Introduction
Inflammation is a protective mechanism that can
counteract diverse biological stimuli; however, chronic
inflammation can play significant roles in the progress of various
disorders such as cancer, chronic respiratory diseases, heart
disorders, and diabetes (1,2). Excessively generated inflammatory
mediators, including nitric oxide (NO), prostaglandin E2
(PGE2), and inflammatory cytokines, are involved in the
development of inflammatory responses (3). Among them, PGE2 can be
generated by the rate-limiting enzyme cyclooxygenase-2 (COX-2) from
arachidonic acid, which is usually elevated in response to
inflammatory stimuli such as chemical injury and tumor promoters
(4). Thus, the arachidonic acid
pathway has been regarded as one of the hallmarks of chronic
inflammation. Moreover, the increased production of PGE2
induced by the upregulated expression of COX-2 can be observed in
various premalignant and malignant tissues (5). It has also been reported that the
upregulation of COX-2 can lead to inhibited apoptosis and
accelerated malignant cell invasion (6), which are reversed by non-steroidal
anti-inflammatory agents. Therefore, inhibitors of COX-2 expression
may be considered as promising therapeutics acting as preventive
agents against cancer and chronic inflammation (7).
Chrysoeriol is a flavone that is found in Flos
Lonicerae (Lonicera japonica flowers), Tanacetum
vulgare, Artemisia arborescens, Salix matsudana leaves,
Aspalathus linearis, and Coronopus didymus (8-12).
Furthermore, chrysoeriol has numerous pharmacological properties,
such as anti-inflammation, antioxidation, relaxing smooth muscle,
reducing obesity, and regulating the immune system (8-14).
While a previous study has revealed the anti-inflammatory activity
of chrysoeriol in the RAW 264.7 cell line (13), its exact mechanism involving COX-2
inhibition is not fully understood. Therefore, the present study
aimed to investigate the molecular mechanisms of chrysoeriol on
lipopolysaccharide (LPS)-induced inflammation in the RAW 264.7 cell
line.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) were obtained from Cytiva. Chrysoeriol was
purchased from ChromaDex (analytical grade verified by HPLC or GC
analysis; cat. no. ASB-00003630-005) and dissolved in dimethyl
sulfoxide (DMSO; cat. no. D8418; Sigma-Aldrich). LY294002 (purity:
≥98%; cat. no. L9908), SP600125 (purity: ≥98%; cat. no. S5567) and
SB202190 (purity: ≥98%; cat. no. S7067) were obtained from
Sigma-Aldrich; Merck KGaA which was applied as selective inhibitor
for phosphoinositde 3-kinase (PI3K)/Akt, c-Jun
NH2-terminal kinase (JNK) and p38, respectively.
Cell culture
RAW 264.7 cell line was purchased from American Type
Culture Collection (cat. no. TIB-71) and was cultured in DMEM
supplemented with 10% FBS and 2 mM L-glutamine (Hyclone; Cytiva).
Cells were seeded in 100 mm dishes (5x106 cells/dish)
and preincubated with indicated concentrations of chrysoeriol for 2
h and then incubated with LPS (1 µg/ml; cat. no. L4516) for
18 h to analyze the expression levels of inflammatory mediators
(15). To identify transcription
factors and upstream signaling molecules, cells were treated with
the indicated concentrations of chrysoeriol for 2 h along with LPS
(1 µg/ml) (16). In
addition, 20 µM of selective inhibitors for PI3K/Akt and
mitogen-activated protein kinases (MAPKs), as well as chrysoeriol,
were pre-incubated for 2 h, and then incubated with LPS (1
µg/ml) for 18 h to investigate which signaling molecules are
related to the anti-inflammatory responses (17).
Cell viability
Cell viability was determined by the CellTiter 96
Aqueous one solution cell proliferation assay (cat. no. G3582;
Promega). RAW 264.7 cells were seeded in 24-well plate
(4x105 cells/well) and incubated with or without various
concentrations of chrysoeriol for 24 h (15). Then, 50 µl of MTS solution
was added to 950 µl of DMEM and incubated for 1 h at 37˚C,
then the absorbance was measured at 490 nm with an xMark Microplate
Absorbance Spectrophotometer (Bio-Rad Laboratories, Inc.).
PGE2 determination
PGE2 concentration was measured using an
enzyme-linked immunosorbent assay (ELISA) kit (cat. no. 500141;
Cayman Chemical), following the manufacturer's instructions.
Briefly, RAW 264.7 cells were seeded in a 24-well plate and
preincubated with indicated concentrations of chrysoeriol for 2 h
and then incubated with LPS (1 µg/ml) for 18 h to analyze
the concentration of PGE2. Briefly, 50 µl of
supernatant of culture medium and the equal volume of
PGE2 tracer were mixed in the PGE2 ELISA
plate and incubated for 18 h at 4˚C. The wells were rinsed for 5
times with wash buffer. Then, 200 µl of Ellman's reagent was
added to the well and incubated in the dark in order to develop.
The absorbance was measured at 405 nm with an xMark Microplate
Absorbance Spectrophotometer (Bio-Rad Laboratories, Inc.) (18).
