Introduction
Schizophrenia is a severe brain disorder
characterized by certain types of delusion, hallucination and
thought disorder (1,2). In addition to these aforementioned
symptoms that may be associated with enhanced brain activity,
schizophrenic patients develop additional symptoms of inhibition,
including avolition, alogia and affective flattening (2). Macroscopically, schizophrenic brains
exhibited enlargement of the ventricles and an overall reduction in
the temporal volume, while microscopical examination of the brain
revealed synaptic and spinal alterations, as well as gliosis, in
various brain areas, including hippocampal formation and the
prefrontal and the entorhinal cortices (3-9).
Additional studies have revealed increasing neuronal packaging
density, as well as decreased neuropil and smaller cell somata of
the pyramidal neurons in layer 3 in different brain areas,
including the primary and association auditory cortices, in
schizophrenia (9-12).
Dendritic spines perform a crucial role in regulating neuronal
excitability while receiving the vast majority of excitatory
synapses in the cortex (13,14).
Deficits in spines are related to impairments in the working
memory, attention, sensory-motor processing and sociability
(15-17).
Spine density has been reported to be significantly reduced in
neurons of the auditory cortex (12) and the basilar dendrites of deep
layer 3 pyramidal neurons (10),
but did not differ for pyramidal neurons in the superficial layer 3
or layers 5 and 6 of area 46 (10,18).
The spinal changes are thought to arise during development and are
probably related to disturbances of the mechanisms underlying the
formation and maintenance of spines.
Visual hallucinations are the second most common
type of hallucinations in schizophrenia (2). Previous studies demonstrated a
critical role for interneurons and cortical connectivity in the
generation of hallucinations (19,20).
At the same time, studies on the visual cortex of schizophrenic
brains have revealed impaired synaptic plasticity and reduced
gamma-aminobutyric acid (GABA) levels (21-23).
In the present study, the morphology of the
pyramidal cells and interneurons in the visual cortex from brains
of schizophrenic patients were examined. It was attempted to
describe any possible dendritic and spinal alterations compared to
normal control brains.
Patients and methods
Subjects
Brain samples were obtained from 10 neurologically
normal individuals with no history of neurological or psychiatric
illness, and 10 patients with schizophrenia, between January 2010
to December 2014 from the Department of Forensic Medicine and
Toxicology, at the Aristotle University of Thessaloniki, Greece.
All of the subjects were aged between 40 and 58 years
(Schizophrenia: Mean age, 48.6±5.7 years; 6 males and 4 females;
Controls: Mean age, 46.3±4.97 years; 7 males and 3 females) and
died from heart attack. The average autolysis time for all subjects
was 12±4 h. After their excision from the skull, all brains were
immersed in 10% neutral buffered formalin for at least 25 days. All
possible information on each subject regarding their previous
physical and illness history was obtained from autopsy reports and
medical records. The mean duration of the disease was 21±7 years
and they had all been prescribed antipsychotic medication. Written
informed consent regarding the use of the tissue for research
purposes was obtained from the relatives of each of the deceased.
The present study was performed according to the legislation of the
Greek Democracy (v.2,472/1997, 2,819/2000, 2,915/2001, 3,235/2004
and 3,471/2006) and the Committee for Research Deontology
Principles of the Aristotle University of Thessaloniki (24). The ethical approval number was
23/4/4521/2018. The brains were macroscopically and microscopically
examined by an independent neuropathologist and did not exhibit any
trauma, oedema or other pathology. Independent psychiatrists based
at the Psychiatric Hospital of Thessaloniki, made the diagnosis of
schizophrenia based on the criteria of the Diagnostic and
Statistical Manual of Mental Disorders Text Revision 5. All of the
patients exhibited visual hallucinations in their disease
history.
Tissue selection and processing
A tissue block measuring 10x5x20 mm was excised from
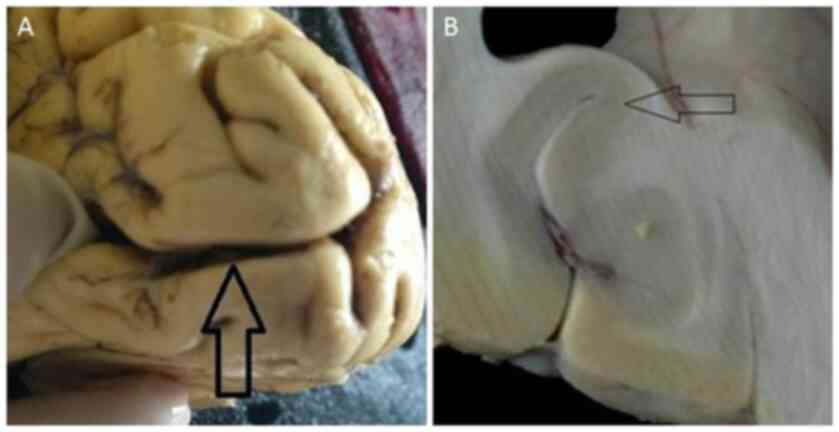
the primary visual cortex or V1 area (calcarine sulcus) (25) (Fig.
1A). The primary visual cortex may be easily recognized by a
band of myelinated axons that run parallel to the surface (line of
Gennari; Fig. 1B). The tissue
blocks were coded to prevent experimental bias and were processed
with the Golgi method and subjected to Nissl staining (26).
Cell selection criteria
Two neuronal types were selected for the present
study; the first one corresponds to the third cortical layer's
pyramidal neurons and the second to the inhibitory aspiny stellate
interneurons of the visual cortex. All neurons chosen for the study
were uniformly stained, there was no precipitated debris around
them and a good contrast between them and background were present
(27).
Golgi method
For silver impregnation, the specimens were
immediately immersed in a dilution of potassium dichromate (7 g of
potassium dichromate and 20 ml of formaldehyde solution 37% in 300
ml of tap water) at room temperature. They remained in that
solution for one week and were then immersed in an aqueous solution
of 1% silver nitrate, where they remained for one more week at a
temperature of 15˚C.
After fixation, the specimens were immersed in
paraffin and cut into thick sections ~120 µm thick, as neuronal
fields can be seen at their whole thickness at ~120 µm (25), using a Reichert slicing microtome. A
total of 5 randomly selected sections were obtained, with a 480 µm
distance between each sample. All of the specimens were examined
with an Axiostar Plus bright field microscope (Zeiss AG).
Nissl staining
Adjacent sections were cut at a thickness of 20 µm
and used for Nissl staining to evaluate the neuronal population and
define the depth of cortical layers and measure the thickness of
the cortex (25).
Neuronal tracing and dendritic
quantification
For each one of the 20 brains, 50 pyramidal cells
and 50 interneurons were selected. The neurons were then analyzed
based on the method described in a previous study by our group
(26).
Dendritic measures and Sholl
analysis
For the morphometric estimation, soma size, total
dendritic length, cell contraction, dendritic field asymmetry, the
total number of dendritic segments and bifurcations, and the length
and number of dendritic segments per order were measured.
Furthermore, the tracings were quantitatively analyzed with Fiji
software (version 2017; Fiji) and the Simple Neurite Tracer plugin
based on Sholl's method of concentric spheres (28,29).
Concentric spheres were drawn at intervals of 10 µm centred on the
cell bodies and dendritic intersections within each sphere were
counted (25).
