Introduction
Coronaviruses are an ancient group of viruses so
named after a crown-like spike which decorates their surface, able
to cross the species barrier. Common human coronaviruses (229E,
NL63, OC43, HKU1) are responsible for the flu-like syndrome, and
only in rare cases induce lower respiratory tract manifestations.
Subtypes that have crossed the species barrier have been shown to
be responsible for severe forms of the diseases in humans (1).
The best-known viruses that can make this leap
include Middle East respiratory syndrome coronavirus (MERS-CoV),
severe acute respiratory syndrome coronavirus (SARS-CoV), and more
recently severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2). SARS-CoV-2 is responsible for the pandemic that
started on March 2020. The clinical scenario is variable and
individualized. Clinical manifestations are found in about half of
the cases as flu-like syndromes, and only 10-15% from them evolve
into life-threatening complications such as acute pneumonia, acute
respiratory distress syndrome, disseminated intravascular
coagulation, and even death (2,3).
Although the case fatality rate (2.29%) (4) remains much lower compared to MERS-CoV
(37.1%) (5) and SARS-CoV (9.6%)
(6), COVID-19 is still an unsolved
issue because of the mortality rate which ranges proportional to
age (4), and the high rate of
transmission of the virus (7).
Antiviral treatment for COVID-19 is still a
challenge. To date, studies have not been able to identify a
sufficiently potent antiviral drug for infections with SARS-CoV-2.
Yet, several molecules have been studied to identify effective
treatment.
Hydroxychloroquine has been approved for the
treatment of malaria and autoimmune diseases, with an antiviral
effect by inhibiting fusion of the virus with the cellular membrane
of the host, by blocking the release of the viral genome and by
immunomodulatory effects. Yet, results regarding its effectiveness
for COVID-19 are contradictory until now (8-10).
Hydroxychloroquine combined with azithromycin has also been
proposed for the treatment of COVID-19. This combination has
antiviral effects by decreasing viral replication (via interferon),
by decreasing the effect of inflammatory cytokines and stimulating
neutrophil activation. Also, the effect of hydroxychloroquine is
reinforced by the presence of the antibiotic. The combination of
the two has been intensively studied, but the results are
contradictory to date (8).
HIV protease inhibitors [lopinavir (LPV) with
ritonavir (r), and darunavir (DRV) with cobicistat (c) or ritonavir
(r)] used until now in the treatment of HIV infection has also
attracted the attention of the international medical community as
possible potent drugs in the fight against SARS-CoV-2. Their effect
could be mediated by inhibiting the viral 3-chymotrypsin-like
protease, necessary for viral replication. So far, the DRV/r and
LPV/r combinations have been shown to have the same mechanism for
inhibiting the HIV replication, but DRV/r appears to be more
effective and with fewer adverse effects (11). Starting from these premises, the
present study aimed to investigate the effect of these drugs on
SARS-CoV-2 infection. To date, there are 2 therapeutic trials that
use DRV/r as a treatment for COVID-19 (ClinicalTrials.gov Identifier: NCT04252274;
NCT04425382) and 42 that use LPV/r (12). Although some studies support the
ineffectiveness of HIV protease inhibitor treatment, many studies
are still ongoing, thus the conclusions are still distant (13-15).
Remdesivir is a nucleotide analogue with favorable
results in the case of COVID-19, by inhibiting viral replication
(16). The drug was recently
approved by the US Food and Drug Administration for use in severe
forms of infection (17). Although
the drug has the most promising results among the molecules
investigated for SARS-CoV-2 infection, the high price and limited
access for most patients make it practically unusable at this
moment on a large scale (16).
Therapeutic options for patients with COVID-19 are
limited, expensive, and with molecules whose efficacy is
contradictory in current studies.
The geographical area of Romania, southeastern
Europe, is characterized by various infectious pathologies
(18-20).
In a field often exposed to infectious agents, the current pandemic
with COVID-19 was initially associated with a lower severity
compared to other parts of the world. The therapeutic approach of
the present study, in this context, was made considering the
particularities of our geographical area.
Based on the data available to date and considering
the fact that the combinations of LPV/r and DRV/r have the same
mechanism of action, this study aimed to evaluate the efficacy of
the two combinations in COVID-19 patients with moderate form of the
disease.
Patients and methods
Protocol
A 3-month retrospective study from April 1, 2020,
until June 30, 2020 was conducted at the Department of Infectious
Disease, Municipal Hospital Oradea ‘Gavril Curteanu’, Oradea, Bihor
County, Romania. All adult patients, diagnosed with COVID-19 with
imaging of pulmonary infiltrates on chest computed tomography (CT),
and who had no history of previous antiretroviral therapy were
included in the study. The diagnosis and the therapy were based on
the guidance of the World Health Organization (21), diagnosis and treatment guidelines
for COVID-19 and local hospital guide (7,22).
