Introduction
Adipose tissue is an important endocrine organ that
regulates energy metabolism and nutrition homeostasis (1). There are at least two distinct types
of adipose tissue in mammals: White adipose tissue (WAT) and brown
adipose tissue (BAT) (2). The main
function of WAT is to store energy in the form of triglycerides,
and WAT plays an important role in regulating systemic metabolism
and insulin sensitivity (3). In
contrast, BAT burns energy through non-shivering thermogenesis and
visually appears brown because it contains a high number of
mitochondria (4). In particular,
uncoupling protein-1 (UCP-1) is expressed specifically in BAT and
controlled by the concerted action of neural and hormonal signals
to activate the program responsible for fuel oxidation and
thermogenesis (5). It is worth
mentioning that high-resolution imaging technology suggested that
BAT not only exists in rodents but also assumes certain biological
functions in adult bodies (6,7). This
new discovery provides scientists with insight into the role of BAT
in regulating energy consumption, restoring glucose homeostasis and
decreasing obesity. Therefore, the molecular regulatory mechanism
of the differentiation of brown adipocytes has been a research
hotspot.
As a member of secreted frizzled-related proteins
(SFRPs), SFRP4 has a cysteine-rich region at the C-terminal and a
netrin-like domain motif at the N-terminal, which is highly
conserved and binds to frizzled or Wnt membrane proteins (8). As a secreted cytokine, SFRP4 plays an
important role in obesity (9),
insulin resistance (10) and type 2
diabetes (11). In addition,
bioinformatics data analysis showed that the expression level of
SFRP4 in fat tissue of obese pigs was significantly higher compared
with that of lean pigs (12).
Thirdly, previously published research findings demonstrated that
the expression pattern and function of SFRP4 has adipose tissue
depot specificity in subcutaneous and visceral adipose tissue in
mice (13). However, little is
known about the role and function of SFRP4 in the formation and
thermogenesis of BAT which plays an important role in lipid
metabolism, obesity and energy consumption (4).
Considering the involvement of SFRP4 in obesity,
insulin resistance and type 2 diabetes, the present study aimed to
determine the role of SFRP4 in brown adipocyte differentiation.
Hence, the effects of SFRP4 on cell morphology, lipid droplet
accumulation, and adipogenesis-specific protein expression in brown
adipocytes were examined. The present study demonstrated that SFRP4
expression is associated with inflammatory markers, and its release
from islets is stimulated by interleukin-1β (IL-1β).
Material and methods
Brown preadipocyte isolation and
culture
Brown preadipocytes were obtained from the
interscapular BAT of mice (age, 3 weeks old; C57BL/6J mice) and
isolated by digestion with collagenase dispersion as described
previously (14). In the present
study, the cell pool from different animals (20 mice each time)
were used and the experiment was repeated three times. In detail,
mice were purchased from Beijing Vital River Laboratory Animal
Technology (Beijing Vital River Laboratory Animal Technology Co.,
Ltd.) and monitored the health of animals every day before
execution. All animals housed in an air-conditioned room under a
12-h light and 12-h dark cycle. Food and water were allowed ad
libitum. Five mice in a cage were euthanized by injecting
carbon dioxide into the cage at a gas exchange rate of 28%
container volume/min. C57BL/6 mice aged 3 weeks lost consciousness
within 4-5 min, and died within 8-10 min, which was consistent with
the requirements of the National Institutes of Health (NIH)
guidelines (15). The
characteristics of death included no spontaneous breathing for 2-3
min and absence of blink reflex. The cell culture experiment was
continued for six months. BAT was collected from the scapular
region of mice under sterile conditions and washed in
phosphate-buffered saline three times.
BAT was collected from the scapular region of mice
under sterile conditions and washed in phosphate-buffered saline
three times. The tissue mass was cut with scissors into
~1-mm3 sections and digested with 1 mg/ml type I
collagenase at 37˚C for 40 min in a shaking water bath. Then, the
digestion of the cell suspension was stopped by Dulbecco's modified
Eagle medium/nutrient mixture F-12 (DMEM/F12) medium supplemented
with 10% foetal bovine serum (FBS). Isolated cells
(1x106/ml) were seeded in 6-well plates with DMEM/F12
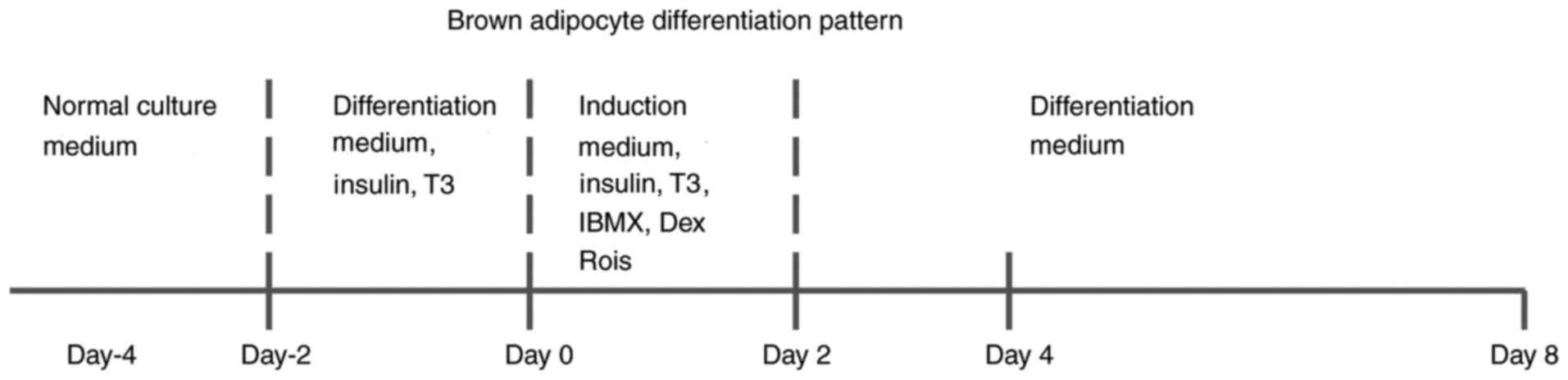
medium supplemented with 10% FBS and 1% Pen/Strep (day 4). The cell
proliferation state is very important for murine adipocyte
differentiation. The cells were seeded in the dish at day-4, and
the cell density was ~70%. At day-2, the percentage of cell
confluence was 80-90%, and the cells reached contact inhibition at
day 0.
For BAT differentiation in vitro, the cells
were treated with differentiation medium containing 1.7 µM insulin
and 1 nM triiodothyronine (T3) from day-2 to 0 and induced with
induction medium supplemented with 1.7 µM insulin, 1 nM T3, 0.5 mM
isobutylmethylxanthine and 1 µM dexamethasone from day 0 to 2.