Western blot analysis
Antibodies for COX-2 (1:1,000; cat. no. 12282),
phospho-p65 (1:1,000; cat. no. 3033), p65 (1:1,000; cat. no. 8242),
phospho-c-jun (1:1,000; cat. no. 3270), c-jun (1:1,000; cat. no.
9165), phospho-Akt (1:1,000; cat. no. 4060), Akt (1:1,000; cat. no.
4691), phospho-extracellular signal-regulated kinase (ERK; 1:1,000;
cat. no. 8544), ERK (1:1,000; cat. no. 4695), phospho-JNK (1:1,000;
cat. no. 4668), JNK (1:1,000; cat. no. 9252), phospho-p38 (1:1,000;
cat. no. 4511), p38 (1:1,000; cat. no. 8690) and actin (1:1,000;
cat. no. 4970) as well as the horseradish peroxidase
(HRP)-conjugated anti-rabbit IgG (1:1,000; cat. no. 7074) were
purchased from Cell Signaling Technology. Antibodies against
Toll-like receptor 4 (TLR4; 1:500; cat. no. ab13356) and myeloid
differentiation primary response 88 (MyD88; 1:500; cat. no. ab2064)
were obtained from Abcam. RAW 264.7 cells were incubated with
indicated concentrations of chrysoeriol for 2 h and then treated
with 1 µg/ml LPS for 18 h. Cells were washed with PBS and
harvested using M-PER™ mammalian protein extraction reagent (cat.
no. 78051; Thermo Fisher Scientific, Inc.) for 10 min at room
temperature. Cell lysis buffer was centrifuged at 13,000 x g for 10
min and the protein concentration was determined by Bradford assay.
Then, 50 µg protein samples were separated on a 10% SDS-PAGE
gel and transferred to a PVDF membrane (Bio-Rad Laboratories,
Inc.). After transfer, the membrane was blocked with 5% skim milk
for 2 h at room temperature. Then, each diluted primary antibody
was incubated with the membranes for overnight at 4˚C. After
