1. Introduction
Pseudoexfoliation syndrome (PEX), first described in
1953 by Dvorak-Theobald (1), is
characterized by the diffuse deposition of grey-white flakes in the
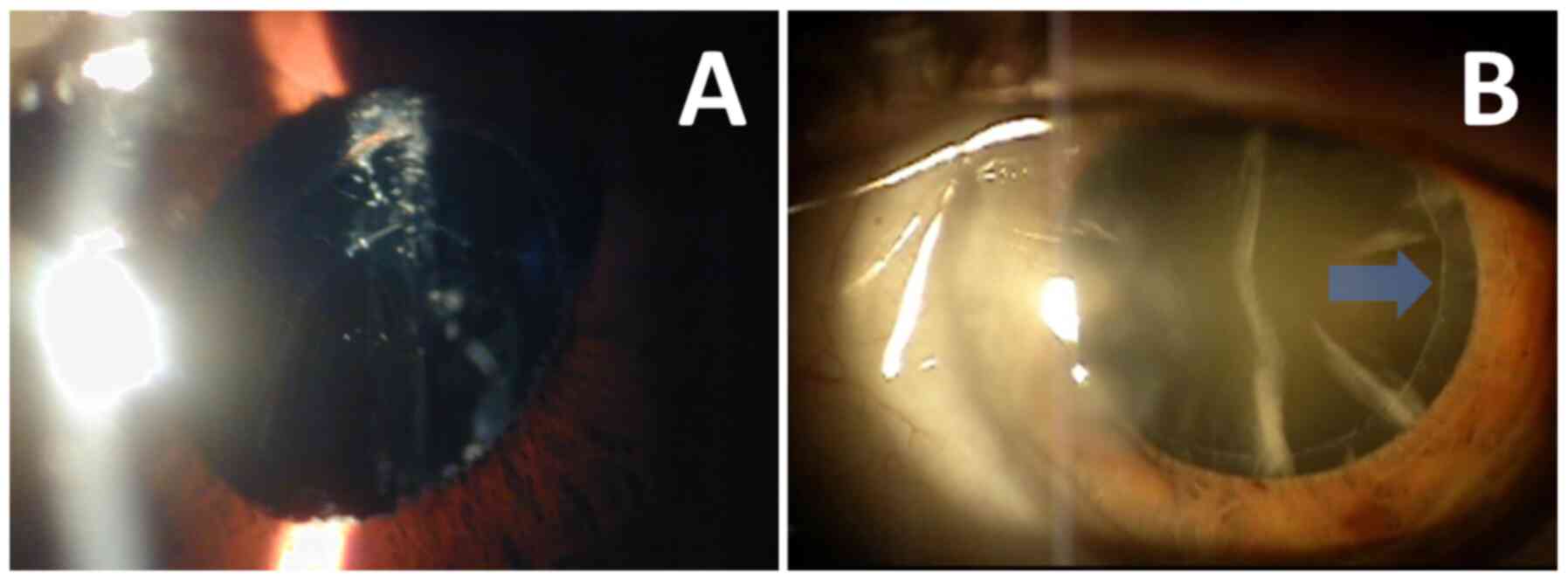
anterior ocular segment (Fig. 1)
without previous exposure to heat (infra-red irradiation), as
opposed to true exfoliation, in which the eye has been exposed to
high heat, often as an occupational hazard (such as in
glassblowers, blacksmiths or bakers) (2). The latter, first described in 1922 by
Elschnig (2), is characterized by
the deposition of material on the anterior lens surface, frequently
in the form of a distinct circular flap, the so-called double-ring
sign or capsulorrhexis masquerade (3). In PEX, the accumulated material
possibly results from a disturbed basal membrane metabolism and is
similar to amyloid (4). Several
previous studies have reported the detection of pseudoexfoliative
material in various extra-ocular sites, giving rise to the concept
that PEX is actually a systemic disease, with multi-organ clinical
implications (5,6). The present review focuses on the
peri-ocular manifestations of PEX, their importance in clinical
management of affected patients and as indicators of the
disease.
2. Epidemiological, pathogenetic and
clinical features
PEX is relatively more prevalent in the areas of
Scandinavia, the Mediterranean basin and the Arab world (7). It is also an age-related condition
with a slight female predominance (8). Although exposure to increased amounts
of ultraviolet (UV) radiation may in part explain this geographic
distribution, other factors, such as genetic predisposition,
auto-immunity or viral infections (including slow-acting viruses or
Herpes simplex infections) may also have pathogenetic roles
(9-11).
Indeed, there have been reports of an increased immunoreactivity in
PEX and PEX-related glaucoma, in comparison with age- and
gender-matched controls (12).
Mutations on the lysil-oxidase-like 1 (LOXL1) gene have been
proposed as a risk factor for PEX, whereas caffeine intake and
vitamin deficiency may also be involved in the pathogenesis of PEX
(13). Genome-wide association
studies employing a DNA-pooling approach have explored the
potential role of genetic variants in PEX and have reported
associations with several single-nucleotide polymorphisms in
various genes, apart from those in the LOXL1 gene, including other
genes such as: OR11L1, CD80, TNIK, CADM2, SORBS2, RNF180, FGF14,
FMN1 and RBFOX1, implying that neuronal development and actin
remodeling are potentially involved in the pathogenesis of PEX
(14).