Differentiating cells were kept in differentiation medium from day
2 to 8(16). The differentiation
pattern of brown adipocytes is shown in Fig. 1. The animal experiments were
strictly following the guidelines of animal experiment in Xi'an
Medical University, which was adapted from the Guide for the Care
and Use of Laboratory Animals (NIH) (15). The Laboratory Animal Administration
Committee of Xi'an Medical University approved all animal
experiments (Institutional Animal Care and Use Committee; approval
no. XYJZS-201806025-8).
Oil Red O staining
Dishes were washed twice with phosphate-buffered
saline and fixed with 4% paraformaldehyde for 30 min at room
temperature. Then, the cells were stained with Oil Red O working
solution at room temperature for 30 min, washed twice with
distilled water, observed and photographed under an inverted
optical microscope (magnification, x200; Nikon Eclipse TE300; Nikon
Corporation). The Oil Red O area was quantified by the image
analysis system (WinRoof Mitani Co.).
Reverse transcription-quantitative
(RT-q)PCR analysis
Total RNA from adipocytes was extracted using TRIzol
plus (Invitrogen; Thermo Fisher Scientific, Inc.), and cDNA was
synthesized with PrimeScript™ RT Master mix using the
following temperature protocol: 37˚C for 15 min, 85˚C for 5 sec and
4˚C for 20 min (Takara Biology Inc.). qPCR analysis was performed
using the StepOne Plus Real-time PCR system (Thermo Fisher
Scientific, Inc.). The number of transcripts was quantified, and
each sample was normalized according to its b-actin content.
Relative expression of the gene of interest (GOI) was determined
using calculation=2(Cq β-actin gene-Cq GOI)
(13). PCR primer sequences are
shown in Table SI.
Western blotting
Brown adipocytes were lysed in cell lysis buffer
(HEPES 50 mM; NaCl 150 mM; Triton X-100 1%;
Na3VO4 1 mM; NaF 30 mM;
Na4P2O7 10 mM; EDTA 10 mM;
aprotinin 1 µg/ml; antipain 1 µg/ml; pepstatin 1 µg/ml; leupeptin 1
µg/ml; benzamidine 2.5 mM; AEBSF 0.5 mM; pH=7.4) at 4˚C, and the
resultant supernatants were subjected to western blotting. As
adipocytes contain high lipid content, an ultrasonic crusher was
used to break up the cells and the protein concentration was
quantified using a BCA test kit, according to the manufacturer's
procedure. Briefly, protein samples (20 µg/lane) were separated on
10% SDS-polyacrylamide gels and then transferred to Sequi-blotting
polyvinylidene fluoride membranes (Bio-Rad Laboratories, Inc.). The
blot membranes were incubated with blocking buffer [5% non-fat milk
powder dissolved in TBST (0.1% Tween-20) buffer solution] for 2 h
at room temperature and then with the following primary antibodies
for 2 h at room temperature (Ab): Anti-adiponectin (cat. no.
AF1119; R&D Systems, Inc.; 1:1,000), anti-leptin (cat. no.
AF498; R&D; 1:1,000), anti-fatty acid-binding protein (FABP4)
(cat. no. AF1443; R&D; 1:1,000), anti-UCP-1 (cat. no. MAB6158;
R&D; 1:1,000), or anti-β-tubulin (cat. no. ab15246; Abcam;
1:2,000). The membranes were washed and incubated with horseradish
peroxidase-conjugated secondary Ab for 2 h at room temperature.
Then, Immobilon reagent (EMD Millipore) was used for visualization
with an LAS-400 Lumino image analyser (Fujifilm Wako Pure Chemical
Corporation). Blots were quantitatively analysed by Quantity One
4.5.2 (Bio-Rad Laboratories, Inc.).
SFRP4 and IL-1β treatment
Brown preadipocytes were treated with recombinant
mouse SFRP4 (cat. no. 50053-M08H; 1, 10 or 100 ng/ml) or IL-1β
(cat. no. 50101-MNAE; 25 ng/ml) active protein (Sino Biological
Company) at the dedicated time points as described previously
(17).
Statistical analysis
All data are expressed as the mean ± SEM.
Statistical analyses were performed using either Student's t-test
when the F value was equal or Welch's t-test when the
F value was not equal. Four groups of data were analysed by
one-way ANOVA followed by Tukey's multiple-comparison procedure.
For unequal variances, data were evaluated by the Kruskal-Wallis
test. In order to avoid the false positive error in the data
analysis, Bonferroni correction was performed as the post-hoc tests
following Kruskal-Wallis tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression pattern of SFRP4 in brown
adipocyte differentiation
Primary cultured brown preadipocytes were isolated
from the scapular region of mice, and the addition of
differentiation medium promoted the expression of gene cascade and
induced differentiation into mature brown adipocytes. The isolated
cells exhibited increased numbers of Oil red O stained lipid
vesicles with culturing, and most preadipocytes were filled with
numerous lipids after 8 days of differentiation; this suggests that
in the process of culturing, brown adipocytes mature from
precursors (data not shown). Next, RT-PCR was performed to
investigate the brown adipocyte-specific gene expression pattern.
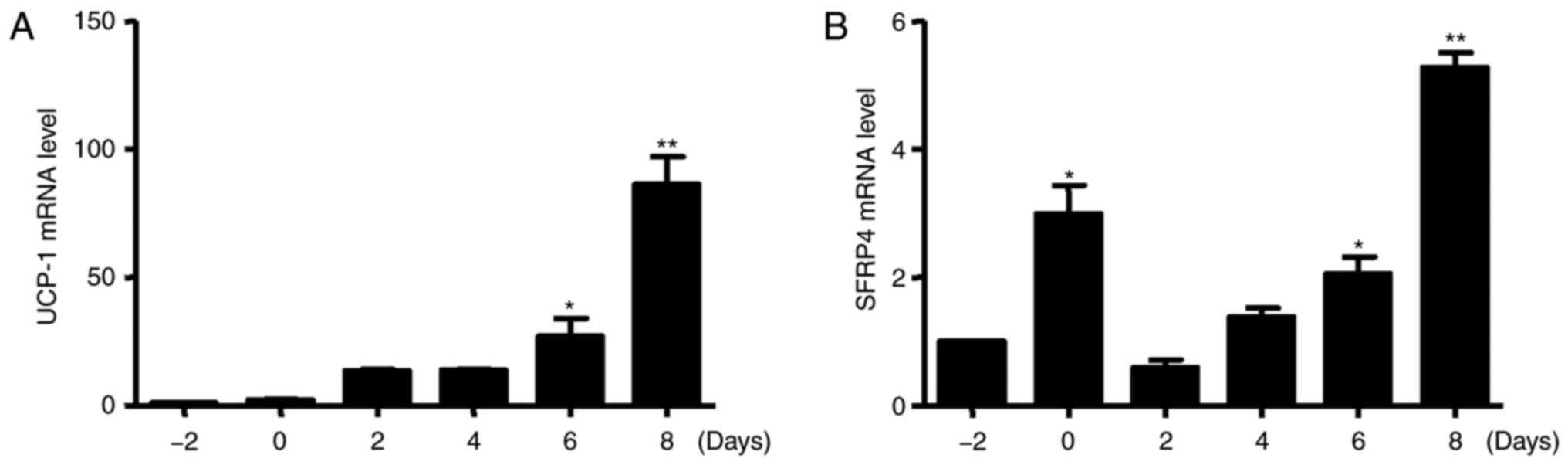
As expected, the expression of UCP-1 was markedly increased during
brown adipogenesis (Fig. 2A). At
the same time, the level of SFRP4 mRNA was significantly increased
after incubation with inducers from day-2 to day 0, but decreased
at day 2. The level of SFRP4 mRNA was significantly increased after
incubation with inducers from day 6 to 8 (Fig. 2B).