washing the membranes with PBST, they were incubated with
horseradish peroxidase-conjugated anti-rabbit IgG secondary
antibody for 2 h at room temperature. The membrane was developed
with ECL substrate solution (Santa Cruz Biotechnology, Inc.) and
western blotting data were quantified using a Gel Doc EQ System
(Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± SD. Statistical
analyses were performed using SPSS version 25.0 (IBM Corp.).
One-way ANOVA with Tukey's multiple comparison test was used to
analyze the difference between each group. P<0.05 was considered
to indicate a statistically significant difference.
Results
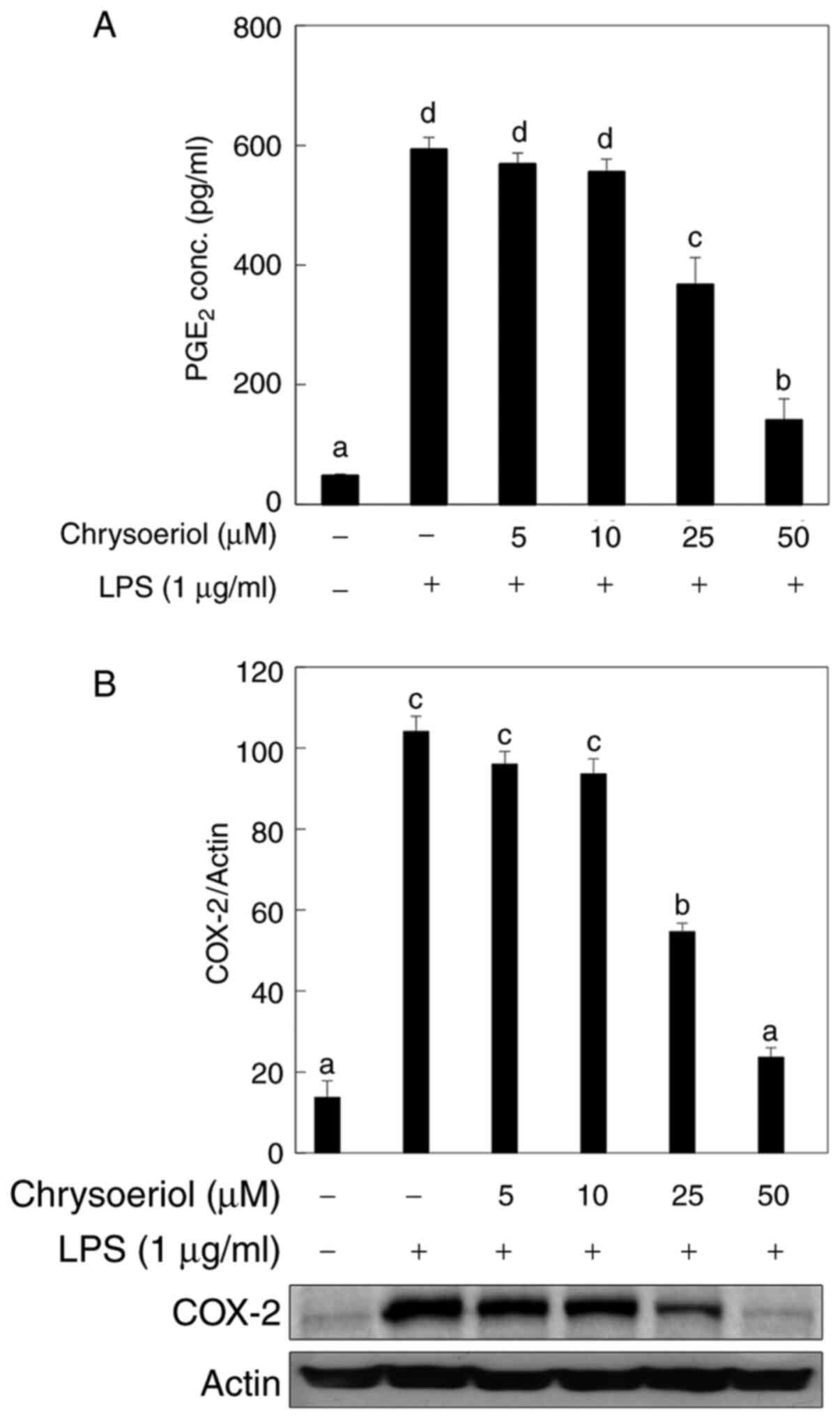
Chrysoeriol inhibits PGE2
secretion and COX-2 expression in LPS-treated RAW 264.7 cells
The anti-inflammatory effect of chrysoeriol was
investigated in LPS-stimulated RAW 264.7 cells. As shown in
Fig. 1A, LPS-induced
PGE2 production was significantly mitigated by
chrysoeriol treatment in a dose-dependent manner, without causing
cytotoxicity (Fig. S1). Moreover,
the corresponding enzyme of PGE2 formation, COX-2, was
also significantly attenuated by chrysoeriol treatment (Fig. 1B).
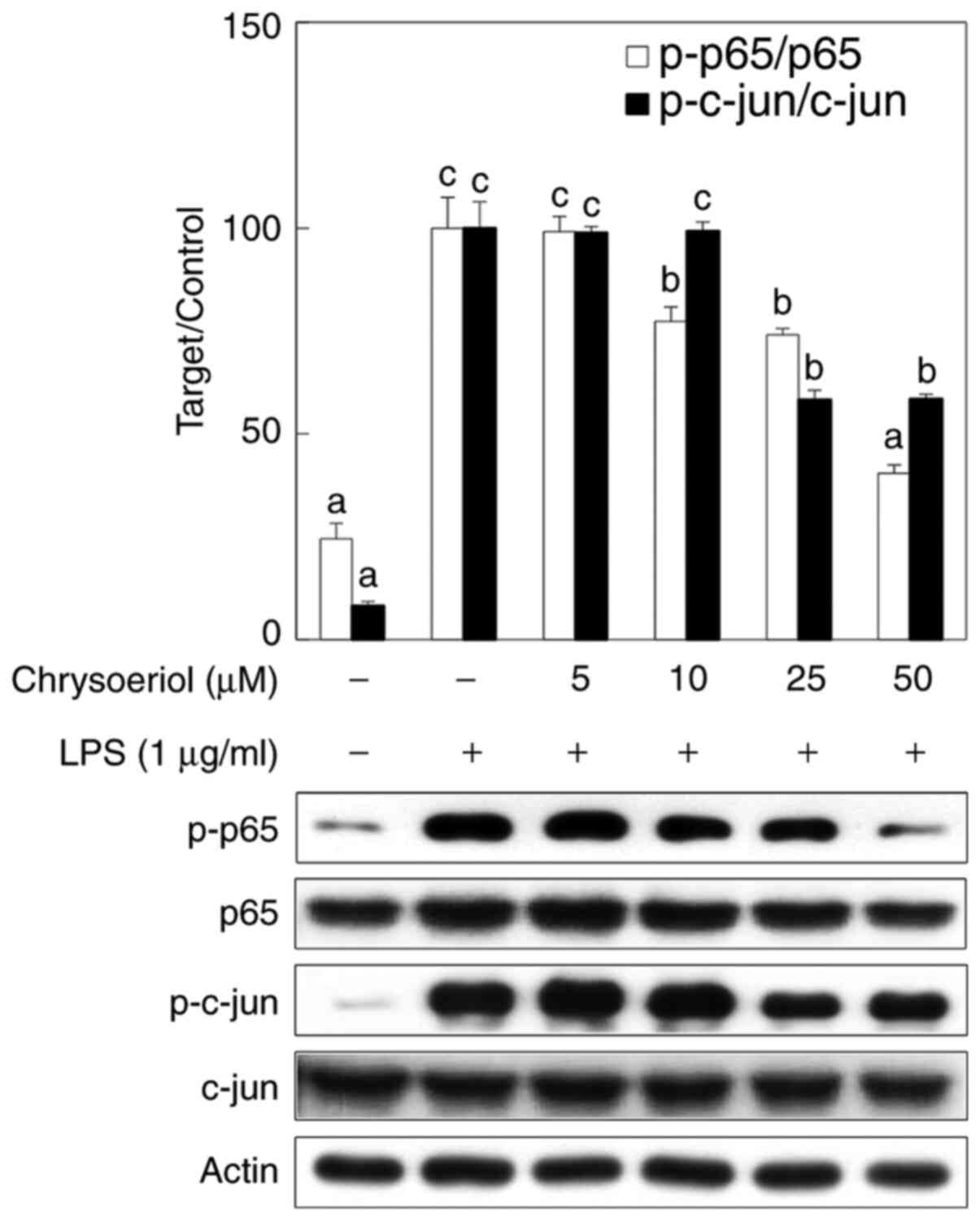
Chrysoeriol suppresses nuclear factor
(NF)-κB and activator protein (AP)-1 activation in LPS-treated RAW
264.7 cells
Western blot analysis was applied in order to
analyze the activated status of NF-κB and AP-1, and phosphorylation
of each subunit of both transcription factors p65 and c-jun was
significantly inhibited by chrysoeriol treatment in a
dose-dependent manner (Fig. 2).
