 6 months (Fig. 3B). The pattern
of BV/TV variation in the medial trabecular bone of male animals
was consistent with that in the female animals. In both male and
female guinea pigs, the thickness of the trabecular bone gradually
increased from 1 to 6 months, and then declined. The trabecular
number in the subchondral trabecular bone showed a slight
increasing trend (Fig. 3C and
D). Trabecular SMI and Tb. Pf
exhibited a general decreasing trend with a slight increase during
the middle stage of growth (Fig. 3E
and F). With age, the subchondral
trabecular bone became less anisotropic. No significant changes
were observed for trabecular separation as the animals aged
(Fig. 3G and H). These results showed that spatial and
temporal ultrastructural changes occur in the subchondral
trabecular bone of both male and female guinea pigs during aging
(detailed comparison results are shown in Tables SI and II).
Identification of differentially
expressed proteins in tibial subchondral bone
To identify proteomic differences in the tibial
articular subchondral bone of both male and female guinea pigs
during the early growth stages, high-accuracy LC-MS/MS integrated
with iTRAQ analysis was used to determine which proteins were
differentially expressed between 1 and 3 months of age. A total of
12,313 unique peptides matching 2,316 non-redundant proteins were
identified in the tibial articular subchondral bone of guinea pigs
with high confidence (≥1 unique peptide with an FDR ≤1%). The list
of candidates was narrowed down to 138 proteins in male guinea pigs
and 113 proteins in female guinea pigs using a threshold of
>1.2-fold change and P<0.05. After characterizing the
expression patterns of these differentially expressed proteins, the
proteins were further grouped into two clusters: Upregulated and
downregulated expression. Amongst these, half of them were
upregulated (69/138) and the other half (69/138) downregulated in
the male animals, whereas 60 out of 113 proteins were upregulated
and 53 proteins (53/113) were downregulated in the female animals
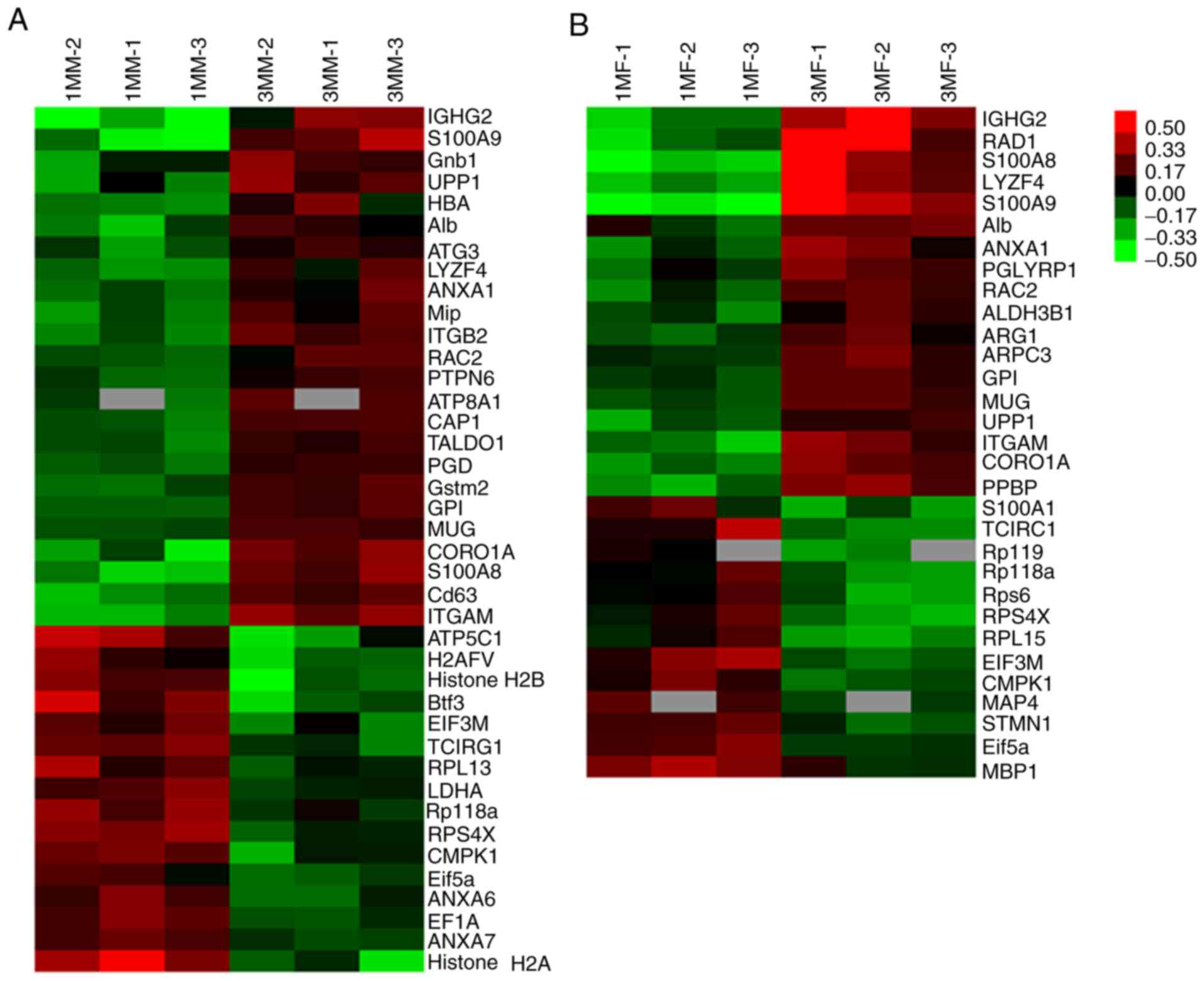
(Fig. S2). Two independent heat
maps were produced to show the accumulation patterns of these
differentially modulated identity-determined proteins found in the
tibial articular subchondral bone tissues from both male and female
guinea pigs between different age groups (Fig. 4).
