Introduction
Breast cancer is the most frequently diagnosed
malignancy among women in Romania and worldwide. According to
GLOBOCAN data, in 2018, ~2.1 million women were diagnosed with
breast cancer, which accounts for ~11.6% of all malignancies,
meaning that 1 out of 4 women with a neoplasm currently has breast
cancer and the incidence continues to be on the increase (1,2). The
American Cancer Society reported that the breast cancer incidence
rate increased by 0.3% per year between 2012 and 2016(3). In addition, there is an estimation
that in 2020, breast cancer will affect 276,480 women in USA alone
(4). In Romania, there were 9,629
newly diagnosed breast cancer cases in 2018, i.e., 11.5% of all
cancer cases among women. Furthermore, over the last three decades,
the breast cancer incidence rate has more than doubled and the
highest growth has been observed in women aged 50-69 years
(2).
Regarding malignancy-related deaths worldwide,
breast cancer is the second most common cause, just after lung
cancer (626,679 cases, which accounts for 6.6%) (1). The same percentage was observed in
Romania, in 2018(2).
The proportion of women diagnosed with breast
neoplasm in the premenopausal age is relatively small. In 2019, all
ductal carcinoma in situ (DCIS) cases among women under 40
years of age accounted for only 2% of all cases, and invasive
breast cancers account for 4% of all age groups (3). Nevertheless, American data have shown
that breast cancer is, not only the most commonly diagnosed cancer
among women aged 20-49 years, but it is also the leading cause of
death in the same group (5). This
situation is similar to that of Romania (2).
Although age is a significant risk factor for breast
cancer, several studies have revealed an increase in the incidence
of this type of cancer among premenopausal women (6-11).
Concerning the figures mentioned above, the
increasing number of younger women who have breast cancer is
becoming a public concern, including in our country. That is
because younger women are not in the scope of breast cancer
screening programs in Romania, which are primarily focused on women
aged 50-69 years. Breast cancer among young women is also a severe
psychosocial problem. Cancer diagnosis and oncological treatment
may impact quality of life, as it causes premature menopause and
impaired fertility (12,13).
The rationale for dose-dense chemotherapy regimens
is based on the hypothesis that maximal chemotherapy effectiveness
can be achieved by scheduling the interval of chemotherapy to
correspond to the period of most rapid tumor growth, as predicted
by preclinical models. The granulocyte-colony stimulating factor
(G-CSF) support has permitted the safe delivery of chemotherapy at
shorter (‘dose-dense’) inter-treatment intervals. Randomized trials
have been conducted to test the feasibility and effectiveness of
anthracycline and/or taxane-based dose-dense strategies, being
associated with a modest impact on disease recurrence and overall
survival (OS) of patients with early-stage breast cancer (14).
The dose-dense neoadjuvant
doxorubicin-cyclophosphamide (AC), followed by paclitaxel (T)
regimen, significantly improves disease-free survival (DFS) and OS,
but can also lead to hematological toxicity, resulting in a higher
number of red blood cell (RBC) transfusions (CALGB C9741,
AGO-ETC).
G-CSF support has permitted the safe delivery of
dose-dense chemotherapy regimens, which, as predicted by
preclinical models, have further improved survival. Recently,
insights into tumor biology have led to the development of targeted
therapies, such as trastuzumab for HER2-positive disease, and it
has now been successfully incorporated into dose-dense therapy
(15). Newer targeted agents may be
similarly incorporated in order to improve patient outcomes
further.
The aim of the study was to evaluate the variation
of the magnitude of the limiting dose parameters in dose-dense vs.
regular chemotherapy, in order to choose the optimal chemotherapy
frequency. In addition, it aims to outline the framework for the
dose-dense chemotherapy concept within neoadjuvant breast cancer
treatment and discusses its implications for clinical practice.