Confirmation of COVID-19 was performed using a single positive test
which highlighted the RNA of the virus in the upper respiratory
tract specimens (nasopharyngeal and oropharyngeal) using real-time
polymerase chain reaction (qPCR) assay.
Patients included in the study were treated with
lopinavir/ritonavir (LPV/r) [Kaletra (K)] (200/50 mg) (2 tablets
q12 h) for 10 or 14 days (K group), or darunavir/ritonavir (DRV/r);
800 mg DRV (1 tablet qDay) plus ritonavir 100 mg (1 tablet qDay)
for 10 or 14 days (D group). All treatment regimens had associated
Plaquenil, antibiotic and anticoagulant. The duration of antiviral
therapy was a maximum of 14 days (optionally 10 if patients had 2
consecutive negative qPCR tests at 24-h intervals, at 10 day
checks). Patients were discharged after two consecutive negative
qPCR tests, collected at 24-h intervals.
The demographic data (age, sex, residence), past
medical history [obesity, cardiovascular disease comorbidities
(CVC), chronic pulmonary diseases (CPD), digestive comorbidities
(DC), diabetes mellitus (DM), neoplasm (N), chronic kidney disease
(CKD)], toxic abuse (smoker), clinical manifestations (stomatitis,
abdominal pain, nausea, vomiting, diarrhea, tachycardia), values
for complete blood count and liver function tests (transaminase),
qPCR test results of nasal or pharyngeal exudate, imaging aspects
on chest-CT, and length of hospital admission (LHA) (calculated
from the first positive qPCR test to discharge), period from onset
to hospitalization (POH), and Brescia-COVID Respiratory Severity
Scale (BCRSS) for all patients included in the study were collected
and subsequently analyzed. BCRSS was applied for the first time in
Italy, for patients with COVID-19 and pneumonia, and aims to assess
the clinical severity of each patient admitted to the hospital
(23). Dyspnea, tachypnoea, chest
imaging and oxygen saturation levels (SpO2)/partial
pressure of arterial oxygen (PaO2), Horowitz Index for
Lung Function [PaO2/fraction of inspired oxygen
(FiO2) ratio], non-invasive or invasive ventilation are
parameters used for the scoring. Score values can range from 0 to
8, directly proportional to the severity of the case (24). Unsatisfactory clinical evolution was
considered if patients had a decrease in oxygen saturation, and
PaO2/FiO2 ratio <300. BCRSS was calculated
at the admission to the hospital for all cases and was repeated at
the moment when unsatisfactory clinical evolution was suspected.
Liver injury was considered in the case of alanine aminotransferase
(ALAT) level elevated more than three times above the upper limit
of normal. Patients with HIV infection, chronic liver diseases,
dermatological diseases, inflammatory bowel diseases, with diarrhea
or stomatitis, those previously treated with antiretroviral
therapy, re-admitted for COVID-19, treated <10 days previously
with antivirals, or received dual treatment with LPV and DRV, or
BCRSS at admission to the hospital >3, were excluded from the
study.
All patients signed an informed consent at admission
in the hospital and before the start of the antiviral treatment.
The study was approved by the Ethics Commission of Medicine and
Pharmacy Faculty, University of Oradea (no. 5/09.21.2020) and
followed the World Medical Association Code of Ethics (Declaration
of Helsinki, 1967).
Diagnosis of CDI
All patients were tested according to World Health
Organization (WHO) recommendations by the qPCR assay (25). The test available in our clinic,
during the time period followed was Allplex™ 2019-nCoV
Assay (CFX96™ RT-PCR Detection Systems, Bio-Rad
Laboratories, Inc.) (limit of detection 4167 copies/ml). Target
genes were E gene (Sarbecovirus), RdRP gene (SARS-CoV-2) and N gene
(SARS-CoV-2). The positive percent agreement of the technique was
100% (95% CI: 92.75-100), and the negative percent agreement was
96.84% (95% CI: 90.39-99.18) (26).
If at least one target sequence of the SARS-CoV-2 viral genome was
identified, then the sample was considered positive. The
nasopharyngeal and oropharyngeal swabs were collected, stored and
transported according to WHO recommendations (25).