Spine counts
The dendritic spine density was measured in 360
images, which were taken at a magnification of x1,000. Two
different investigators (FP and SC) independently counted visible
spines on three random segments of the dendritic tree of 20-30 µm
in length, the first one being on a first-order dendrite, the
second on a second-order dendrite and the third on a tertiary
branch (30).
Statistical analysis
Statistical analysis was performed using Student's
t-test based on 320 cells in R Studio (v. 4.04). To make sure that
the autolysis time did not affect dendritic measurements,
two-tailed Pearson product correlation analyses were performed
between all dependent measures and autolysis time (31). P<0.05 was considered to indicate
a statistically significant difference.
Results
Dendritic changes of pyramidal
neurons
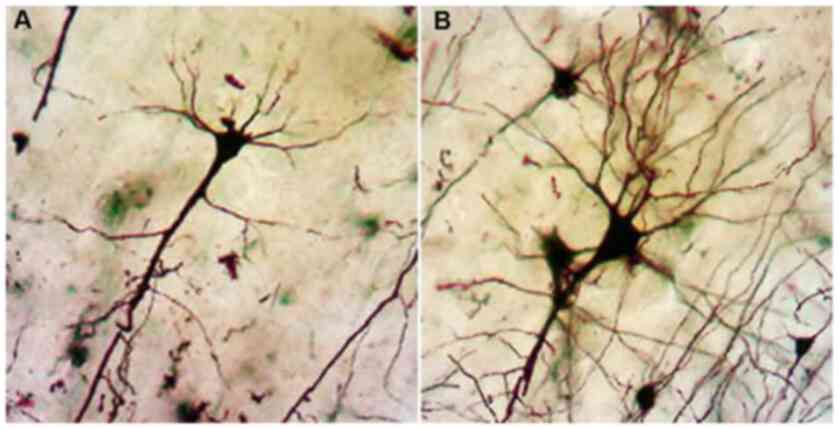
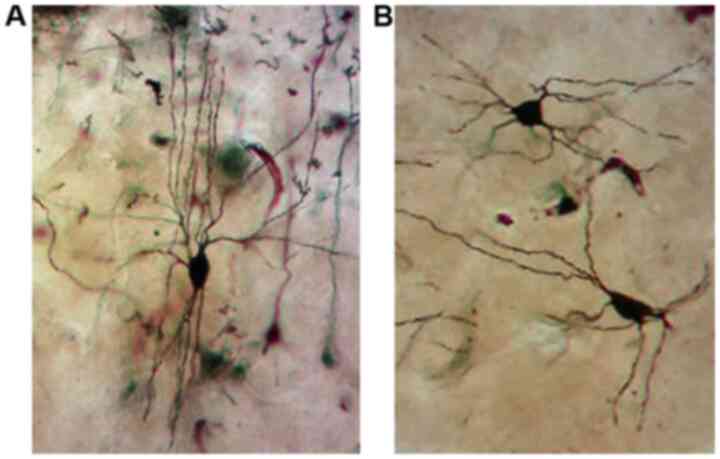
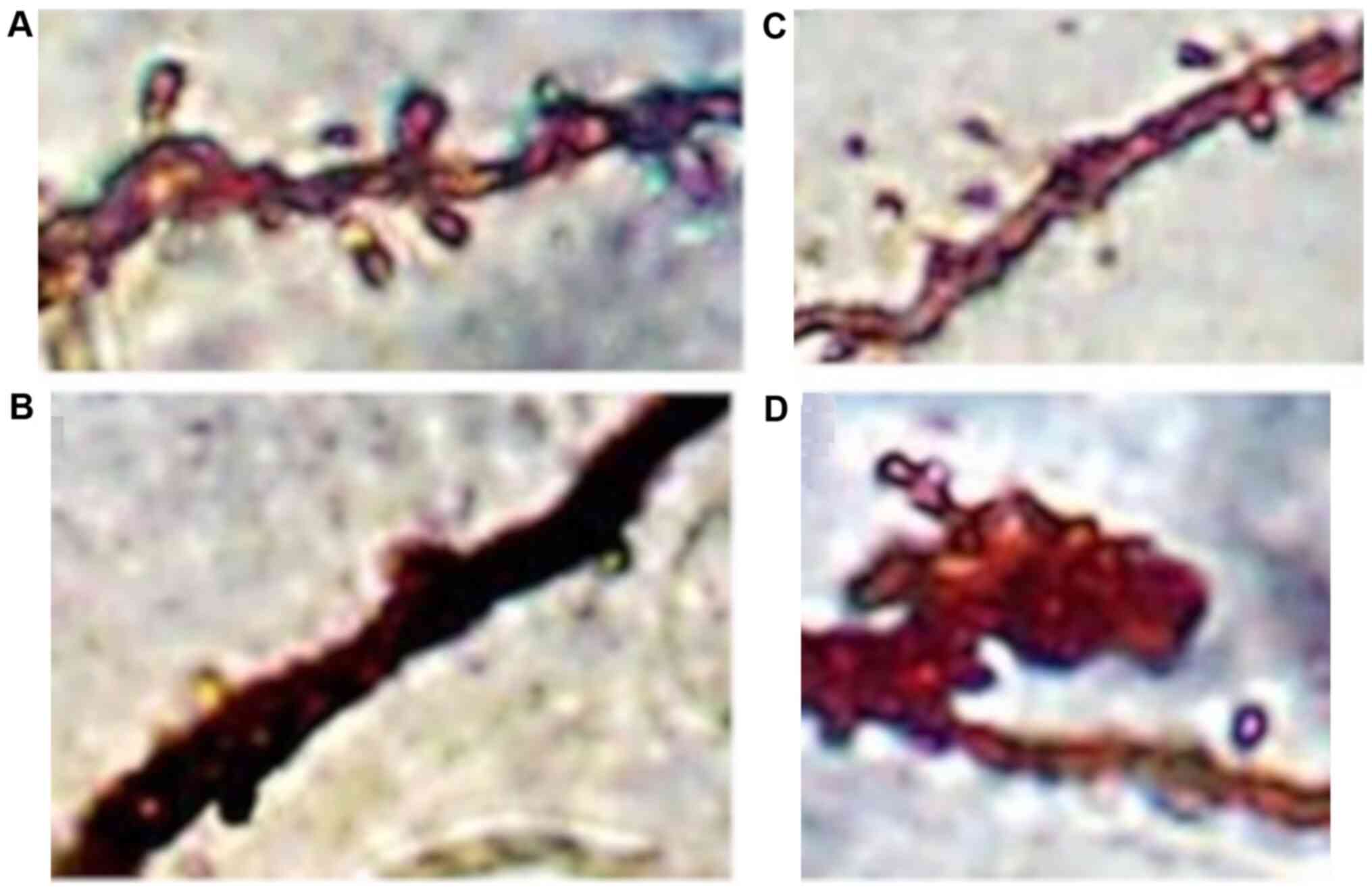
Analysis with the Golgi impregnation technique
revealed a significant loss of distal dendritic segments, tortuous
branches and varicosities and an overall restriction of the
dendritic field in the schizophrenic brains compared to the normal
brains (Fig. 2A and B).