The importance in PEX diagnosis, as confirmed by
clinical examination, from an ophthalmological standpoint, lies in
the fact that it has been correlated with several ophthalmic
pathologies, more importantly glaucoma, age-related macular
degeneration and complications of anterior ocular segment surgery
(15-17).
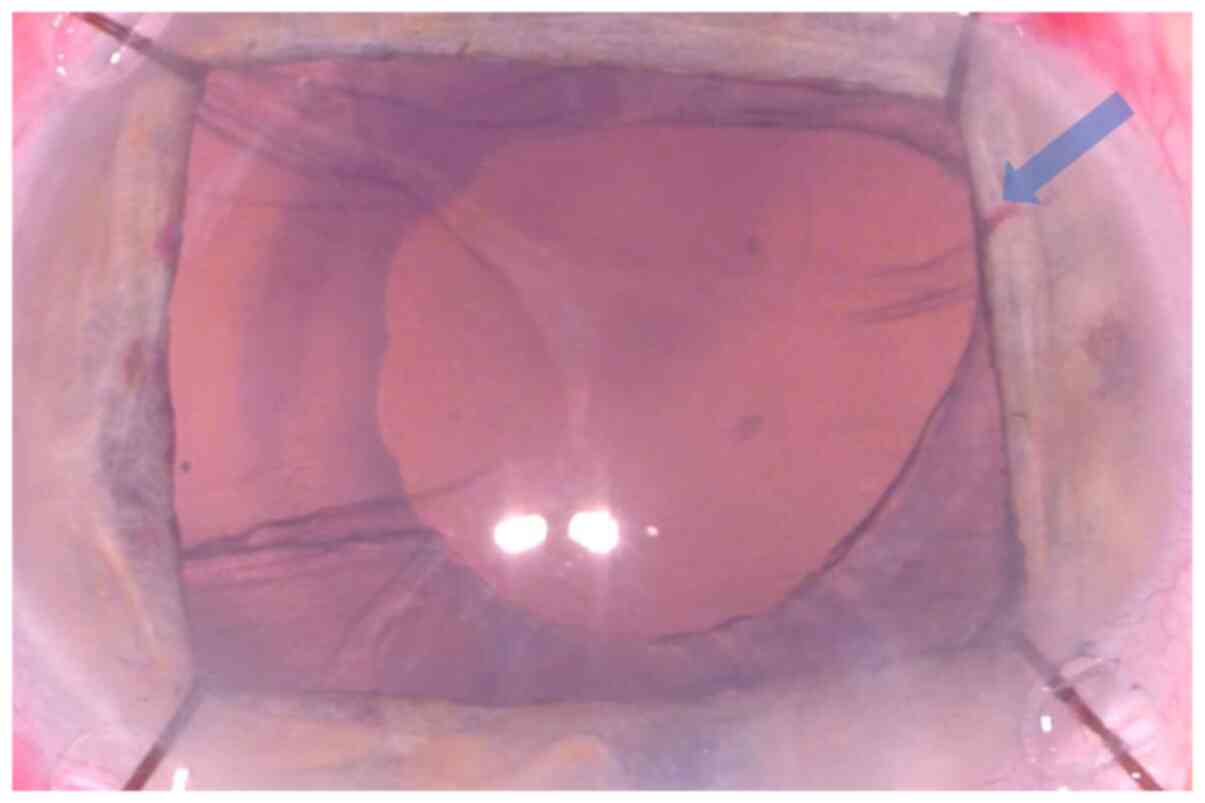
Pseudoexfoliative glaucoma is an aggressive form of open-angle
glaucoma, frequently displaying non-linear progression and
necessitating surgical intervention for adequate intraocular
pressure control (18), whereas
poor mydriasis, Zinn zonule instability and floppy iris behavior
are frequent causes of complications in cataract surgery performed
on eyes with PEX (Fig. 2) (17).
3. Extra-ocular manifestations
Of note, pseudoexfoliative material or associated
abnormalities have been detected in several extra-ocular sites,
such as visceral organs (including the lung, heart, liver, kidneys
and gallbladder) (19), vascular
tissue (20,21) and the brain (22). Based on these observations, it has
been suggested that PEX is a systemic condition, which may cause
functional disturbances at the affected sites (5,6). So
far, associations between PEX and various systemic pathologies have
been described, including acoustic impairment (evaluated by both
audiometric and tympanometric examinations) (23), obstructive sleep apnea (24), chronic obstructive pulmonary disease
(25), indirect inguinal hernia
(26) and pelvic organ prolapse in
female patients (27). One of the
most intriguing clinical associations described is the potential
connection between PEX and Alzheimer's disease (AD) (28,29).