SpO2 was evaluated at least twice a day,
and whenever the patient's clinical condition had worsened, using a
Hunan Accurate pulse oximeter. In case of a patients with a
decrease in oxygen saturation, PaO2/FiO2
ratio was performed, using EPOC Blood Analysis System (Siemens
Medical Solutions) from arterial blood. CBC determination was
performed using venous blood samples collected in Tri-potassium
ethylenediaminetetraacetic acid tubes. All specimens were
immediately transported to the hospital laboratory. The test was
performed using Beckman Coulter 628134 UniCel DxH 800 Haematology
analyzer (Beckman Coulter International S.A.). Reference values
were interpreted according to age and sex of the patients. To
determine the blood transaminase level, the venous blood samples
were collected in tubes without anticoagulant, after a fasting
period, and transported immediately to the hospital laboratory. The
test was performed using Beckman Coulter AU5811 Chemistry analyzer
(Beckman Coulter International S.A.). The reference values were
processed according to age, sex, and assay used. All patients were
evaluated by chest computed tomography (CT) at admission and on day
10.
Statistical analysis
The Statistical Package for the Social Sciences
(SPSS), version 26 (IBM Corp.) was used to process data and perform
statistical analysis. Quantitative data are presented as means and
standard deviation (SD), and qualitative data as numbers (N) and
proportions (%). The calculation of the P-value was realized using
Student's t-test, Chi-square test, Mann-Whitney U test, and
log-rank test for Kaplan-Meier method. Statistical significance was
considered for a P-value of less than 0.05.
Results
During the study period, 205 patients with COVID-19
were diagnosed at the Department of Infectious Disease, Municipal
Hospital Oradea ‘Gavril Curteanu’, Oradea, Bihor County, Romania.
From these, only 105 met the criteria of inclusion in the study. A
total of 52 patients were included in the K group and 53 in D
group. No patient required discontinuation of antiviral therapy.
The demographic characteristics of the two groups did not present
statistically significantly differences (Table I).
 | Table IBaseline characteristics of the
patients with COVID-19. |