Functional classification and Kyoto
encyclopedia of genes and genomes (KEGG) analysis of differentially
regulated proteins
To further understand these differentially expressed
proteins identified by the iTRAQ analysis, the proteomic results
were analyzed using Gene Ontology (GO), a universally recognized
and standardized classification system for gene function. However,
since only a small percentage of the guinea pig proteome has been
characterized in the Uniprot database, this classification places a
particular emphasis on proteins with definite identities. With this
caveat, 40 proteins (24 upregulated and 16 downregulated) were
identified in male guinea pigs and 31 proteins (18 upregulated and
13 downregulated) were identified in female animals. These proteins
were subsequently annotated as three key categories; namely,
biological processes, molecular function and cellular components
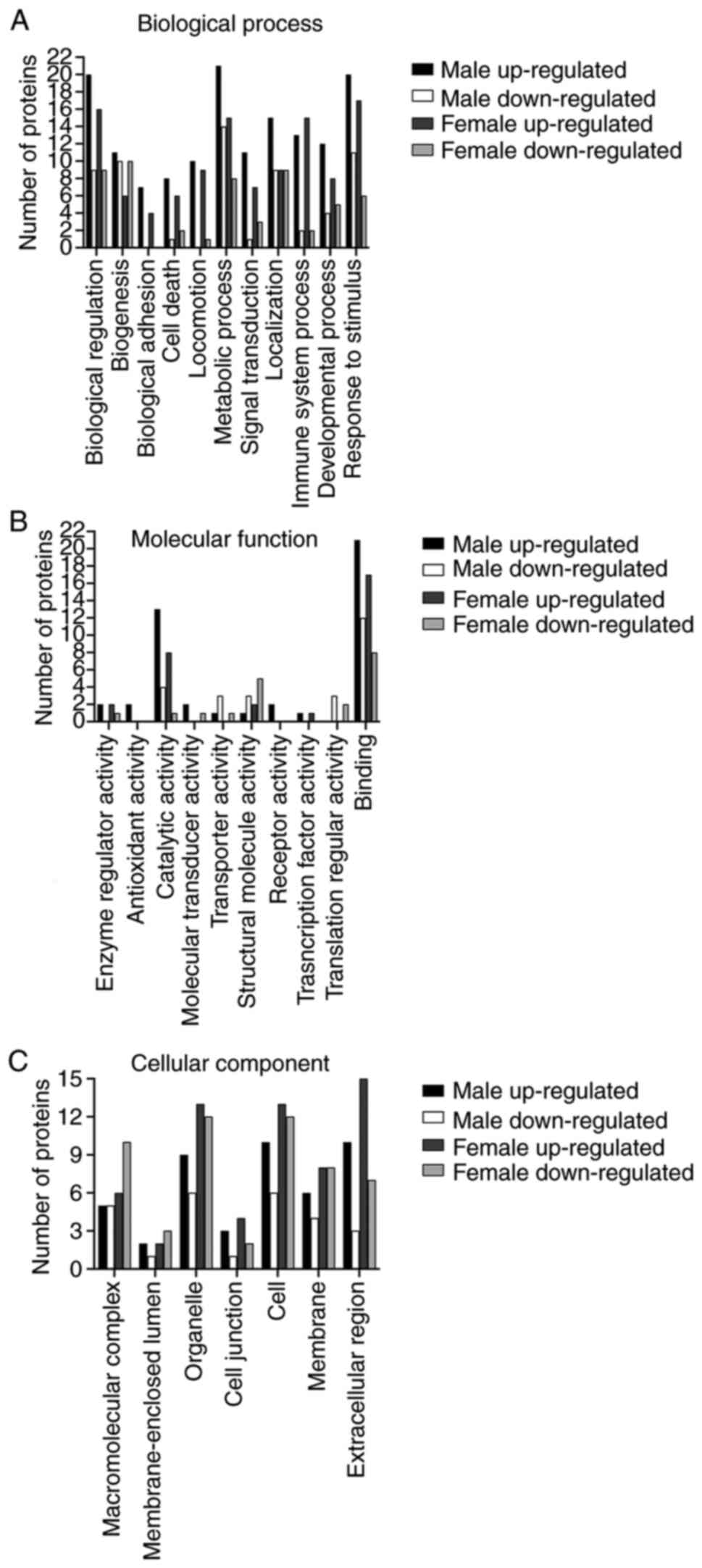
based on GO enrichment analysis. These proteins were mapped onto 11
biological functional categories and annotated with 10 molecular
functional terms (Fig. 5A and
B). These differentially expressed
proteins were also mapped onto 7 cellular components annotations:
‘Macromolecular complex’ (male, 10, 25.0%; female, 16, 51.6%),
‘membrane-enclosed lumen’ (male, 3, 7.5%; female, 5, 16.1%),
‘organelle’ (male, 15, 37.5%; female, 25, 80.6%), ‘cell junction’
(male, 4, 10.0%; female, 6, 19.4%), ‘cell’ (male, 16, 40.0%;
female, 25, 80.6%), ‘membrane’ (male, 10, 25.0%; female, 16,
51.6%), and ‘extracellular region’ (male, 13, 32.5%; female, 22,
71.0%) (Fig. 5C). The numbers of
upregulated and downregulated proteins for each category are shown
in Fig. 5.
To further investigate the functions and signaling
pathways in which these identified proteins were involved in,
KEGG-based pathway analysis was performed. Several important
biological pathways that may be potentially affected by the
differentially expressed proteins with definite identities are
summarized in Table I. These
results reveal that protein signature can be alerted in tibial