Materials and methods
General
Patients with non-metastatic breast cancer received
doxorubicin (60 mg/m2) + cyclophosphamide (600
mg/m2) (AC), either four cycles every two weeks,
followed by the same regimen of paclitaxel (175 mg/m2)
(T) (arm A) or four cycles every three weeks, followed by the same
regimen of paclitaxel (175 mg/m2) (arm B). All patients
in arm A received prophylactic G-CSF support.
Criteria
A search within the OncoHelp Association breast
cancer database was performed in order to identify all patients who
underwent an initial consultation with non-metastatic breast cancer
at a medical oncologist between March 2016 and April 2020. A
retrospective chart review was performed and the following
including criteria were analyzed: i) Sex, ii) follow-up care
obtained at OncoHelp Association, iii) intent to treat with
neoadjuvant dose-dense AC-T every two weeks for four cycles
followed by paclitaxel every two weeks for four cycles, with white
blood cell growth factor support, and iv) regular
anthracycline-based AC-T every three weeks for four cycles followed
by paclitaxel every three weeks for four cycles, iv) weight, v)
height, vi) Eastern Cooperative Oncology Group (ECOG) performance
status, vii) hemoglobin (Hb) level, viii) platelet count, ix)
neutrophil count. In order to collect the data, the patient
observation sheets were analyzed, and data were subsequently
entered in electronic format using the Microsoft Excel 2016
program.
Regarding age, this study included two age groups,
under 60 and over 60 years. In the latter group, 11 patients out of
60 received dose-dense chemotherapy, with the maximum age of 68
years, while 65 patients out of 133 received standard chemotherapy,
with the maximum age of 78 years.
Statistical analysis
Non-parametric test (Mann-Whitney) was used for
variables with non-normal distribution. P<0.05 was used as the
threshold for statistical significance.
Results
Parameters
The evolution of clinical and biological parameters
of 168 patients was followed in this retrospective study, 60 in arm
A and 108 in arm B (Table I). Out
of 739 chemotherapy settings, 254 were dose-dense regimens and 485
were regular schedules of neoadjuvant regimens.
 | Table IAverage parameters depending on
chemotherapy regimens. |
Table I
Average parameters depending on
chemotherapy regimens.
| Chemotherapy
regimen | Average age | Average weight | Average height | Average ECOG | Average Hb | Average Plt | Average ANC | Cases |
|---|
| Dose-dense | 48.1 | 69.1 | 160.9 | 0.1 | 13.3 | 296.5 | 4.8 | 60 |
| Normal | 58.4 | 74.9 | 150.7 | 0.3 | 13.3 | 275.4 | 4.5 | 108 |
Chemotherapy settings
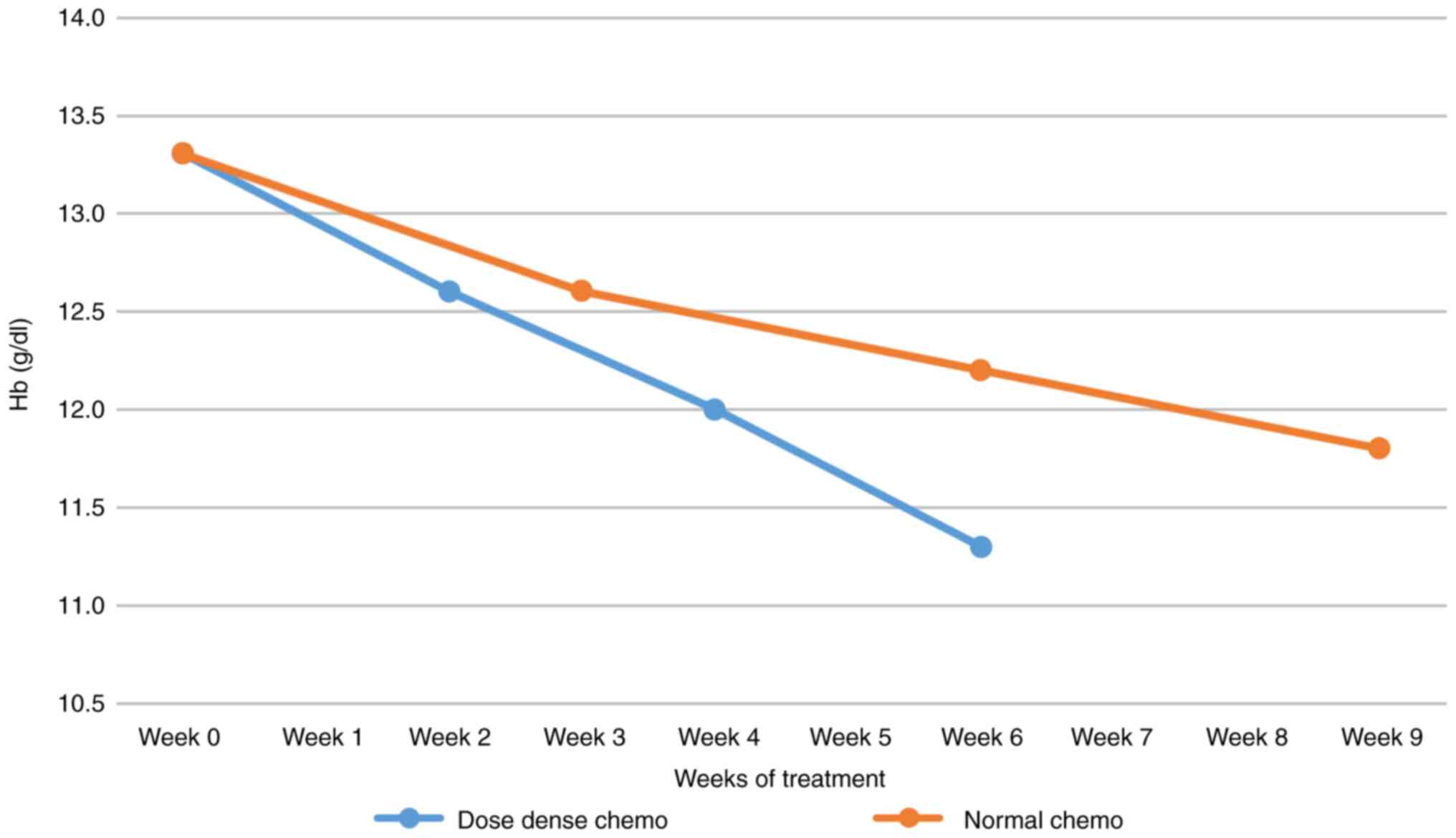