Table I
Baseline characteristics of the
patients with COVID-19.
| Parameter | Group K | Group D | P-value |
|---|
| DD | | | |
|
Age, years,
mean ± SD | 36.45±14.99 | 36.73±13.80 | 0.922a |
|
Female, n
(%) | 35 (67.30%) | 26 (49.05%) | 0.058b |
|
Rural area,
n (%) | 26 (50%) | 31 (58.49%) | 0.383b |
|
SpO2,
mean ± SD | 95.29±1.97 | 95.27±2.21 | 0.952a |
|
Smoker, N
(%) | 18 (34.61%) | 15 (28.30%) | 0.486b |
|
POH, mean ±
SD | 3.31±1.05 | 3.27±1.03 | 0.828a |
| PMH, n (%) | | | |
|
Obesity | 14 (26.92%) | 10 (18.86%) | 0.326b |
|
CVC | 16 (30.76%) | 21 (39.62%) | 0.342b |
|
CPD | 9 (17.30%) | 7 (13.20%) | 0.559b |
|
DC | 9 (17.30%) | 12 (22.64%) | 0.495b |
|
DM | 8 (15.38%) | 6 (11.32%) | 0.540b |
|
N | 5 (9.61%) | 7 (13.20%) | 0.563b |
|
CKD | 18 (34.61%) | 15 (28.30%) | 0.387b |
| Clinical
manifestations, n (%) | | | |
|
Stomatitis | 0 (0%) | 1 (0.01%) | 0.320b |
|
Diarrhea | 3 (0.05%) | 4 (0.07%) | 0.715b |
|
Tachycardia | 2 (0.03%) | 1 (0.01%) | 0.481b |
|
Abdominal
pain | 4 (7.69%) | 6 (11.32%) | 0.527b |
|
Nausea | 6 (11.54%) | 2 (3.77%) | 0.157b |
|
Vomiting | 3 (5.77%) | 0 (0.00%) | 0.083b |
| CBC, mean ± SD | | | |
|
WBC
(x103/mm3) | 9.74±2.42 | 10.79±3.67 | 0.084a |
|
RBC
(x106/mm3) | 4.30±0.84 | 4.18±0.90 | 0.506a |
|
PLT
(x105/mm3) | 3.63±1.34 | 4.04±1.15 | 0.096a |
|
LFT, mean ±
SD | | | |
|
ALAT
(mg%) | 32.87±15.34 | 27.92±16.99 | 0.120a |
|
BCRSS | 0.06±0.24 | 0.13±0.34 | 0.196a |
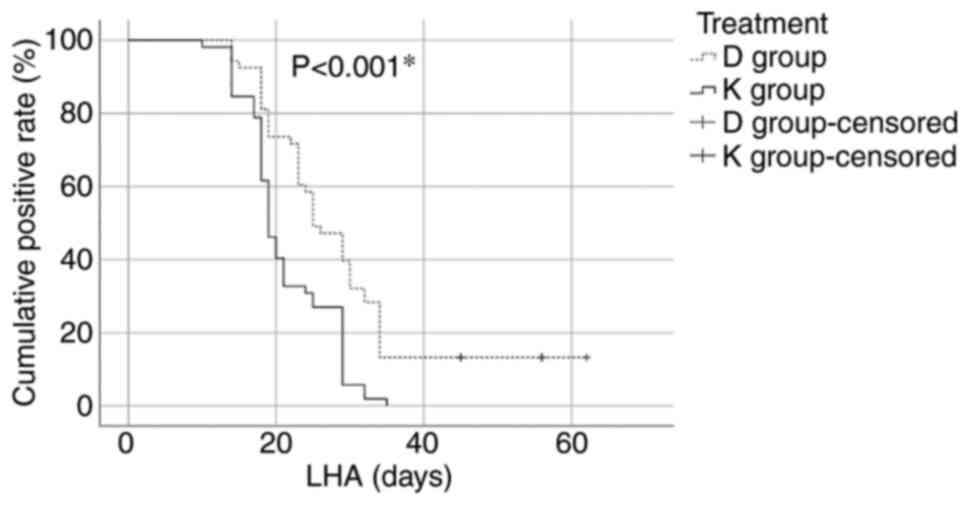
The length of hospital admission (LHA) value was
statistically significantly higher in the D group compared with the
K group (28.71±11.78 vs. 21.25±5.99, P<0.001). The number of
patients with viral clearance differed significantly after 21 days
from hospital admission [35 (67.30%) in the K group vs. 14 (26.41%)
in the D group, P<0.001) (Fig.
1).
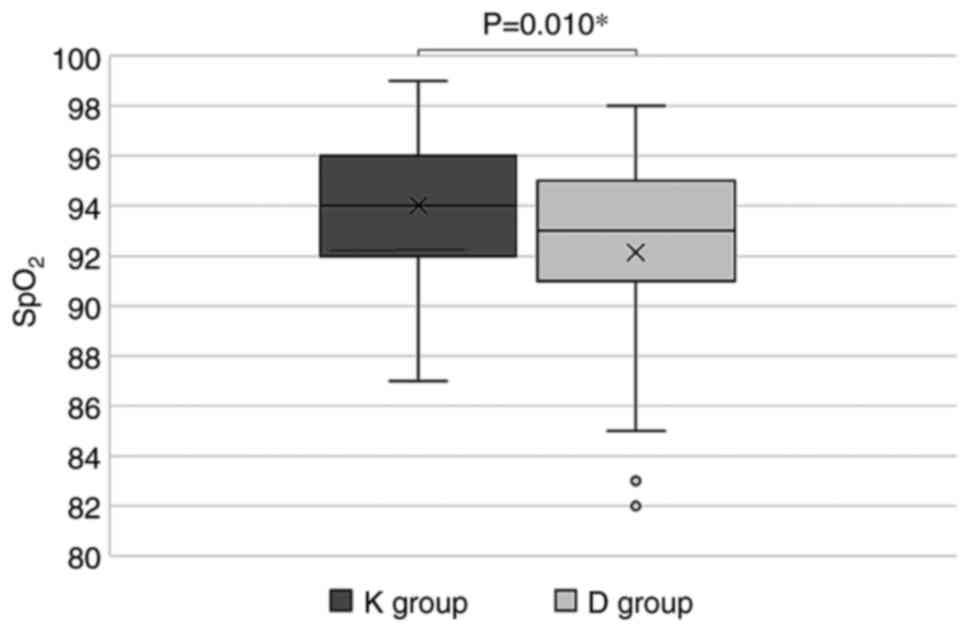
During hospitalization, the lowest blood oxygen
saturation values observed in the K group were statistically
significantly higher compared to the D group (94.02±3.12% vs.
92.13±4.24%, P=0.010) (Fig. 2) but
the number of patients with unsatisfactory clinical evolution did
not differ statistically significantly in the two groups [12
(23.08%) in the K group vs. 20 (37.74%) in the D group,
P=0.157].