Although there have been reports linking the 2 entities based on
epidemiological and clinical observations, a 30-year
population-based study has failed to confirm this hypothesis
(28). However, converging evidence
points towards biochemical and genetic similarities between PEX and
so-called conformational diseases, which are characterized by the
accumulation of abnormal proteinaceous amyloid-like material and
include AD, Parkinson's disease and amyotrophic lateral sclerosis
(29-32).
4. Eyelid skin involvement
Previous studies have reported the detection of PEX
material in biopsies from eyelid skin and have suggested that
peri-ocular skin changes may actually precede the development of
frank intra-ocular PEX (33).
Periocular skin is particularly susceptible to senile degenerative
changes due to the fact that it is thin, undergoes constant
mechanical stress associated with blinking movements and, more
importantly, it is exposed to the oxidative effects of UV solar
radiation (34). Exposure to UV
light results in the activation of degrading enzymes from cutaneous
lysosomes such as cathepsin K (a potent elastase), resulting in
photo-aging and age-related elastosis (35). In fact, epidermal photo-desquamation
depends on two distinct proteolytic activities, one of which is an
analogue of chymotrypsin and the other one is cathepsin D, with
corresponding proteinases being the stratum corneum chymotryptic
enzyme and the mature active form of cathepsin D (36). Such skin changes have also been
associated with PEX, which shares common confounders with senile
eyelid skin elastosis, such as advanced age and exposure to UV
radiation (37).
5. Tarsal and canthal tendon
involvement
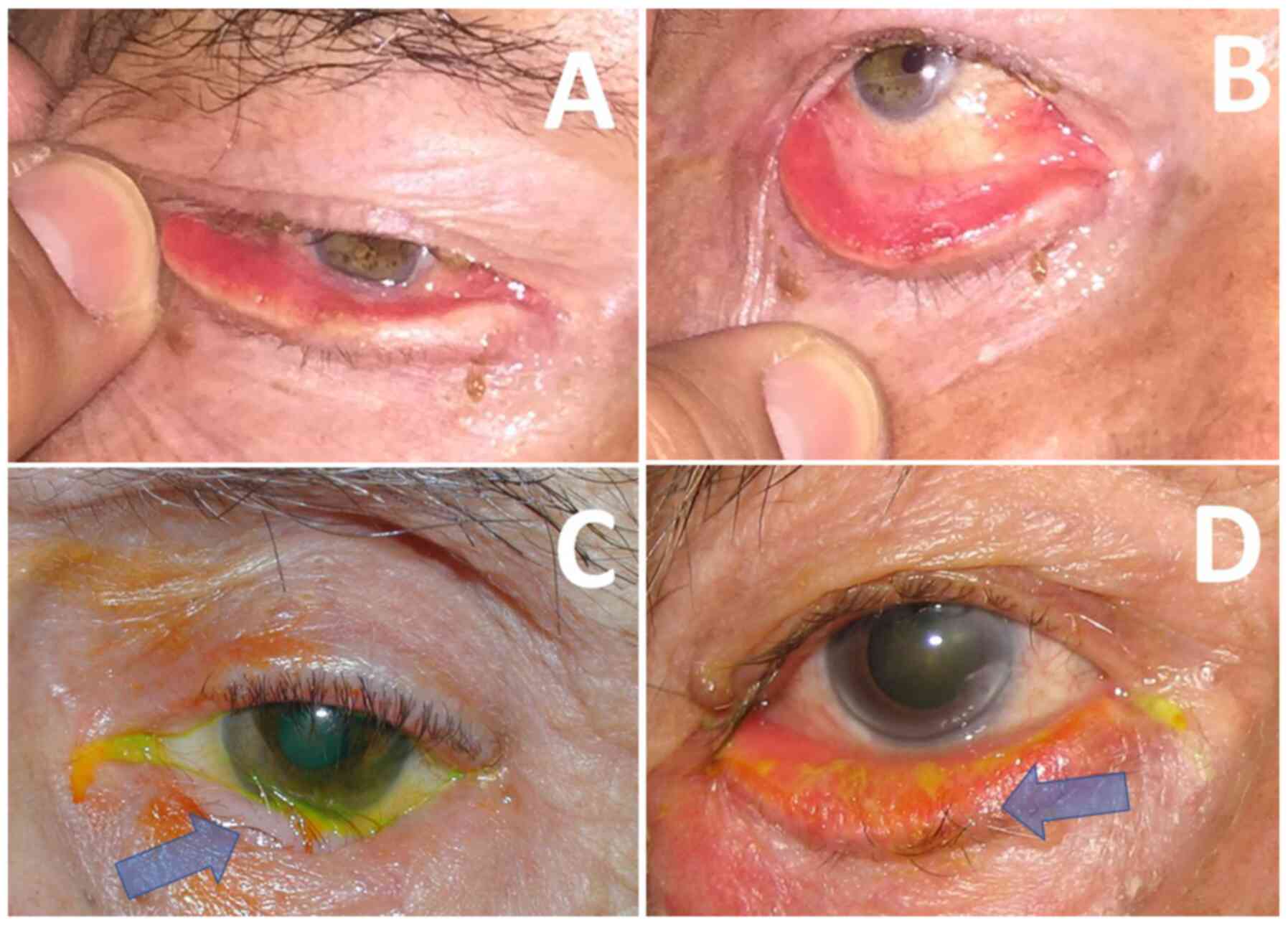
The accumulation of pseudoexfoliative material in
the peri-ocular connective tissues includes the medial and lateral
canthal tendons, tarsal plates and orbicularis oculi muscle
(33,38). A study has reported that PEX may be
associated with atonic changes of the orbicularis oculi and
compromise the stability of medial and lateral canthal tendons,
resulting in horizontal and vertical lid laxity and predisposing to
the development of eyelid margin malpositions, such as entropion or
ectropion (Fig. 3) (38). Furthermore, the tarsal attachments
of the lower eyelid retractors may be weakened. On the other hand,
PEX has not been associated with upper eyelid ptosis, possibly due
to the fact that the upper eyelid retractors (levator palpebrae
superioris, Muller's muscle) are comparatively stronger structures
that are less likely to be affected by PEX fibrilopathy (38). Alteration in the anatomical
stability of peri-ocular connective tissue elements complies with
reports for similar extra-ocular changes, such as inguinal hernias
(26) or pelvic organ prolapse in
female patients (27).