subchondral bone.
 | Table IKyoto Encyclopedia of Genes and
Genomes analysis of the differentially regulated proteins with
known identities in the tibial articular subchondral bone of guinea
pigs. |
Table I
Kyoto Encyclopedia of Genes and
Genomes analysis of the differentially regulated proteins with
known identities in the tibial articular subchondral bone of guinea
pigs.
| Pathway term | Associated
proteins |
|---|
| ‘Ribosome’ | H0W636, H0VST8,
H0W953, H0V1L6, H0VUY3, H0UY44 |
| ‘Phagosome’ | H0V1Y3, H0VVX0,
P11578, H0UW57 |
| ‘Carbon
metabolism’ | H0VLR7, H0UXA8,
H0VCM3, H0VZ24 |
| ‘Rap1 signaling
pathway’ | H0VVP9, H0VVX0,
P11578 |
| ‘Natural killer
cell mediated cytotoxicity’ | H0VVP9, H0VDD2,
H0VVX0 |
| ‘Glycolysis /
Gluconeogenesis’ | H0VCM3, H0VKB0 |
| ‘Ras signaling
pathway’ | A9LS48, H0VVP9 |
| ‘Chemokine
signaling pathway’ | A9LS48, H0WD04 |
| ‘PI3K-Akt signaling
pathway’ | A9LS48, H0V1L6 |
| ‘Lysosome’ | H0VDY4, H0UW57 |
| ‘Regulation of
autophagy’ | H0V7Z3 |
Verification of differentially
expressed proteins using western blotting
To validate the key proteomic changes identified by
the untargeted iTRAQ-based proteomic analysis, western blotting was
used to assess the expression of selected candidates. Specific
attention was given to those proteins involved in the processes of
bone formation and resorption, as these may serve a major role in
OA bone remodeling (18).
Expression of S100A8, CORO1A and TCIRG1, possibly affect the
process of bone remodeling (19-21)
(Fig. 6A). As shown in Fig. 6B, the western blotting analysis
results precisely mirrored the proteomic findings. During OA
progression, there was a continued increase in the expression of
CORO1A and S100A8 in the subchondral bone in both male and female
guinea pigs from 1 to 6 months. Following an initial decrease, the
abundance of TCIRG1 increased from months 3 to 6 in female guinea
pigs, whereas its expression levels remained relatively stable in
male guinea pigs. Together the data suggest that these proteins may
serve a role in subchondral bone homeostasis during spontaneous
OA.