Therefore, chrysoeriol treatment ameliorated LPS-induced
inflammatory circumstances in RAW 264.7 cells.
Chrysoeriol inhibits PI3K and p38
phosphorylation levels via TLR4/MyD88 inactivation in LPS-treated
RAW 264.7 cells
To identify the upstream signaling molecules that
can regulate NF-κB and AP-1 activation, the phosphorylation of PI3K
and MAPK was measured by western blot analysis. Chrysoeriol
significantly inhibited Akt and p38 phosphorylation, while slightly
affecting JNK activation (Fig. 3A).
Furthermore, ERK was not affected by chrysoeriol treatment in RAW
264.7 cells.
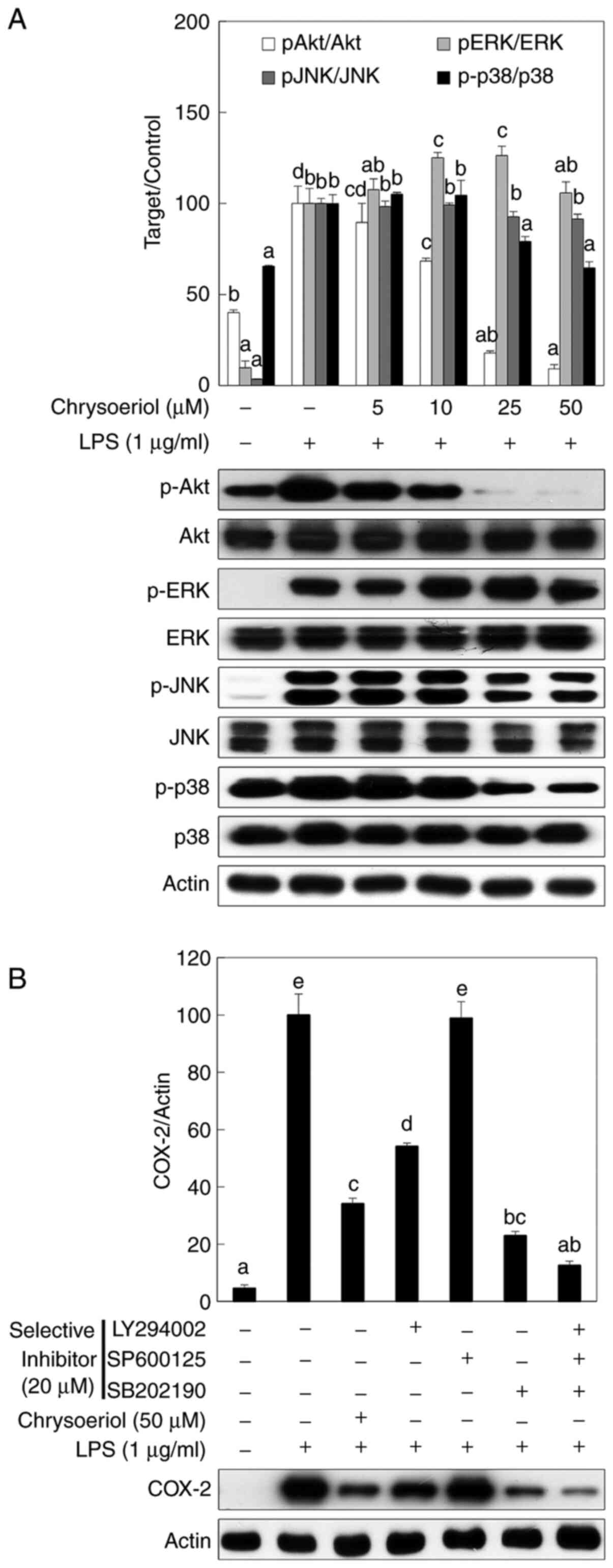 | Figure 3Chrysoeriol inhibited the
phosphorylation of Akt and p38, which was confirmed by selective
inhibitors in LPS-stimulated RAW 264.7 cells. Cells were incubated
with or without LPS (1 µg/ml) and with the indicated concentrations
of chrysoeriol for 2 h at 37˚C in a humidified atmosphere
containing 5% CO2. (A) Protein expression levels of
p-Akt, p-ERK, p-JNK, and p-p38 were assessed following chrysoeriol
treatment. Unphosphorylated forms of signaling molecules and actin
were used as internal controls. Akt, ERK, JNK and p38
phosphorylation was quantified by densitometry and unphosphorylated
forms of each signaling molecule were used as an internal control.