6. Conjunctival, ocular surface and lacrimal
involvement
As in the case of eyelid skin and tendinous
anatomical elements of the peri-ocular area, the conjunctiva has
also been reported to be affected in PEX, resulting in conjunctival
chalasis (39) and subsequent
development or deterioration of pre-existing ocular surface disease
(OSD). The latter is a chronic malfunction of the physiological
elements, contributing to the effectiveness of the ocular surface
in supporting eyeball integrity and preserving visual function
(40). It has been associated with
several pathogenetic mechanisms, including Dry Eye Disease (DED),
drug toxicity, particularly from anti-glaucomatous medications, and
viral infections, such as the human papillomavirus (40,41).
Growing evidence has suggested that PEX may also be involved
through combined pathological effects on all layers, including the
tear film, corneal and conjunctival epithelium, as well as eyelid
apposition to the eyeball (38).
PEX has been reported to be implicated in OSD by several previous
studies (40,42). Of note, PEX has been reported to
compromise both the quality and quantity of the tear film, as
indicated by the defective Schirmer, tear break-up time and tear
osmolarity tests (42-45),
as well as defective conjunctival goblet cell activity (46), contributing to the development of
DED.
7. Orbital involvement
The presence of PEX material in the orbits has been
reported by several previous studies (4,47).
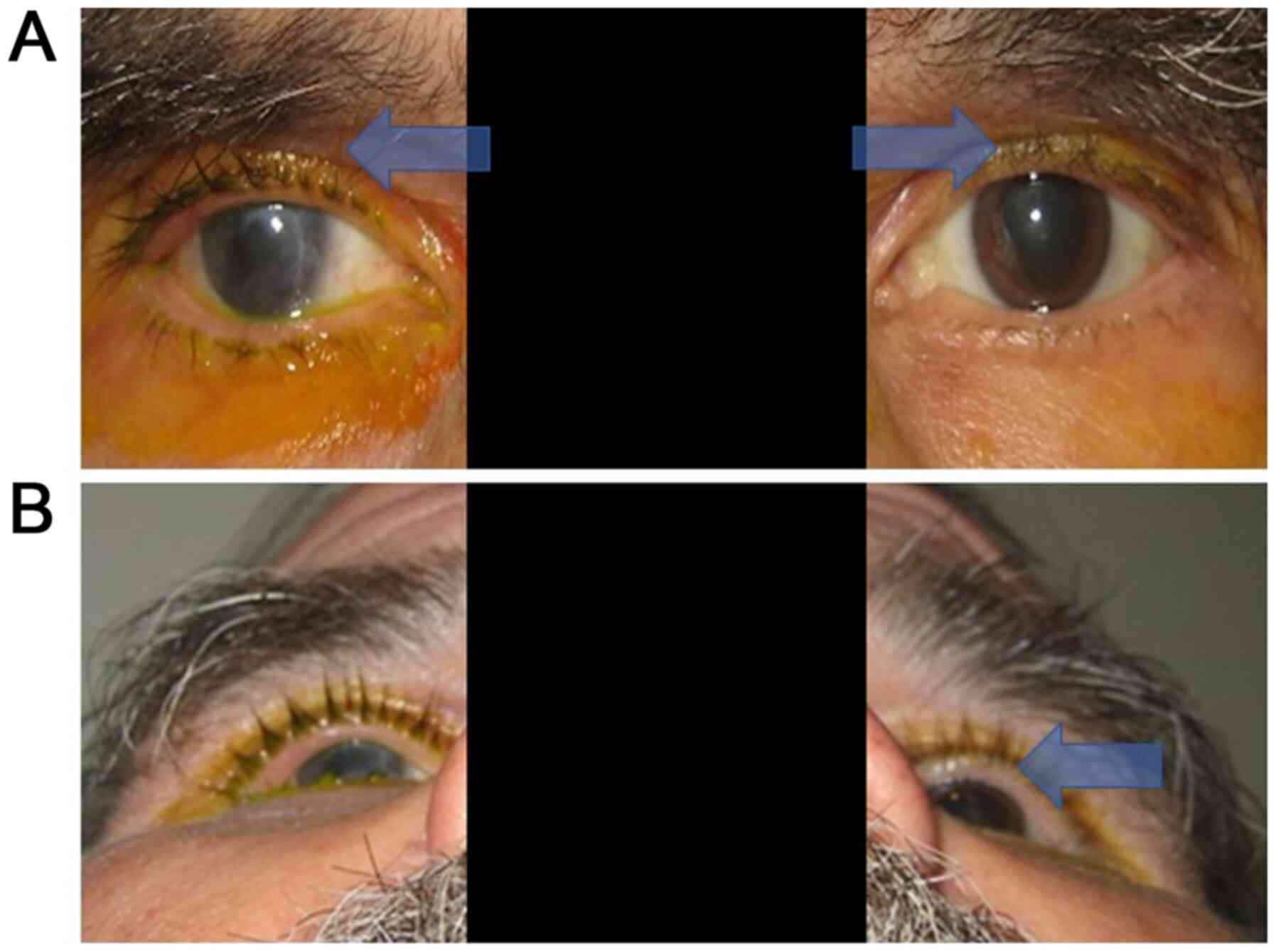
Orbital fat pads, particularly those of the superior eyelid, may be
particularly susceptible to atrophic changes induced by bimatoprost
(frequently administered as an anti-glaucomatous medication in
pseudoexfoliative glaucoma), resulting in enophthalmos (Fig. 4) (48). Apart from the detection of
pseudoexfoliative material in the orbital soft tissues, the effect
of PEX is particularly pronounced in the orbital vasculature
(49,50). Reduced end-diastolic blood velocity
at the long posterior ciliary arteries (measured by color Doppler
ultrasound imaging) has been detected in eyes with PEX compared
with that in normal eyes or even eyes with primary open-angle
glaucoma (POAG), implying PEX-induced ischemic stress at the
anterior ocular segment (51). Such
hemodynamic and hemorheological changes may have important roles in
the pathogenesis and clinical course of pseudoexfoliative glaucoma
(49,51).
8. Optic nerve changes
The importance of studying the potential detrimental
effects of PEX on the optic nerve is due to its connection with a
particularly aggressive form of glaucoma (pseudoexfoliative
glaucoma) (17). The adverse
effects of PEX on the optic nerve blood supply are supported by
findings of reduced optic nerve head vessel density and reduced
peri-papillary capillary vessel density in PEX compared to eyes not
affected by PEX (52-54).
Of note, PEX may also affect the biomechanical behavior of both
