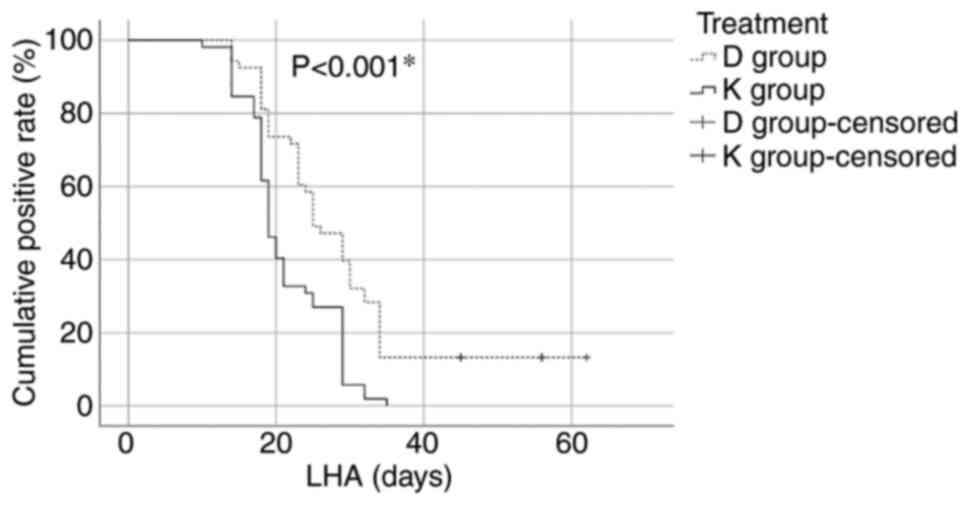
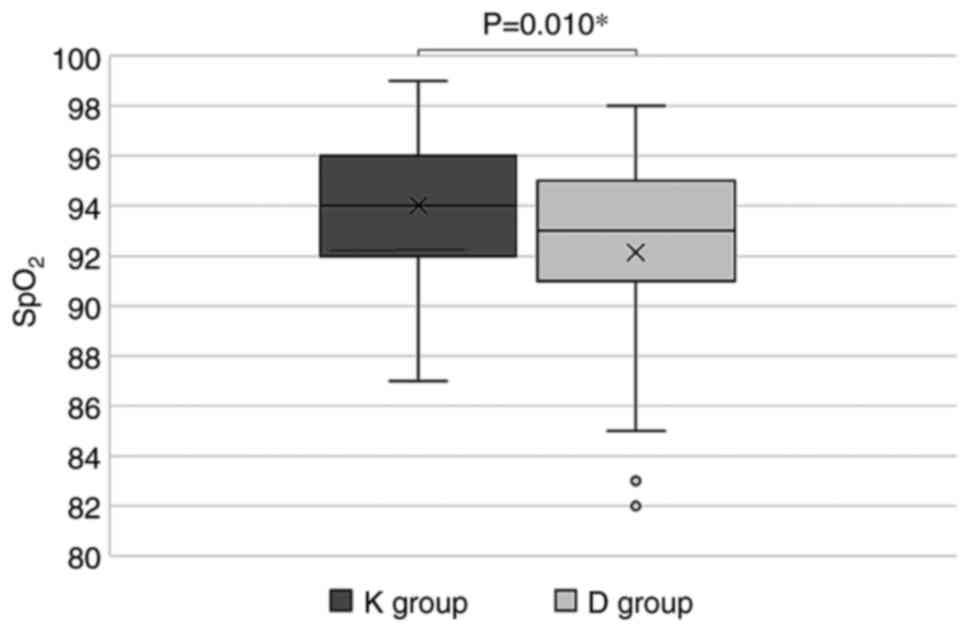
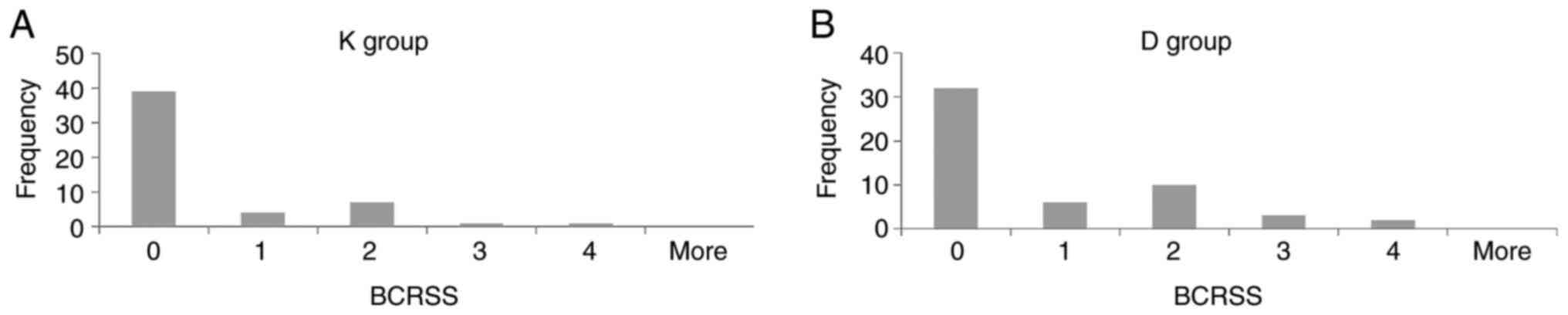
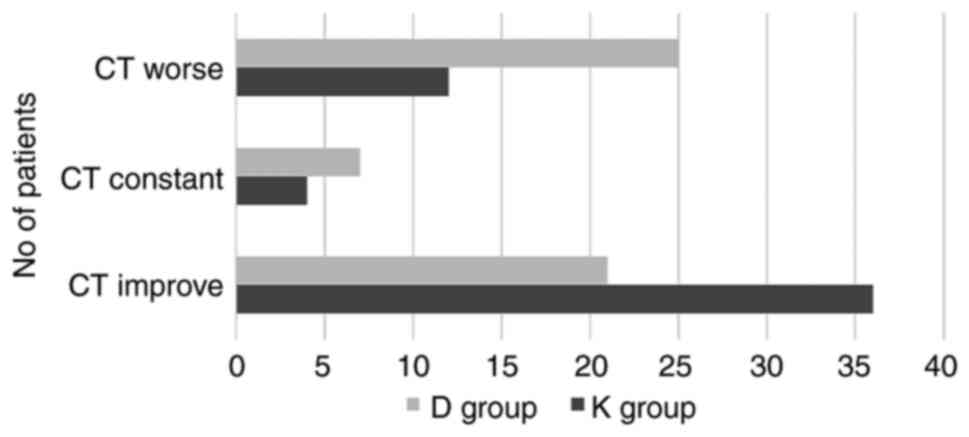
|
1
|
Docea AO, Tsatsakis A, Albulescu D,
Cristea O, Zlatian O, Vinceti M, Moschos SA, Tsoukalas D, Goumenou
M, Drakoulis N, et al: A new threat from an old enemy: Re-emergence
of coronavirus (Review). Int J Mol Med. 45:1631–1643.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
He W, Yi GY and Zhu Y: Estimation of the
basic reproduction number, average incubation time, asymptomatic
infection rate, and case fatality rate for COVID-19: Meta-analysis
and sensitivity analysis. J Med Virol. 92:2543–2550.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Acuti Martellucci C, Flacco ME, Cappadona
R, Bravi F, Mantovani L and Manzoli L: SARS-CoV-2 pandemic: An
overview. Adv Biol Regul. 77(10073)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Verity R, Okell LC, Dorigatti I, Winskill
P, Whittaker C, Imai N, Cuomo-Dannenburg G, Thompson H, Walker PGT,
Fu H, et al: Estimates of the severity of coronavirus disease 2019:
A model-based analysis. Lancet Infect Dis. 20:669–677.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
WHO: Middle East respiratory syndrome
coronavirus (MERS-CoV) 2019. Available from: https://www.who.int/emergencies/mers-cov/en/.