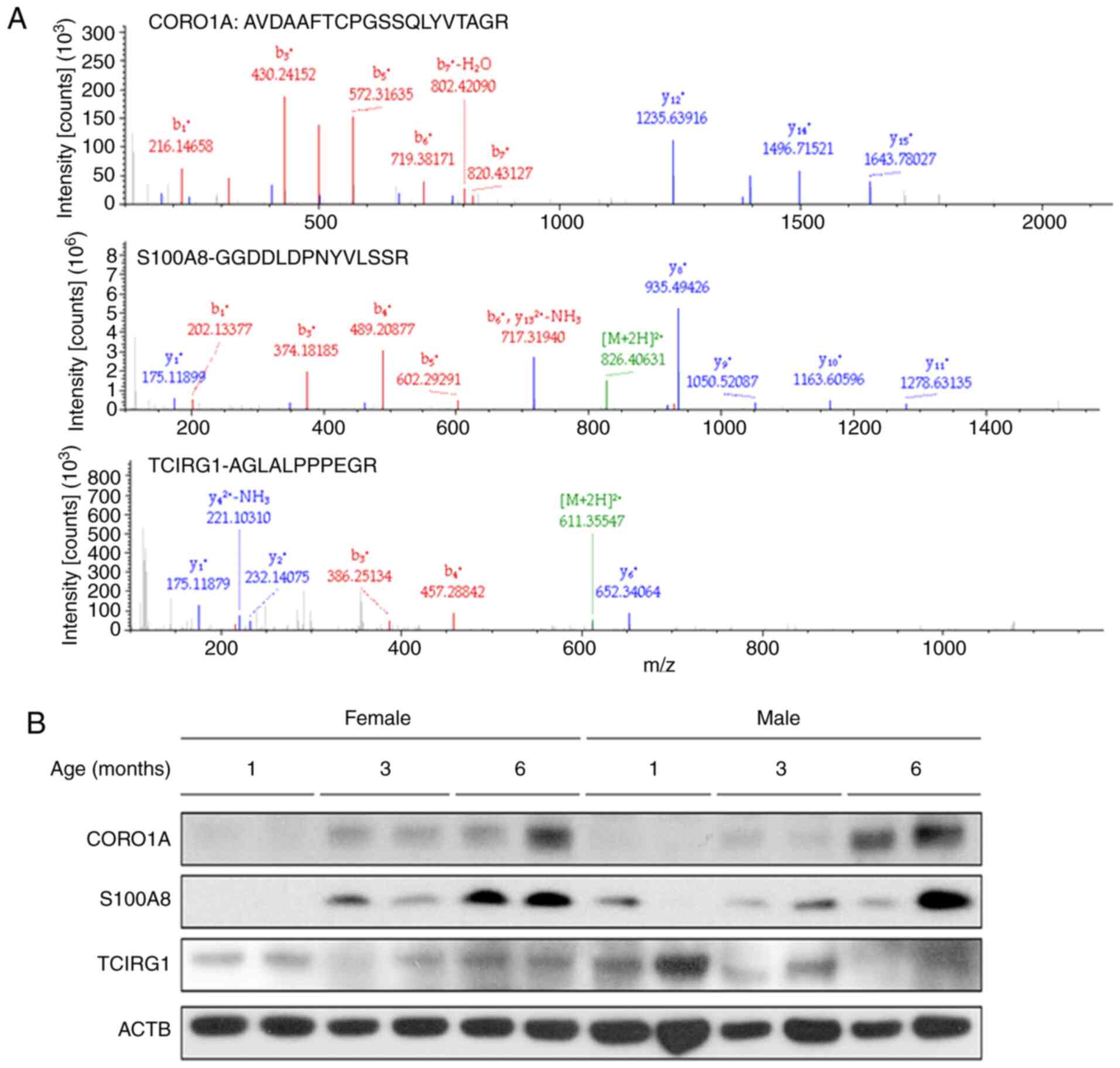 | Figure 6Validation of the three candidate
differentially expressed proteins, CORO1A, S100A8 and TCIRG1, by
western blotting. (A) Representative mass spectra of CORO1A, S100A8
and TCIRG1-derived peptides (AVDAAFTCPGSSQLYVTAGR, GGDDLDPNYVLSSR
and AGLALPPPEGR, respectively) isolated from subchondral bone
tissues from 3-month old guinea pigs. (B) Protein samples extracted
from the subchondral bone tissues of the indicated age groups were
subjected to western blotting. There was a continuous increase in
the expression of CORO1A and S100A8 in the subchondral bone in both
male and female guinea pigs from 1 to 6 months. Following an
initial decrease, the abundance of TCIRG1 increased from month 3 to
6 in the female guinea pigs, whereas its expression levels remained
relatively stable in the male guinea pigs. ACTB was used as the
loading control. ACTB, β-actin; S100A8, S100 calcium-binding
protein A8; CORO1A, coronin 1A; TCIRG1, T-cell immune regulator
1. |
Discussion
Involvement of the subchondral bone in the
pathogenesis of OA has been increasingly recognized by recent
advances in research. Alterations in the metabolic properties of
the subchondral bone during OA progression are more poignant than
secondary manifestations and may serve as the potential cause of
the disease (18,22-25).
In the present study, it was confirmed that both male and female
Hartley guinea pigs could be used as a suitable model to
investigate the etiology and pathogenesis of OA, consistent with
previous studies (26-28).