SFRP4 promotes primary brown
preadipocyte differentiation
To explore whether SFRP4 is involved in the
management of brown adipogenic differentiation, the effect of SFRP4
during brown adipogenesis was detected. Brown preadipocytes were
isolated from murine BAT and SFRP4 was overexpressed using three
different concentrations of recombinant active protein (1, 10 and
100 ng/ml). Interestingly, primary brown adipocytes formed more
lipid droplets, and the differentiation rate was up to ~90% with
the treatment of high-dose SFRP4 recombinant protein, as the
differentiation rate of the control group was just ~70% (Fig. 3A and B). Adipogenic markers of brown adipocytes
were assessed by RT-qPCR and western blotting (Figs. 3 and 4). Solute carrier family 2 (facilitated
glucose transporter), member 4 (GLUT4), cell death-inducing
dffa-like effector a (Cidea), CCAAT/enhancer binding protein α
(C/EBPα), C/EBPβ, UCP-1, peroxisome proliferator-activated receptor
γ (PPARγ), coactivator 1α (PGC-1α) and PR domain containing 16
(PRDM16) mRNAs were significantly increased. For a more detailed
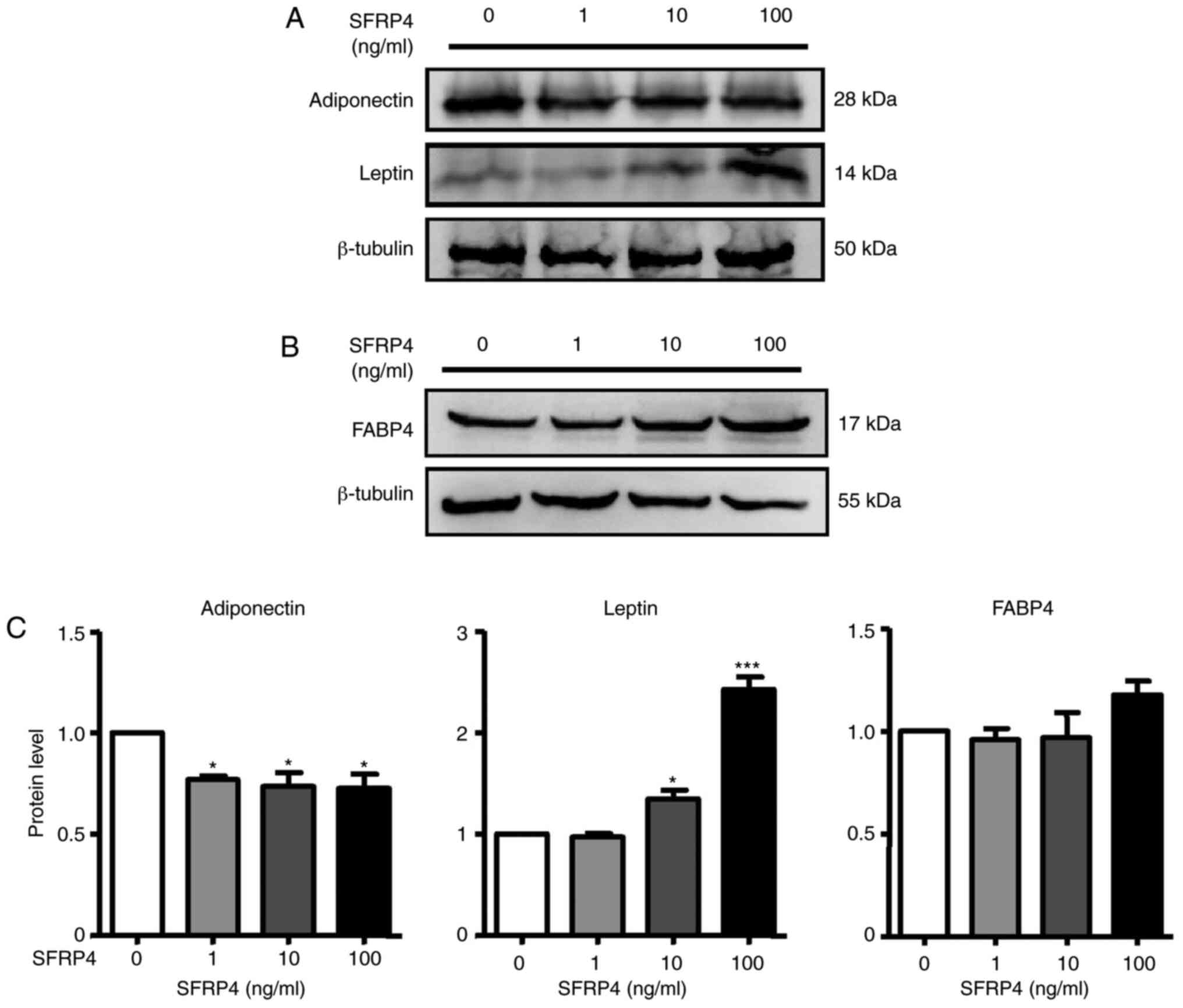
examination of SFRP4, western blotting we also performed to analyse
protein expression. The results revealed that SFRP4 significantly
inhibited adiponectin protein expression in a dose-independent
manner but increased leptin secretion at concentrations of 10 and
100 ng/ml (Fig. 4A and C). These results clearly implied that
SFRP4 induces brown preadipocyte differentiation into mature
adipocytes.