anterior and posterior ocular segments (including the
peri-papillary sclera and lamina cribrosa) (55,56),
which may offer potential explanatory mechanisms for the connection
between PEX and glaucoma (57).
Biomechanical changes of the optic nerve per se have also been
reported by a study applying ultrasound elastography, which
detected different strain ratios of orbital fat to the optic nerve
head in glaucoma patients with PEX as compared with those in the
POAG and control groups, implying that such changes may be involved
in the pathogenesis of pseudoexfoliative glaucoma (58).
9. Clinical implications of peri-ocular
involvement in PEX
The effects of PEX material accumulation in
peri-ocular tissues may be important concerning the clinical
management of affected patients (Fig.
5). Apart from the risks for cataract surgery associated with
intraocular PEX, enophthalmic changes (the so-called ‘deep-set
eyes’) due to the use of prostaglandin analogues (particularly
bimatoprost), frequently administered in pseudoexfoliative
glaucoma, may be an additional source of surgical difficulty and
potential intra-operative complications. Eyelid laxity, often
resulting in changes of the eyelid margin position such as
entropion or ectropion, as well as in lacrimal deficiencies, such
as DED or OSD, may also be a source of both intra-operative and
post-operative complications in cataract or other forms of
intra-ocular surgery in the presence of PEX. Such changes may be
more detrimental on the ocular surface, taking into account the
reported mechanical corneal sensitivity defects in PEX (59).
10. Conclusions
The PEX enigma has yet to be unraveled in the field
of Ophthalmology. However, there is growing evidence connecting PEX
with other systemic diseases, particularly neurological conditions,
associated with tissue and cellular deposition of altered
proteinaceous material, such as AD. Several studies have indicated
peri-ocular tissue involvement in PEX, including the eyelid skin,
tarsus, canthal ligaments, lacrimal secretions, orbital soft
tissue, orbital vasculature and optic nerve. Apart from the
intraocular clinical effects of PEX, which have been comparatively
more extensively studied, the peri-ocular tissues may also have an
important clinical role in the overall management of patients with
PEX.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
ETD, DAS and GB conceptualized the study. ETD and GB
wrote and prepared the draft of the manuscript. EED and DAS
provided critical revisions. ETD and GB drew the figures. All
authors contributed to manuscript revision and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor in Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Dvorak-Theobald G: Pseudoexfoliation of
the lens capsule: Relation to true exfoliation of the lens capsule
as reported in the literature, and role in the production of
glaucoma capsulocuticulare. Trans Am Ophthalmol Soc. 51:385–407.