More importantly, to the best of our knowledge, the present study
was the first to document a differentially modulated proteome in
the tibial articular subchondral bone in male and female guinea
pigs using an unbiased proteomic approach followed by western
blotting for confirmation. The identification of critical proteins
using mass spectrometry-based strategies will serve a key role in
elucidating the underlying mechanism of OA, which will not only
facilitate further investigation of the pathological process of OA
development, but also help develop novel nonsurgical treatment
strategies for management of this disease.
In patients with OA, the degree of subchondral
trabecular remodeling is correlated with body weight (13). A similar positive correlation
between body weight and BMD of subchondral trabecular bone was
observed in our 11-month longitudinal study of cartilage
degeneration and bone remodeling in male and female guinea pigs.
Additionally in the present study, whilst cartilage degeneration
occurred in both male and female guinea pigs, male animals tended
to exhibit more severe pathological alterations, which may be
attributable to their faster growth rate, resulting in their
reaching greater body weights earlier than the female counterparts
(26).
Research findings from both clinical and animal
studies indicate that the pathological remodeling of the
subchondral bone occurs in the early stage of OA progression, prior
to cartilage degeneration (13,29-32).
Consistent with these findings, it was shown that elevated bone
turnover occurred as early as 2 months of age, prior to cartilage
degeneration in both male and female Hartley guinea pigs. In the
early growth stage, it is normally expected that the tibial
subchondral trabecular bone shows an age-related increase in BMD,
bone volume, trabecular thickness and trabecular number, indicating
continual bone remodeling. However, the subchondral trabecular bone
in the present study was shown to be more plate-like, as indicated
by the continuous decrease in SMI and Tb. Pf., unlike what is
observed during the normal aging process (33). Changes from rod-like to plate-like
morphology may increase the stiffness of the subchondral trabecular
bone (34), which is detrimental to
the overlying articular cartilage. Additionally, the subchondral
trabecular bone was found to be inferior in its ability to transfer
load from cartilage, based on the evidence of lower DA. Moreover,
the medial side of the subchondral trabecular bone had a lower SMI
and Tb. Pf compared with the lateral sides in both male and female
guinea pigs. Such differences between the medial and lateral
compartments of the tibial plateau indicate increased mechanical
strength on the medial side, which may explain why cartilage
degeneration initially occurred in the medial tibial plateau,
particularly in the region not covered by the meniscus, as observed
in both our current and previous study (26).
A decrease in Tb. N and an increase in Tb. Sp in
subchondral trabeculae have been reported in OA (35). However, an increasing trend in Tb. N
and no significant difference in Tb. Sp was observed based on the
micro-CT analysis in the present study. This discrepancy could be
due to factors such as individual variances between animals, or
differences in diet and housing conditions (36). Such variations can interfere with
disease development and lead to inconsistencies between OA animal
experiments (36). In the present
study, this issue was circumvented by employing a non-targeted
proteomic approach using iTRAQ-integrated LC-MS/MS technology.
Instead of investigating only one or a few specific proteins at a
time, it was possible to simultaneously identify multiple proteins
with this unbiased approach. Non-targeted methods not only directly
determine the expression levels of proteins but also provide
insights into the functional properties of a larger number of
potential candidates under both physiological and pathological
conditions. Whilst previous studies (15,16,37)
have revealed a large number of cytokines and proteases in the
articular cartilage, synovial fluid and serum in patients with OA
and in animal models, limited information is currently available
regarding the protein profile in the osteoarthritic subchondral
bone (38). Microarray technology
has revealed the gene-expression profile of the subchondral bone
obtained from the knee joint of an experimental rat OA model
(39), demonstrating the importance
and necessity of research on subchondral bone using high-throughput
protein analysis. In the present study, iTRAQ-based LC-MS/MS
technology was used to identify proteins involved in the
development of OA in the subchondral bone using a spontaneous
guinea pig OA model.
The present proteomic analysis showed that 6.6 and
8% of all proteins were significantly altered in the tibial
subchondral bone of female and male guinea pigs, respectively,
suggesting that further analysis of these differentially expressed
proteins may increase our understanding of the role these
subchondral bone microstructural changes may serve in OA. Owing to
the lack of information on the proteome of guinea pigs, it was only
possible to identify 40 proteins (24 upregulated and 16
downregulated) and 31 proteins (18 upregulated and 13
downregulated) with characterized identity, amongst these
significantly altered proteins in male and female guinea pigs,
respectively.