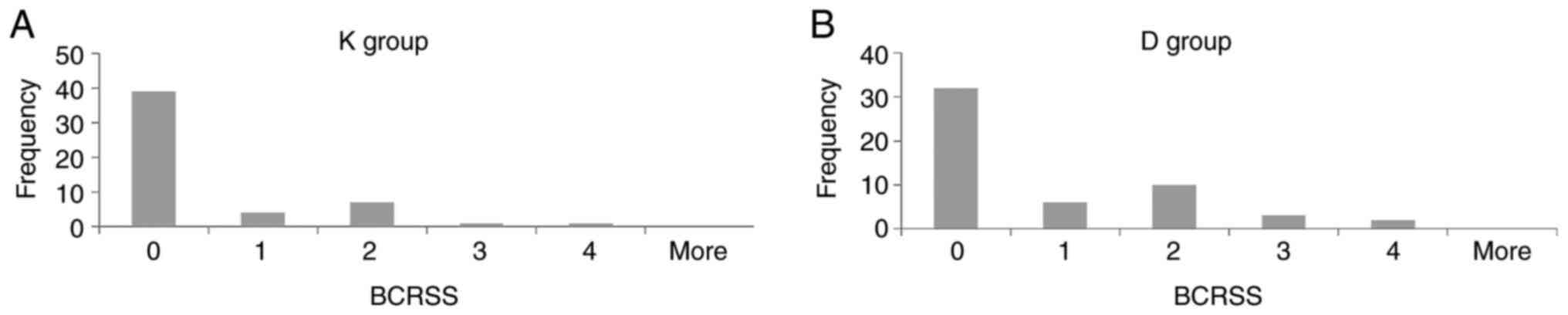
There were no statistically significant differences
between the BCRSS of the patients with unsatisfactory clinical
evolution for the two groups (0.48±0.95 in the K group vs.
0.81±1.16 in the D group, P=0.111). For all the data we plotted the
distribution by using histograms (Fig.
3).
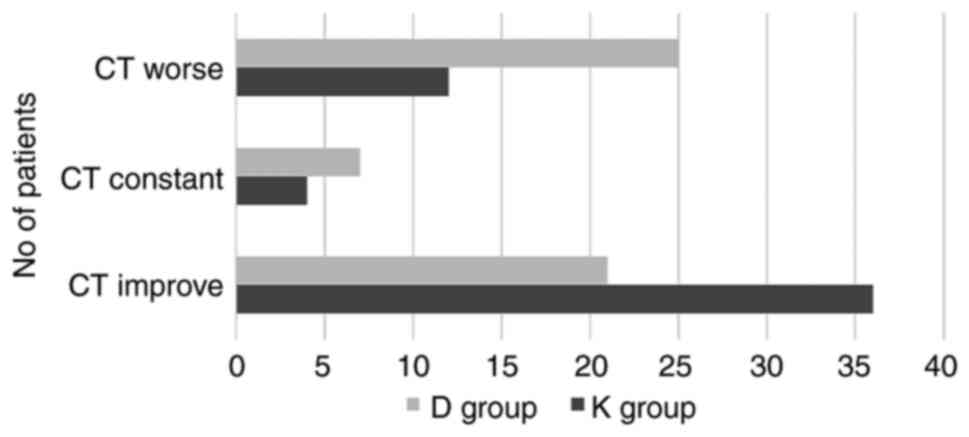
The evolution of chest CT at 10 days in the K group
improved in 69.23% patients, remained constant for 7.69%, and
became worse for 23.08% patients, compared to the D group where
39.62% patients had an improved evolution, 13.21% remained constant
and 47.17% became worse. The results were not statistically
significantly different (P=0.075) (Fig.
4). Stomatitis, diarrhea, abdominal pain, nausea, vomiting,
tachycardia and liver injury were identified as adverse effects
(AE) during the hospitalization of the patients (Table II). AE were more common in group K
compared to group D, but the difference was not statistically
significant (P=0.101), except for diarrhea (P=0.002).
 | Table IIAdverse effects in patients with
COVID-19 during the treatment period. |
Table II
Adverse effects in patients with
COVID-19 during the treatment period.
| | Treatment
groups | |
|---|
| AE, n (%) | K group (n=52)
(%) | D group (n=53)
(%) |
P-valuea |
|---|
| Stomatitis | 8 (15.38) | 5 (9.43) | 0.405 |
| Diarrhea | 22 (42.31) | 6 (11.32) | 0.002 |
| Abdominal pain | 14 (26.92) | 7 (13.21) | 0.126 |
| Nausea | 7 (13.46) | 5 (9.43) | 0.563 |
| Vomiting | 3 (5.77) | 0 (0.00) | 0.083 |
| Tachycardia | 6 (11.54) | 5 (9.43) | 0.763 |
| Liver injury | 12 (23.08) | 9 (16.98) | 0.512 |
| Total | 28 (53.85) | 17 (32.08) | 0.101 |
Discussion
Treatment for COVID-19 is still far from being
standardized. Therapeutic trials conducted have not been able to
present an effective antiviral drug against SARS-CoV-2, to date. To
our knowledge, there is only one ongoing study in the world
comparing the effectiveness of the two antivirals (LPV vs. DRV) to
date (12).