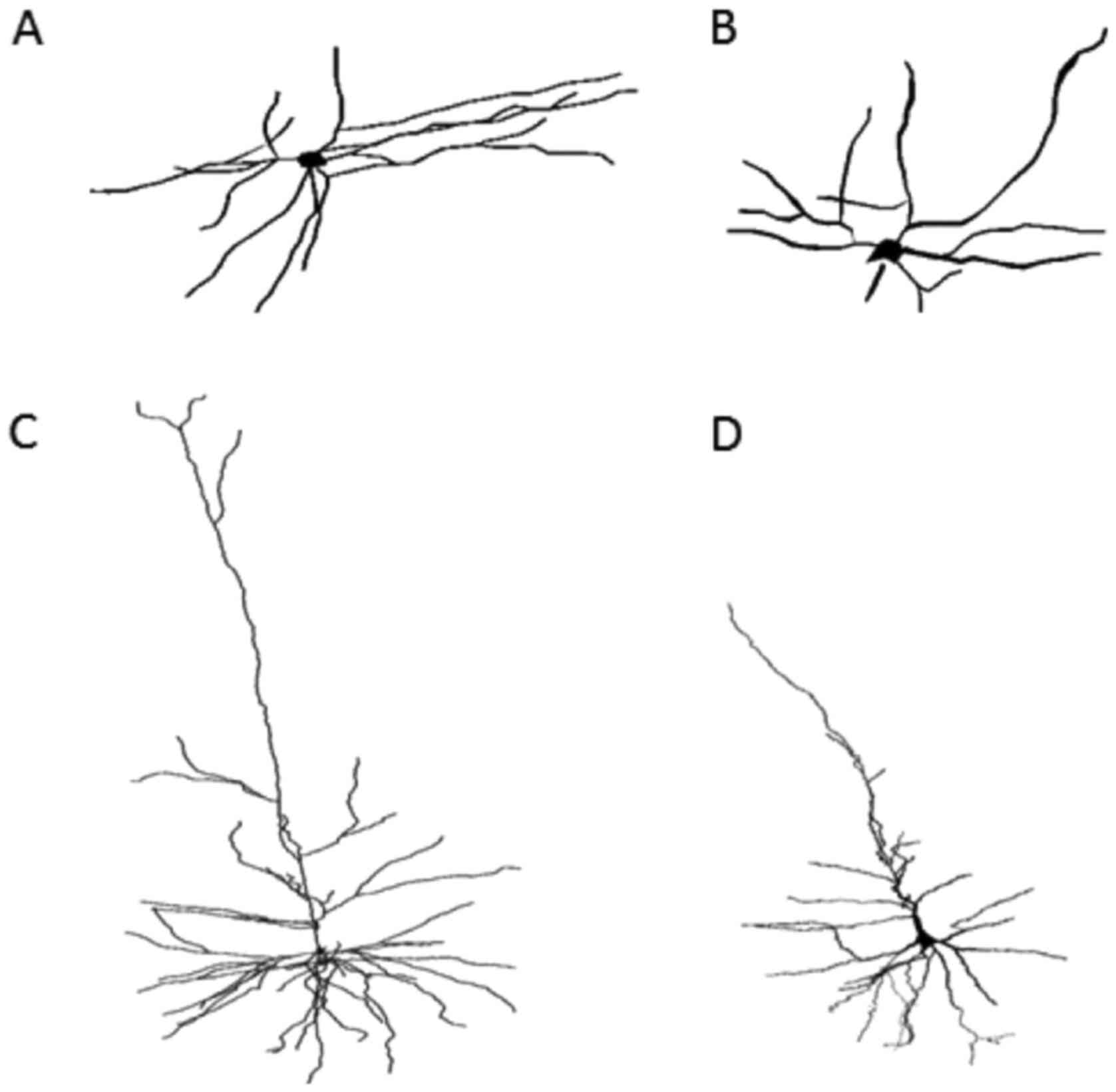
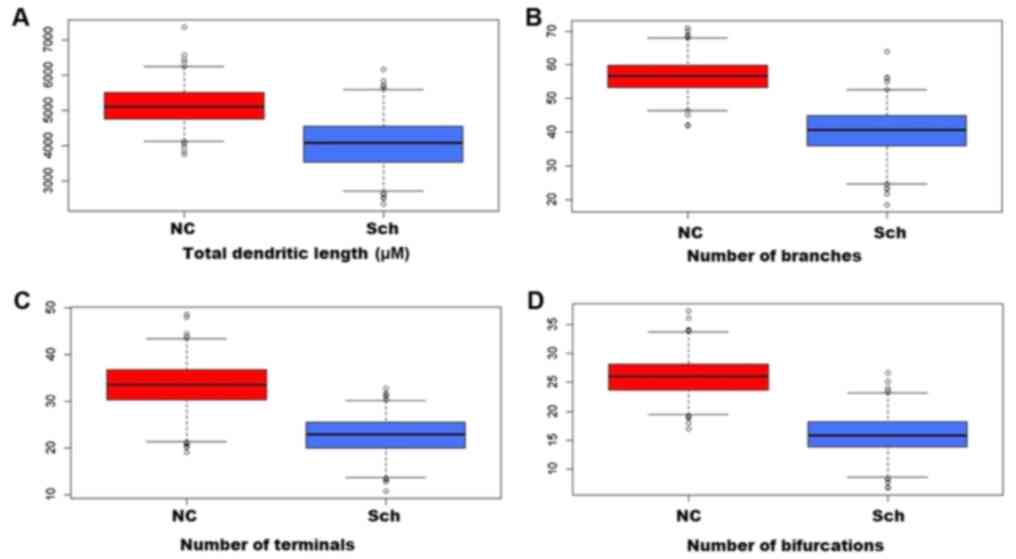
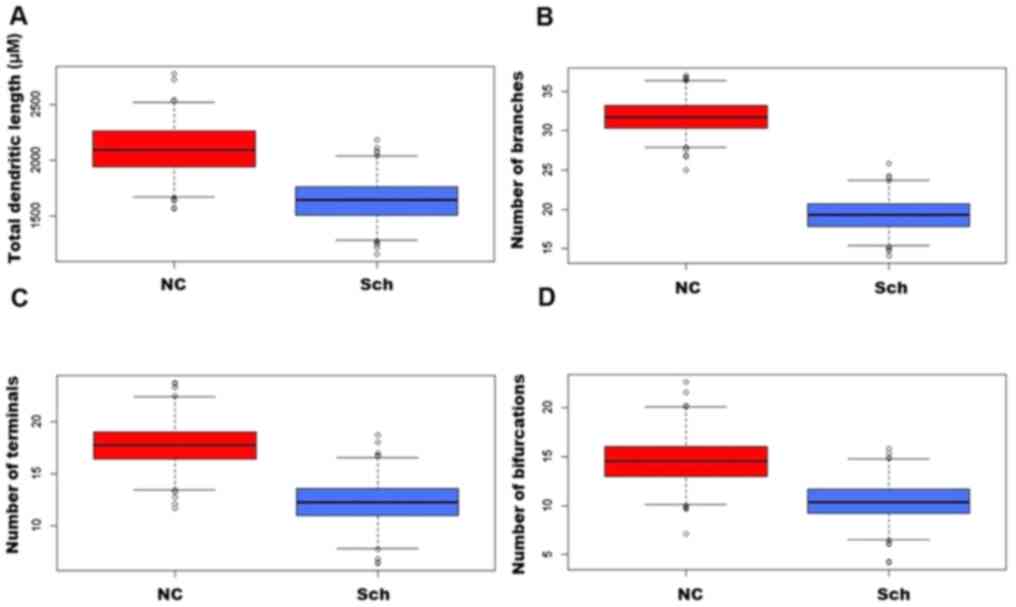
In the brains of schizophrenic subjects, the
dendritic field's total length was significantly decreased as
compared with that of normal subjects (Figs. 3A-D and 4A). A severe loss of distal dendritic
branches (quaternary) (Fig. 4B),
and a decrease in the number of terminal branches (Fig. 4C) and dendritic bifurcations were
also noted (Fig. 4D).