One of the most exciting parameter dynamics was the
evolution of average Hb values for each administration cycle, both
in dose-dense or in regular chemotherapy settings. Relative Hb
decrease was -15% after three cycles of chemotherapy. The Hb value
starts from the same average, but the decrease in Hb in the case of
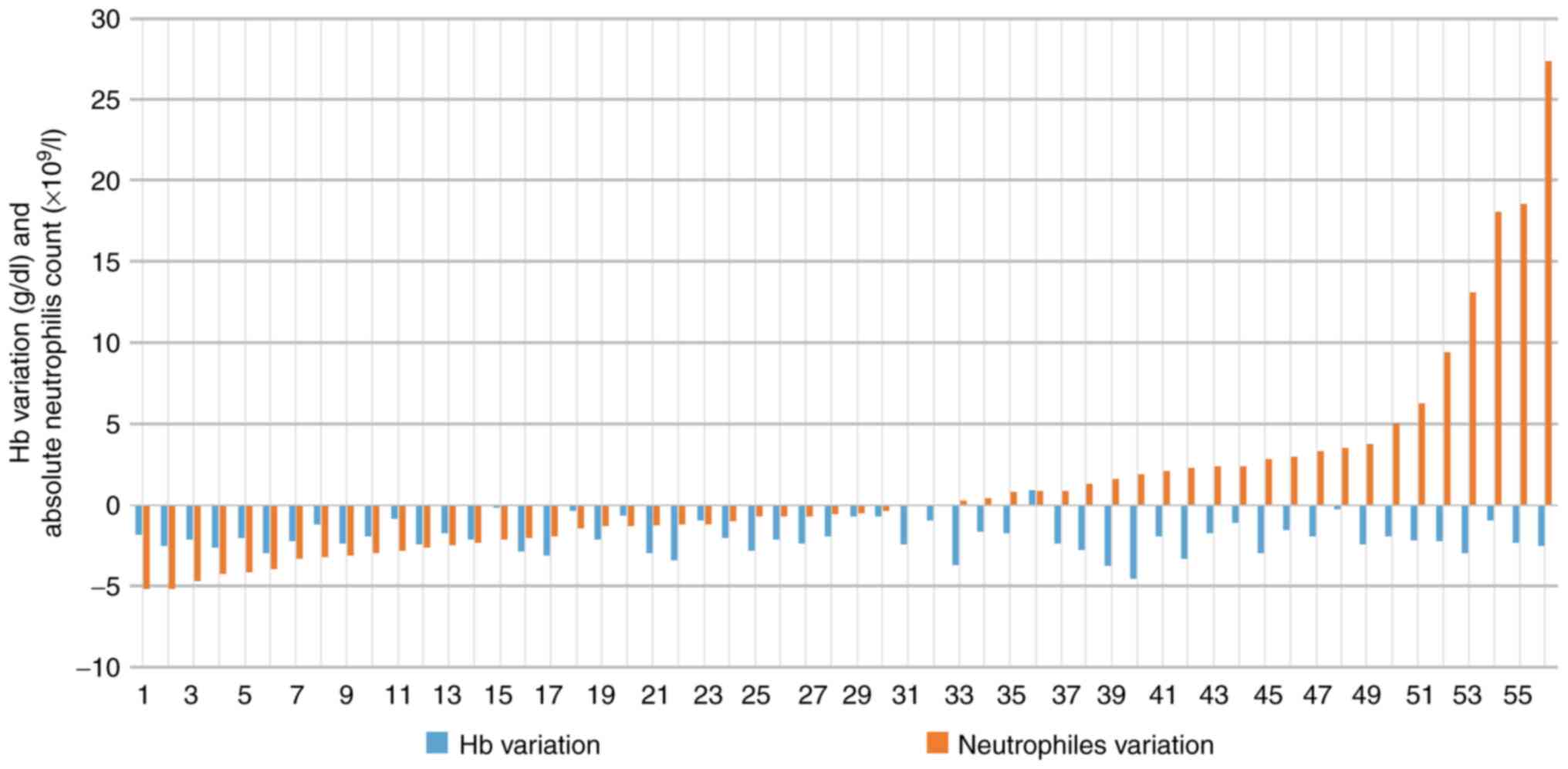
dose-dense chemotherapy was faster and more profound (Fig. 1). Regarding dose-dense chemotherapy,
individual variations of neutrophils and Hb cycle (C) 1 to C4, are
presented in Fig. 2.
Dose-dense vs. standard
chemotherapy
For individual variations of Hb from C1 to C4, the
differences between dose-dense and standard chemotherapy were
statistically significant. The z-score was 2.55645 and the P-value
was 0.01046. In addition, for individual variations of Hb, the
difference between dose-dense and standard chemotherapy in the over
60 years of age group was statistically significant
(z-score=2.41663; P=0.01552). Similarly, the difference was
statistically significant in the group under 60 years of age, also
(z-score=2.43779; P=0.01468). The group over 60 years of age
presented a variation of Hb at average -2.41 for dose-dense
compared to -1.70 for standard chemotherapy, while the group under
60 years presented a variation of Hb at average -1.71 for
dose-dense compared to -1.17 for standard chemotherapy.