(B) A selective inhibitor of each signaling molecule was applied to
RAW 264.7 cells. The relative inhibition of COX-2 was quantified by
densitometry and actin was used as an internal control. Data are
presented as the mean ± SD of triplicate experiments. Values
sharing the same superscript letter were not significantly
different at P<0.05. LPS, lipopolysaccharide; p, phosphorylated;
ERK, extracellular signal-regulated kinase; JNK, c-Jun
NH2-terminal kinase; COX-2, cyclooxygenase-2. |
A selective inhibitor of each signaling molecule was
used to assess the role of PI3K and MAPK signaling molecules in
LPS-stimulated inflammatory cascades. LY294002 and SB202190,
selective inhibitors of PI3K and p38, respectively, significantly
inhibited COX-2 expression, but SP600125 did not affect COX-2
expression in LPS-stimulated RAW 264.7 cells (Fig. 3B). Moreover, COX-2 expression was
most highly inhibited when LY294002, SB202190, and SP600125
treatments were applied together in RAW 264.7 cells.
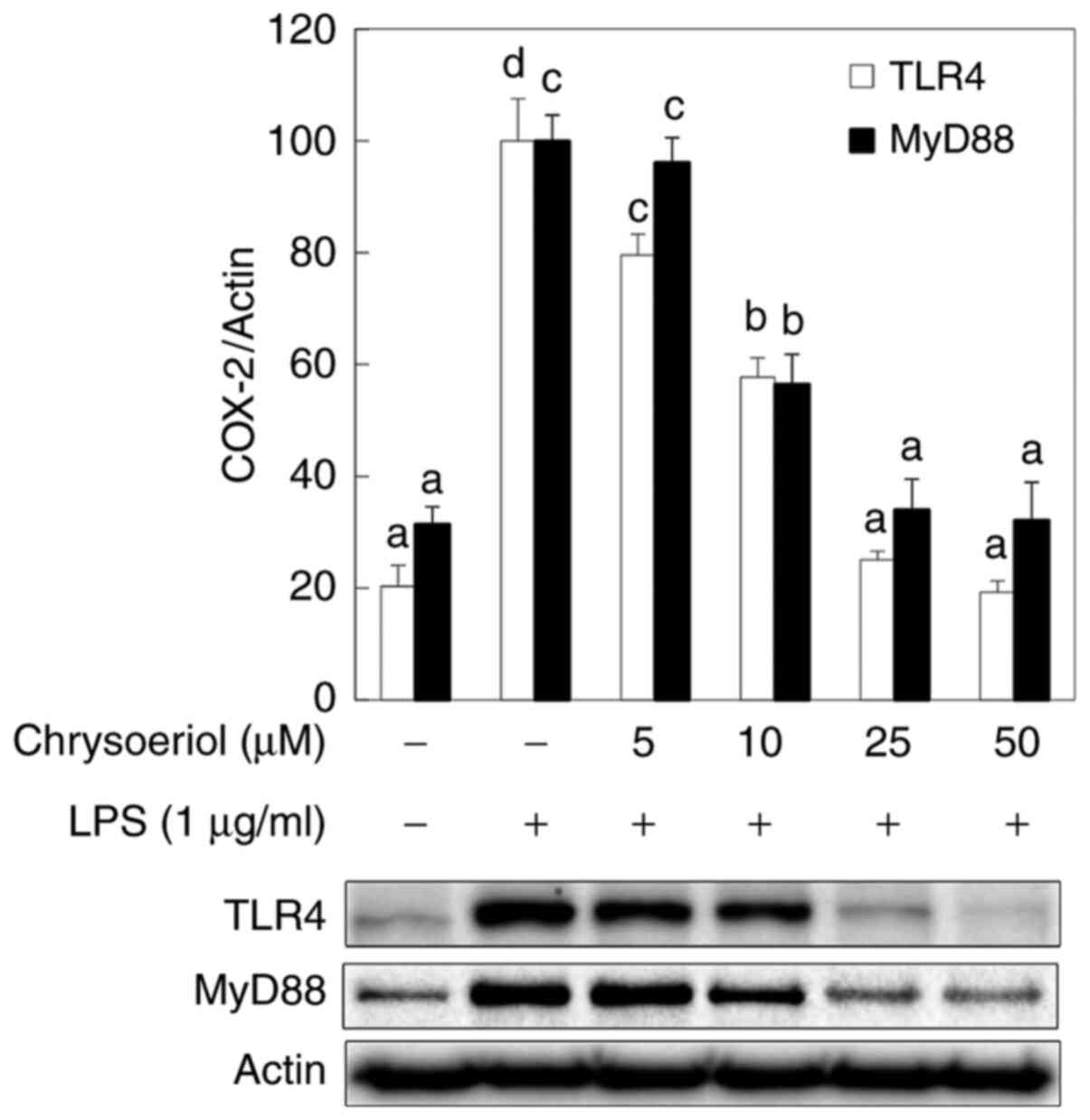
In addition, the present study also investigated the
effect of chrysoeriol on TLR4 and MyD88, based on their roles as
adaptor molecules in the development of NF-κB, AP-1, PI3K, and
MAPKs (14,15) in LPS-stimulated RAW 264.7 cells. It
was indicated that chrysoeriol mitigated the activation of TLR4 and
MyD88 in a dose-dependent manner, in accordance with the inhibited
NF-κB, AP-1, PI3K, and p38 MAPK expression levels, in
LPS-stimulated RAW 264.7 cells (Fig.