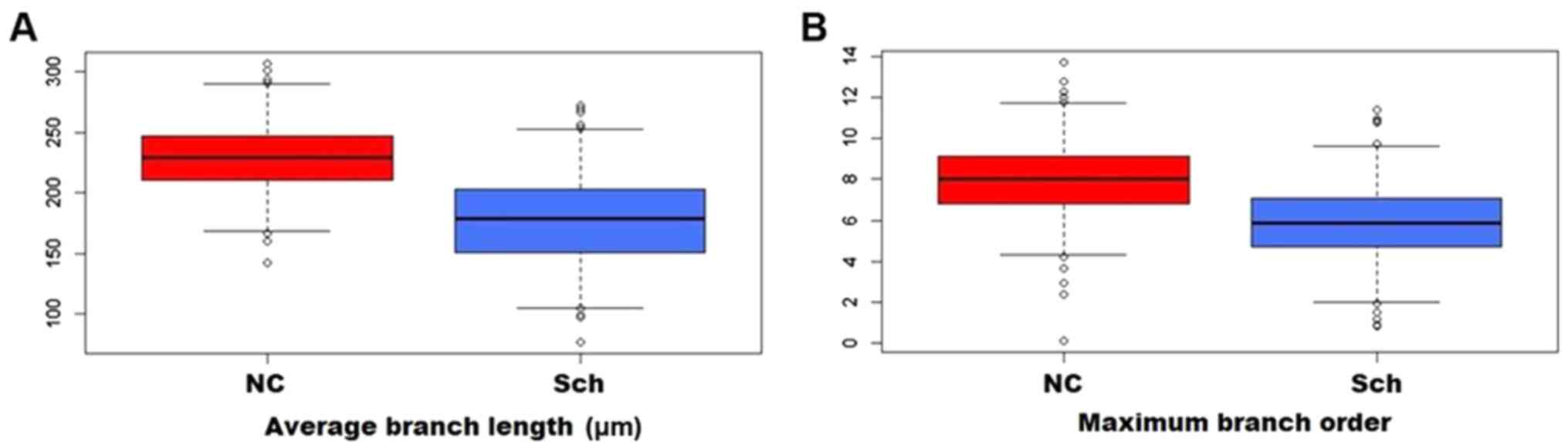
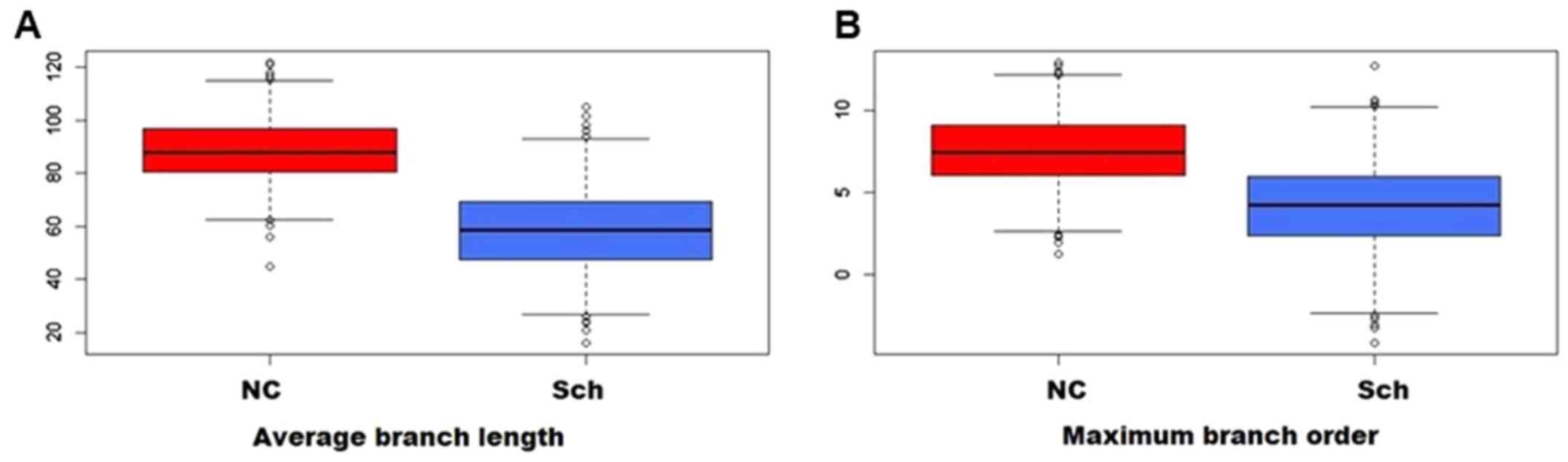
Compared to the normal controls, the branching ratio
was reduced in the schizophrenic group, and the average branch
length (Fig. 5A) and the maximum
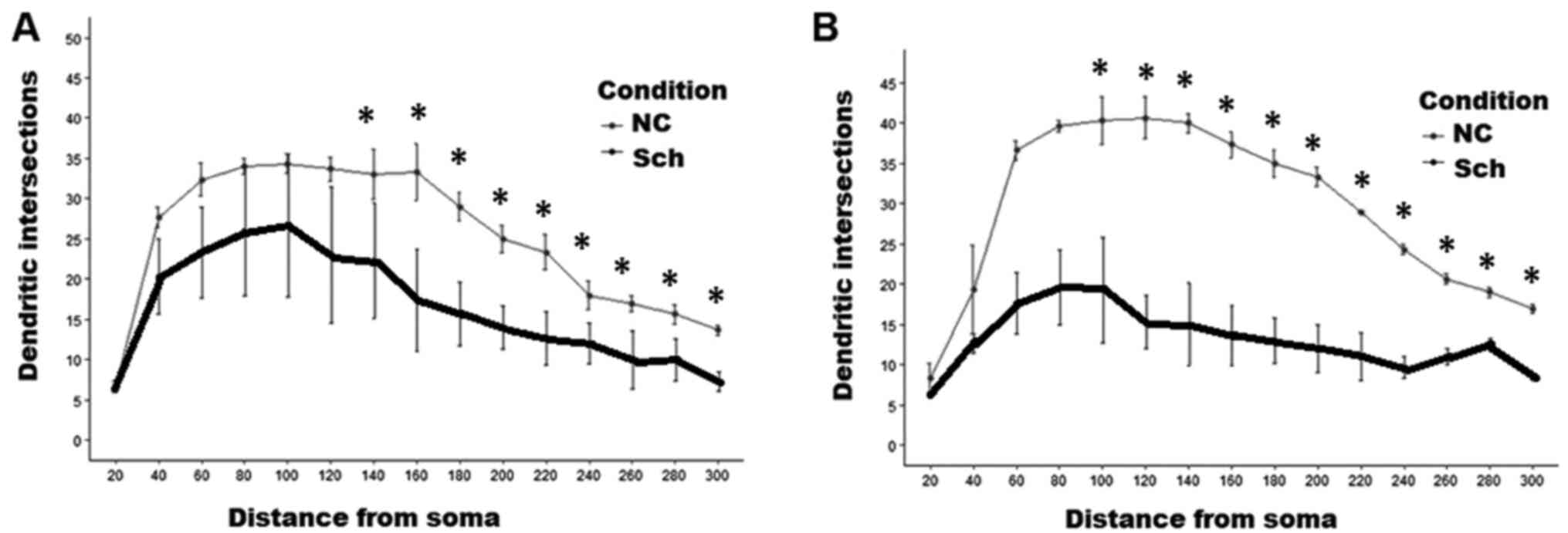
branching order were likewise affected (Fig. 5B). As presented in Fig. 6, Sholl analysis indicated a
restriction of the dendritic field due to the loss of distal
branches, although the proximal ones in the pyramidal cells
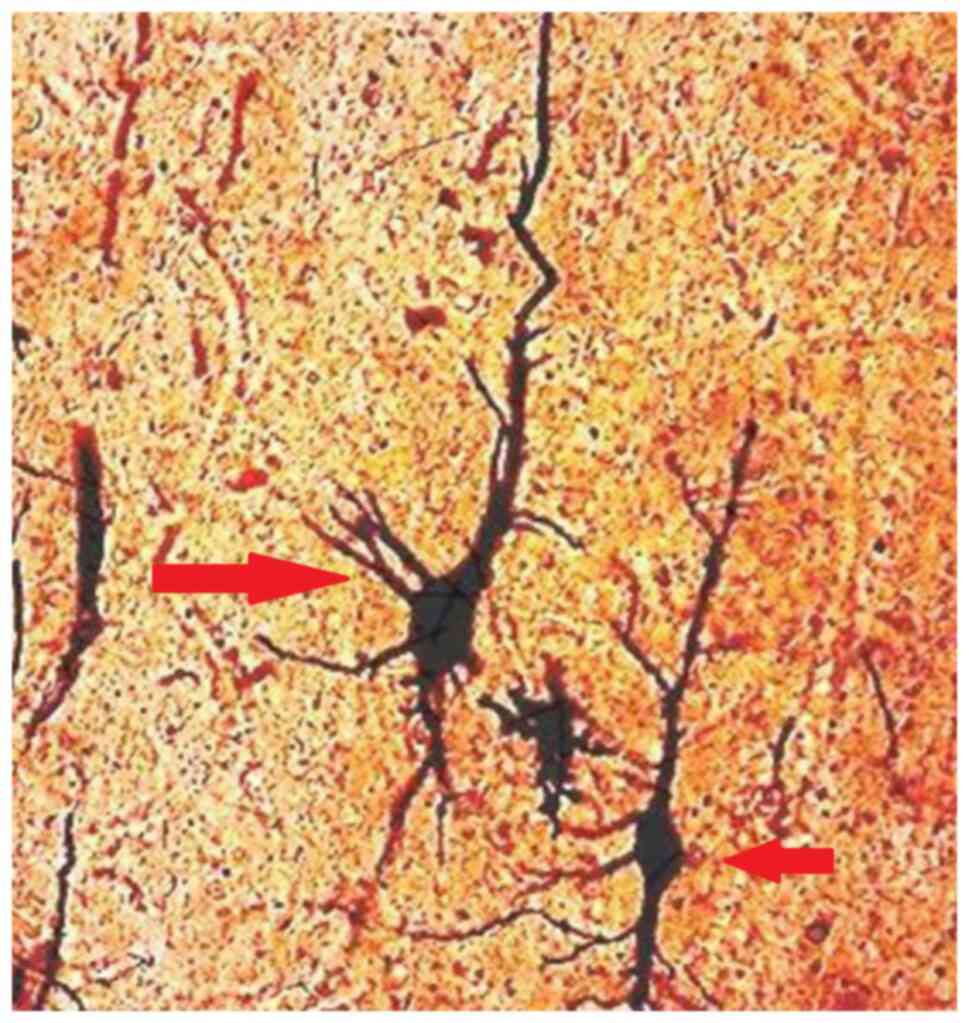
remained intact (Fig. 6A). A small
amount of degenerated pyramidal neurons was also noted in the
schizophrenic brains, and none were identified in the control group
(Fig. 7).
Interneurons
Aspiny stellate interneurons of the visual cortex
from the schizophrenic brains exhibited a significant decrease of