1953.PubMed/NCBI
|
|
2
|
Elschnig A: A Detachment of the zonular
lamellae in glassblowers. Klin Monatsbl Augenheilkd. 69:732–734.
1922.
|
|
3
|
Braude LS and Edward DP: Partial splitting
of the anterior lens capsule giving a ‘double-ring’ sign. Arch
Ophthalmol. 113:705–708. 1995.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ritch R and Schlötzer-Schrehardt U:
Exfoliation syndrome. Surv Ophthalmol. 45:265–315. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Naumann GO, Schlötzer-Schrehardt U and
Küchle M: Pseudoexfoliation syndrome for the comprehensive
ophthalmologist. Intraocular and systemic manifestations.
Ophthalmology. 105:951–968. 1998.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Schlötzer-Schrehardt UM, Koca MR, Naumann
GOH and Volkholz H: Pseudoexfoliation syndrome. Ocular
manifestation of a systemic disorder? Arch Ophthalmol.
110:1752–1756. 1992.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bialasiewicz AA, Wali U, Shenoy R and
Al-Saeidi R: Patients with secondary open-angle glaucoma in
pseudoexfoliation (PEX) syndrome among a population with high
prevalence of PEX. Clinical findings and morphological and surgical
characteristics. Ophthalmologe. 102:1064–1068. 2005.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
8
|
Kozart DM and Yanoff M: Intraocular
pressure status in 100 consecutive patients with exfoliation
syndrome. Ophthalmology. 89:214–218. 1982.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Damji KF, Bains HS, Stefansson E,
Loftsdottir M, Sverrisson T, Thorgeirsson E, Jonasson F,
Gottfredsdottir M and Allingham RR: Is pseudoexfoliation syndrome
inherited? A review of genetic and nongenetic factors and a new
observation. Ophthalmic Genet. 19:175–185. 1998.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kozobolis VP, Detorakis ET, Sourvinos G,
Pallikaris IG and Spandidos DA: Loss of heterozygosity in
pseudoexfoliation syndrome. Invest Ophthalmol Vis Sci.
40:1255–1260. 1999.PubMed/NCBI
|
|
11
|
Detorakis ET, Kozobolis VP, Pallikaris IG
and Spandidos DA: Detection of herpes simplex virus in
pseudoexfoliation syndrome and exfoliation glaucoma. Acta
Ophthalmol Scand. 80:612–616. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Joachim SC, Wuenschig D, Pfeiffer N and
Grus FH: IgG antibody patterns in aqueous humor of patients with
primary open angle glaucoma and pseudoexfoliation glaucoma. Mol
Vis. 13:1573–1579. 2007.PubMed/NCBI
|
|
13
|
Miglior S and Bertuzzi F: Exfoliative
glaucoma: New evidence in the pathogenesis and treatment. Prog
Brain Res. 221:233–241. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zagajewska K, Piątkowska M, Goryca K,
Bałabas A, Kluska A, Paziewska A, Pośpiech E, Grabska-Liberek I and
Hennig EE: GWAS links variants in neuronal development and actin
remodeling related loci with pseudoexfoliation syndrome without
glaucoma. Exp Eye Res. 168:138–148. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kozobolis VP, Detorakis ET, Tsilimbaris M,
Siganos DS, Vlachonikolis IG and Pallikaris IG: Crete, Greece
glaucoma study. J Glaucoma. 9:143–149. 2000.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kozobolis VP, Detorakis ET, Tsilimbaris
MK, Vlachonikolis IG, Tsambarlakis IC and Pallikaris IG:
Correlation between age-related macular degeneration and
pseudoexfoliation syndrome in the population of Crete (Greece).
Arch Ophthalmol. 117:664–669. 1999.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sangal N and Chen TC: Cataract surgery in
pseudoexfoliation syndrome. Semin Ophthalmol. 29:403–408.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Schweitzer C: Pseudoexfoliation syndrome
and pseudoexfoliation glaucoma. J Fr Ophtalmol. 41:78–90.
2018.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
19
|
Streeten BW, Li ZY, Wallace RN, Eagle RC
Jr and Keshgegian AA: Pseudoexfoliative fibrillopathy in visceral
organs of a patient with pseudoexfoliation syndrome. Arch
Ophthalmol. 110:1757–1762. 1992.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chung H, Arora S, Damji KF and Weis E:
Association of pseudoexfoliation syndrome with cardiovascular and
cerebrovascular disease: A systematic review and meta-analysis. Can
J Ophthalmol. 53:365–372. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Siordia JA, Franco J, Golden TR and Dar B:
Ocular Pseudoexfoliation syndrome linkage to cardiovascular
disease. Curr Cardiol Rep. 18(61)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zikou AK, Kitsos G, Astrakas LG, Xydis VG,
Spiliopoulos K, Bagli E and Argyropoulou MI: Pseudoexfoliation
syndrome without glaucoma: White matter abnormalities detected by
conventional MRI and diffusion tensor imaging. Eur J Radiol.