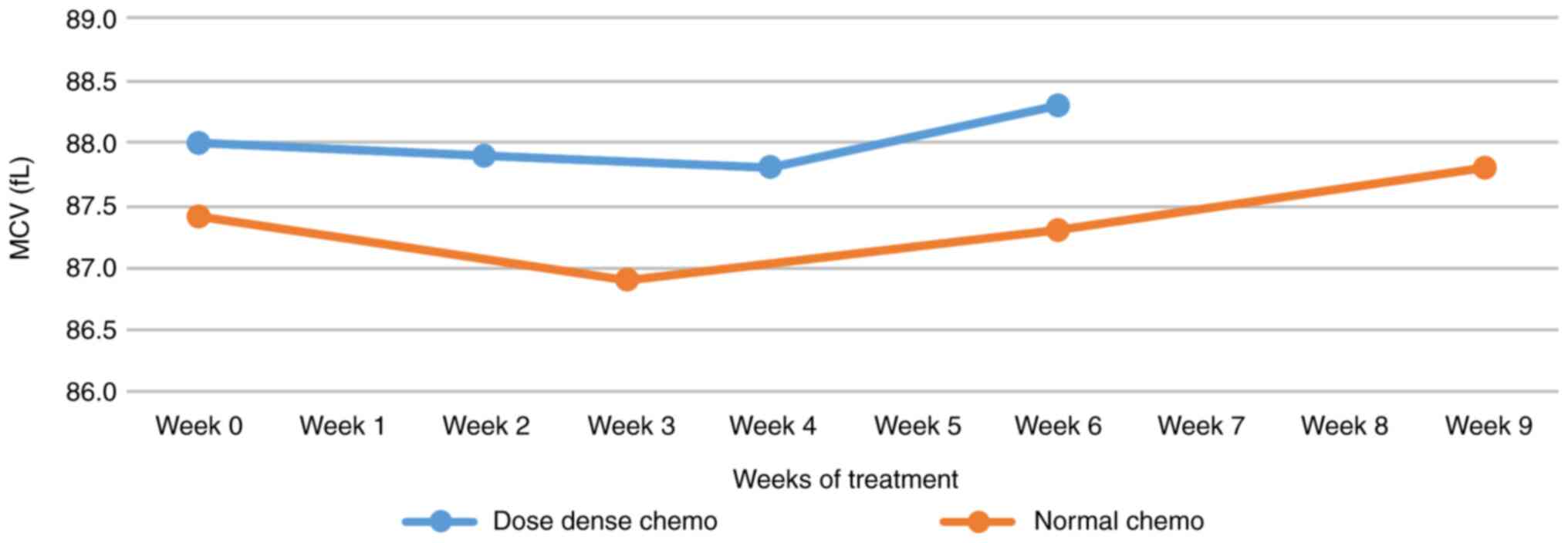
Mean corpuscular volume
The mean corpuscular volume (MCV) started at higher
values for dense doses and takes a steeper upward slope after an
initial decrease lasting four weeks. In regular latent
chemotherapy, the initiation of growth was shorter, but the growth
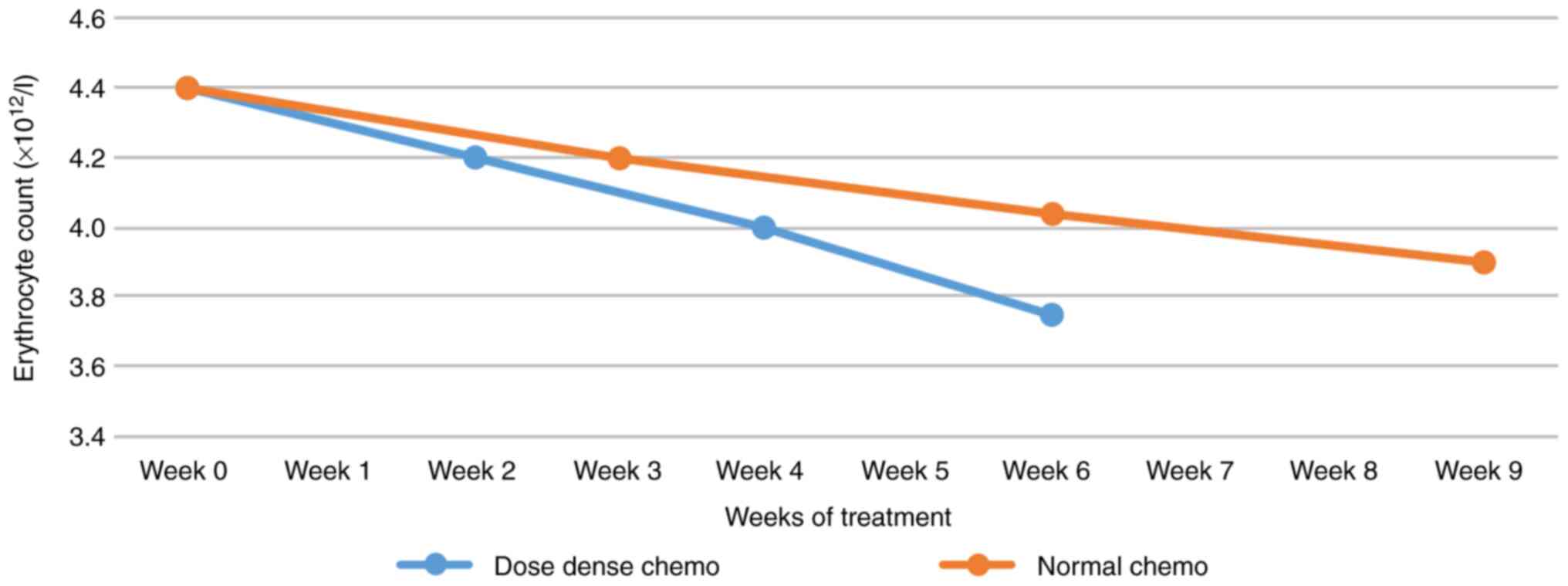
was more sustained (Fig. 3). Unlike