Accessed September 16, 2020.
|
|
6
|
WHO: Summary of probable SARS cases with
onset of illness from 1 November 2002 to 31 July 2003 2019.
Available from: https://www.who.int/csr/sars/country/table2003_09_23/en/.
Accessed September 16, 2020.
|
|
7
|
Kabir MT, Uddin MS, Hossain MF, Abdulhakim
JA, Alam MA, Ashraf GM, Bungau SG, Bin-Jumah MN, Abdel-Daim MM and
Aleya L: nCOVID-19 pandemic: From molecular pathogenesis to
potential investigational therapeutics. Front Cell Dev Biol.
8(616)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gautret P, Lagier JC, Parola P, Hoang VT,
Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE,
et al: Hydroxychloroquine and azithromycin as a treatment of
COVID-19: Results of an open-label non-randomized clinical trial.
Int J Antimicrob Agents. 56(105949)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Singh AK, Singh A, Shaikh A, Singh R and
Misra A: Chloroquine and hydroxychloroquine in the treatment of
COVID-19 with or without diabetes: A systematic search and a
narrative review with a special reference to India and other
developing countries. Diabetes Metab Syndr. 14:241–246.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mahévas M, Tran VT, Roumier M, Chabrol A,
Paule R, Guillaud C, Fois E, Lepeule R, Szwebel TA, Lescure FX, et
al: Clinical efficacy of hydroxychloroquine in patients with
covid-19 pneumonia who require oxygen: Observational comparative
study using routine care data. BMJ. 369(m1844)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Orkin C, DeJesus E, Khanlou H, Stoehr A,
Supparatpinyo K, Lathouwers E, Lefebvre E, Opsomer M, Van de
Casteele T and Tomaka F: Final 192-week efficacy and safety of
once-daily darunavir/ritonavir compared with lopinavir/ritonavir in
HIV-1-infected treatment-naïve patients in the ARTEMIS trial. HIV
Med. 14:49–59. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
U.S. National library of medicine:
Clinical trials. Available from: https://clinicaltrials.gov/ct2/home. Accessed
September 16, 2020.
|
|
13
|
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan
G, Ruan L, Song B, Cai Y, Wei M, et al: A trial of
lopinavir-ritonavir in adults hospitalized with severe Covid-19. N
Engl J Med. 382:1787–1799. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
De Meyer S, Bojkova D, Cinatl J, Van Damme
E, Buyck C, Van Loock M, Woodfall B and Ciesek S: Lack of antiviral
activity of darunavir against SARS-CoV-2. Int J Infect Dis.
97:7–10. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen J, Xia L, Liu L, Xu Q, Ling Y, Huang
D, Huang W, Song S, Xu S, Shen Y and Lu H: Antiviral activity and
safety of darunavir/cobicistat for the treatment of COVID-19. Open
Forum Infect Dis. 7(ofaa241)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Beigel JH, Tomashek KM, Dodd LE, Mehta AK,
Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, et
al: Remdesivir for the treatment of Covid-19-preliminary report. N
Engl J Med. 383:1813–1826. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
FDA: Fact sheet for health care providers
emergency use authorization (EUA) of remdesivir
(GS-5734™) 2020. Available from: https://www.fda.gov/media/137566/download. Accessed
September 16, 2020.
|
|
18
|
Negrut N, Nistor-Cseppento DC, Khan SA,
Pantis C, Maghiar TA, Maghiar O, Aleya S, Rus M, Tit DM, Aleya L,
et al: Clostridium difficile infection epidemiology over a period
of 8 years-a single centre study. Sustainability. 12(4439)2020.