the total dendritic length (Figs.
8A and B and 9A), severe loss of dendritic branches
(Fig. 9B) and a substantial
reduction of the number of terminal dendritic branches (Fig. 9C). The branching ratio was grossly
reduced, and the average branch length and the maximum branch order
were significantly affected (Fig.
10A and B). Sholl analysis of
interneurons indicated extensive loss of distal dendritic branches
and decline of the dendritic field density at a distance >100 µm
from the cell soma (Fig. 6B). The
number of bifurcations was markedly lower in schizophrenic brains
compared with controls (Fig.
9D).
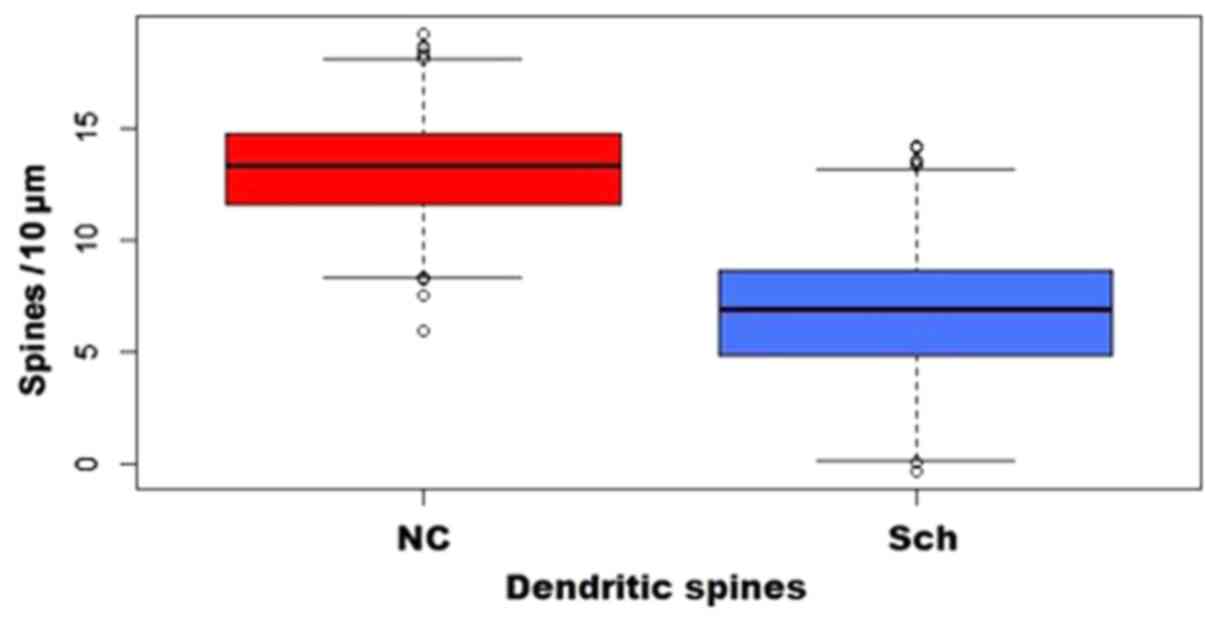
Spinal changes
Pyramidal neurons exhibited a significant decrease
in spinal density, affecting mainly the distal dendritic segments,
while dystrophic and giant spines were also observed (Figs. 11 and 12).