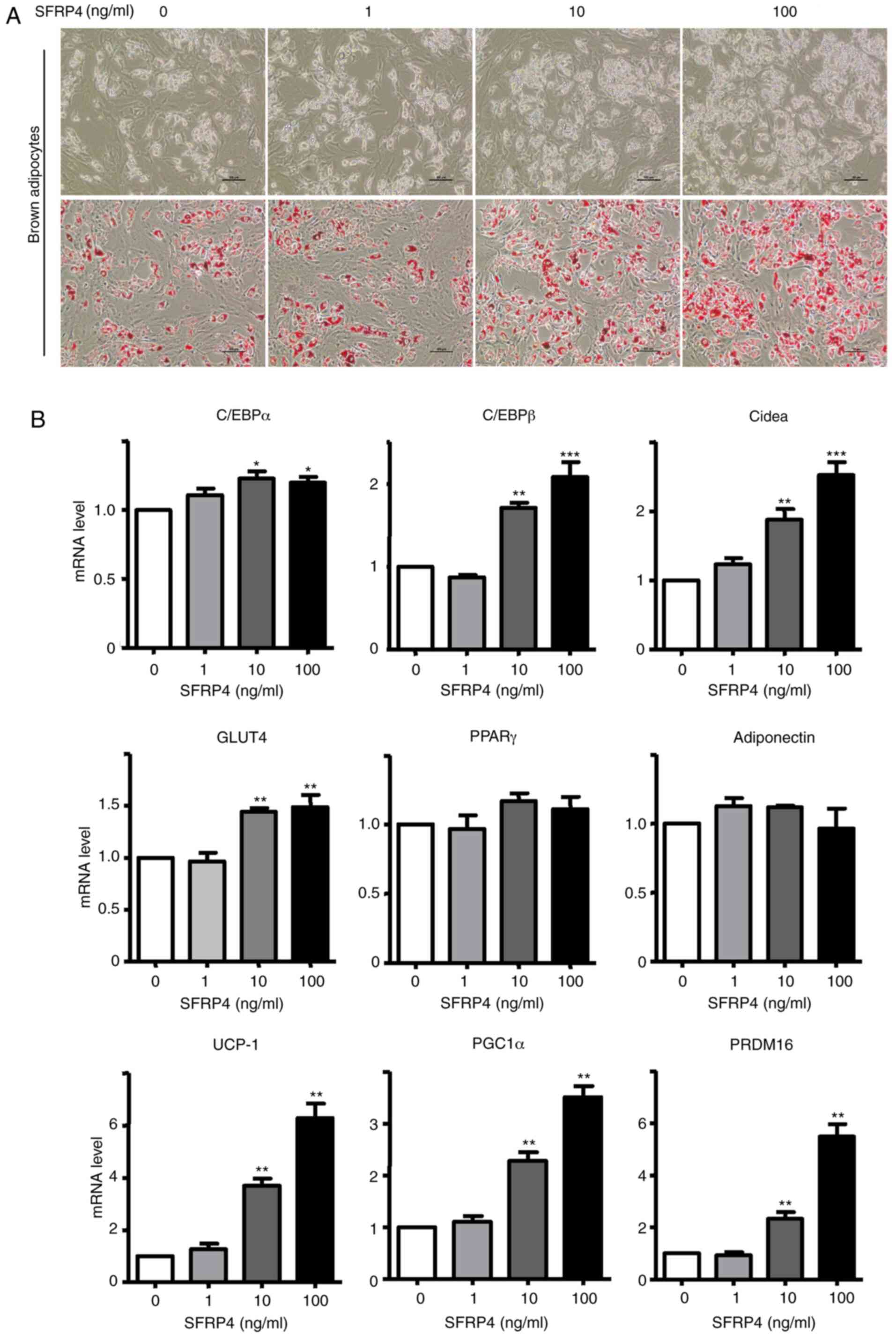 | Figure 3SFRP4 promotes brown preadipocyte
differentiation in a dose-dependent manner. Brown preadipocytes
incubated with SFRP4 during maintenance and differentiation in
different concentration gradients of 1, 10 and 100 ng/ml. (A)
Photomicrographs show Oil Red O staining of cells on day 8 of
differentiation. Magnification, x200. (B) mRNA expression of the
brown adipocyte adipogenic markers C/EBPα, C/EBPβ, GLUT4 and Cidea,
UCP-1, PGC-1α and PRDM16 were assessed by reverse
transcription-quantitative PCR. At a concentration of 100 ng/ml
SFRP4, the levels of C/EBPα, C/EBPβ, GLUT4 and Cidea were all
1.5-fold higher compared with those in the absence of SFRP4. Data
are expressed as the mean ± SEM; n=4 for each group.
*P<0.05; **P<0.01;
***P<0.001 vs. control. SFRP4, secreted frizzled
related protein 4; UCP-1, uncoupling protein 1; C/EBPα/β,
CCAAT/enhancer-binding protein α/β; GLUT4, solute carrier family 2
(facilitated glucose transporter), member 4; Cidea, cell
death-inducing dffa-like effector a; PGC-1α, peroxisome
proliferator-activated receptor γ, coactivator 1α; PRDM16, PR
domain containing 16. |
IL-1β has no effect on SFRP4
expression enhancement in brown adipocytes
A previous study demonstrated that SFRP4 expression
correlated with inflammatory markers and was stimulated by IL-1β in
islets (11). To further establish
the regulation of IL-1β in the context of SFRP4, the effect of
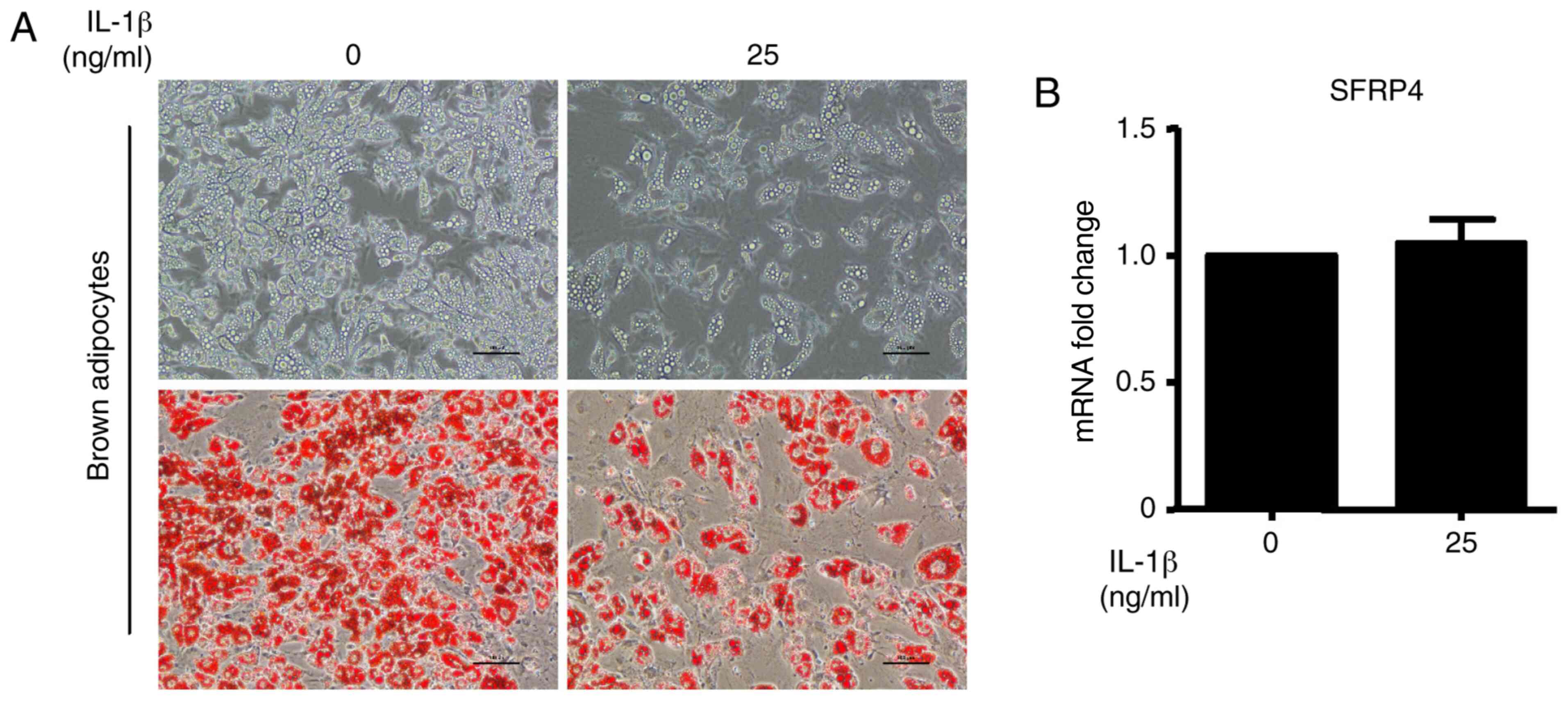
SFRP4 on the sensitivity to IL-1β was evaluated. The results show
that the brown adipocyte differentiation efficiency was
significantly decreased after incubation with IL-1β at a
concentration of 25 ng/ml. The upper images of Fig. 5A show the cell morphology by
inverted phase contrast microscopy. To evaluate the accumulation of
lipid droplets during brown adipocyte differentiation, Oil Red O
staining was performed (Fig. 5A).