The median duration of viral shedding varies
depending on the form of the disease and the treatment used from 11
days for mild cases (27) to 31
days in patients with severe illness (28). In our study, patients treated with
lopinavir/ritonavir (LPV/r) had a viral shedding period of 21.25
days, and those with darunavir/ritonavir (DRV/r) had 28.71 days,
but the number of patients who became negative on qPCR testing was
significantly different between the two groups only after day 21 of
hospitalization. The results obtained by us fall within the time
periods presented by the medical literature. Their importance lies
in the fact that although until now it is known that the presence
of viral RNA in a test is not necessarily identically with the
infectivity of the case, we do not have exact data about the
latter.
In this study, patients treated with LPV/r required
7.5 days less compared to those treated with DRV/r, until viral
clearance was obtained. Considering that patients were discharged
at the time of obtaining viral clearance, the decrease in the
hospitalization within a week leads to cost reduction and a higher
turnover of patients, in the moment which the number of places
available for hospitalization of COVID-19 patients is insufficient.
In a study published in 2020, performed on 30 Chinese patients
diagnosed with COVID-19, it was claimed that a 5-day treatment with
DRV did not increase the proportion of negative test results
compared to standard care alone (15). Cao et al concluded, in a
study of 199 Chinese patients with SARS-Cov-2 in Wuhan, Hubei
Province, China, that 10 days of LPV/r treatment did not reduce the
duration of viremia detection compared to standard care alone
(13).
Our study identified that in cases of COVID-19,
LPV/r treatment maintained statistically significantly higher
oxygen saturation values compared with those measured for the group
with DRV/r therapy, with a difference of almost 1.89%; but the
number of cases that developed acute respiratory failure did not
differ significantly between the two groups. Shen et al
demonstrated that placing a COVID-19 patient in an nocturnal
oxygen-rich environment slowed down viral replication, decreased
angiotensin-converting enzyme 2 receptor expression at the target
cell, improved antiviral immune response via interferons, T and
natural killer cells, and prevented disease progression to the
severe form (29).
The prompt modification of therapy according to the
changes in the clinical condition of COVID-19 patients is important
for the further evolution of the disease (7,30).
Based on relatively few elements, but specific to lung damage, the
Brescia-COVID Respiratory Severity Scale (BCRSS) quickly alerts the
clinician about the increasing levels of respiratory severity. The
study did not identify statistically significant results for the
BCRSS in the two treatment groups. The results obtained by us are
in accordance with the medical literature. Thus, in the study by
Cao et al, it was confirmed that LPV/r treatment in severe
forms of the disease did not show obvious benefits (13).
The radiological aspect of the lung in SARS-Cov2
infection has a critical role in establishing the diagnosis and
follow-up of patients. Pneumonia in cases of COVID-19 patients
requires follow-up during evolution, to provide quick management in
the case of aggravation (31). In
our study, although treatment with LPV/r determined a satisfactory
evolution of the chest CT in a higher percentage of patients,
compared to the group treated with LPV/r, we did not register a
statistically significant difference. The results obtained by us
are the same as the current medical literature. Similarly, in a
study by Li et al on 86 Chinese patients with mild/moderate
COVID-19, the authors did not find improvement of chest CT after
LPV/r treatment, compared with patients without antiviral therapy
or those treated with Arbidol (32). To date, no data are available in the
medical literature referring to chest CT in COVID-19 patients
treated with DRV/r.
Adverse effects (AEs) affect treatment compliance,
and some may even make it impossible for their administration.
SARS-CoV-2 infection is responsible for multi-organ damage;
therefore overlapping post-medication AEs are not desirable.
Gastrointestinal manifestations, changes in renal function,
hypersensitivity reactions, prolongation of the QT interval, blood
dyscrasias are the most frequent AE of LPV/r administration in case
of patients with COVID-19, cited in the medical literature
(33). In the present study,
diarrhea was more frequently reported in patients treated with
LPV/r compared to DRV/r (42.31% vs. 11.32%), but the number of
patients with AEs after antiviral treatment did not statistically
significantly differ in the two groups (53.85% vs. 32.08%). Both
drugs were well tolerated, and it was not necessary to stop their
administration due to AEs. The studies with DRV/r in COVID-19
patients do not report any notable AEs for the drug (15). Osborne et al, in a systematic
benefit-risk assessment made on 143 papers in 2020, concluded that
the benefit-risk profile of the LPV/r therapy for patients infected
with SARS-CoV-2 is still not positive without other data (33).
In conclusion, although treatment with LPV/r or
DRV/r is still recommended, due to the lack of therapeutic
alternatives in SARS-CoV-2 infection, the effects of the two
therapies are far from that which one could expect. Considering our
experience, except for a few AEs that could be managed
conservatively without interruption of administration, the
treatment did not show notable AEs.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data are registered at the Municipal Hospital
Oradea ‘Gavril Curteanu’, Oradea, Bihor County, Romania.