4). Collectively, these results suggested that inhibited PI3K
and p38 MAPK phosphorylation levels via TLR4/MyD88 mitigation by
chrysoeriol treatment may contribute to reducing LPS-induced NF-κB
and AP-1 activation, resulting in reduced COX-2 expression and
PGE2 production in RAW 264.7 cells.
Discussion
After the discovery of COX-2 in 1991, a variety of
pharmaceutical candidates were tested to identify their potential
uses as selective inhibitors of COX-2 and PG in inflammatory
lesions (19). A large number of
plant-derived compounds have been examined as candidates as a COX-2
selective inhibitor. Among them, chrysoeriol, which is a flavonoid,
has numerous pharmaceutical properties, including
anti-inflammatory, antioxidative, and anticarcinogenic activities
(8-13,20).
It has been reported that the anti-inflammatory activity of
chrysoeriol may occur via the inhibition of NO production caused by
AP-1 blockage in RAW 264.7 cells (13). However, to the best of our
knowledge, there are no previous studies analyzing chrysoeriol as a
COX-2 inhibitor and identifying its underlying mechanism in
LPS-induced inflammatory responses. Therefore, the present study
aimed to investigate the anti-inflammatory mechanism of
chrysoeriol, focused on COX-2 regulation, in LPS-stimulated murine
macrophage cells.
Inflammation is a type of defense mechanism
occurring in the immune system against tissue injuries, infections,
or toxic materials (21,22). Several pathophysiological disorders,
such as cancer, arthritis, cardiovascular disease, atherosclerosis,
and neurodegenerative diseases, may be caused by prolonged
inflammatory responses when acute inflammation cannot be controlled
in the early-stage (23,24). Among various immune cells,
macrophages play important roles in host defense mechanisms via the
regulation of NO and PGE2, as well as pro-inflammatory
cytokines including tumor necrosis factor (TNF)-α, interleukin
(IL)-6, and IL-1β (25). LPS, found
in the outer membrane of some types of Gram-negative bacteria,
activates immune responses by interacting with TLR4 associated with
CD14, which induces phosphorylation of MAPKs and subsequently
initiates the stimulation of transcription factors, including NF-κB
and AP-1 (26-29).
NF-κB and AP-1 are critical transcription factors that regulate the
expression of inflammatory mediators such as iNOS and COX-2. P65
and c-jun, each respective subunits of NF-κB and AP-1, are
phosphorylated and changed to active forms when inflammatory
stimuli reach the cells. Then, these subunits translocate into the
nucleus and bind to the promoter regions of inflammatory mediators
(30). Therefore, the
downregulation of inflammatory mediators as well as inflammatory
signaling molecules is one of the major targets for ameliorating
inflammation and its associated various disorders. In RAW 264.7
cells, LPS induced PGE2 overproduction and upregulation
of its corresponding enzyme, COX-2, and this was significantly
ameliorated by chrysoeriol treatment in a dose-dependent manner
without cytotoxicity (Fig. 1A and
B). Along with the effect on
inflammatory signaling cascades, activation of NF-κB and AP-1 is
highly associated with the upregulation of inflammatory mediators,
including iNOS and COX-2. Previous studies have also reported that
both transcription factors NF-κB and AP-1 are critical regulators
of inflammatory signaling pathways (30). NF-κB ubiquitously exists in the
cytoplasm and consists of p50 and p65 subunits bound to IκBα, while
AP-1 resides as homo- or heterodimers with the c-jun and c-fos
families (30). In response to LPS
stimulation, NF-κB and AP-1 become active forms via phosphorylated
IκBα, resulting in release from the NF-κB dimer and c-jun
phosphorylation, respectively (30). In this study as shown in Fig. 2, the phosphorylated status of p65
and c-jun, each subunit of NF-κB and AP-1, was measured by western
blot analysis, which was the phosphorylation of both transcription
factors was significantly inhibited by chrysoeriol treatment in a
dose-dependent manner. Moreover, activated MAPKs or PI3K/Akt can
lead to a series of inflammatory signaling cascades via the
regulation of NF-κB and AP-1 activation (31). As shown in Fig. 3A and B, chrysoeriol significantly inhibited
PI3K/Akt and p38 phosphorylation levels, as well as slightly
mitigated JNK activation, while ERK was not affected. In addition,
selective inhibitors were applied to confirm the role of signaling
molecules in LPS-stimulated inflammatory cascades. Each selective
inhibitor of PI3K and p38 significantly attenuated COX-2 expression
while JNK did not give any effect on COX-2 expression in this
experiment. Cotreatment of selective inhibitors of these signaling
molecules was the most potent inhibitory effect in LPS stimulated
RAW 264.7 cells.