As expected, lipid accumulation was decreased by treatment with
IL-1β (Fig. 5A). However, no
changes were observed in SFRP4 mRNA expression (Fig. 5B). Quantification of this effect
revealed a significant fact that IL-1β cannot influence SFRP4
expression in brown adipocytes.
C/EBPα, C/EBPβ and PPARγ mRNA expression levels were
not changed after treatment, while IL-1β, Cidea and UCP-1 mRNA
expression were significantly decreased after treatment compared
with the control condition (Fig.
6A). After treatment with IL-1β, UCP-1 protein expression was
consistent with the change in mRNA expression. However, adiponectin
protein expression was significantly increased compared with the
control group in brown adipocytes (Fig.
6B). These data demonstrated that although the inflammatory
factor IL-1β has antiadipogenic function, it did not promote SFRP4
expression in adipocytes as it did in islet cells.
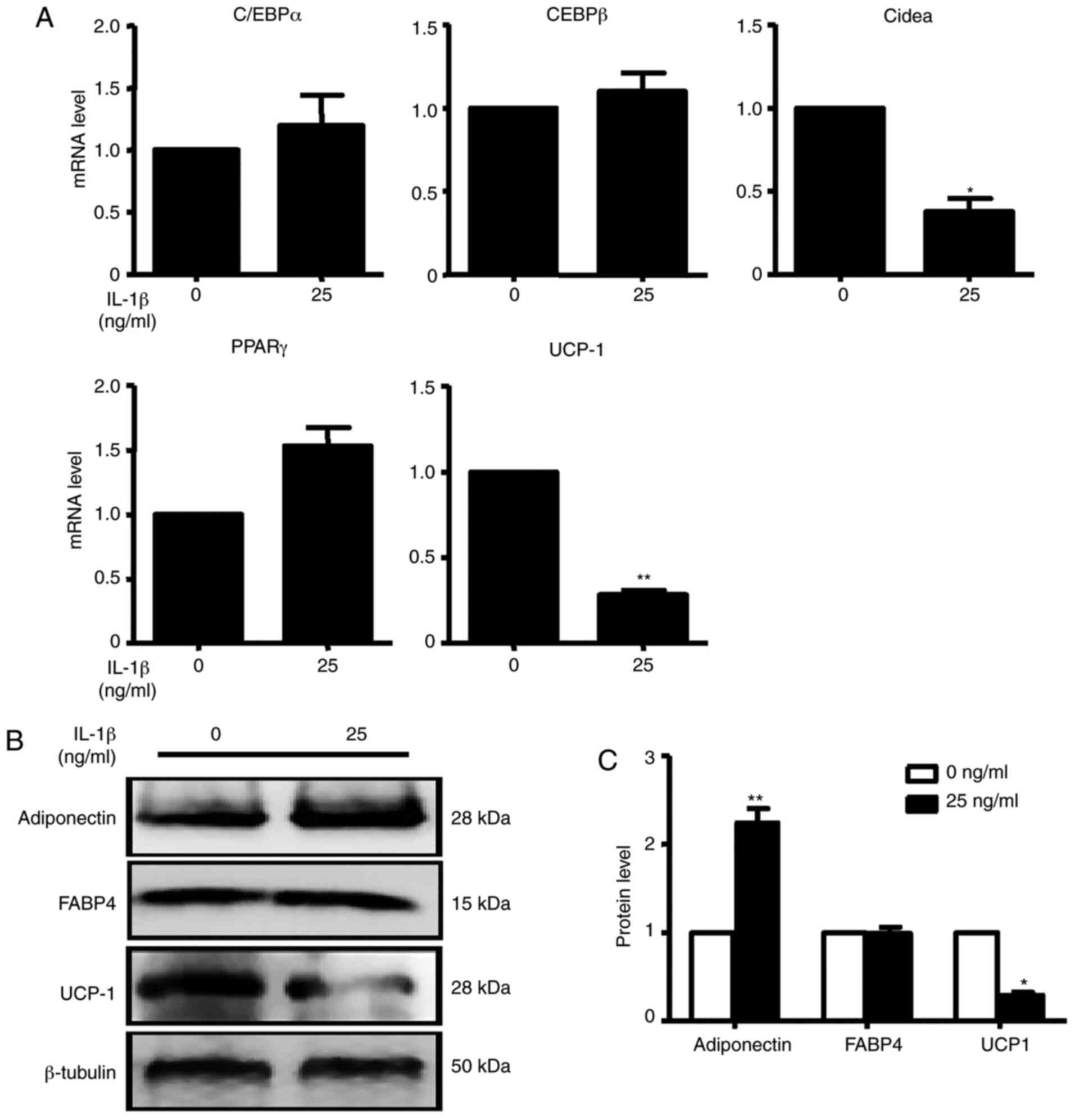 | Figure 6IL-1β inhibits the mRNA and protein
expression of adipogenic markers. (A) C/EBPα, C/EBPβ, Cidea, PPARγ
and UCP-1 were assessed by reverse transcription-quantitative PCR.
