Introduction
Ischemic stroke is a common clinical cerebrovascular
disease that is accompanied by significant disability and
mortality, with survivors often suffering from various degrees of
neurological dysfunction (1). The
mechanism of cerebral ischemic injury involves a number of
pathophysiological processes, with accumulating evidence suggesting
that inflammation is involved (2,3).
Therefore, the regulation of inflammatory signaling may represent a
potential treatment strategy for ischemic stroke.
Microglia are a type of glial cell, which are the
resident macrophages of the brain (4). Microglia are the first and most
important line of defense in the central nervous system (CNS). As
the immune effector cells of the CNS, microglia have been
demonstrated to serve an important role in the processes behind
cerebral injury (5). For example,
activated microglia have been found in the surrounding lesions of
various neurodegenerative diseases, including Alzheimer's disease,
Parkinson's disease, muscular amyotrophic lateral sclerosis and
multiple sclerosis (6). Activated
microglia can release a number of molecules, including cytotoxic
substances such as nitric oxide (NO), oxygen free radicals and
proteolytic enzymes, as well as inflammatory factors such as IL-1,
TNF-α and IFN-γ, which are essential for the pathological process
of cerebral ischemic injury (7).
Microglia exist in a dynamic equilibrium between the
pro-inflammatory (M1) and anti-inflammatory (M2) types, and the
polarization state is associated with the local microenvironment
(8). In vitro, M0 type
microglia have been revealed to be induced to the M1 type through
lipopolysaccharide or IFN-γ, or induced to the M2 type through
IL-4, IL-10 and TGF-α (8). The
excessive activation of M1 type microglia leads to the secretion of
large amounts of inflammatory factors and free radicals, such as
macrophage colony-stimulatory factor, TNF-α, IL-1 and IL-6, which
subsequently promote a major inflammatory response (5). Therefore, it remains necessary to
avoid the occurrence of neuroinflammatory reactions by regulating
the balance of the polarization state of M1 and M2 microglia.
Nuclear factor (erythroid-derived 2)-like 2 (Nrf2)
is a major regulator of the cell defense response against chemical
or oxidative stress (9). Nrf2
modulation is achieved by various proteins and signaling pathways
at both the cytoplasmic and nuclear level (10). The pharmacological activation of
Nrf2 has been suggested as a promising therapeutic approach for
several chronic diseases, including Alzheimer's and Parkinson's
diseases (11). Interestingly, a
previous study revealed that sinomenine (SINO) provided protection
against ischemic/reperfusion-associated liver damage in a heme
oxygenase-1 (HO-1) dependent manner (12), indicating that SINO may exert its
protective effects through the Nrf2 signaling pathway, since HO-1
is mainly regulated by Nrf2 signaling (13).
SINO is an active alkaloid extracted from the
Chinese medical plant, Sinomenium acutum (14). Previous studies have reported that
SINO possesses anti-inflammatory and immunoregulatory properties,
as well as exhibiting significant therapeutic efficacy for
rheumatoid arthritis (15,16). In addition, SINO is protective
against various autoimmune and inflammation-associated diseases
(17,18). SINO has been found to modulate
immune responses mainly through downregulating the expression of
inflammation-related molecules, including NO, TNF-α, IL-1β and
prostaglandin E3, both in vitro and in vivo (19-21).
Other studies have demonstrated that SINO exerts its
anti-inflammatory role by inhibiting IκB phosphorylation and
subsequent NF-κB transcription (22,23).
However, the underlying mechanism of action behind the protective
effect of SINO in cerebral ischemic injury remains unclear.
Therefore, the present study aimed to investigate the role of SINO
and its potential mechanism of action in cerebral ischemic injury.
The results indicated that SINO may inhibit neuroinflammation by
targeting the microglia Nrf2 signaling pathway, both in vivo
and in vitro, which provided a novel target for the
treatment of ischemic stroke.
Materials and methods
Chemicals and regents
The following antibodies were used in the present
study: SINO (MedChemExpress); ML385 (MedChemExpress); Nrf2, Keap1,
NAD(P)H: Quinoneoxidoreductase (NQO1), Lamin B and HO-1 antibodies
(Abcam); β-actin antibodies (Bioworld Technology, Inc.); NF-κB p65,
Phosphorylated (p-)IκBα and IκBα antibodies (Cell Signaling
Technology, Inc.); HRP-conjugated secondary antibodies (Bioworld
Technology, Inc.); and Dylight488 conjugated goat anti-rabbit IgG
secondary antibodies (Bioworld Technology, Inc.).
Cell culture and treatment
BV2 cells (Shanghai Kang Lang Biological Technology
Co. Ltd.) were cultured in DMEM (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) and maintained at 37˚C in a 5% CO2 incubator
(Thermo Fisher Scientific, Inc.). Oxygen glucose deprivation (OGD)
was induced according to a previously described method (24). BV2 cells were cultured at 37˚C with
deoxygenated DMEM without glucose and FBS, in the presence of a
premixed gas (1% O2, 94% N2 and 5%
CO2) for 4 h. The cells were subsequently cultured in
normal DMEM supplemented with 10% FBS and maintained at 37˚C in a
5% CO2 incubator. Cells in the control group were
cultured with normal DMEM and 10% FBS throughout.
Cell viability analysis
BV2 cells were cultured on a 96-well plate and
treated with increasing concentrations (0-200 µM) of SINO for 24 h.
Cell viability was then measured using a Cell Counting Kit-8
(ProteinTech Group, Inc.) according to the manufacturer's
instructions. In total, 1 h was the duration of the incubation with
the CCK-8 reagent at 37˚C.
Establishment of middle cerebral
artery occlusion (MCAO)
The present study was approved by the Ethics
Committee of the Medical College of Xi'an Peihua University. A
total of 48 male C57BL/6 mice (age, 12 weeks; weight, 25±2 g) were
obtained from Xi'an Jiaotong University Experimental Animal Center.
They were housed in the specific pathogen-free conditions with
standard temperature (22±1˚C), humidity (50-60%) and light
conditions (12 h light/dark cycle), with access to food and water
ad libitum.
MCAO surgery was performed as previously described
(25). Mice were anesthetized with
an intraperitoneal injection of 5% chloral hydrate (400 mg/kg). A
silicone-coated 6-0 suture (Covidien; Medtronic, Ltd.) was slowly
inserted from the exposed external carotid artery to the internal
carotid artery and wedged into the circle of Willis to obstruct the
opening of the middle cerebral artery. The distance from the
bifurcation of the internal/external carotid artery to the middle
cerebral artery was 9±1.0 mm. The suture was withdrawn following 60
min of obstruction. Mice were divided into four groups (n=6) as
follows: i) The sham group that was identical with MCAO but did not
include the occlusion of the middle cerebral artery; ii) SINO group
that received SINO at a dose of 20 mg/kg daily for 3 days; iii)
MCAO group; and iv) SINO/MCAO group in which SINO was subsequently
injected intraperitoneally into mice 6 h after MCAO surgery at a
dose of 20 mg/kg daily for 3 days. Notably, 20 mg/kg SINO used were
performed as previously described (26,27).
The sham group received the same volume of saline
intraperitoneally. After completion of the experiment, mice were
sacrificed by exsanguination under deep anesthesia (sodium
pentobarbital intraperitoneal injection, 50 mg/kg). Death was
confirmed by cessation of the heartbeat.
Histological analysis
For histological analysis, brain tissues were fixed
in 4% paraformaldehyde overnight at 4˚C and embedded in paraffin.
Paraffin-embedded tissues were cut into 4-µm-thick sections and
stained with H&E using standard procedures (28). Stained sections from 6 animals were
visualized in 10 randomly selected fields of view using a light
microscope with x20 magnification (Nikon Corporation).