99:82–87. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Detorakis ET, Chrysochoou F, Paliobei V,
Konstas AG, Daniilidis V, Balatsouras D, Kefalidis G and Kozobolis
VP: Evaluation of the acoustic function in pseudoexfoliation
syndrome and exfoliation glaucoma: Audiometric and tympanometric
findings. Eur J Ophthalmol. 18:71–76. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shumway C, Curtin K, Taylor S, Sundar KM,
Wirostko BM and Ritch R: Association between Obstructive Sleep
Apnea and Exfoliation Syndrome: The Utah Project on Exfoliation
Syndrome. Ophthalmol Glaucoma: Sep 30, 2020 (Epud ahead of
print).
|
|
25
|
Taylor SC, Bernhisel AA, Curtin K,
Allingham RR, Ritch R and Wirostko BM: Association between Chronic
Obstructive Pulmonary Disease and Exfoliation Syndrome: The Utah
Project on Exfoliation Syndrome. Ophthalmol Glaucoma. 2:3–10.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Besch BM, Curtin K, Ritch R, Allingham RR
and Wirostko BM: Association of Exfoliation Syndrome With Risk of
Indirect Inguinal Hernia: The Utah Project on Exfoliation Syndrome.
JAMA Ophthalmol. 136:1368–1374. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wirostko BM, Curtin K, Ritch R, Thomas S,
Allen-Brady K, Smith KR, Hageman GS and Allingham RR: Risk for
Exfoliation Syndrome in Women With Pelvic Organ Prolapse: A Utah
Project on Exfoliation Syndrome (UPEXS) Study. JAMA Ophthalmol.
134:1255–1262. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cumurcu T, Dorak F, Cumurcu BE, Erbay LG
and Ozsoy E: Is there any relation between pseudoexfoliation
syndrome and Alzheimer's type dementia? Semin Ophthalmol.
28:224–229. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ekström C and Kilander L:
Pseudoexfoliation and Alzheimer's disease: A population-based
30-year follow-up study. Acta Ophthalmol. 92:355–358.
2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Davanger M: On the molecular composition
and physico-chemical properties of the pseudo-exfoliation material.
Acta Ophthalmol (Copenh). 55:621–633. 1977.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ziangirova GG and Antonova OV: Local
senile ocular amyloidosis in the pathogenesis of open-angle
glaucoma and pseudo-exfoliative syndrome. Vestn Ross Akad Med Nauk.
2:40–43. 2003.PubMed/NCBI(In Russian).
|
|
32
|
Reniewska B, Mulak M, Misiuk-Hojło M and
Kostuś E: Coexistence of Alzheimer's disease with pseudoexfoliation
syndrome PEX. Klin Oczna. 106:107–109. 2004.PubMed/NCBI(In Polish).
|
|
33
|
Schlötzer-Schredhardt U, Küchle M, Dörfler
S and Naumann GO: Pseudoexfoliative material in the eyelid skin of
pseudoexfoliation-suspect patients: A clinico-histopathological
correlation. Ger J Ophthalmol. 2:51–60. 1993.PubMed/NCBI
|
|
34
|
Offret G and Haye C: Senile elastosis of
the eyelids and of the conjunctiva. Bull Soc Ophtalmol Fr.
4:263–264. 1959.PubMed/NCBI(In French).
|
|
35
|
Codriansky KA, Quintanilla-Dieck MJ, Gan
S, Keady M, Bhawan J and Rünger TM: Intracellular degradation of
elastin by cathepsin K in skin fibroblasts--a possible role in
photoaging. Photochem Photobiol. 85:1356–1363. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Horikoshi T, Igarashi S, Uchiwa H, Brysk H
and Brysk MM: Role of endogenous cathepsin D-like and
chymotrypsin-like proteolysis in human epidermal desquamation. Br J
Dermatol. 141:453–459. 1999.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Streeten BW, Dark AJ, Wallace RN, Li ZY
and Hoepner JA: Pseudoexfoliative fibrillopathy in the skin of
patients with ocular pseudoexfoliation. Am J Ophthalmol.
110:490–499. 1990.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Potemkin VV, Rakhmanov VV, Ageeva EV,
Alchinova AS and Meshveliani EV: Pseudoexfoliation syndrome and
ocular adnexa. Ophthalmol J. 9:15–21. 2016.
|
|
39
|
Erdoğan H, Arici DS, Toker MI, Arici MK,
Fariz G and Topalkara A: Conjunctival impression cytology in
pseudoexfoliative glaucoma and pseudoexfoliation syndrome. Clin Exp
Ophthalmol. 34:108–113. 2006.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Fogagnolo P, Torregrossa G, Tranchina L,
Ferreras A, De Cillá S, Labbé A, Figus M, Ottobelli L and Rossetti
L: Tear film osmolarity, ocular surface disease and glaucoma: a
review. Curr Med Chem. 26:4241–4252. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chalkia AK, Bontzos G, Spandidos DA and
Detorakis ET: Human papillomavirus infection and ocular surface
disease (Review). Int J Oncol. 54:1503–1510. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Škegro I, Suić SP, Kordić R, Jandroković
S, Petriček I, Kuzman T, Kalauz M, Perić S and Masnec S: Ocular
surface disease in pseudoexfoliation syndrome. Coll Antropol.