At the concentration of 25 ng/ml IL-1β, C/EBPα, C/EBPβ and PPARγ
were not changed compared with the absence of IL-1β. Cidea and
UCP-1 mRNA expression levels were all significantly decreased in
the presence of IL-1β compared with the absence of IL-1β. (B)
Adiponectin, FABP4 and UCP-1 were analysed by western blotting. (C)
Quantification of western blotting analysis. With the treatment of
IL-1β, adiponectin protein expression was significantly increased
with the treatment of IL-1β. UCP-1 protein expression was decreased
0.5-fold compared with the absence of IL-1β. Data are expressed as
the mean ± SEM. n=4 for each group. *P<0.05;
**P<0.01 vs. control. UCP-1, uncoupling protein 1;
C/EBPα/β, CCAAT/enhancer-binding protein α/β; Cidea, cell
death-inducing dffa-like effector a; PPARγ, peroxisome
proliferator-activated receptor γ; FABP4, fatty acid-binding
protein 4. |
Discussion
As the worldwide rates of obesity and associated
metabolic disorders have increased, better understanding of the
fundamental mechanisms underlying adipocyte formation and fat
tissue expansion is required (18).
The present study demonstrated increasing expression pattern of
SFRP4 in mouse preadipocytes during differentiation. In brown
preadipocytes, incubation with gradient doses of SFRP4 (0, 1, 10
and 100 ng/ml) promoted the formation of lipid droplets in brown
adipocytes by morphological observation. Furthermore, it was
discovered that high-dose SFRP4 significantly accelerated the
thermogenic metabolism of brown adipocytes by regulating the
secretion of leptin and adiponectin. Additionally, the potential of
IL-1β as an upstream factor to regulate SFRP4 expression in brown
adipocytes was investigated. The results showed that in addition to
inhibiting the differentiation of brown adipocytes, IL-1β has no
effect on the expression of SFRP4.
BAT is the main source of non-shivering
thermogenesis and heat production in mammals, containing a large
number of mitochondria with strong oxidation capacity (19). UCP-1, existing in the inner membrane
of mitochondria and providing heat for cells in the form of an
uncoupled respiratory chain, is expressed specifically in brown
adipocytes (5). In rodents, brown
adipocytes are found in specific brown adipocyte depots, such as
the scapula, neck and the chest of mice (20). In addition to heat production, BAT
also has some physiological functions. Firstly, the thermogenic
activity of BAT can be added to the clearance of triglycerides in
plasma, regulate the homeostasis of vascular lipoproteins, and
effectively alleviate the formation of atherosclerotic plaques
(21). Secondly, BAT allows the
body adapt to the cold external environment and promotes energy
consumption to prevent obesity and type 2 diabetes (22). According to this characteristic of
BAT, the excess energy stored is consumed spontaneously, which was
also applicable to BAT transplantation individuals (23). Thirdly, as an endocrine organ in the
mammalian body, in addition to adipocytokine secretion, brown
adipocytes also regulate the inflammatory response of the body by
secreting inflammatory factors to the extracellular space (24). In the present study, it was found
that SFRP4 promoted brown adipocyte differentiation, indicating
that overexpression of SFRP4 significantly increased the number of
mature brown adipocytes in the brown adipose depot, which is
conducive to maintaining endocrine homeostasis and energy
metabolism balance in mice.
In the present study, the SFRP4 expression pattern
was ‘S’ style during the brown adipocyte differentiation, as the
SFRP4 mRNA expression was increased at day 0, decreased at day 2,
then increased again from day 2 to day 8 following the brown
adipocyte differentiation; which was an interesting phenomenon
worthy of attention. Firstly, the preadipocytes were seeded in
dishes and the time point was set as day-4, and the cell density
was ~70%. After incubation for two days, the cells proliferated to
~80-90% at day-2. At time point 0, the cells proliferated and fused
to differentiate into mature adipocytes. It was speculated that in
the cell proliferation phase, SFRP4 promoted cell proliferation.
However, at days 0 to 4, induction medium was added to induce cell
differentiation, and the preadipocytes exhibited contact inhibition
and cell proliferation arrest. The expression of SFRP4 had no
effect on cell differentiation at the early stage. It was
noteworthy that on days 6 to 8, the brown adipocytes differentiated
into mature adipocytes and formed lipid droplets, and SFRP4
promoted brown adipocyte differentiation and lipid formation.
It has been confirmed that PPARγ, as a marker gene
of adipocyte differentiation, is not only essential for the
development of all types of adipocytes but also plays an important
role in the process of adipocyte de novo differentiation
(25). Moreover, PPARγ increases
the capacity of mitochondria and the potential for uncoupling,
promoting the browning of white adipocytes (26). Based on this, C/EBP family members
play a major role in maintaining PPARγ expression and coregulating
transcription to intensify and maintain adipocyte differentiation
(27). A previous study reported
that the absence of C/EBPα in mice prevents the development of
white adipocytes and has no effect on brown adipocyte formation,
which suggests that the absence of C/EBPα may be compensated by
upregulating the expression of C/EBPβ in brown adipose tissue
(28). However, the present study
confirmed that overexpression of SFRP4 promoted C/EBPα and C/EBPβ
expression in brown adipocytes rather than PPARγ.
Importantly, Seale et al (29) demonstrated that zinc-finger protein
PRDM16 is highly expressed in BAT compared with the WAT and
activated the brown adipocyte identity by direct binding to PGC-1α
and PGC-1β to control the determination of brown adipocyte fate.
Meanwhile, the expression level of PGC-1α was elevated upon cold
exposure and controlled mitochondrial biogenesis and respiration
regulated by UCP-1(30).
Specifically, overexpression of PGC-1α promoted the brown adipocyte
phenotype formation (31).
In Fig. 3, after
incubation with SFRP4, the C/EBPα, C/EBPβ, Cidea and GLUT4 mRNA
expression levels were significantly increased. However, only PPARγ
and adiponectin mRNA expression were unchanged with SFRP4
treatment. Firstly, as previously reported, C/EBPα and C/EBPβ are
adipocyte markers used to evaluate differentiation (32). In the present study, it was
demonstrated that C/EBPα and C/EBPβ were increased significantly
after incubation with SFRP4 (10 and 100 ng/ml). Secondly, Cidea, a
lipid droplet-associated protein, was highly expressed after
induction by high concentrations of recombinant SFRP4 in the
present study. Additionally, Cidea specifically strengthened and
regulated the transcription of UCP-1 in the nucleus of human fat
cells (33). Thirdly, in the
present study, the mRNA expression of PPARγ and adiponectin were
measured in mature brown adipocytes at day 8. It was speculated
that PPARγ was expressed at the early stage of brown adipocyte
differentiation, and no difference in PPARγ mRNA expression could
be detected at the terminal differentiation stage of brown
adipocytes. Despite the statement that SFRP4 promotes brown
adipocyte differentiation in vitro was strong, additional
in vivo experiments were needed to elucidate the potential
mechanism of this subject in the future.