Brain water content measurement
Mice were deeply anesthetized and decapitated 3 days
following MCAO surgery. Brain edema was analyzed according to a
previously described method (29).
Wet brains were weighed and then immediately dried at 95˚C
overnight. The brain water content was calculated using the
following formula: [(Wet tissue weight-dry tissue weight)/wet
tissue weight] x100%.
Measurement of cerebral antioxidant
enzyme activities
The activities of cerebral enzymes, glutathione
(GSH) peroxidase (GPx) and superoxide dismutase (SOD), were
measured using their respective colorimetric assay kits (Abcam),
according to the manufacturers' protocols. Briefly, for the GPx
assay, the tissue homogenates were incubated with GSH reductase
(GR), GSH, tert-butyl hydroperoxide and NADPH at 25˚C for 3 min and
the absorbance was measured at wavelength of 340 nm. In this assay,
the generated GSH disulfide was reduced to GSH following the
consumption of NADPH by GR. GPx activity was proportional to the
decrease of NADPH (which is measured at 340 nm). For the SOD assay,
tissue homogenates were treated with water-soluble tetrazolium at
37˚C for 30 min and the absorbance was measured at a wavelength of
560 nm. In this assay, the water-soluble tetrazolium was converted
by the superoxide radical anion to a formazan dye. SOD in the
tissue lysates reduced O2-levels, thereby decreasing the
dye formation.
Reverse
transcription-semi-quantitative PCR (RT-sqPCR)
Total RNA was extracted from the tissues or BV2
cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Total
RNA was reverse transcribed into cDNA using a Reverse
Transcriptase-kit (Qiagen, Inc.), according to the manufacturer's
protocol. The RT-sqPCR was performed using a 2x Taq Plus MasterMix
(Cwbiotech, Inc.) and products were analyzed on 1% agarose gel and
the intensity of each band was quantified by ImageJ software
version 1.8.0.112 (National Institutes of Health). qPCR was
subsequently performed using a SuperReal PreMix Plus (SYBR-Green)
kit (Qiagen, Inc.) on a CFX96™ Real-Time PCR Detection System
(Bio-Rad Laboratories, Inc.). The thermocycling conditions for the
sqPCR reaction included an initial step of 15 min at 95˚C to
activate the chemically modified hot-start Taq DNA polymerase,
followed by 40 cycles of a 15-sec denaturation at 95˚C and then 30
sec annealing and extension at 60˚C. GAPDH was used as the internal
reference control. Primer sequences were as follows (28,30):
TNFα forward, 5'-CATCTTCTCAAAATTCGAGT GAC-3' and reverse,
5'-TGGGAGTAGACAAGGTACAACCC-3'; IL-1 forward,
5'-TGGAAAAGCGGTTTGTCTTC-3' and reverse, 5'-TACCAGTTGGGGAACTCTGC-3';
NO synthase 2 (NOS2) forward, 5'-CAGCTGGGCTGTACAAACCT T-3' and
reverse, 5'-CATTGGAAGTGAAGCGTTTCG-3'; IL-6 forward,
5'-GCTGG-TGACAACCACGGCCT-3' and reverse,
5'-AGCCTCGACTTGTGAAGTGGT-3'; IL-10 forward,
5'-GCTCTTACTGACTGGCATGAG-3' and reverse,
5'-CGCAGCTCTAGGAGCATGTG-3'; arginase-1 (Arg-1) forwards,
5'-GTGAAGAACCCACGGTCTGT-3' and reverse, 5'-GCCAGAGATGCTTCCAACTG-3';
GAPDH forwards, 5'-GGCCCGGTGCTGAGTATGTC-3' and reverse,
5'-TGCCTGCTTCACCACCTTCT-3'. Relative expression levels were
analyzed using the 2-ΔΔCq method (31).
Western blotting
Protein was extracted with RIPA Lysis Buffer
(Beyotime Institute of Biotechnology) from the tissues and cells,
and quantified using a BCA protein assay kit (Thermo Scientific,
Inc.). The cytoplasmic/nuclear fractions were separated using
NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher
Scientific, Inc.) according to the manufacturer's manual. Proteins
(40 µg) were separated via 12% SDS-PAGE and transferred to PVDF
membranes, which were blocked with 5% bovine serum albumin
(Sigma-Aldrich; Merck KGaA) for 2 h at room temperature. The
membranes were then incubated at 4˚C overnight with the following
primary antibodies: Anti-β-actin (cat. no. AP0060, 1:5,000),
anti-Nrf2 (cat. no. ab92946, 1:1,000), anti-NQO1 (cat. no. ab80588,
1:1,000), anti-Lamin B (cat. no. ab194109, 1:1,000), anti-HO-1
(cat. no. ab68477, 1:2,000), anti-NF-κB p65 (cat. no. 8242
1:2,000), anti-p-IκBα (cat. no. 5209, 1:2,000), anti-IκBα (cat. no.
4812 1:1,000). Following the primary antibody incubation, the
membranes were washed with TBST (0.1% Tween 20) and incubated with
a HRP-conjugated secondary antibody (cat. no. BS13278, 1:5,000) for
1 h at room temperature. Protein bands were visualized using an ECL
Plus Western Blotting Detection system (Bio-Rad Laboratories,
Inc.).
Immunofluorescence staining
BV2 cells were cultured at 37˚C in a 24-well chamber
and treated with SINO and/or OGD for 4 h. Immunofluorescence
staining of NF-κB (cat. no. 8242, 1:500; Abcam) and subsequent
nuclear staining with DAPI (2 µg/ml; Sigma-Aldrich; Merck KGaA)
were performed as previously described (32). Briefly, the cells were fixed with 4%
paraformaldehyde for 30 min and then permeabilized in PBS
containing 2% Triton X-100 for 5 min at room temperature. The cells
were blocked with 5% bovine serum albumin (Sigma-Aldrich; Merck
KGaA) for 2 h at room temperature and subsequently incubated with
the anti-NF-κB antibody at 4˚C overnight and a DyLight
594-conjugated AffiniPure donkey anti-rabbit IgG secondary antibody
(cat. no. BS10030, 1:100) at 1 h room temperature. The cells were
also stained with DAPI for 5 min and observed under a confocal
microscope with x40 magnification.
Statistical analysis
All data are presented as the mean ± SEM and
statistical analysis was performed using SPSS Version 18.0 (SPSS,
Inc.). Each experiment was repeated in triplicate and statistical
differences between 2 groups were analyzed using a two-tailed
Student's t-test, while statistical differences between >2
groups were determined using one-way ANOVA followed by Tukey's
post-hoc tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
SINO treatment relieves MCAO-induced
cerebral injuries
The cerebral protective role of SINO in a MCAO model
mice was analyzed. Briefly, 20 mg/kg SINO was injected
intraperitoneally into mice for 3 days following MCAO surgery,
after which the cerebral histological changes and the brain water
content were determined. H&E staining revealed that mice in the
sham and SINO treatment groups did not have altered cerebral
morphology or brain water content, while the brains of MCAO model
mice exhibited a marked reduction in the number of nerve cells, as
well as an increased brain water content (Fig. 1A and B). Notably, treatment with SINO following
MCAO reduced the degree of pathological damage in the cerebral
tissue, as well as the brain water content (Fig. 1A and B). A previous study reported that
SINO-induced HO-1 serves an important role in attenuating cold
ischemia/reperfusion injury in rats (12) and that HO-1 is an Nrf2 target gene
(33). As such, the translocation
of nuclear of Nrf2 and the expression levels of HO-1 and NQO1 were
investigated. Compared with the sham group, Nrf2 translocation and
HO-1 and NQO1 expression levels were increased following MCAO
surgery. This result was and further enhanced by SINO treatment
(Fig. 1C and D). These results indicated that SINO may
protect against cerebral injury in MCAO model mice and the
protective effect may be related to the activation of the Nrf2
signaling pathway.