TLRs are a growing family of pattern recognition
receptors (PRRs) which can stimulate innate immunity and
inflammatory responses upon the interaction with numerous
pathogen-associated molecular patterns including bacterial LPS,
viral RNA, and flagellin (32). As
a ligand for TLR4, LPS can bind to the extracellular domain of TLR4
and form intracellular adaptor molecules including MyD88 and
Toll-interleukin 1 receptor domain-containing adaptor protein
(TIRAP) (17,32,33).
Accelerated production of MyD88 can lead to the activation of
NF-κB, AP-1, PI3K/Akt, and MAPKs and the production of inflammatory
mediators (17,33). As shown in Fig. 4, chrysoeriol attenuated
dose-dependently LPS initiated TLR4 and MyD88 activation in
accordance with the inhibited NF-κB, AP-1, PI3K/Akt, and p38 MAPK
expression levels, in LPS-stimulated RAW 264.7 cells.
In conclusion, the present results suggest that
chrysoeriol signigicantly ameliorates LPS induced PGE2
production and COX-2 expression through the regulation of TLR4 and
MyD88 mediated NF-κB, AP-1, PI3K/Akt, and MAPKs in RAW 264.7
cells.
Supplementary Material
Chrysoeriol didn’t exhibit any
cytotoxic effect in LPS-stimulated RAW 264.7 cells. Cells were
pre-incubated with or without the indicated concentrations of
chrysoeriol for 2 h, then incubated with LPS (1 μg/ml) for 18 h at
37°C in a humidified atmosphere containing 5% CO2. Data represent
the mean±SD of triplicate experiments. Values sharing the same
superscript are not significantly different at P<0.05 by Tukey’s
multiple comparison tests.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
HSY and CMP made substantial contributions to the
conceptualization and design of the study. HSY performed the
experiments for data acquisition and conducted statistical
analysis. HSY and CMP confirmed the authenticity of all the raw
data. CMP interpreted the experimental results and wrote the
manuscript; HSY and CMP revised the final manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kunnumakkara AB, Sailo BL, Banik K, Harsha
C, Prasad S, Gupta SC, Bharti AC and Aggarwal BB: Chronic diseases,
inflammation, and spices: How are they linked? J Transl Med.
16(14)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kundu JK and Surh YJ: Inflammation:
Gearing the journey to cancer. Mutat Res. 659:15–30.
2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sukketsiri W, Tanasawet S, Moolsap F,
Tantisira MH, Hutamekalin P and Tipmanee V: ECa 233 suppresses
LPS-induced proinflammatory responses in macrophages via
suppressing ERK1/2, p38 MAPK and akt pathways. Biol Pharm Bull.
42:1358–1365. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Park SA, Kim EH, Na HK and Surh YJ: KG-135
inhibits COX-2 expression by blocking the activation of JNK and
AP-1 in phorbol ester-stimulated human breast epithelial cells. Ann
NY Acad Sci. 1095:545–553. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Na HK and Surh YJ: Intracellular signaling
network as a prime chemopreventive target of (-)-epigallocatechin
gallate. Mol Nutr Food Res. 50:152–159. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kim JH, Na HK, Pak YK, Lee YS, Lee SJ,
Moon A and Surh YJ: Roles of ERK and p38 mitogen-activated protein
kinases in phorbol ester-induced NF-kappaB activation and COX-2
expression in human breast epithelial cells. Chem Biol Interact.
171:133–141. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kawamori T, Rao CV, Seibert K and Reddy
BS: Chemopreventive activity of celecoxib, a specific
cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer
Res. 58:409–412. 1998.PubMed/NCBI
|
|
8
|
Choi CW, Jung HA, Kang SS and Choi JS:
Antioxidant constituents and a new triterpenoid glycoside from flos
lonicerae. Arch Pharm Res. 30:1–7. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Schinella GR, Giner RM, Recio MC,
Mordujovich de Buschiazzo P, Rios JL and Manez S: Anti-inflammatory
effects of South American Tanacetum vulgare. J Pharm Pharmacol.
50:1069–1074. 1998.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Abu Zarga M, Qauasmeh R, Sabri S, Munsoor
M and Abdalla S: Chemical constituents of artemisia arborescens and
the effect of the aqueous extract on rat isolated smooth muscle.
Planta Med. 61:242–245. 1995.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Han LK, Sumiyoshi M, Zheng YN, Okuda H and
Kimura Y: Anti-obesity action of salix matsudana leaves (Part 2).