As an important endocrine organ, BAT secretes
several types of adipocytokines, such as leptin and adiponectin, to
participate in physiological metabolism (34). Leptin, an important secretory
protein, regulates food intake and energy consumption (35). For example, Wang et al
(36) discovered that leptin
promoted the browning of white adipocytes by inhibiting the Hh
signalling pathway to decrease obesity in mice. In terms of energy
balance, leptin also maintains the thermogenesis of brown
adipocytes to achieve a steady state and further participate in and
support the regulation of brown adipocytes in energy balance
(37), which was consistent with
the present finding that a high concentration of SFRP4
significantly upregulated the expression of leptin secretion. In
contrast, as a polypeptide hormone, a previous study revealed that
adiponectin decreases thermogenesis by inhibiting the activity of
BAT in mice (38), which was
sufficient to explain why even low-dose SFRP4 treatment
significantly inhibited the secretion of adiponectin in brown
adipocytes. Obviously, the mRNA expression of adiponectin was not
consistent with protein expression after treatment with SFRP4 in
brown adipocytes.
Gene expression regulation is complex and
changeable, especially during dynamic transition (39). The mRNA encoding protein undergoes
transcription, translation and posttranslational processes;
however, the spatiotemporal variability of genes and the limitation
of protein biosynthesis resources strongly affect the association
between protein levels and their encoded transcripts (40).
There are many comorbidities of obesity, including
chronic and low-grade systemic inflammation, increased adipose
tissue, fatty cell hypertrophy and hyperplasia (17). The local infiltration of immune
cells and the release of proinflammatory cytokines decrease the
activity of metabolism in obese patients (41). Brown adipocytes are responsible for
energy consumption and are less prone to local inflammation
compared with white adipocytes; however, strong damage from obesity
eventually leads to a local BAT proinflammatory environment, which
directly changes the thermogenic activity of brown adipocytes,
impairs the energy consumption mechanism and fuel matrix glucose
uptake (42). There is evidence
that IL-1β significantly downregulated the expression of UCP-1 when
incubated with brown adipocytes (43). Moreover, IL-1β derived from
macrophages significantly inhibited the expression of UCP-1 in
C3H10T1/2 cells (38), which was
consistent with the present findings. Previous studies have
confirmed that the release of SFRP4 in islets is stimulated by the
inflammatory marker IL-1β, which provides an association between
islet inflammation and impaired insulin secretion (11). However, IL-1β had no contribution to
the stimulation of SFRP4 expression in brown adipocytes in the
present study, revealing that brown and white adipocytes are
heterogeneous and that the expression patterns are quite different
(44).
In summary, the present study identified and
characterized the role of SFRP4, a secreted inhibitor of Wnt
signalling, in various functions in different types of adipose
tissue expansion. Future studies demonstrated that the inflammatory
factor IL-1β has no effect on SFRP4 expression and secretion in
brown adipocytes. Collectively, the present data support the
concept that SFRP4 regulates murine brown adipocyte
differentiation.
Supplementary Material
Sequences of the primers for reverse
transcription-quantitative PCR.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by grants from National Natural
Science Foundation of China (grant no. 81900399), Natural Science
Foundation Project of Shaanxi Province (grant nos. 19JS060 and
2016JM8122), State Administration of Traditional Chinese Medicine
of Shaanxi Province (grant no. 2019-ZZ-JC034) and the Key Research
and Development Plan of Shaanxi Province (grant no.
2018SF-266).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HG, LX, EL and QY confirm the authenticity of all
the raw data. HG, HYZ and AX performed the experiments. YL and LX
analyzed the data. HG, EL and QY designed the study and wrote the
manuscript. JZ and HDZ performed the RT-qPCR and analysis of the
data in the revision manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The Laboratory Animal Administration Committee of
Xi'an Medical University approved all animal experiments
(Institutional Animal Care and Use Committee; approval no. XYJZS
201806025-8).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Murawska-Cialowicz E: Adipose
tissue-morphological and biochemical characteristic of different
depots. Postepy Hig Med Dosw (Online). 71:466–484. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cinti S: The adipose organ at a glance.
Dis Model Mech. 5:588–594. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ibrahim MM: Subcutaneous and visceral
adipose tissue: Structural and functional differences. Obes Rev.
11:11–18. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Marlatt KL and Ravussin E: Brown adipose
tissue: An update on recent findings. Curr Obes Rep. 6:389–396.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yu Q, Wang F, Meng X, Gong Y, Wang Y, Xu C
and Wang S: Short-term use of atorvastatin affects glucose
homeostasis and suppresses the expression of LDL receptors in the
pancreas of mice. Mol Med Rep. 18:2780–2788. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Virtanen KA, Lidell ME, Orava J, Heglind
M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ,
Enerbäck S and Nuutila P: Functional brown adipose tissue in
healthy adults. N Engl J Med. 360:1518–1525. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ode KL, Frohnert BI and Nathan BM:
Identification and treatment of metabolic complications in
pediatric obesity. Rev Endocr Metab Disord. 10:167–188.
2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pawar NM and Rao P: Secreted frizzled
related protein 4 (sFRP4) update: A brief review. Cell Signal.
45:63–70. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mastaitis J, Eckersdorff M, Min S, Xin Y,
Cavino K, Aglione J, Okamoto H, Na E, Stitt T, Dominguez MG, et al:
Loss of SFRP4 alters body size, food intake and energy expenditure
in diet-induced obese male mice. Endocrinology. 156:4502–4510.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu F, Qu H, Li Y, Tang Q, Yang Z, Wang H
and Deng H: Relationship between serum secreted frizzled-related
protein 4 levels and the first-phase of glucose-stimulated insulin
secretion in individuals with different glucose tolerance. Endocr
J. 62:733–740. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mahdi T, Hänzelmann S, Salehi A, Muhammed
SJ, Reinbothe TM, Tang Y, Axelsson AS, Zhou Y, Jing X, Almgren P,
et al: Secreted frizzled-related protein 4 reduces insulin
secretion and is overexpressed in type 2 diabetes. Cell Metab.
16:625–633. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li XJ, Yang H, Li GX, Zhang GH, Cheng J,
Guan H and Yang GS: Transcriptome profile analysis of porcine
adipose tissue by high-throughput sequencing. Anim Genet.