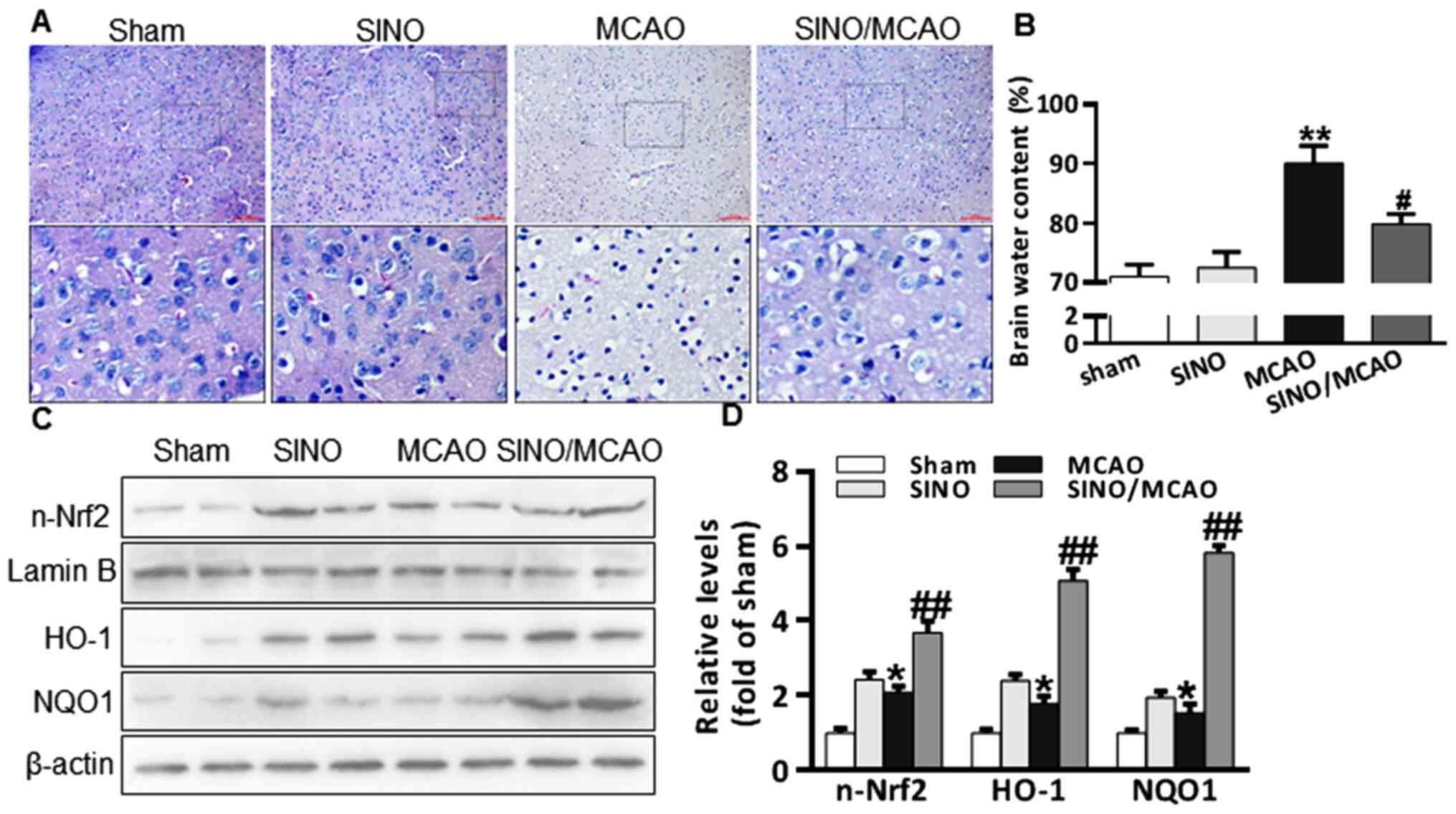 | Figure 1SINO treatment relieves MCAO-induced
cerebral injuries. (A) Representative micrographs of H&E
staining of brain sections (x20 magnification; scale bar, 50 µm).
(B) Brain water content analysis in brain tissues from Sham
(sham-operated, n=6), SINO (treatment only, n=6), MCAO
(MCAO-operated, n=6) and SINO/MCAO (MCAO operated plus SINO
treatment, n=6). (C) Western blot analysis of n-Nrf2, HO-1 and NQO1
protein expression levels from brain tissue. The samples from two
randomly selected brains in each group were presented. (D)
Quantification of brain protein expression levels. All the
experiments were repeated at least three times. The data are
presented as the mean ± SEM. *P<0.05 and
**P<0.01 vs. Sham; #P<0.05 and
##P<0.01 vs. MCAO. HO-1, heme oxygenase-1; MCAO,
middle cerebral artery occlusion; n-Nrf2, nuclear-nuclear
factor-erythroid 2-related factor; NQO1, NAD(P)H:
Quinoneoxidoreductase 1; SINO, sinomenine. |
SINO treatment activates the Nrf2
signaling pathway
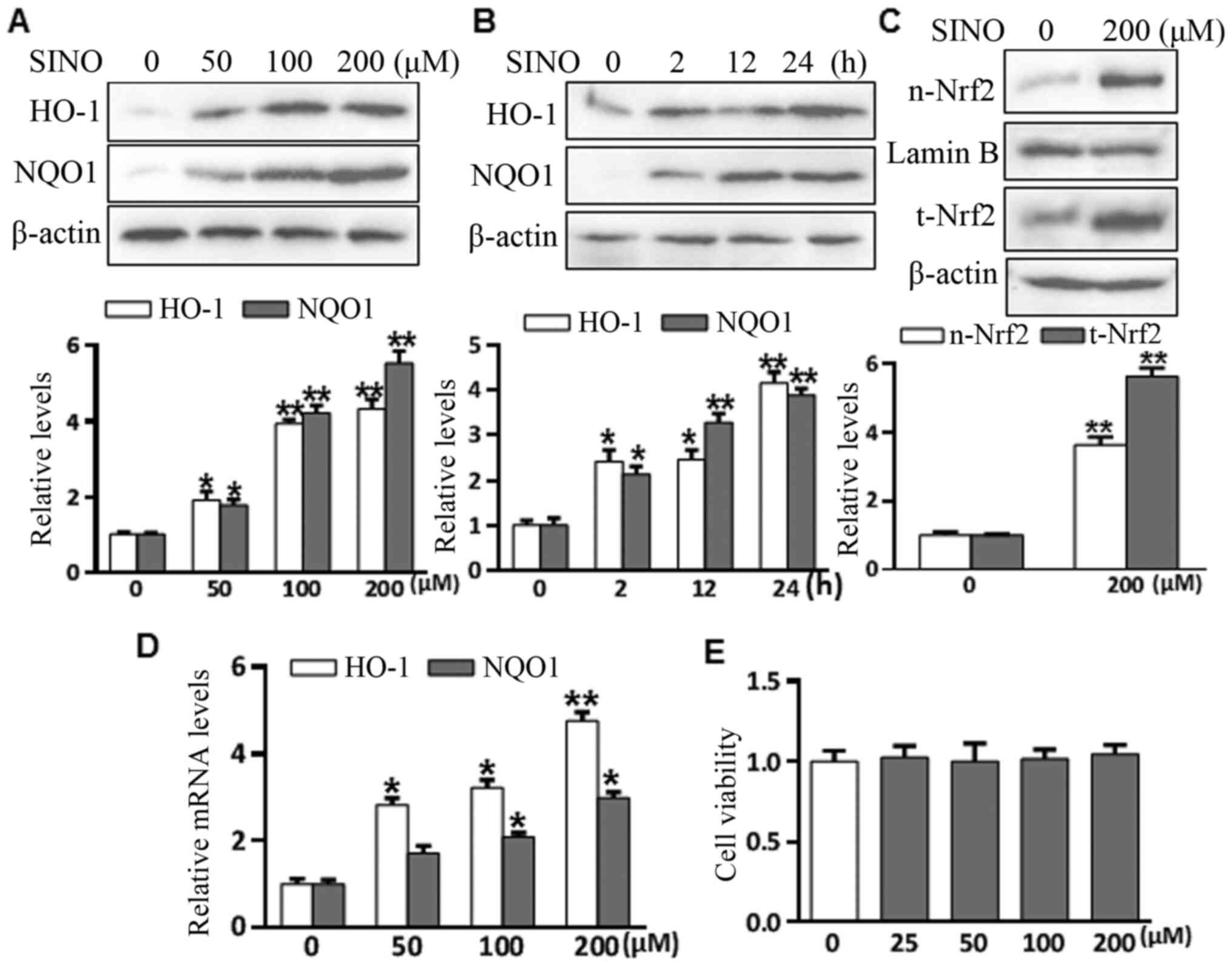
To investigate the effect of SINO treatment on Nrf2
signaling, microglia BV2 cells were treated with 50-200 µM SINO for
various durations (2-24 h) and the levels of HO-1 and NQO1 were
analyzed using western blotting. As presented in Fig. 2A and B, SINO treatment significantly upregulated
HO-1 and NQO1 expression levels in a concentration- and
time-dependent manner. Nrf2 nuclear translocation is a necessary
step for Nrf2 activation (30).