the MCV values, the number of erythrocytes shows a continuous
decrease, with a steeper slope for dose-dense chemotherapy
(Fig. 4).
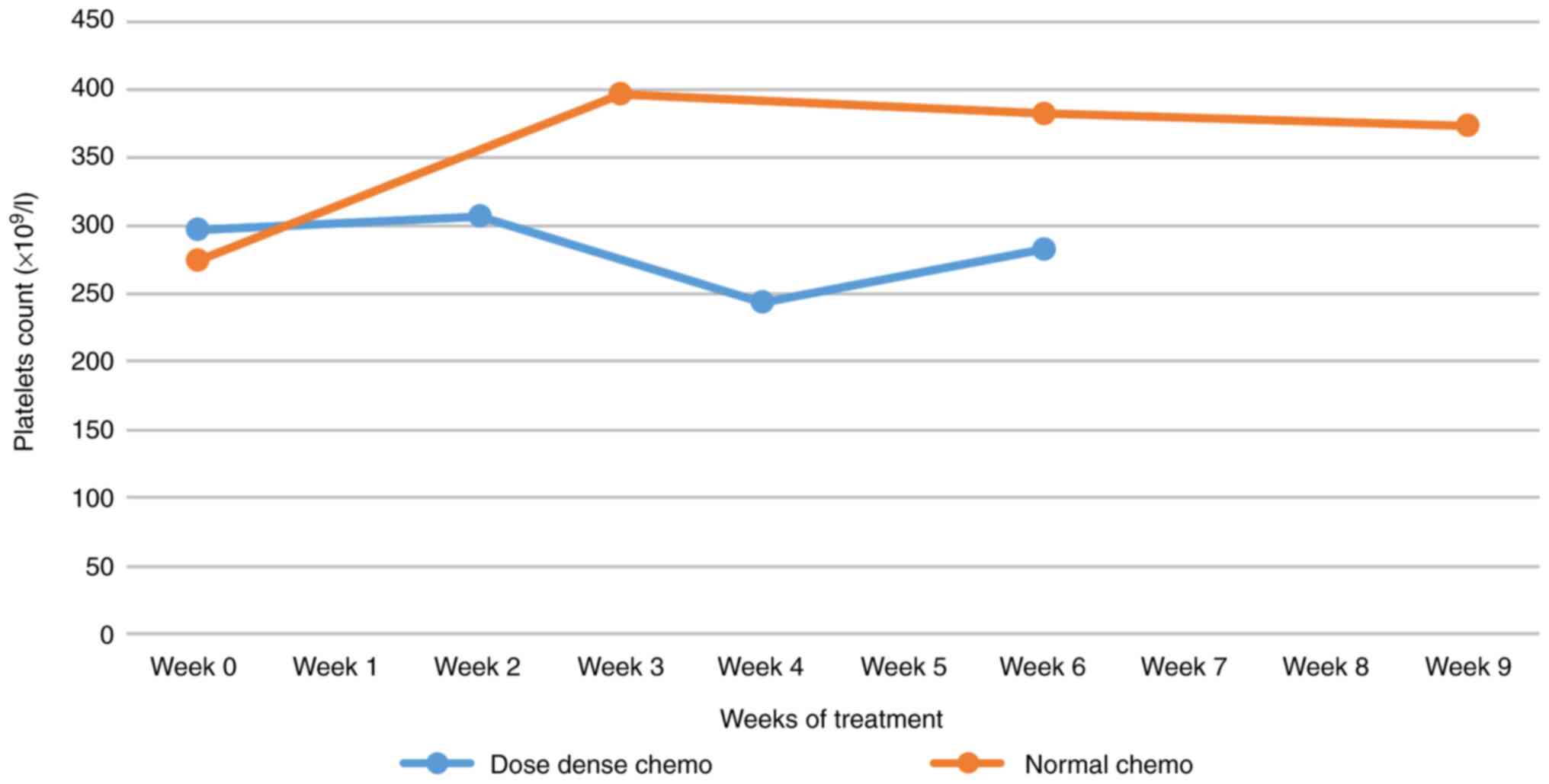
Platelet count
Dose-dense chemotherapy leads to a 15% decrease in
platelet count (possibly in the context of the use of granulocyte
growth factors), while regular chemotherapy shows an exhaustible
tendency to thrombocytosis (Fig.
5). For individual platelet variations from C1 to C4, the
differences between dense and regular doses were statistically
significant. The z-score was 7.48347 and the P-value <0.00001.
At dense doses, the number of platelets slightly decreased, as long
as the number of neutrophils usually increased from C1 to C4.