43:144–152. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Guan H, Zhang Y, Gao S, Bai L, Zhao S,
Cheng XW, Fan J and Liu E: Differential patterns of secreted
frizzled-related protein 4 (SFRP4) in adipocyte differentiation:
Adipose depot specificity. Cell Physiol Biochem. 46:2149–2164.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gao W, Kong X and Yang Q: Isolation,
primary culture, and differentiation of preadipocytes from mouse
brown adipose tissue. Methods Mol Biol. 1566:3–8. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Barthold SW, Bayne KA, Davis MA, Everitt
JI, Fox JG, Garnett NL, Gauda EB, Kemnitz JW, Clark JAM, McClintock
MK, et al: Guide for the care and use of laboratory animals. 8th
edition. National Institutes of Health (NIH), 2008.
|
|
16
|
Frontini A and Cinti S: Distribution and
development of brown adipocytes in the murine and human adipose
organ. Cell Metab. 11:253–256. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Alomar SY, Zaibi MS, Kępczyńska MA,
Gentili A, Alkhuriji A, Mansour L, Dar JA and Trayhurn P: PCR array
and protein array studies demonstrate that IL-1β (interleukin-1β)
stimulates the expression and secretion of multiple cytokines and
chemokines in human adipocytes. Arch Physiol Biochem. 121:187–193.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Withrow D and Alter DA: The economic
burden of obesity worldwide: A systematic review of the direct
costs of obesity. Obes Rev. 12:131–141. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hankir MK: Loading and firing the brown
adipocyte. Adipocyte. 7:4–11. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Giralt M and Villarroya F: White, brown,
beige/brite: Different adipose cells for different functions?
Endocrinology. 154:2992–3000. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xiong W, Zhao X, Villacorta L, Rom O,
Garcia-Barrio MT, Guo Y, Fan Y, Zhu T, Zhang J, Zeng R, et al:
Brown adipocyte-specific PPARγ (peroxisome proliferator-activated
receptor γ) deletion impairs perivascular adipose tissue
development and enhances atherosclerosis in mice. Arterioscler
Thromb Vasc Biol. 38:1738–1747. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Scheideler M, Herzig S and Georgiadi A:
Endocrine and autocrine/paracrine modulators of brown adipose
tissue mass and activity as novel therapeutic strategies against
obesity and type 2 diabetes. Horm Mol Biol Clin Investig: Aug 29,
2017 (Epub ahead of print). doi: 10.1515/hmbci-2017-0043.
|
|
23
|
White JD, Dewal RS and Stanford KI: The
beneficial effects of brown adipose tissue transplantation. Mol
Aspects Med. 68:74–81. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Villarroya F, Cereijo R, Villarroya J and
Giralt M: Brown adipose tissue as a secretory organ. Nat Rev
Endocrinol. 13:26–35. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tsai YS and Maeda N: PPARgamma: A critical
determinant of body fat distribution in humans and mice. Trends
Cardiovasc Med. 15:81–85. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nedergaard J, Petrovic N, Lindgren EM,
Jacobsson A and Cannon B: PPARgamma in the control of brown
adipocyte differentiation. Biochim Biophys Acta. 1740:293–304.
2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ramji DP and Foka P:
CCAAT/enhancer-binding proteins: Structure, function and
regulation. Biochem J. 365:561–575. 2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yubero P, Manchado C, Cassard-Doulcier AM,
Mampel T, Vinas O, Iglesias R, Giralt M and Villarroya F:
CCAAT/enhancer binding proteins alpha and beta are transcriptional
activators of the brown fat uncoupling protein gene promoter.
Biochem Biophys Res Commun. 198:653–659. 1994.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Seale P, Kajimura S, Yang W, Chin S, Rohas
LM, Uldry M, Tavernier G, Langin D and Spiegelman BM:
Transcriptional control of brown fat determination by PRDM16. Cell
Meta. 6:38–54. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Puigserver P, Wu Z, Park CW, Graves R,
Wright M and Spiegelman BM: A cold-inducible coactivator of nuclear
receptors linked to adaptive thermogenesis. Cell. 92:829–839.
1998.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tiraby C, Tavernier G, Lefort C, Larrouy
D, Bouillaud F, Ricquier D and Langin D: Acquirement of brown fat
cell features by human white adipocytes. J Biol Chem.
278:33370–33376. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kiess W, Petzold S, Töpfer M, Garten A,
Blüher S, Kapellen T, Körner A and Kratzsch J: Adipocytes and
adipose tissue. Best Pract Res Clin Endocrinol Metab. 22:135–153.
2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jash S, Banerjee S, Lee MJ, Farmer SR and
Puri V: CIDEA transcriptionally regulates UCP1 for britening and
thermogenesis in human fat cells. iScience. 20:73–89.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Villarroya J, Cereijo R, Gavalda-Navarro
A, Peyrou M, Giralt M and Villarroya F: New insights into the
secretory functions of brown adipose tissue. J Endocrinol.
243:R19–R27. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Paz G, Mastronardi CA and Licinio J:
Leptin treatment: Facts and expectations. Metabolism. 64:146–156.
2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang J, Ge J, Cao H, Zhang X, Guo Y, Li X,
Xia B, Yang G and Shi X: Leptin promotes white adipocyte browning
by inhibiting the HH signaling pathway. Cells.
8(372)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pandit R, Beerens S and Adan RAH: Role of
leptin in energy expenditure: The hypothalamic perspective. Am J
Physiol Regul Integr Comp Physiol. 312:R938–R947. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Qiao L, Yoo HS, Bosco C, Lee B, Feng GS,
Schaack J, Chi NW and Shao J: Adiponectin reduces thermogenesis by
inhibiting brown adipose tissue activation in mice. Diabetologia.
57:1027–1036. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Song G, Zong C, Zhang Z, Yu Y, Yao S, Jiao
P, Tian H, Zhai L, Zhao H, Tian S, et al: Molecular hydrogen
stabilizes atherosclerotic plaque in low-density lipoprotein
receptor-knockout mice. Free Radic Biol Med. 87:58–68.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jing L, Wang Y, Zhao XM, Zhao B, Han JJ,
Qin SC and Sun XJ: Cardioprotective effect of hydrogen-rich saline
on isoproterenol-induced myocardial infarction in rats. Heart Lung
Circ. 24:602–610. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Stolarczyk E: Adipose tissue inflammation
in obesity: A metabolic or immune response? Curr Opin Pharmacol.
37:35–40. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Villarroya F, Cereijo R, Gavalda-Navarro
A, Villarroya J and Giralt M: Inflammation of brown/beige adipose
tissues in obesity and metabolic disease. J Intern Med.
284:492–504. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Nøhr MK, Bobba N, Richelsen B, Lund S and
Pedersen SB: Inflammation downregulates UCP1 expression in brown
adipocytes potentially via SIRT1 and DBC1 interaction. Int J Mol
Sci. 18(1006)2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Goto T, Naknukool S, Yoshitake R, Hanafusa
Y, Tokiwa S, Li Y, Sakamoto T, Nitta T, Kim M, Takahashi N, et al:
Proinflammatory cytokine interleukin-1β suppresses cold-induced
thermogenesis in adipocytes. Cytokine. 77:107–114. 2016.PubMed/NCBI View Article : Google Scholar
|