SINO treatment markedly induced Nrf2 nuclear accumulation after 4 h
(Fig. 2C). SINO treatment also
upregulated the mRNA expression of HO-1 and NQO1 in a
dose-dependent manner (Fig. 2D),
suggesting that SINO may upregulate HO-1 and NQO1 at the
transcriptional or post-transcription level. The cytotoxic effects
of SINO were analyzed using a Cell Counting Kit 8 assay, which
revealed that treatment with ≤200 µM SINO for 24 h was unable to
induce cell death, with no obvious changes in the amount of cell
death following treatment with between 0-200 µM SINO (Fig. 2E). Taken together, the results
suggested that SINO may be an activator of the Nrf2 signaling
pathway in microglial BV2 cells.
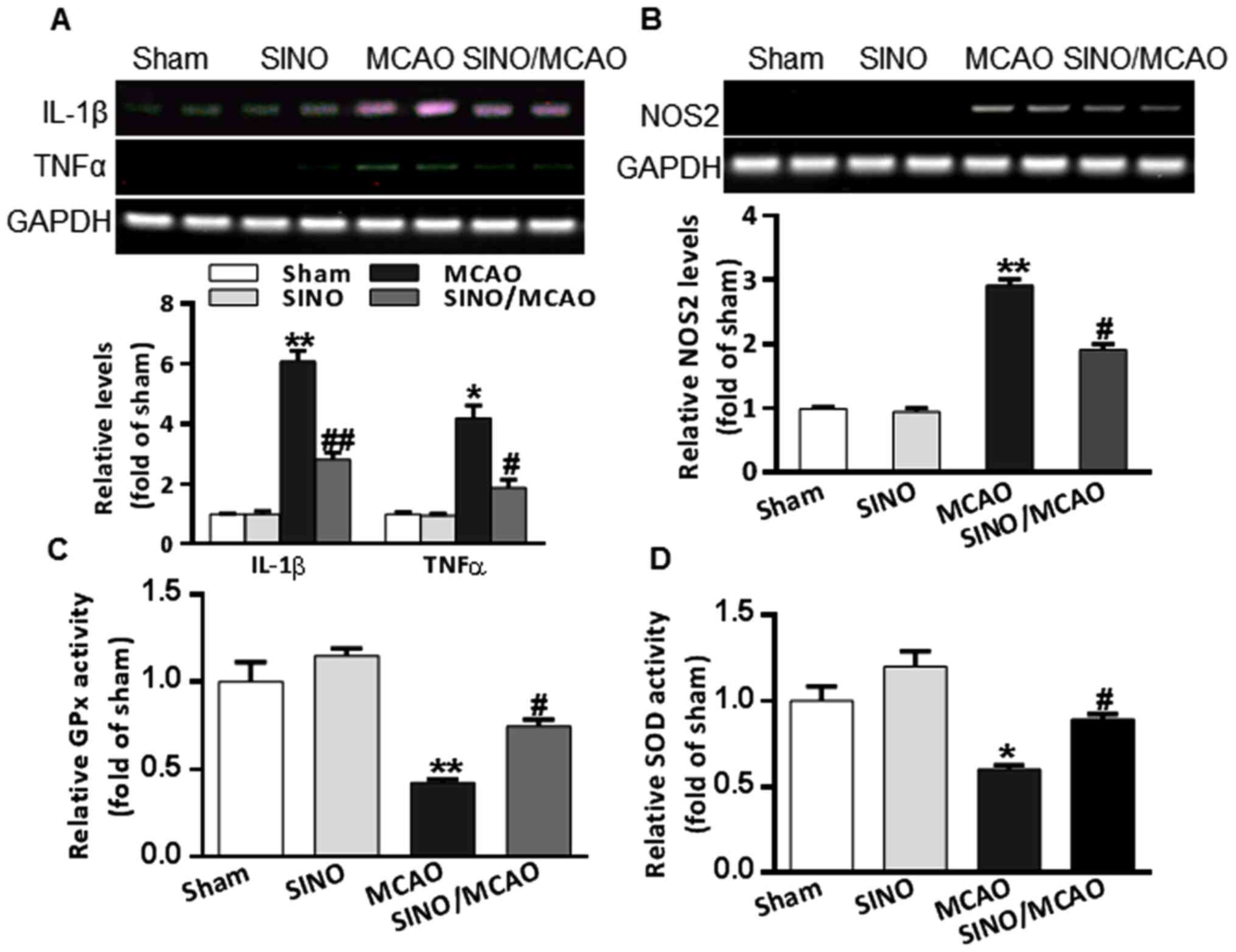
SINO treatment mitigates
MCAO-associated inflammation and oxidative stress
Cerebral inflammation and oxidative stress are
critical pathological processes associated with cerebral
pathogenesis in MCAO model mice (34). MCAO model mice had a markedly
upregulated expression of TNF-α and IL-1β compared with the sham
group. However, SINO treatment significantly inhibited the
production of both TNF-α and IL-1β (Fig. 3A). NOS2 is not only considered to be
a pro-inflammatory mediator, but also the key enzyme that increases
peroxynitrite production, a major reactive oxygen species (35). The brains of MCAO model mice had
significantly upregulated mRNA expression levels of NOS2 (Fig. 3B). SOD and GPx are two important
antioxidant enzymes (36). The
enzymatic activities of SOD and GPx in the brains of MCAO model
mice were found to be decreased (Fig.
3C and D); however, treatment
with SINO significantly reversed the upregulation of NOS2 mRNA
expression levels (Fig. 3B) and the
reduction in SOD and GPx enzyme activities (Fig. 3C and D). Collectively, these results indicated
that SINO may exert strong anti-inflammatory and anti-oxidative
stress functions in MCAO model mice.