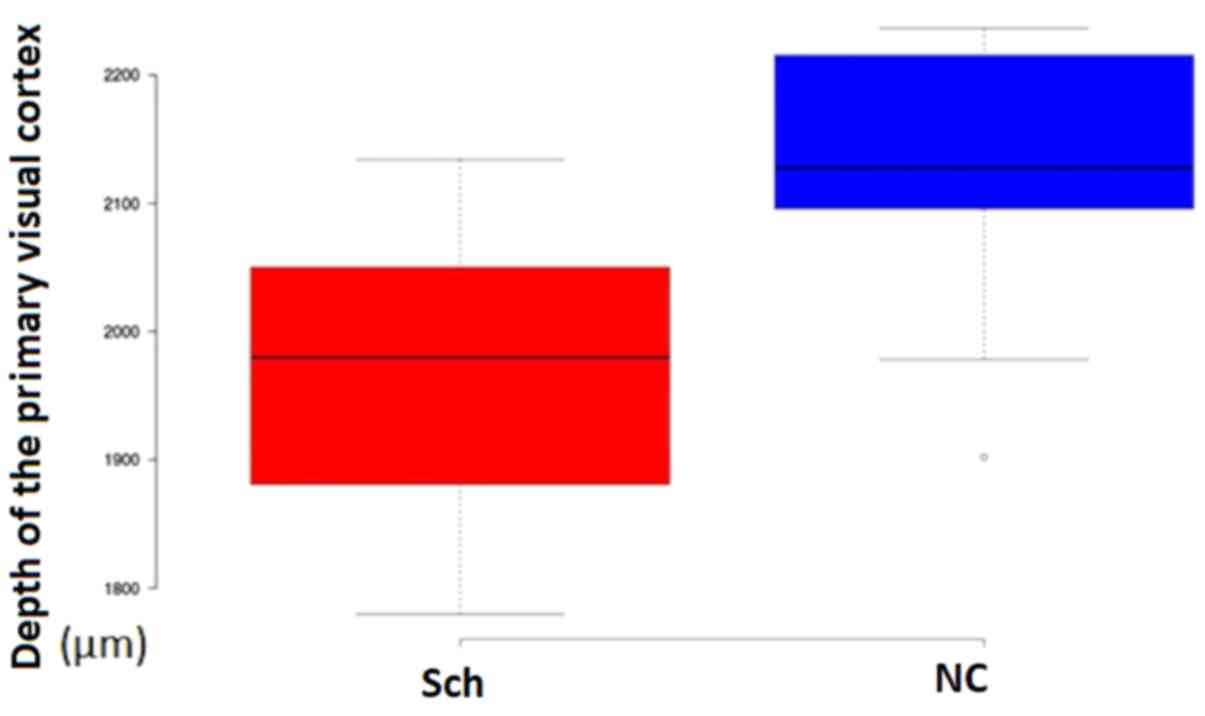
Cortical thickness
The thickness of the primary visual cortex measured
in Nissl preparations was significantly different between the
groups of the study (Schizophrenia, 1,956±105 µm; Controls,
2,124±96 µm; P=0.023; Fig.
13).
Discussion
There remains a lack of consensus or set of
quantified patient characteristics in regards to Schizophrenia and
it has remained an enigma to neuropathologists (32). Accumulating evidence from
macroscopic and microscopic pathology has been provided in the last
20 years. The main macroscopic findings include a decrease in brain
weight (33-35),
brain length (36) and volume of
the cerebral hemispheres (37). An
additional enlargement of the lateral ventricles (36,37),
changes to limbic structures (38),
reduced size of temporal lobe structures (39-41),
decreased thalamic volume (34,42)
and enlarged basal ganglia (43)
have also been described. Certain findings regarding synaptic and
spinal pathology, cell orientation, neuronal density, neuronal
size, protein expression and neurotransmitter deficits have also
been consistently reported by numerous studies (6).
The majority of existing studies are focused on
hippocampal formation, the temporal lobe, prefrontal cortex and
basal ganglia. To the best of our knowledge, no previous study has
reported on the morphological changes of the pyramidal and stellate
neurons of the occipital lobe. In 1998, Garey et al
(44) reported decreased spinal
density on the pyramidal cells of lamina III from Brodmann areas 11
and 38 observed on Golgi staining, and in the same year, Woo et
al (45) indicated a selective
decrease of terminal branches in brodmann areas 9 and 46 chandelier
neurons.
In 1996, Roberts et al (46) revealed changes of the dendritic
spines at the striatum, and in the same year, Uranova et al
(47) described certain changes in
the postsynaptic density of axo-spinous synapses.
In addition, Dorph-Petersen et al (22) reported significantly decreased
neuronal volume with no significant reduction of the neuronal
density in the primary visual cortex of schizophrenic brains. These
results were confirmed by neuroimaging studies using voxel-based
morphometry, which reported a significant reduction in the
occipital lobe's overall volume with a decrease in grey matter in
schizophrenic patients (48-52).
In the present study, no significant changes in the
neuronal density of the primary visual cortex were obtained, but
the overall thickness of the primary visual cortex was
substantially decreased in schizophrenic brains, corroborating the
findings of the earlier study by Dorph-Petersen et al
(22). Golgi silver staining and 3D
reconstruction of neurons revealed several morphological changes on
both cortical aspiny interneurons and pyramidal cells. The total
neuronal volume was decreased in both populations. The aspiny
interneurons exhibited a severe restriction of their dendritic
field areas, along with a loss of distal and terminal dendritic
branches. Pyramidal neurons from lamina III similarly exhibited a
significant loss of terminal branches and substantially lower
dendritic spines, mainly on the distal branches.
Regarding the clinical significance of the present
results, visual hallucinations are amongst the most common symptoms
associated with increased brain activity in patients with
schizophrenia (48). They have been
correlated to GABA deficits and functional impairment of cortical
interneurons, as well as a disturbance of cortico-thalamic or
intracortical connections (19).
Furthermore, studies have revealed certain functional deficits of
the visual cortex in schizophrenic patients, including early-stage
visual processing, contrast sensitivity abnormalities, surround
suppression and motor processing disturbance (53,54).
Lamina III pyramidal neurons contribute to reception, elaboration
and transmission of the visual information to other cortical areas,
while the aspiny interneurons provide inhibitory control and
modulate the synchronized oscillations (55). Both neuronal populations are
critical for the integrity of the cortico-thalamic and
intracortical circuits. The loss of dendrites and dendritic spines
of both pyramidal cells and interneurons leads to a substantial
decrease of the synaptic contacts and a significant impairment of
the pyramidal-interneuronal connectivity, as well as of the
connections of the cells of the visual cortex with the neurons of
other cortical and subcortical areas, which may be implicated in
the modulation of the visual information (30,55).