Isolation of anti-obesity effectors from polyphenol fractions of
salix matsudana. Phytother Res. 17:1195–1198. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Mishra B, Priyadarsini KI, Kumar MS,
Unnikrishnan MK and Mohan H: Effect of O-glycosilation on the
antioxidant activity and free radical reactions of a plant
flavonoid, chrysoeriol. Bioorg Med Chem. 11:2677–2685.
2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Choi DY, Lee JY, Kim MR, Woo ER, Kim YG
and Kang KW: Chrysoeriol potently inhibits the induction of nitric
oxide synthase by blocking AP-1 activation. J Biomed Sci.
12:949–959. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim JH, Cho YH, Park SM, Lee KE, Lee JJ,
Lee BC, Pyo HB, Song KS, Park HD and Yun YP: Antioxidants and
inhibitor of matrix metalloproteinase-1 expression from leaves of
Zostera marina L. Arch Pharm Res. 27:177–183. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Park CM and Song YS: Luteolin and
luteolin-7-O-glucoside inhibit lipopolysaccharide-induced
inflammatory responses through modulation of NF-kB/AP-1/PI3K-Akt
signaling cascades in RAW 264.7 cells. Nutr Res Pract. 7:423–429.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bak MJ, Truong VL, Kang HS, Jun M and
Jeong WS: Anti-inflammatory effect of procyanidins from wild grape
(Vitis amurensis) seeds in LPS-induced RAW 264.7 cells. Oxid Med
Cell Longev. 2013(409321)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chun J, Choi RJ, Khan S, Lee DS, Kim YC,
Nam YJ, Lee DU and Kim YS: Alantolactone suppresses inducible
nitric oxide synthase and cyclooxygenase-2 expression by
down-regulating NF-kB, MAPK and AP-1 via the MyD88 signaling
pathway in LPS-activated RAW 264.7 cells. Int Immunopharmacol.
14:375–383. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jeong D, Dong GZ, Lee HJ and Ryu JH:
Anti-inflammatory compounds from atractylodes macrocephala.
Molecules. 24(1859)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xie WL, Chipman JG, Robertson DL, Erikson
RL and Simmons DL: Expression of a mitogen-responsive gene encoding
prostaglandin synthase is regulated by mRNA splicing. Proc Natl
Acad Sci USA. 88:2692–2696. 1991.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yang Y, Zhou X, Xiao M, Hong Z, Gong Q,
Jiang L and Zhou J: Discovery of chrysoeriol, a PI3K-AKT-mTOR
pathway inhibitor with potent antitumor activity against human
multiple myeloma cells in vitro. J Huazhong Univ Sci Technolog Med
Sci. 30:734–740. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Medzhitov R: Origin and physiological
roles of inflammation. Nature. 454:428–435. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tabas I and Glass CK: Anti-inflammatory
therapy in chronic disease: Challenges and opportunities. Science.
339:166–172. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Qian C, Liu J and Cao X: Innate signaling
in the inflammatory immune disorders. Cytokine Growth Factor Rev.
25:731–738. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Schindler SM, Little JP and Klegeris A:
Microparticles: A new perspective in central nervous system
disorders. Biomed Res Int. 2014(756327)2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Van Dyke TE and van Winkelhoff AJ:
Infection and inflammatory mechanisms. J Periodontol. 84 (Suppl
4):S1–S7. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Meng F and Lowell CA: Lipopolysaccharide
(LPS)-induced macrophage activation and signal transduction in the
absence of Src-family kinases Hck, Fgr, and Lyn. J Exp Med.
185:1661–1670. 1997.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Roy A, Srivastava M, Saqib U, Liu D,
Faisal SM, Sugathan S, Bishnoi S and Baig MS: Potential therapeutic
targets for inflammation in toll-like receptor 4 (TLR4)-mediated
signaling pathways. Int Immunopharmacol. 40:79–89. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hu W, Wu L, Qiang Q, Ji L, Wang X, Luo H,
Wu H, Jiang Y, Wang G and Shen T: The dichloromethane fraction from
Mahonia bealei (Fort.) Carr. leaves exerts an anti-inflammatory
effect both in vitro and in vivo. J Ethnopharmacol. 188:134–143.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang J, Kang YX, Pan W, Lei W, Feng B and
Wang XJ: Enhancement of anti-inflammatory activity of curcumin
using phosphatidylserine-containing nanoparticles in cultured
macrophages. Int J Mol Sci. 17(969)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Endale M, Park SC, Kim S, Kim SH, Yang Y,
Cho JY and Rhee MH: Quercetin disrupts tyrosine-phosphorylated
phosphatidylinositol 3-kinase and myeloid differentiation factor-88
association, and inhibits MAPK/AP-1 and IKK/NF-kB-induced
inflammatory mediators production in RAW 264.7 cells.
Immunobiology. 218:1452–1467. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen K, Huang J, Gong W, Iribarren P,
Dunlop NM and Wang JM: Toll-like receptors in inflammation,
infection and cancer. Int Immunopharmacol. 7:1271–1285.
2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dauphinee SM and Karsan A:
Lipopolysaccharide signaling in endothelial cells. Lab Invest.
86:9–22. 2006.PubMed/NCBI View Article : Google Scholar
|