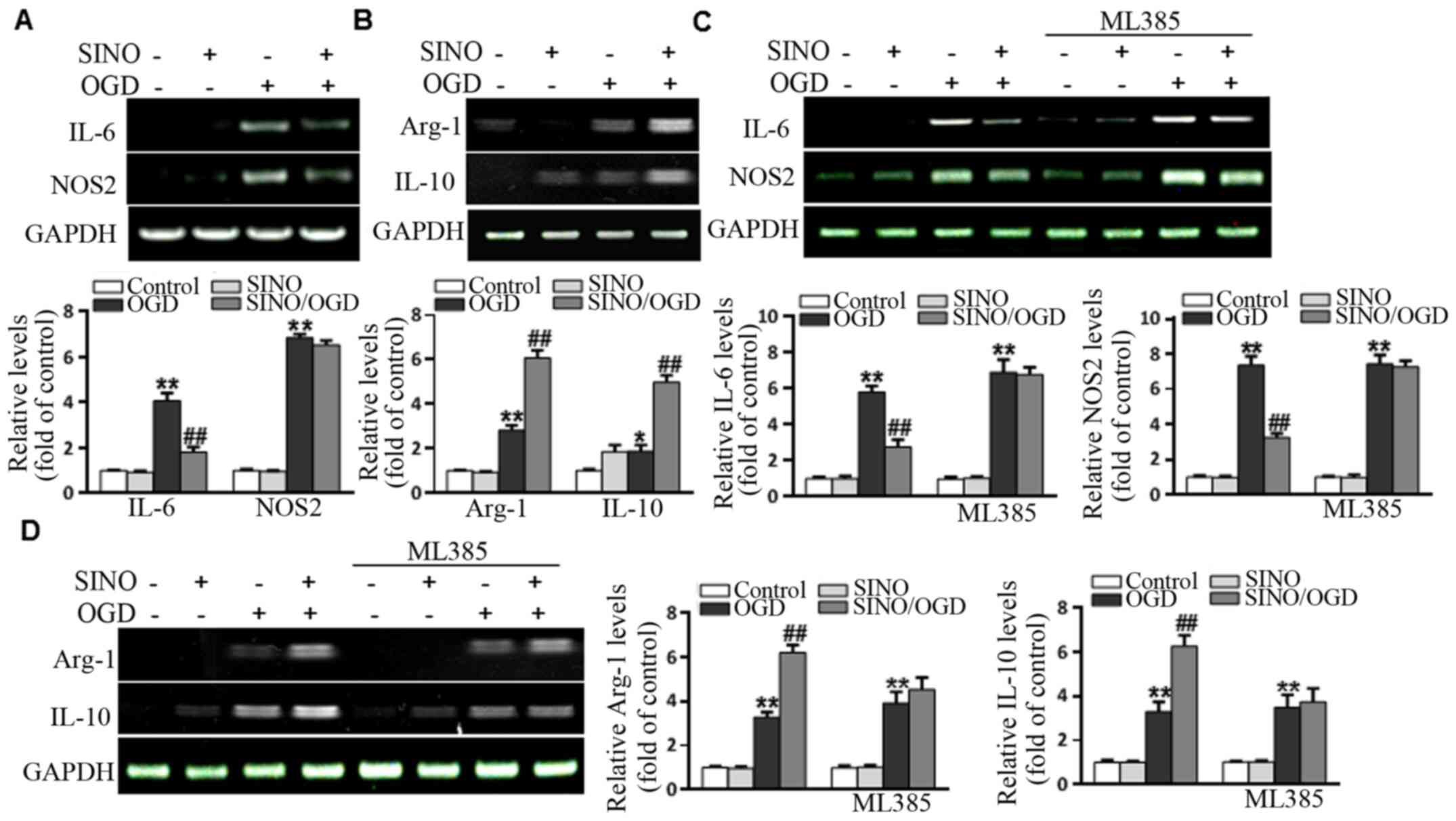
SINO treatment regulates microglia
polarization and inflammation in an Nrf2-dependent manner
Although SINO exerts strong anti-inflammatory
properties, its regulatory role in microglial polarization has not
been fully determined. Thus, whether SINO affected the expression
levels of M1 markers (NOS2 and IL-6) and M2 markers (Arg-1 and
IL-10) in BV2 cells was investigated. The results demonstrated that
OGD upregulated the expression levels of M1 markers, while SINO
treatment significantly inhibited the OGD-induced increases in IL-6
and NOS2 levels (Fig. 4A). In
addition, OGD upregulated the expression levels of M2 markers,
while SINO treatment markedly enhanced the OGD-induced expression
of Arg-1 and IL-10 compared with the control group (Fig. 4B), suggesting that SINO may have
dual functions in regulating microglial polarization. To further
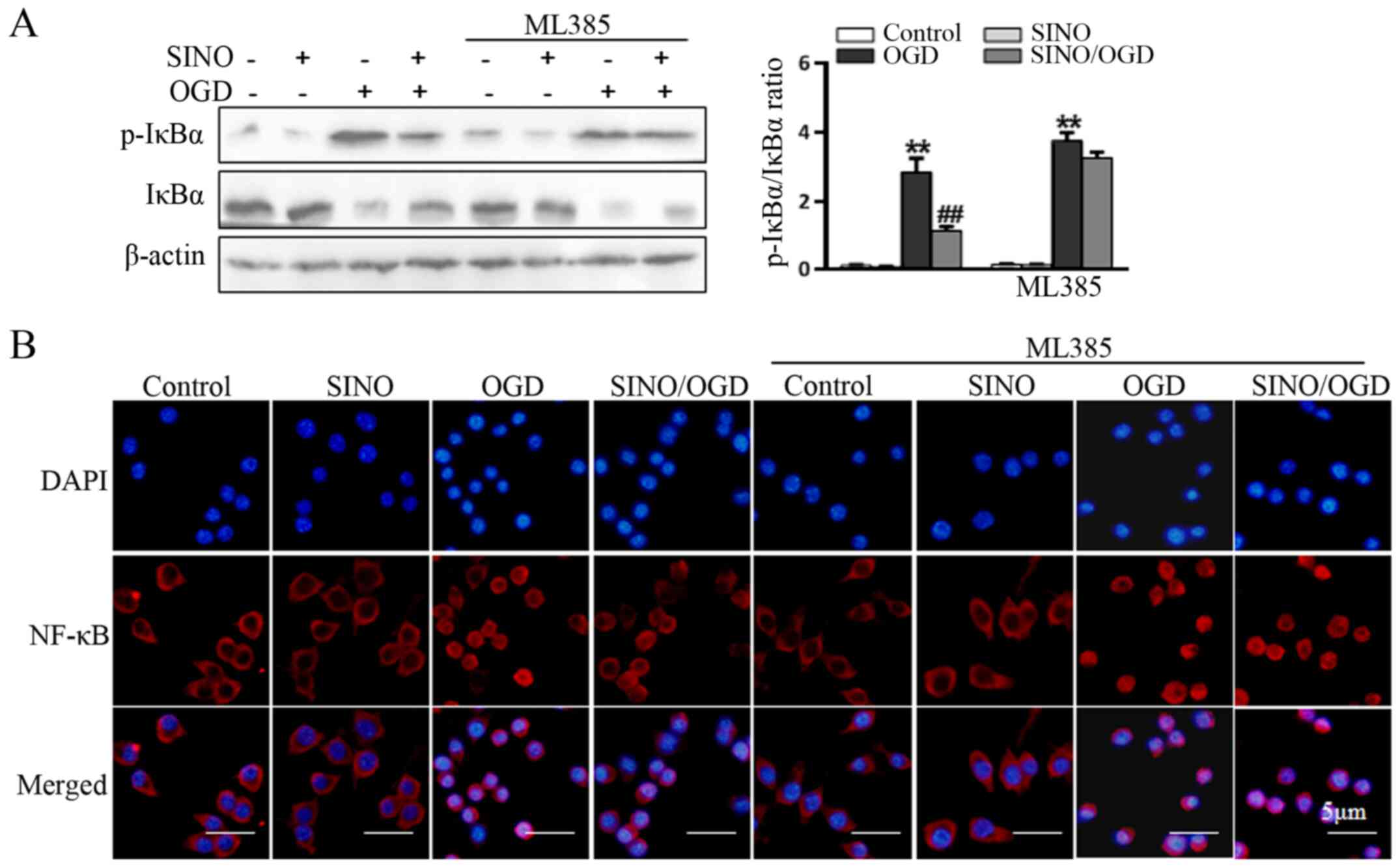
determine whether SINO exerted its effects through Nrf2, BV2 cells
were treated with 5 µM of the Nrf2 inhibitor, ML385, for 48 h. As
shown in Fig. 4C and D, the effect of SINO treatment on the
OGD-induced upregulation of NOS2, IL-6, Arg-1 and IL-10 expression
levels was markedly reduced following the inhibition of Nrf2. In
addition, the SINO-induced inhibition of OGD-induced IκBα
phosphorylation (Fig. 5A) and NF-κB
p65 nuclear translocation (Fig. 5B)
was also markedly reduced following the inhibition of Nrf2,
suggesting that Nrf2 signaling may be a crucial upstream event
involved in mediating the SINO-induced inhibition of NF-κB
signaling and inflammatory responses.