Although other cortical areas beyond the primary visual cortex are
related to the production of visual hallucinations, the
interruption of connectivity of the primary visual cortex with
secondary visual, temporal and parietal areas may have a crucial
role in the pathophysiology of visual hallucinations and other
functional deficits of the visual cortex in schizophrenia.
To the best of our knowledge, the present study was
the first to describe the morphological alterations in pyramidal
and spinal stellate neurons on the primary visual cortex in
patients with schizophrenia. The results may provide novel insights
into the brain changes exhibited by patients with schizophrenia. It
may be concluded that the present observations may be related to
certain clinical phenomena associated with the visual cortex
usually encountered in schizophrenia.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
IM prepared the manuscript and supervised the data
collection and analysis. FP, SC, EK and DK collected and analyzed
the data, and prepared the specimens. AC, ACI, RD and CT prepared
the manuscript and analyzed the data. SN, VC and SB made
substantial contributions to conception and design, acquisition of
data and analysis and interpretation of data. IM and SB confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed according to the
legislation of the Greek Democracy (v.2,472/1997, 2,819/2000,
2,915/2001, 3,235/2004 and 3,471/2006) and the Committee for
Research Deontology Principles of the Aristotle University of
Thessaloniki (24) (approval no.
23/4/4521/2018). Written informed consent was obtained from
relatives.
Patient consent for publication
For each of the patients and controls, and written
informed consent was obtained from their relatives and power of
attorneys regarding the publication of data and associated
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McKenna K, Gordon C and Rapoport J:
Childhood-onset schizophrenia: Timely neurobiological research. J
Am Acad Child Adolesc Psychiatry. 33:771–781. 1994.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Andreasen N: Symptoms, signs, and
diagnosis of schizophrenia. Lancet. 346:477–481. 1995.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Flaum M, Arndt S and Andreasen NC: The
role of gender in studies of ventricle enlargement in
schizophrenia: A predominantly male effect. Am J Psychiatry.
147:1327–1332. 1990.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jellinger K: Neuromorphological Background
of Pathochemical Studies in Major Psychoses. 1st Edition, Springer,
Berlin, pp1-23, 1985.
|
|
5
|
Johnstone EC, Bruton CJ, Crow TJ, Frith CD
and Owens DG: Clinical correlates of postmortem brain changes in
schizophrenia: Decreased brain weight and length correlate with
indices of early impairment. J Neurol Neurosurg Psychiatry.
57:474–479. 1994.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Harrison PJ: The neuropathology of
schizophrenia. A critical review of the data and their
interpretation. Brain. 122:593–624. 1999.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Haug JO: Pneumoencephalographic evidence
of brain atrophy in acute and chronic schizophrenic patients. Acta
Psychiatr Scand. 66:374–383. 1982.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Porter RH, Eastwood SL and Harrison PJ:
Distribution of kainite receptor subunit mRNAs in human
hippocampus, neocortex and cerebellum, and bilateral reduction of
hippocampal GluR6 and KA2 transcripts in schizophrenia. Brain Res.
751:217–231. 1997.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rajkowska G, Selemon LD and Goldman-Rakic
PS: Neuronal and glial somal size in the prefrontal cortex: A
postmortem morphometric study of schizophrenia and Huntington
disease. Arch Gen Psychiatry. 55:215–224. 1998.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Glantz LA and Lewis DA: Decreased
dendritic spine density on prefrontal cortical pyramidal neurons in
schizophrenia. Arch Gen Psychiatry. 57:65–73. 2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pierri JN, Volk CL, Auh S, Sampson A and
Lewis DA: Decreased somal size of deep layer 3 pyramidal neurons in
the prefrontal cortex of subjects with schizophrenia. Arch Gen
Psychiatry. 58:466–473. 2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sweet RA, Pierri JN, Auh S, Sampson AR and
Lewis DA: Reduced pyramidal cell somal volume in auditory
association cortex of subjects with schizophrenia.
Neuropsychopharmacology. 28:599–609. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
DeFelipe J and Fariñas I: The pyramidal
neuron of the cerebral cortex: Morphological and chemical
characteristics of the synaptic inputs. Prog Neurobiol. 39:563–607.
1992.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wilson CJ: GABAergic inhibition in the
neostriatum. Prog Brain Res. 160:91–110. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liston C, Miller MM, Goldwater DS, Radley
JJ, Rocher AB, Hof PR, Morrison JH and McEwen BS: Stress-induced
alterations in prefrontal cortical dendritic morphology predict
selective impairments in perceptual attentional set-shifting. J
Neurosci. 26:7870–7874. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cahill ME, Xie Z, Day M, Photowala H,
Barbolina MV, Miller CA, Weiss C, Radulovic J, Sweatt JD,
Disterhoft JF, et al: Kalirin regulates cortical spine
morphogenesis and disease-related behavioral phenotypes. Proc Natl
Acad Sci USA. 106:13058–13063. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Brennaman LH, Kochlamazashvili G, Stoenica
L, Nonneman RJ, Moy SS, Schachner M, Dityatev A and Maness PF:
Transgenic mice overexpressing the extracellular domain of NCAM are
impaired in working memory and cortical plasticity. Neurobiol Dis.
43:372–378. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kolluri N, Sun Z, Sampson AR and Lewis DA:
Lamina-specific reductions in dendritic spine density in the
prefrontal cortex of subjects with schizophrenia. Am J Psychiatry.
162:1200–1202. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Boksa P: On the neurobiology of
hallucinations. J Psychiatry Neurosci. 34:260–262. 2009.PubMed/NCBI
|
|
20
|
de la Iglesia-Vaya M, Escartí MJ,
Molina-Mateo J, Martí-Bonmatí L, Gadea M, Castellanos FX, Aguilar
García-Iturrospe EJ, Robles M, Biswal BB and Sanjuan J: Abnormal
synchrony and effective connectivity in patients with schizophrenia
and auditory hallucinations. Neuroimage Clin. 6:171–179.
2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cavus I, Reinhart RM, Roach BJ,
Gueorguieva R, Teyler TJ, Clapp WC, Ford JM, Krystal JH and
Mathalon DH: Impaired visual cortical plasticity in schizophrenia.
Biol Psychiatry. 71:512–520. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dorph-Petersen KA, Pierri JN, Wu Q,
Sampson AR and Lewis DA: Primary visual cortex volume and total
neuron number are reduced in schizophrenia. J Comp Neurol.
501:290–301. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yoon JH, Maddock RJ, Rokem A, Silver MA,
Minzenberg MJ, Ragland JD and Carter CS: GABA concentration is
reduced in visual cortex in schizophrenia and correlates with
orientation-specific surround suppression. J Neurosci.
30:3777–3781. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Research Committee. Research Deontology
Principles. 2nd edition, Aristotle University of Thessaloniki,
Thessaloniki, pp22-25, 2010.
|
|
25
|
Mavroudis IA, Fotiou DF, Manani MG, Njaou
SN, Frangou D, Costa VG and Baloyannis SJ: Dendritic pathology and
spinal loss in the visual cortex in Alzheimer's disease a Golgi
study in pathology. Int J Neurosci. 121:347–354. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mavroudis IA, Petrides F, Manani M,
Chatzinikolaou F, Ciobica AS, Padurariu M, Kazis D, Njau SN, Costa
VG and Baloyannis SJ: Purkinje cells pathology in schizophrenia. A
morphometric approach. Rom J Morphol Embryol. 58:419–424.