Authors' contributions
NN, AC, IH and DCNC collected, analyzed and
interpreted the patient data. NN, SB, DMT, RM, TB, CCD, and FB made
substantial contributions to the conception of the work and
interpretation of the data; also, they drafted the manuscript and
were major contributors in writing the manuscript. All authors read
and approved the final manuscript to be published. All the authors
agreed to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All patients signed an informed consent at the time
of admission to the hospital and before the start of the antiviral
treatment. The study was approved by the Ethics Commission of
Medicine and Pharmacy Faculty, University of Oradea (no.
5/09.21.2020) and followed the World Medical Association Code of
Ethics (Declaration of Helsinki, 1967).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Docea AO, Tsatsakis A, Albulescu D,
Cristea O, Zlatian O, Vinceti M, Moschos SA, Tsoukalas D, Goumenou
M, Drakoulis N, et al: A new threat from an old enemy: Re-emergence
of coronavirus (Review). Int J Mol Med. 45:1631–1643.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
He W, Yi GY and Zhu Y: Estimation of the
basic reproduction number, average incubation time, asymptomatic
infection rate, and case fatality rate for COVID-19: Meta-analysis
and sensitivity analysis. J Med Virol. 92:2543–2550.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Acuti Martellucci C, Flacco ME, Cappadona
R, Bravi F, Mantovani L and Manzoli L: SARS-CoV-2 pandemic: An
overview. Adv Biol Regul. 77(10073)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Verity R, Okell LC, Dorigatti I, Winskill
P, Whittaker C, Imai N, Cuomo-Dannenburg G, Thompson H, Walker PGT,
Fu H, et al: Estimates of the severity of coronavirus disease 2019:
A model-based analysis. Lancet Infect Dis. 20:669–677.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
WHO: Middle East respiratory syndrome
coronavirus (MERS-CoV) 2019. Available from: https://www.who.int/emergencies/mers-cov/en/.
Accessed September 16, 2020.
|
|
6
|
WHO: Summary of probable SARS cases with
onset of illness from 1 November 2002 to 31 July 2003 2019.
Available from: https://www.who.int/csr/sars/country/table2003_09_23/en/.
Accessed September 16, 2020.
|
|
7
|
Kabir MT, Uddin MS, Hossain MF, Abdulhakim
JA, Alam MA, Ashraf GM, Bungau SG, Bin-Jumah MN, Abdel-Daim MM and
Aleya L: nCOVID-19 pandemic: From molecular pathogenesis to
potential investigational therapeutics. Front Cell Dev Biol.
8(616)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gautret P, Lagier JC, Parola P, Hoang VT,
Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE,
et al: Hydroxychloroquine and azithromycin as a treatment of
COVID-19: Results of an open-label non-randomized clinical trial.
Int J Antimicrob Agents. 56(105949)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Singh AK, Singh A, Shaikh A, Singh R and
Misra A: Chloroquine and hydroxychloroquine in the treatment of
COVID-19 with or without diabetes: A systematic search and a
narrative review with a special reference to India and other
developing countries. Diabetes Metab Syndr. 14:241–246.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mahévas M, Tran VT, Roumier M, Chabrol A,
Paule R, Guillaud C, Fois E, Lepeule R, Szwebel TA, Lescure FX, et
al: Clinical efficacy of hydroxychloroquine in patients with
covid-19 pneumonia who require oxygen: Observational comparative
study using routine care data. BMJ. 369(m1844)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Orkin C, DeJesus E, Khanlou H, Stoehr A,
Supparatpinyo K, Lathouwers E, Lefebvre E, Opsomer M, Van de
Casteele T and Tomaka F: Final 192-week efficacy and safety of
once-daily darunavir/ritonavir compared with lopinavir/ritonavir in
HIV-1-infected treatment-naïve patients in the ARTEMIS trial. HIV
Med. 14:49–59. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
U.S. National library of medicine:
Clinical trials. Available from: https://clinicaltrials.gov/ct2/home. Accessed
September 16, 2020.
|
|
13
|
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan
G, Ruan L, Song B, Cai Y, Wei M, et al: A trial of
lopinavir-ritonavir in adults hospitalized with severe Covid-19. N
Engl J Med. 382:1787–1799. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
De Meyer S, Bojkova D, Cinatl J, Van Damme
E, Buyck C, Van Loock M, Woodfall B and Ciesek S: Lack of antiviral
activity of darunavir against SARS-CoV-2. Int J Infect Dis.