Discussion
The present study used MCAO model mice to establish
an in vivo model of ischemic stroke to investigate the role
of Nrf2 signaling in SINO-induced cerebral protection. The results
of the present study revealed that SINO served dual functions in
regulating inflammatory responses in microglia, in that SINO
downregulated NOS2 and IL-6 expression levels and promoted Arg-1
and IL-10 expression levels. This subsequently suppressed NF-κB
signaling, suggesting that the SINO-induced activation of Nrf2 may
act upstream of its inhibition of NF-κB signaling.
Stroke is the main cause of disability in adults
worldwide (37). Ischemic stroke
accounts for 85% of strokes (38,39)
and is a clinical syndrome which leads to neurological deficits due
to the ischemic and anoxic necrosis of the local tissue (40). Inflammation is an important response
in the pathological process of ischemic stroke. Previous studies
have reported that inflammation is the main factor that determines
the outcome and long-term prognosis of patients with ischemic
strokes (41). Microglia are
macrophages that are widespread throughout the CNS and participate
in the cellular immune process (42). At present, therapies that inhibit
the M1 polarization of microglia cells through physical and
pharmaceutical intervention, to promote anti-inflammatory
functions, have been applied in the clinic (43). Physical methods to inhibit M1
polarization include electro-acupuncture guidance and hyperbaric
oxygen treatment. Pharmaceutical interventions include exenatide
acetate and minocycline. Exenatide acetate has been found to
promote the change of microglia from the M1 to M2 type (44,45).
Minocycline has been found to inhibit the polarization of microglia
to the M1 type, but has no effect on the M2 type (46-48).
Kata et al (49) reported
that rosuvastatin inhibits the proliferation and adhesion of
microglia and upregulates the expression levels of certain
anti-inflammatory genes, including C-X-C motif chemokine ligand 1,
C-C motif chemokine ligand 5 and mannose binding lectin 2.
Concurrently, rosuvastatin also downregulated the expression levels
of the pro-inflammatory cytokines, IL-1β and TNF-α, upregulates the
expression levels of the anti-inflammatory factor, IL-10, and
promotes the polarization of microglia to the M2 type. The results
of the present study confirmed that SINO treatment effectively
inhibited the OGD-induced upregulation of the expression levels of
M1 markers, IL-6 and NOS2, while it markedly upregulated the
expression levels of the M2 markers, Arg-1 and IL-10, suggesting
that SINO may have dual functions in modulating microglia
polarization.
Accumulating evidence has revealed that Nrf2 serves
a crucial role behind the processes of oxidative stress and
inflammation (50,51). Therefore, Nrf2 may represent a
promising target for stroke intervention (50). However, the present understanding of
the functions of Nrf2 and its application for stroke-targeted
therapy remains limited (50). In
the present study, SINO was demonstrated to protect against
cerebral injury in MCAO mice. The protective effects were
associated with the ability of SINO to activate the Nrf2 signaling
pathway. The functional importance of Nrf2 has been reported in
several pathological conditions, most of which are from studies
performed investigating Nrf2-/- mice (52,53).
Protopanaxtriols, as a potent Nrf2 natural inducer extracted from
the root of Panax ginseng, C.A. Mey. has been widely used in East
Asia for thousands of years, exhibiting potent anti-inflammatory
and antioxidative properties (54,55).
Previous research has reported that Nrf2 deficiency causes a
significant increase in infarct volume following MCAO for 3 days,
which indicates that Nrf2 may exert a beneficial role in ischemic
injury during the acute development and progression (56-58).
The findings of the present study confirmed that SINO treatment
effectively reversed the MCAO-associated induction of the
inflammatory cytokines, TNF-α and IL-1β, and the activities of the
antioxidant enzymes, GPx and SOD. In addition, the SINO-induced
activation of Nrf2 signaling was found to be essential for
SINO-induced cerebral protection, as the inhibition of Nrf2
eliminated the protective effects of SINO on MCAO-associated
cerebral injury. In theory, the activation of Nrf2 should
upregulate SOD; however, in the current study, the enzymatic
activity of SOD in the brains of MCAO model mice was found to be
decreased and Nrf2 was induced to act against brain tissue damage
in MCAO model mice. Therefore, the light upregulation of Nrf2 in
MCAO model mice may not prevent the downregulation of SOD.
According to a previous study, the Nrf2-target genes
were revealed to be preferentially activated in glial cells, which
produces a more effective antioxidant effect than neurons (59). Over the past decade, previous
findings have suggested that SINO may exert a significant potential
for the treatment of strokes (27,60-62).
Glial cells contribute to the expansion and resolution of
infarctions, and affect the process of ischemic injury. However,
the long-term effects of Nrf2 function and SINO neuroprotection on
ischemic injury remain unclear (50). Previous studies have revealed the
mechanism of Nrf2 pathway (63,64).
The present study suggested that SINO-induced regulation of Nrf2
signaling may be an important pathway through which SINO protects
against MCAO-associated cerebral injury, although the precise
mechanism of action requires further investigation.
In conclusion, the findings of the present study
suggested that the SINO-induced regulation of Nrf2 signaling may be
an important pathway through which SINO protects against
MCAO-associated cerebral injury. The results indicated that SINO
treatment may activate Nrf2 in microglia and subsequently modulate
microglia polarization. The SINO-induced inhibition of M1
polarization and promotion of M2 polarization in microglia
contributed to its anti-inflammatory and cerebral protective
properties. Since the current MCAO model shares its pathogenesis
with numerous other cerebral diseases, these findings may provide
novel insights into the potential application of SINO for the
treatment of brain or other inflammatory diseases.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a grant from the
Natural Science Basic Research Program of Shaanxi (grant no.