Bone remodeling is a physiological process governed
by an equilibrium between bone formation through osteoblast
activity and bone resorption through osteoclast activity (40). Disruption of this balance, which may
be caused by chronic inflammation (41), may lead to abnormal changes in bone
architecture and quality. OA subchondral bone impairment is
characterized by abnormal bone remodeling and altered
osteoblast-osteoclast coupling. Based on the present and previous
studies (13,29-31),
during the pathogenesis of OA, abnormal remodeling of the
subchondral bone occurs in the early stage prior to cartilage
degeneration, suggesting a vital role of subchondral bone
remodeling in OA onset and development. As a result, proteins that
are associated with either bone formation or bone resorption have
been the subjects of extensive research. From the analysis of
differentially expressed proteins identified in the proteomic
profiling in the present study, it was decided to focus on three
particular proteins, S100A8, CORO1A and TCIRG1, which exhibited a
high association with biological processes GO terms, including
‘bone formation’ and ‘bone resorption’. The expression of these
proteins was first verified using western blotting. S100A8, which
is associated with osteoblast differentiation (19) and osteoarthritic osteophyte
formation (42), was upregulated in
the osteoarthritic subchondral bone of both male and female guinea
pigs. CORO1A, a negative regulator of bone resorption activity
through inhibition of the release of cathepsin K, is an important
factor in bone resorption by degrading bone matrix proteins
(20). The abundance of CORO1A was
shown to continuously increase with age in the present study.
TCIRG1, a component of the osteoclast vacuolar proton pump, was
downregulated at 3 months of age in both male and female guinea
pigs, followed by a distinct expression pattern at 6 months of age
in guinea pigs of both sexes. Increasing evidence has shown that
mutational inactivation of TCIRG1 causes autosomal recessive
osteopetrosis, a disorder of bone metabolism caused by subnormal
osteoclast function (43-45).
In addition, osteoclast function can be restored via lentiviral
vector-mediated overexpression of TCIRG1(21). These results further add to the
increasing amount of evidence that these proteins may mediate the
abnormal formation of subchondral bone in the early growth stage.
In future studies, it will be both interesting and important to
elucidate their specific functions in initiation and progression of
OA.
In conclusion, the results of the present study
provide novel insights into the potential mechanisms underlying the
malformation of subchondral bone during OA development and
progression. Future studies using various cell lines, animal models
and human osteoarthritic specimens are required to further
elucidate the roles of these differentially expressed proteins in
the osteoarthritic subchondral bone identified in the present
study, as they may provide a mechanistic basis for development of
novel OA treatments.
Supplementary Material
Detailed body weight comparisons of
male and female animals from 1 to 11 months of age. There was a
significantly higher growth rate in male guinea pigs compare with
the female guinea pigs. n=6 per group.
Heat maps of identified proteins with
definite identities detected in the subchondral bone of (A) male
and (B) female Hartley guinea pigs. Amongst these, half of the
proteins were upregulated (69/138) and the other half (69/138) were
downregulated in the male animals, whereas 60 out of 113 proteins
were upregulated and 53 proteins were downregulated in the female
animals. 1MM, 1-month-old male guinea pig; 1MF, 1-month-old female
guinea pig; 3MM, 3-month-old male guinea pig; 3MF, 3-month-old
female guinea pig.
Medial or lateral microarchitecture of
the subchondral trabecular bone alterations between ages.
Raw values of microarchitecture
factors of the subchondral trabecular bone.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81330043 and
81071499).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to compliance with the
Inner Confidentiality Policy of Scientific Data by Beijing Research
Institute of Traumatology and Orthopedics but are available from
the corresponding author on reasonable request.
Authors' contributions
CW, XJ and WT designed the experiments. YW performed
the animal experiments. YW, XJ and CW analyzed the data. DZ
facilitated animal breeding, acquired images and interpreted the
data. JT performed image analysis. YW and CW wrote the manuscript.
CW, YW and XJ confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Beijing
Jishuitan Hospital Animal Care and Use Committee, Beijing,
China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hunter DJ and Bierma-Zeinstra S:
Osteoarthritis. Lancet. 393:1745–1759. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mobasheri A: The future of osteoarthritis
therapeutics: Targeted pharmacological therapy. Curr Rheumatol Rep.
15(364)2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Roman-Blas JA, Bizzi E, Largo R, Migliore
A and Herrero-Beaumont G: An update on the up and coming therapies
to treat osteoarthritis, a multifaceted disease. Expert Opin
Pharmacother. 17:1745–1756. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Parrish WR, Byers BA, Su D, Geesin J,
Herzberg U, Wadsworth S, Bendele A and Story B: Intra-articular
therapy with recombinant human GDF5 arrests disease progression and
stimulates cartilage repair in the rat medial meniscus transection
(MMT) model of osteoarthritis. Osteoarthritis Cartilage.
25:554–560. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wei Y and Bai L: Recent advances in the
understanding of molecular mechanisms of cartilage degeneration,
synovitis and subchondral bone changes in osteoarthritis. Connect
Tissue Res. 57:245–261. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Neogi T: Clinical significance of bone
changes in osteoarthritis. Ther Adv Musculoskelet Dis. 4:259–267.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Martel-Pelletier J and Pelletier JP: Is
osteoarthritis a disease involving only cartilage or other
articular tissues? Eklem Hastalik Cerrahisi. 21:2–14.