39:43–45. 2015.PubMed/NCBI
|
|
43
|
Kozobolis VP, Detorakis ET, Tsopakis GM
and Pallikaris IG: Evaluation of tear secretion and tear film
stability in pseudoexfoliation syndrome. Acta Ophthalmol Scand.
77:406–409. 1999.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Noori S, Sati A, Moulick PS, Kaushik J,
Shankar S and Bose R: Tear film abnormalities in pseudoexfoliation
syndrome and normal healthy participants: A comparative analysis.
Med J Armed Forces India. 76:303–306. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Öncel BA, Pinarci E and Akova YA: Tear
osmolarity in unilateral pseudoexfoliation syndrome. Clin Exp
Optom. 95:506–509. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kozobolis VP, Christodoulakis EV, Naoumidi
II, Siganos CS, Detorakis ET and Pallikaris LG: Study of
conjunctival goblet cell morphology and tear film stability in
pseudoexfoliation syndrome. Graefes Arch Clin Exp Ophthalmol.
242:478–483. 2004.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ringvold A: On the occurrence of
pseudo-exfoliation material in extrabulbar tissue from patients
with pseudo-exfoliation syndrome of the eye. Acta Ophthalmol
(Copenh). 51:411–418. 1973.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Aydin S, Işikligil I, Tekşen YA and Kir E:
Recovery of orbital fat pad prolapsus and deepening of the lid
sulcus from topical bimatoprost therapy: 2 case reports and review
of the literature. Cutan Ocul Toxicol. 29:212–216. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Galassi F, Giambene B and Menchini U:
Ocular perfusion pressure and retrobulbar haemodynamics in
pseudoexfoliative glaucoma. Graefes Arch Clin Exp Ophthalmol.
246:411–416. 2008.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Dayanir V, Topaloğlu A, Ozsunar Y, Keceli
M, Okyay P and Harris A: Orbital blood flow parameters in
unilateral pseudoexfoliation syndrome. Int Ophthalmol. 29:27–32.
2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Detorakis ET, Achtaropoulos AK, Drakonaki
EE and Kozobolis VP: Hemodynamic evaluation of the posterior
ciliary circulation in exfoliation syndrome and exfoliation
glaucoma. Graefes Arch Clin Exp Ophthalmol. 245:516–521.
2007.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sekeroglu MA, Irkec M, Mocan MC, Ileri E,
Dikmenoglu N, Seringec N, Karaosmanoglu D and Orhan M: The
association of ocular blood flow with haemorheological parameters
in primary open-angle and exfoliative glaucoma. Acta Ophthalmol.
89:429–434. 2011.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Simsek M, Kocer AM, Cevik S, Sen E and
Elgin U: Evaluation of the optic nerve head vessel density in the
patients with asymmetric pseudoexfoliative glaucoma: An OCT
angiography study. Graefes Arch Clin Exp Ophthalmol. 258:1493–1501.
2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Goker YS and Kızıltoprak H: Quantitative
analysis of radial peripapillary capillary plexuses in patients
with clinically unilateral pseudoexfoliation syndrome. Graefes Arch
Clin Exp Ophthalmol. 258:1217–1225. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Grammenandi E, Detorakis ET, Pallikaris IG
and Tsilimbaris MK: Differences between Goldmann Applanation
Tonometry and Dynamic Contour Tonometry in pseudoexfoliation
syndrome. Clin Exp Ophthalmol. 38:444–448. 2010.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Braunsmann C, Hammer CM, Rheinlaender J,
Kruse FE, Schäffer TE and Schlötzer-Schrehardt U: Evaluation of
lamina cribrosa and peripapillary sclera stiffness in
pseudoexfoliation and normal eyes by atomic force microscopy.
Invest Ophthalmol Vis Sci. 53:2960–2967. 2012.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Ritch R, Schlötzer-Schrehardt U and
Konstas AG: Why is glaucoma associated with exfoliation syndrome?
Prog Retin Eye Res. 22:253–275. 2003.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Unal O, Caglayan M, Kosekahya P, Yulek F
and Taslipinar G: Evaluation of optic nerve head biomechanical
properties in pseudoexfoliation glaucoma with real-time
elastography. Curr Med Imaging Rev. 15:637–644. 2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Detorakis ET, Koukoula S, Chrisohoou F,
Konstas AG and Kozobolis VP: Central corneal mechanical sensitivity
in pseudoexfoliation syndrome. Cornea. 24:688–691. 2005.PubMed/NCBI View Article : Google Scholar
|