2018JM7140).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FB, YZ and WL carried out the experimental work, as
well as the data collection and interpretation. FB and WL
participated in the design and coordination of experimental work
and acquisition of data. WL and KX carried out the study design;
the analysis and interpretation of data; and drafted the
manuscript. WL and KX confirmed the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Medical College of Xi'an Peihua University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jeon JH, Jung HW, Jang HM, Moon JH, Park
KT, Lee HC, Lim HY, Sur JH, Kang BT, Ha J and Jung DI: Canine model
of ischemic stroke with permanent middle cerebral artery occlusion:
Clinical features, magnetic resonance imaging, histopathology, and
immunohistochemistry. J Vet Sci. 16:75–85. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ito M, Shichita T, Okada M, Komine R,
Noguchi Y, Yoshimura A and Morita R: Bruton's tyrosine kinase is
essential for NLRP3 inflammasome activation and contributes to
ischaemic brain injury. Nat Commun. 6(7360)2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fu Y, Liu Q, Anrather J and Shi FD: Immune
interventions in stroke. Nat Rev Neurol. 11:524–535.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pont-Lezica L, Bechade C, Belarif-Cantaut
Y, Pascual O and Bessis A: Physiological roles of microglia during
development. J Neurochem. 119:901–908. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Subhramanyam CS, Wang C, Hu Q and Dheen
ST: Microglia-mediated neuroinflammation in neurodegenerative
diseases. Semin Cell Dev Biol. 94:112–120. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xu L, He D and Bai Y: Microglia-Mediated
inflammation and neurodegenerative disease. Mol Neurobiol.
53:6709–6715. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dong YF, Chen ZZ, Zhao Z, Yang DD, Yan H,
Ji J and Sun XL: Potential role of microRNA-7 in the
anti-neuroinflammation effects of nicorandil in astrocytes induced
by oxygen-glucose deprivation. J Neuroinflammation.
13(60)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Qin C, Zhou LQ, Ma XT, Hu ZW, Yang S, Chen
M, Bosco DB, Wu LJ and Tian DS: Dual functions of microglia in
ischemic stroke. Neurosci Bull. 35:921–933. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Skowron MA, Niegisch G, Albrecht P, van
Koeveringe G, Romano A, Albers P, Schulz WA and Hoffmann MJ:
Various mechanisms involve the nuclear factor (Erythroid-Derived
2)-Like (NRF2) to achieve cytoprotection in long-term
cisplatin-treated urothelial carcinoma cell lines. Int J Mol Sci.
18(1680)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fao L, Mota SI and Rego AC: Shaping the
Nrf2-ARE-related pathways in Alzheimer's and Parkinson's diseases.
Ageing Res Rev. 54(100942)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Robledinos-Anton N, Fernandez-Gines R,
Manda G and Cuadrado A: Activators and Inhibitors of NRF2: A review
of their potential for clinical development. Oxid Med Cell Longev.
2019(9372182)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Song S, Shen X, Tang Y, Wang Z, Guo W,
Ding G, Wang Q and Fu Z: Sinomenine pretreatment attenuates cold
ischemia/reperfusion injury in rats: The role of heme oxygenase-1.
Int Immunopharmacol. 10:679–684. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ko WC, Shieh JM and Wu WB: P38 MAPK and
Nrf2 activation mediated naked gold nanoparticle induced heme
oxygenase-1 expression in rat aortic vascular smooth muscle cells.
Arch Med Res. 51:388–396. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhao Z, Xiao J, Wang J, Dong W, Peng Z and
An D: Anti-inflammatory effects of novel sinomenine derivatives.
Int Immunopharmacol. 29:354–360. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cheng Y, Li F, Wang D, Zhang Y, Yuan F and
Zhang J: Sinomenine inhibits the expression of PD-L1 in the
peripheral blood mononuclear cells of mesangial proliferative
nephritis patients. Mol Med Rep. 7:1223–1228. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shen Q, Zhang X, Qi J, Shu G, Du Y and
Ying X: Sinomenine hydrochloride loaded thermosensitive liposomes
combined with microwave hyperthermia for the treatment of
rheumatoid arthritis. Int J Pharm. 576(119001)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tang J, Raza A, Chen J and Xu H: A
systematic review on the sinomenine derivatives. Mini Rev Med Chem.
18:906–917. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang Q and Li XK: Immunosuppressive and
anti-inflammatory activities of sinomenine. Int Immunopharmacol.
11:373–376. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jiang Y, Gao M, Wang W, Lang Y, Tong Z,
Wang K, Zhang H, Chen G, Liu M, Yao Y and Xiao X: Sinomenine
hydrochloride protects against polymicrobial sepsis via autophagy.
Int J Mol Sci. 16:2559–2573. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Qian L, Xu Z, Zhang W, Wilson B, Hong JS
and Flood PM: Sinomenine, a natural dextrorotatory morphinan
analog, is anti-inflammatory and neuroprotective through inhibition
of microglial NADPH oxidase. J Neuroinflammation.
4(23)2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sun Y, Yao Y and Ding CZ: A combination of
sinomenine and methotrexate reduces joint damage of collagen
induced arthritis in rats by modulating osteoclast-related
cytokines. Int Immunopharmacol. 18:135–141. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu S, Chen Q, Liu J, Yang X, Zhang Y and
Huang F: Sinomenine protects against E. coli-induced acute
lung injury in mice through Nrf2-NF-κB pathway. Biomed
Pharmacother. 107:696–702. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang Y, Yu C and Zhang H:
Lipopolysaccharides-mediated injury to chondrogenic ATDC5 cells can
be relieved by Sinomenine via downregulating microRNA-192.
Phytother Res. 33:1827–1836. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Bickler PE, Clark JP, Gabatto P and
Brosnan H: Hypoxic preconditioning and cell death from
oxygen/glucose deprivation co-opt a subset of the unfolded protein
response in hippocampal neurons. Neuroscience. 310:306–321.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu Y, Lu L, Hettinger CL, Dong G, Zhang
D, Rezvani K, Wang X and Wang H: Ubiquilin-1 protects cells from
oxidative stress and ischemic stroke caused tissue injury in mice.
J Neurosci. 34:2813–2821. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wu WN, Wu PF, Chen XL, Zhang Z, Gu J, Yang
YJ, Xiong QJ, Ni L, Wang F and Chen JG: Sinomenine protects against
ischaemic brain injury: Involvement of co-inhibition of
acid-sensing ion channel 1a and L-type calcium channels. Br J
Pharmacol. 164:1445–1459. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Qiu J, Yan Z, Tao K, Li Y, Li Y, Li J,
Dong Y, Feng D and Chen H: Sinomenine activates astrocytic dopamine
D2 receptors and alleviates neuroinflammatory injury via the
CRYAB/STAT3 pathway after ischemic stroke in mice. J
Neuroinflammation. 13(263)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bi F, Chen F, Li Y, Wei A and Cao W:
Klotho preservation by Rhein promotes toll-like receptor 4
proteolysis and attenuates lipopolysaccharide-induced acute kidney
injury. J Mol Med (Berl). 96:915–927. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang B, Tian S, Wang J, Han F, Zhao L,
Wang R, Ning W, Chen W and Qu Y: Intraperitoneal administration of
thioredoxin decreases brain damage from ischemic stroke. Brain Res.