2010.PubMed/NCBI
|
|
8
|
Muratovic D, Cicuttini F, Wluka A, Findlay
D, Wang Y, Otto S, Taylor D, Humphries J, Lee Y, Labrinidis A, et
al: Bone marrow lesions detected by specific combination of MRI
sequences are associated with severity of osteochondral
degeneration. Arthritis Res Ther. 18(54)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Audrey HX, Abd Razak HR and Andrew TH: The
truth behind subchondral cysts in osteoarthritis of the knee. Open
Orthop J. 8:7–10. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Day JS, Ding M, van der Linden JC, Hvid I,
Sumner DR and Weinans H: A decreased subchondral trabecular bone
tissue elastic modulus is associated with pre-arthritic cartilage
damage. J Orthop Res. 19:914–918. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yu D, Xu J, Liu F, Wang X, Mao Y and Zhu
Z: Subchondral bone changes and the impacts on joint pain and
articular cartilage degeneration in osteoarthritis. Clin Exp
Rheumatol. 34:929–934. 2016.PubMed/NCBI
|
|
12
|
Hunter DJ and Little CB: The great debate:
Should osteoarthritis research focus on ‘mice’ or ‘men’?
Osteoarthritis Cartilage. 24:4–8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Reina N, Cavaignac E, Pailhé R, Pailliser
A, Bonnevialle N, Swider P and Laffosse JM: BMI-related
microstructural changes in the tibial subchondral trabecular bone
of patients with knee osteoarthritis. J Orthop Res. 35:1653–1660.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
van der Kraan P, Matta C and Mobasheri A:
Age-related alterations in signaling pathways in articular
chondrocytes: Implications for the pathogenesis and progression of
osteoarthritis - a mini-review. Gerontology. 63:29–35.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lourido L, Calamia V, Mateos J,
Fernández-Puente P, Fernández-Tajes J, Blanco FJ and Ruiz-Romero C:
Quantitative proteomic profiling of human articular cartilage
degradation in osteoarthritis. J Proteome Res. 13:6096–6106.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tsolis KC, Bei ES, Papathanasiou I,
Kostopoulou F, Gkretsi V, Kalantzaki K, Malizos K, Zervakis M,
Tsezou A and Economou A: Comparative proteomic analysis of
hypertrophic chondrocytes in osteoarthritis. Clin Proteomics.
12(12)2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wisniewski JR, Zougman A, Nagaraj N and
Mann M: Universal sample preparation method for proteome analysis.
Nat Methods. 6:359–362. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhu X, Chan YT, Yung PSH, Tuan RS and
Jiang Y: Subchondral bone remodeling: A therapeutic target for
osteoarthritis. Front Cell Dev Biol. 8(607764)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zreiqat H, Howlett CR, Gronthos S, Hume D
and Geczy CL: S100A8/S100A9 and their association with cartilage
and bone. J Mol Histol. 38:381–391. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ohmae S, Noma N, Toyomoto M, Shinohara M,
Takeiri M, Fuji H, Takemoto K, Iwaisako K, Fujita T, Takeda N, et
al: Actin-binding protein coronin 1A controls osteoclastic bone
resorption by regulating lysosomal secretion of cathepsin K. Sci
Rep. 7(41710)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Moscatelli I, Thudium CS, Flores C, Schulz
A, Askmyr M, Gudmann NS, Andersen NM, Porras O, Karsdal MA, Villa
A, et al: Lentiviral gene transfer of TCIRG1 into peripheral blood
CD34(+) cells restores osteoclast function in infantile malignant
osteopetrosis. Bone. 57:1–9. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen Y, Hu Y, Yu YE, Zhang X, Watts T,
Zhou B, Wang J, Wang T, Zhao W, Chiu KY, et al: Subchondral
trabecular rod loss and plate thickening in the development of
osteoarthritis. J Bone Miner Res. 33:316–327. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ma L, Zhao X, Liu Y, Wu J, Yang X and Jin
Q: Dihydroartemisinin attenuates osteoarthritis by inhibiting
abnormal bone remodeling and angiogenesis in subchondral bone. Int
J Mol Med. 47(1)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cui Z, Crane J, Xie H, Jin X, Zhen G, Li
C, Xie L, Wang L, Bian Q, Qiu T, et al: Halofuginone attenuates
osteoarthritis by inhibition of TGF-β activity and H-type vessel
formation in subchondral bone. Ann Rheum Dis. 75:1714–1721.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Finnilä MAJ, Thevenot J, Aho OM, Tiitu V,
Rautiainen J, Kauppinen S, Nieminen MT, Pritzker K, Valkealahti M,
Lehenkari P and Saarakkala S: Association between subchondral bone
structure and osteoarthritis histopathological grade. J Orthop Res.
35:785–792. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bendele AM: Animal models of
osteoarthritis. J Musculoskelet Neuronal Interact. 1:363–376.