2017.PubMed/NCBI
|
|
27
|
Jacobs B, Driscoll L and Schall M:
Life-span dendritic and spine changes in areas 10 and 18 of human
cortex: A quantitative Golgi study. J Comp Neurol. 386:661–680.
1997.PubMed/NCBI
|
|
28
|
Johannes S, Arganda-Carreras I, Frise E,
Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S,
Schmid B, et al: Fiji: An open-source platform for biological-image
analysis. Nat Methods. 9:676–682. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sholl DA: The organization of the visual
cortex in the cat. J Physiol. 124:23–24. 1954.PubMed/NCBI
|
|
30
|
Anderson K, Bones B, Robinson B, Hass C,
Lee H, Ford K, Roberts TA and Jacobs B: The morphology of
supragranular pyramidal neurons in the human insular cortex: A
quantitative Golgi study. Cerebral Cortex. 19:2131–2144.
2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Plum F: Prospects for research on
schizophrenia. 3. Neurophysiology. Neuropathological findings.
Neurosci Res Program Bull. 10:384–388. 1972.PubMed/NCBI
|
|
32
|
Brown R, Colter N, Corsellis JA, Crow TJ,
Frith CD, Jagoe R, Johnstone EC and Marsh L: Postmortem evidence of
structural brain changes in schizophrenia. Differences in brain
weight, temporal horn area, and parahippocampal gyrus compared with
affective disorder. Arch Gen Psychiatry. 43:36–42. 1986.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Pakkenberg B: The volume of the
mediodorsal thalamic nucleus in treated and untreated
schizophrenics. Schizophr Res. 7:95–100. 1992.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bruton CJ, Crow TJ, Frith CD, Johnstone
EC, Owens DG and Roberts GW: Schizophrenia and the brain: A
prospective cliniconeuropathological study. Psycholo Med.
20:285–304. 1990.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Crow TJ, Ball J, Bloom SR, Brown R, Bruton
CJ, Colter N, Frith CD, Johnstone EC, Owens DG and Roberts GW:
Schizophrenia as an anomaly of development of cerebral asymmetry.
Arch Gen Psychiatry. 46:1145–1150. 1989.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Pakkenberg B: Post-mortem study of chronic
schizophrenic brains. Br J Psychiatry. 151:744–752. 1987.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bogerts B, Falkai P, Haupts M, Greve B,
Ernst S, Tapernon-Franz U and Heinzmann U: Post-mortem volume
measurements of limbic system and basal ganglia structures in
chronic schizophrenics. Initial results from a new brain
collection. Schizophr Res. 3:295–301. 1990.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Falkai P, Bogerts B and Rozumek M: Limbic
pathology in schizophrenia: The entorhinal region-a morphometric
study. Biol Psychiatry. 24:515–521. 1998.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Falkai P and Bogerts B: Cell loss in the
hippocampus of schizophrenics. Eur Arch Psychiatry Neurol Sci.
236:154–161. 1986.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Altshuler LL, Casanova MF, Goldberg TE and
Kleinman JE: The hippocampus and parahippocampus in schizophrenia,
suicide, and control brains. Arch Gen Psychiatry. 47:1029–1034.
1990.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Vogeley K, Hobson T, Schneider-Axmann T,
Honer WG, Bogerts B and Falkai P: Compartmental volumetry of the
superior temporal gyrus reveals sex differences in schizophrenia-a
post-mortem study. Schizophr Res. 31:83–87. 1998.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Danos P, Baumann B, Bernstein HG, Franz M,
Stauch R, Northoff G, Krell D, Falkai P and Bogerts B:
Schizophrenia and anteroventral thalamic nucleus: Selective
decrease of parvalbumin-immunoreactive thalamocortical projection
neurons. Psychiatry Res. 82:1–10. 1989.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Heckers S, Heinsen H and Heinsen YC:
Limbic structures and lateral ventricle in schizophrenia: A
quantitative post-mortem study. Arch Gen Psychiatry. 47:1016–1022.
1991.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Garey LJ, Ong WY, Patel TS, Kanani M,
Davis A, Mortimer AM, Barnes TR and Hirsch S: Reduced dendritic
spine density on cerebral cortical pyramidal neurons in
schizophrenia. J Neurol Neurosurg Psychiatry. 65:446–453.
1998.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Woo TU, Whitehead RE, Melchitzky DS and
Lewis DA: A subclass of prefrontal gamma- aminobutyric acid axon
terminals are selectively altered in schizophrenia. Proc Natl Acad
Sci USA. 95:5341–5346. 1998.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Roberts RC, Conley R, Kung L, Peretti FJ
and Chute DJ: Reduced striatal spine size in schizophrenia: A
post-mortem ultrastructural study. Neuroreport. 7:1214–1218.
1996.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Uranova NA, Casanova MF, DeVaughn NM,
Orlovskaya DD and Denisov DV: Ultrastructural alterations of
synaptic contacts and astrocytes in postmortem caudate nucleus of
schizophrenic patients (letter). Schizophr Res. 22:81–83.
1996.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Andreasen NC, Flashman L, Flaum M, Arndt
S, Swayze V and O'Leary DS: Regional brain abnormalities in
schizophrenia measured with magnetic resonance imaging. JAMA.
272:1763–1769. 1994.PubMed/NCBI
|
|
49
|
Bilder RM, Wu H, Bogerts B, Ashtari M,
Robinson D and Woerner M: Cerebral volume asymmetries in
schizophrenia and mood disorders: A quantitative magnetic resonance
imaging study. Int J Psychophysiol. 34:197–205. 1999.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Bilder RM, Wu H, Bogerts B, Degreef G,
Ashtari M and Alvir JM: Absence of regional hemispheric volume
asymmetries in first-episode schizophrenia. Am J Psychiatry.
151:1437–1447. 1994.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Goldstein JM, Goodman JM, Seidman LJ,
Kennedy DN, Makris N and Lee H: Cortical abnormalities in
schizophrenia identified by structural magnetic resonance imaging.
Arch Gen Psychiatry. 56:537–547. 1999.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zipursky RB, Lim KO, Sullivan EV, Brown BW
and Pfefferbaum A: Widespread cerebral gray matter volume deficits
in schizophrenia. Arch Gen Psychiatry. 49:195–205. 1992.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Butler P, Silverstein S and Dakin S:
Visual perception and its impairment in schizophrenia. Biol
Psychiatry. 64:40–47. 2008.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Slaghuis W: Contrast sensitivity for
stationary and drifting spatial frequency gratings in positive and
negative-symptom schizophrenia. J Abnorm Psychol. 107:49–62.
1998.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Jones KS, Corbin JG and Huntsman MM:
Neonatal NMDA receptor blockade disrupts spike timing and
glutamatergic synapses in fast spiking interneurons in a NMDA
receptor hypofunction model of schizophrenia. PLoS One.
9(e109303)2014.PubMed/NCBI View Article : Google Scholar
|