1615:89–97. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Qin T, Du R, Huang F, Yin S, Yang J, Qin S
and Cao W: Sinomenine activation of Nrf2 signaling prevents
hyperactive inflammation and kidney injury in a mouse model of
obstructive nephropathy. Free Radic Biol Med. 92:90–99.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yin S and Cao W: Toll-like receptor
signaling induces Nrf2 pathway activation through p62-Triggered
Keap1 degradation. Mol Cell Biol. 35:2673–2683. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu Q, Zhang F, Zhang X, Cheng R, Ma JX,
Yi J and Li J: Fenofibrate ameliorates diabetic retinopathy by
modulating Nrf2 signaling and NLRP3 inflammasome activation. Mol
Cell Biochem. 445:105–115. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wan JJ, Wang PY, Zhang Y, Qin Z, Sun Y, Hu
BH, Su DF, Xu DP and Liu X: Role of acute-phase protein ORM in a
mice model of ischemic stroke. J Cell Physiol. 234:20533–20545.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang F, Zhang JG, Yang W, Xu P, Xiao YL
and Zhang HT: 6-Gingerol attenuates LPS-induced neuroinflammation
and cognitive impairment partially via suppressing astrocyte
overactivation. Biomed Pharmacother. 107:1523–1529. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zalewska-Ziob M, Adamek B, Kasperczyk J,
Romuk E, Hudziec E, Chwalińska E, Dobija-Kubica K, Rogoziński P and
Bruliński K: Activity of antioxidant enzymes in the tumor and
adjacent noncancerous tissues of non-small-cell lung cancer. Oxid
Med Cell Longev. 2019(2901840)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Virani SS, Alonso A, Benjamin EJ,
Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR,
Cheng S, Delling FN, et al: Heart disease and stroke
statistics-2020 update: A report from the American heart
association. Circulation. 141:e139–e596. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kernan WN, Ovbiagele B, Black HR, Bravata
DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck
DV, et al: Guidelines for the prevention of stroke in patients with
stroke and transient ischemic attack: A guideline for healthcare
professionals from the American heart association/American stroke
association. Stroke. 45:2160–2236. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Go AS, Mozaffarian D, Roger VL, Benjamin
EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al:
Heart disease and stroke statistics-2014 update: A report from the
American heart association. Circulation. 129:e28–e292.
2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Khoshnam SE, Winlow W, Farbood Y,
Moghaddam HF and Farzaneh M: Emerging roles of microRNAs in
ischemic stroke: As possible therapeutic agents. J Stroke.
19:166–187. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li WX, Qi F, Liu JQ, Li GH, Dai SX, Zhang
T, Cheng F, Liu D and Zheng SG: Different impairment of immune and
inflammation functions in short and long-term after ischemic
stroke. Am J Transl Res. 9:736–745. 2017.PubMed/NCBI
|
|
42
|
Cowan M and Petri WA Jr: Microglia: Immune
regulators of neurodevelopment. Front Immunol.
9(2576)2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lee JH, Wei ZZ, Cao W, Won S, Gu X, Winter
M, Dix TA, Wei L and Yu SP: Regulation of therapeutic hypothermia
on inflammatory cytokines, microglia polarization, migration and
functional recovery after ischemic stroke in mice. Neurobiol Dis.
96:248–260. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wu HY, Tang XQ, Liu H, Mao XF and Wang YX:
Both classic Gs-cAMP/PKA/CREB and alternative
Gs-cAMP/PKA/p38beta/CREB signal pathways mediate
exenatide-stimulated expression of M2 microglial markers. J
Neuroimmunol. 316:17–22. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wu HY, Tang XQ, Mao XF and Wang YX:
Autocrine interleukin-10 mediates glucagon-like peptide-1
receptor-induced spinal microglial β-endorphin expression. J
Neurosci. 37:11701–11714. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Miao H, Li R, Han C, Lu X and Zhang H:
Minocycline promotes posthemorrhagic neurogenesis via M2 microglia
polarization via upregulation of the TrkB/BDNF pathway in rats. J
Neurophysiol. 120:1307–1317. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Dai J, Ding Z, Zhang J, Xu W, Guo Q, Zou
W, Xiong Y, Weng Y, Yang Y, Chen S, et al: Minocycline relieves
depressive-like behaviors in rats with bone cancer pain by
inhibiting microglia activation in hippocampus. Anesth Analg.
129:1733–1741. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Michels M, Abatti MR, Avila P, Vieira A,
Borges H, Carvalho C Jr, Wendhausen D, Gasparotto J, Tiefensee
Ribeiro C, Moreira JC, et al: Characterization and modulation of
microglial phenotypes in an animal model of severe sepsis. J Cell
Mol Med. 24:88–97. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kata D, Foldesi I, Feher LZ, Hackler L Jr,
Puskas LG and Gulya K: Rosuvastatin enhances anti-inflammatory and
inhibits pro-inflammatory functions in cultured microglial cells.
Neuroscience. 314:47–63. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Liu L, Locascio LM and Dore S: Critical
role of Nrf2 in experimental ischemic stroke. Front Pharmacol.
10(153)2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhang R, Xu M, Wang Y, Xie F, Zhang G and
Qin X: Nrf2-a promising therapeutic target for defensing against
oxidative stress in stroke. Mol Neurobiol. 54:6006–6017.
2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hayes JD and Dinkova-Kostova AT: The Nrf2
regulatory network provides an interface between redox and
intermediary metabolism. Trends Biochem Sci. 39:199–218.
2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Benarroch EE: Nrf2, cellular redox
regulation, and neurologic implications. Neurology. 88:1942–1950.
2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Rajabian A, Rameshrad M and Hosseinzadeh
H: Therapeutic potential of Panax ginseng and its constituents,
ginsenosides and gintonin, in neurological and neurodegenerative
disorders: A patent review. Expert Opin Ther Pat. 29:55–72.
2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Kim KH, Lee D, Lee HL, Kim CE, Jung K and
Kang KS: Beneficial effects of Panax ginseng for the treatment and
prevention of neurodegenerative diseases: Past findings and future
directions. J Ginseng Res. 42:239–247. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Liu L, Vollmer MK, Fernandez VM, Dweik Y,
Kim H and Dore S: Korean red ginseng pretreatment protects against
long-term sensorimotor deficits after ischemic stroke likely
through Nrf2. Front Cell Neurosci. 12(74)2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Liu L, Vollmer MK, Ahmad AS, Fernandez VM,
Kim H and Dore S: Pretreatment with Korean red ginseng or dimethyl
fumarate attenuates reactive gliosis and confers sustained
neuroprotection against cerebral hypoxic-ischemic damage by an
Nrf2-dependent mechanism. Free Radic Biol Med. 131:98–114.
2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Shih AY, Li P and Murphy TH: A
small-molecule-inducible Nrf2-mediated antioxidant response
provides effective prophylaxis against cerebral ischemia in vivo. J
Neurosci. 25:10321–10335. 2005.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Vargas MR and Johnson JA: The Nrf2-ARE
cytoprotective pathway in astrocytes. Expert Rev Mol Med.
11(e17)2009.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Yang S, Ning F, Li J, Guo D, Zhang L, Cui
R and Liu Y: Therapeutic effect analysis of sinomenine on rat
cerebral ischemia-reperfusion injury. J Stroke Cerebrovasc Dis.
25:1263–1269. 2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Qiu J, Wang M, Zhang J, Cai Q, Lu D, Li Y,
Dong Y, Zhao T and Chen H: The neuroprotection of Sinomenine
against ischemic stroke in mice by suppressing NLRP3 inflammasome
via AMPK signaling. Int Immunopharmacol. 40:492–500.
2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Rastogi V, Santiago-Moreno J and Dore S:
Ginseng: A promising neuroprotective strategy in stroke. Front Cell
Neurosci. 8(457)2015.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Bartolini D, Dallaglio K, Torquato P,
Piroddi M and Galli F: Nrf2-p62 autophagy pathway and its response
to oxidative stress in hepatocellular carcinoma. Transl Res.
193:54–71. 2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Sun X, Chen L and He Z: PI3K/Akt-Nrf2 and
anti-inflammation effect of macrolides in chronic obstructive
pulmonary disease. Curr Drug Metab. 20:301–304. 2019.PubMed/NCBI View Article : Google Scholar
|