97:7–10. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen J, Xia L, Liu L, Xu Q, Ling Y, Huang
D, Huang W, Song S, Xu S, Shen Y and Lu H: Antiviral activity and
safety of darunavir/cobicistat for the treatment of COVID-19. Open
Forum Infect Dis. 7(ofaa241)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Beigel JH, Tomashek KM, Dodd LE, Mehta AK,
Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, et
al: Remdesivir for the treatment of Covid-19-preliminary report. N
Engl J Med. 383:1813–1826. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
FDA: Fact sheet for health care providers
emergency use authorization (EUA) of remdesivir
(GS-5734™) 2020. Available from: https://www.fda.gov/media/137566/download. Accessed
September 16, 2020.
|
|
18
|
Negrut N, Nistor-Cseppento DC, Khan SA,
Pantis C, Maghiar TA, Maghiar O, Aleya S, Rus M, Tit DM, Aleya L,
et al: Clostridium difficile infection epidemiology over a period
of 8 years-a single centre study. Sustainability. 12(4439)2020.
|
|
19
|
Negruţ N, Khan S, Bungau S, Zaha D, Anca
C, Bratu O, Diaconu C and Ionita-Radu F: Diagnostic challenges in
gastrointestinal infections. Rom J Mil Med. 123:83–90. 2020.
|
|
20
|
Codrean A, Dumitrascu DL, Codrean V, Tit
DM, Bungau S, Aleya S, Rus M, Fratila O, Nistor-Cseppento DC, Aleya
L and Negrut N: Epidemiology of human giardiasis in Romania: A 14
years survey. Sci Total Environ. 705(135784)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
WHO: Clinical management of COVID-19:
Interim guidance 2020. Available from: https://www.who.int/publications/i/item/clinical-management-of-covid-19.
Accessed September 26, 2020.
|
|
22
|
Behl T, Kaur I, Bungau S, Kumar A, Uddin
MS, Kumar C, Pal G, Sahil Shrivastava K, Zengin G and Arora S: The
dual impact of ACE2 in COVID-19 and ironical actions in geriatrics
and pediatrics with possible therapeutic solutions. Life Sci.
257(118075)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rodriguez-Nava G, Yanez-Bello MA,
Trelles-Garcia DP, Chung CW, Friedman HJ and Hines DW: Performance
of the quick COVID-19 severity index and the Brescia-COVID
respiratory severity scale in hospitalized patients with COVID-19
in a community hospital setting. Int J Infect Dis. 102:571–576.
2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dreher M, Kersten A, Bickenbach J, Balfanz
P, Hartmann B, Cornelissen C, Daher A, Stöhr R, Kleines M, Lemmen
SW, et al: The characteristics of 50 hospitalized COVID-19 patients
with and without ARDS. Dtsch Arztebl Int. 117:271–278.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
WHO: Laboratory testing for coronavirus
disease (COVID-19) in suspected human cases: Interim guidance 2020.
Available from: https://apps.who.int/iris/handle/10665/331501,
Accessed September 16, 2020.
|
|
26
|
FDA: Allplex™ 2019-nCoV Assay 2020.
Available from: https://www.fda.gov/media/137178/download. Accessed
September 16, 2020.
|
|
27
|
Li TZ, Cao ZH, Chen Y, Cai MT, Zhang LY,
Xu H, Zhang JY, Ma CH, Liu Y, Gao LJ, et al: Duration of SARS-CoV-2
RNA shedding and factors associated with prolonged viral shedding
in patients with COVID-19. J Med Virol. 93:506–512. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhou B, She J, Wang Y and Ma X: The
duration of viral shedding of discharged patients with severe
COVID-19. Clin Infect Dis. 71:2240–2242. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shen C, Yue X, Wang J, Shi C and Li W:
Nocturnal oxygen therapy as an option for early COVID-19. Int J
Infect Dis. 98:176–179. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Calina D, Docea AO, Petrakis D, Egorov AM,
Ishmukhametov AA, Gabibov AG, Shtilman MI, Kostoff R, Carvalho F,
Vinceti M, et al: Towards effective COVID-19 vaccines: Updates,
perspectives and challenges (Review). Int J Mol Med. 46:3–16.
2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sánchez-Oro R, Torres Nuez J and
Martínez-Sanz G: Radiological findings for diagnosis of SARS-CoV-2
pneumonia (COVID-19). Med Clin (Barc). 155:36–40. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li Y, Xie Z, Lin W, Cai W, Wen C, Guan Y,
Mo X, Wang J, Wang Y, Peng P, et al: Efficacy and safety of
Lopinavir/Ritonavir or Arbidol in adult patients with mild/moderate
COVID-19: An exploratory randomized controlled trial. Med (NY).
1:105–113.e4. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Osborne V, Davies M, Lane S, Evans A,
Denyer J, Dhanda S, Roy D and Shakir S: Lopinavir-ritonavir in the
treatment of COVID-19: A dynamic systematic benefit-risk
assessment. Drug Saf. 43:809–821. 2020.PubMed/NCBI View Article : Google Scholar
|