|
|
19
|
Negruţ N, Khan S, Bungau S, Zaha D, Anca
C, Bratu O, Diaconu C and Ionita-Radu F: Diagnostic challenges in
gastrointestinal infections. Rom J Mil Med. 123:83–90. 2020.
|
|
20
|
Codrean A, Dumitrascu DL, Codrean V, Tit
DM, Bungau S, Aleya S, Rus M, Fratila O, Nistor-Cseppento DC, Aleya
L and Negrut N: Epidemiology of human giardiasis in Romania: A 14
years survey. Sci Total Environ. 705(135784)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
WHO: Clinical management of COVID-19:
Interim guidance 2020. Available from: https://www.who.int/publications/i/item/clinical-management-of-covid-19.
Accessed September 26, 2020.
|
|
22
|
Behl T, Kaur I, Bungau S, Kumar A, Uddin
MS, Kumar C, Pal G, Sahil Shrivastava K, Zengin G and Arora S: The
dual impact of ACE2 in COVID-19 and ironical actions in geriatrics
and pediatrics with possible therapeutic solutions. Life Sci.
257(118075)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rodriguez-Nava G, Yanez-Bello MA,
Trelles-Garcia DP, Chung CW, Friedman HJ and Hines DW: Performance
of the quick COVID-19 severity index and the Brescia-COVID
respiratory severity scale in hospitalized patients with COVID-19
in a community hospital setting. Int J Infect Dis. 102:571–576.
2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dreher M, Kersten A, Bickenbach J, Balfanz
P, Hartmann B, Cornelissen C, Daher A, Stöhr R, Kleines M, Lemmen
SW, et al: The characteristics of 50 hospitalized COVID-19 patients
with and without ARDS. Dtsch Arztebl Int. 117:271–278.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
WHO: Laboratory testing for coronavirus
disease (COVID-19) in suspected human cases: Interim guidance 2020.
Available from: https://apps.who.int/iris/handle/10665/331501,
Accessed September 16, 2020.
|
|
26
|
FDA: Allplex™ 2019-nCoV Assay 2020.
Available from: https://www.fda.gov/media/137178/download. Accessed
September 16, 2020.
|
|
27
|
Li TZ, Cao ZH, Chen Y, Cai MT, Zhang LY,
Xu H, Zhang JY, Ma CH, Liu Y, Gao LJ, et al: Duration of SARS-CoV-2
RNA shedding and factors associated with prolonged viral shedding
in patients with COVID-19. J Med Virol. 93:506–512. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhou B, She J, Wang Y and Ma X: The
duration of viral shedding of discharged patients with severe
COVID-19. Clin Infect Dis. 71:2240–2242. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shen C, Yue X, Wang J, Shi C and Li W:
Nocturnal oxygen therapy as an option for early COVID-19. Int J
Infect Dis. 98:176–179. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Calina D, Docea AO, Petrakis D, Egorov AM,
Ishmukhametov AA, Gabibov AG, Shtilman MI, Kostoff R, Carvalho F,
Vinceti M, et al: Towards effective COVID-19 vaccines: Updates,
perspectives and challenges (Review). Int J Mol Med. 46:3–16.
2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sánchez-Oro R, Torres Nuez J and
Martínez-Sanz G: Radiological findings for diagnosis of SARS-CoV-2
pneumonia (COVID-19). Med Clin (Barc). 155:36–40. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li Y, Xie Z, Lin W, Cai W, Wen C, Guan Y,
Mo X, Wang J, Wang Y, Peng P, et al: Efficacy and safety of
Lopinavir/Ritonavir or Arbidol in adult patients with mild/moderate
COVID-19: An exploratory randomized controlled trial. Med (NY).
1:105–113.e4. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Osborne V, Davies M, Lane S, Evans A,
Denyer J, Dhanda S, Roy D and Shakir S: Lopinavir-ritonavir in the
treatment of COVID-19: A dynamic systematic benefit-risk
assessment. Drug Saf. 43:809–821. 2020.PubMed/NCBI View Article : Google Scholar
|