2001.PubMed/NCBI
|
|
27
|
Lampropoulou-Adamidou K, Lelovas P,
Karadimas EV, Liakou C, Triantafillopoulos IK, Dontas I and
Papaioannou NA: Useful animal models for the research of
osteoarthritis. Eur J Orthop Surg Traumatol. 24:263–271.
2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kuyinu EL, Narayanan G, Nair LS and
Laurencin CT: Animal models of osteoarthritis: Classification,
update, and measurement of outcomes. J Orthop Surg Res.
11(19)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang T, Wen CY, Yan CH, Lu WW and Chiu KY:
Spatial and temporal changes of subchondral bone proceed to
microscopic articular cartilage degeneration in guinea pigs with
spontaneous osteoarthritis. Osteoarthritis Cartilage. 21:574–581.
2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Muraoka T, Hagino H, Okano T, Enokida M
and Teshima R: Role of subchondral bone in osteoarthritis
development: A comparative study of two strains of guinea pigs with
and without spontaneously occurring osteoarthritis. Arthritis
Rheum. 56:3366–3374. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Uasnichka HL, Anderson-MacKenzie JM and
Bailey AJ: Subchondral bone and ligament changes precede cartilage
degradation in guinea pig osteoarthritis. Biorheology. 43:389–397.
2006.PubMed/NCBI
|
|
32
|
Radin EL and Rose RM: Role of subchondral
bone in the initiation and progression of cartilage damage. Clin
Orthop Relat Res: 34-40, 1986.
|
|
33
|
Ding M and Hvid I: Quantification of
age-related changes in the structure model type and trabecular
thickness of human tibial cancellous bone. Bone. 26:291–295.
2000.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ding M, Odgaard A, Danielsen CC and Hvid
I: Mutual associations among microstructural, physical and
mechanical properties of human cancellous bone. J Bone Joint Surg
Br. 84:900–907. 2002.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhao W, Wang T, Luo Q, Chen Y, Leung VY,
Wen C, Shah MF, Pan H, Chiu KY, Cao X and Lu WW: Cartilage
degeneration and excessive subchondral bone formation in
spontaneous osteoarthritis involves altered TGF-β signaling. J
Orthop Res. 34:763–770. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
van der Kraan PM: Factors that influence
outcome in experimental osteoarthritis. Osteoarthritis Cartilage.
25:369–375. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu W, He J, Lin R, Liang J and Luo Q:
Differential proteomics of the synovial membrane between bilateral
and unilateral knee osteoarthritis in surgery-induced rabbit
models. Mol Med Rep. 14:2243–2249. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fellows CR, Matta C and Mobasheri A:
Applying proteomics to study crosstalk at the cartilage-subchondral
bone interface in osteoarthritis: Current status and future
directions. EBioMedicine. 11:2–4. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhang R, Fang H, Chen Y, Shen J, Lu H,
Zeng C, Ren J, Zeng H, Li Z, Chen S, et al: Gene expression
analyses of subchondral bone in early experimental osteoarthritis
by microarray. PLoS One. 7(e32356)2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Andersen TL, Sondergaard TE, Skorzynska
KE, Dagnaes-Hansen F, Plesner TL, Hauge EM, Plesner T and Delaisse
JM: A physical mechanism for coupling bone resorption and formation
in adult human bone. Am J Pathol. 174:239–247. 2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Shaw AT and Gravallese EM: Mediators of
inflammation and bone remodeling in rheumatic disease. Semin Cell
Dev Biol. 49:2–10. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Schelbergen RF, de Munter W, van den Bosch
MH, Lafeber FP, Sloetjes A, Vogl T, Roth J, van den Berg WB, van
der Kraan PM, Blom AB and van Lent PL: Alarmins S100A8/S100A9
aggravate osteophyte formation in experimental osteoarthritis and
predict osteophyte progression in early human symptomatic
osteoarthritis. Ann Rheum Dis. 75:218–225. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Anderson SL, Jalas C, Fedick A, Reid KF,
Carpenter TO, Chirnomas D, Treff NR, Ekstein J and Rubin BY: A
founder mutation in the TCIRG1 gene causes osteopetrosis in the
Ashkenazi Jewish population. Clin Genet. 88:74–79. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Gheorghe G, Galambos C, Jain S,
Krishnamurti L and Jaffe R: A novel TCIRG1 gene mutation leads to
severe osteopetrosis with altered content of monocytes/macrophages
in several organs. Pediatr Dev Pathol. 15:156–159. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Frattini A, Orchard PJ, Sobacchi C,
Giliani S, Abinun M, Mattsson JP, Keeling DJ, Andersson AK,
Wallbrandt P, Zecca L, et al: Defects in TCIRG1 subunit of the
vacuolar proton pump are responsible for a subset of human
autosomal recessive osteopetrosis. Nat Genet. 25:343–346.
2000.PubMed/NCBI View
Article : Google Scholar
|