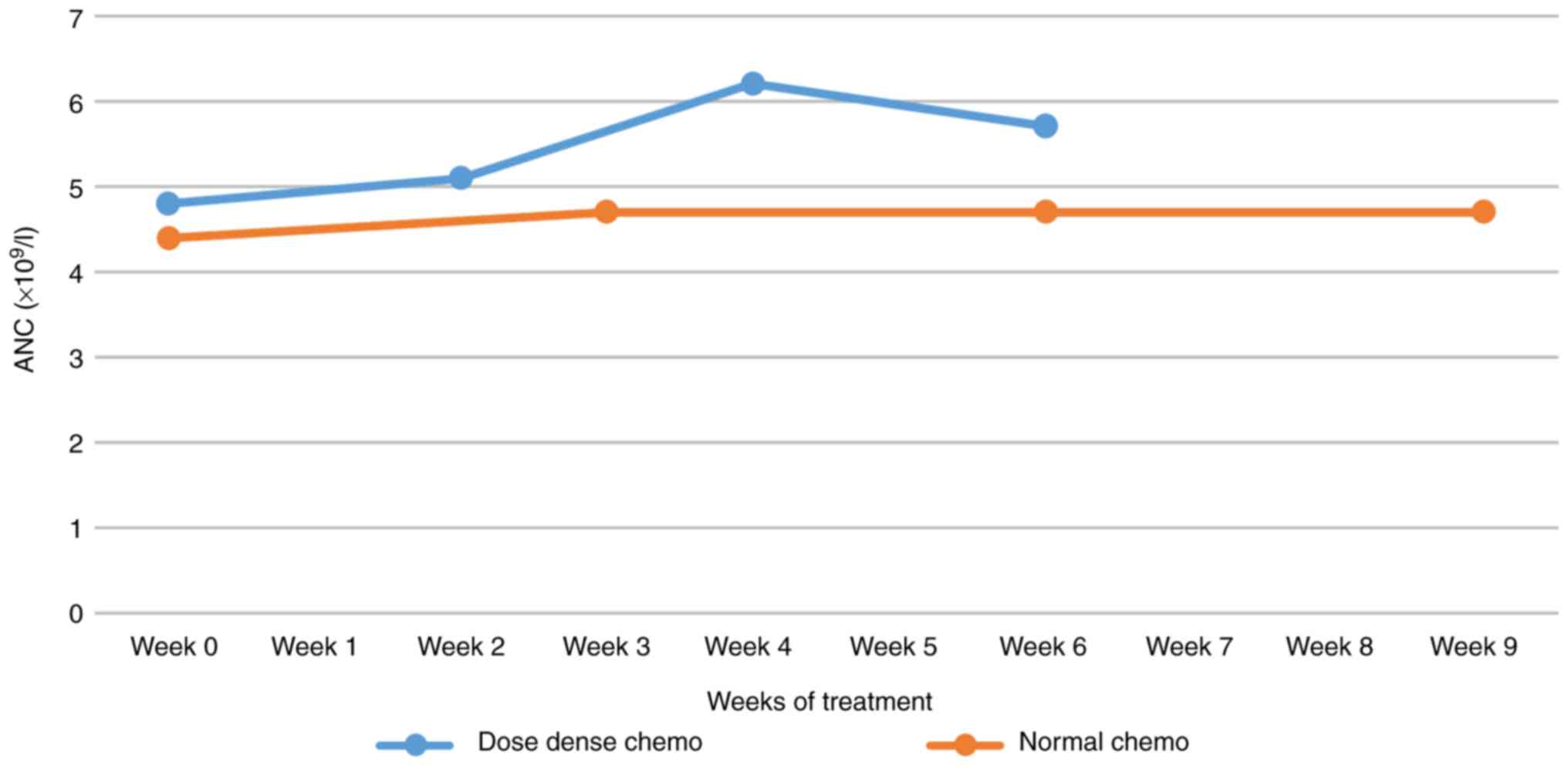
Neutrophil count
Regarding the neutrophil count in dose-dense
settings, there was a tendency towards neutrophilia, probably in
the context of constant use of G-CSF. In regular chemotherapy, a
plateau of absolute neutrophil count (ANC) was obtained, possibly
in the context of the progressive use of G-CSF (Fig. 6).
For individual variations of neutrophils from C1 to
C4, the difference between dense and regular doses was not
statistically significant (Fig. 2).
The z-score was 0.11739 and the P-value was 0.90448. In addition,
for individual variations of neutrophils, the difference between
dose-dense chemotherapy vs. routine chemotherapy in the group over
60 years of age was not statistically significant (z-score=1.71726;
P=0.08544), the same being considered in the group under 60 years
of age (z-score=1.40685; P=0.15854).
The over 60 years of age group presented a variation
of neutrophils at average -1.20 for dose-dense compared to -0.20
for standard chemotherapy, while the under 60 years of age group
presented a variation of neutrophils at average +1.65 for
dose-dense compared to -0.55 for regular chemotherapy.
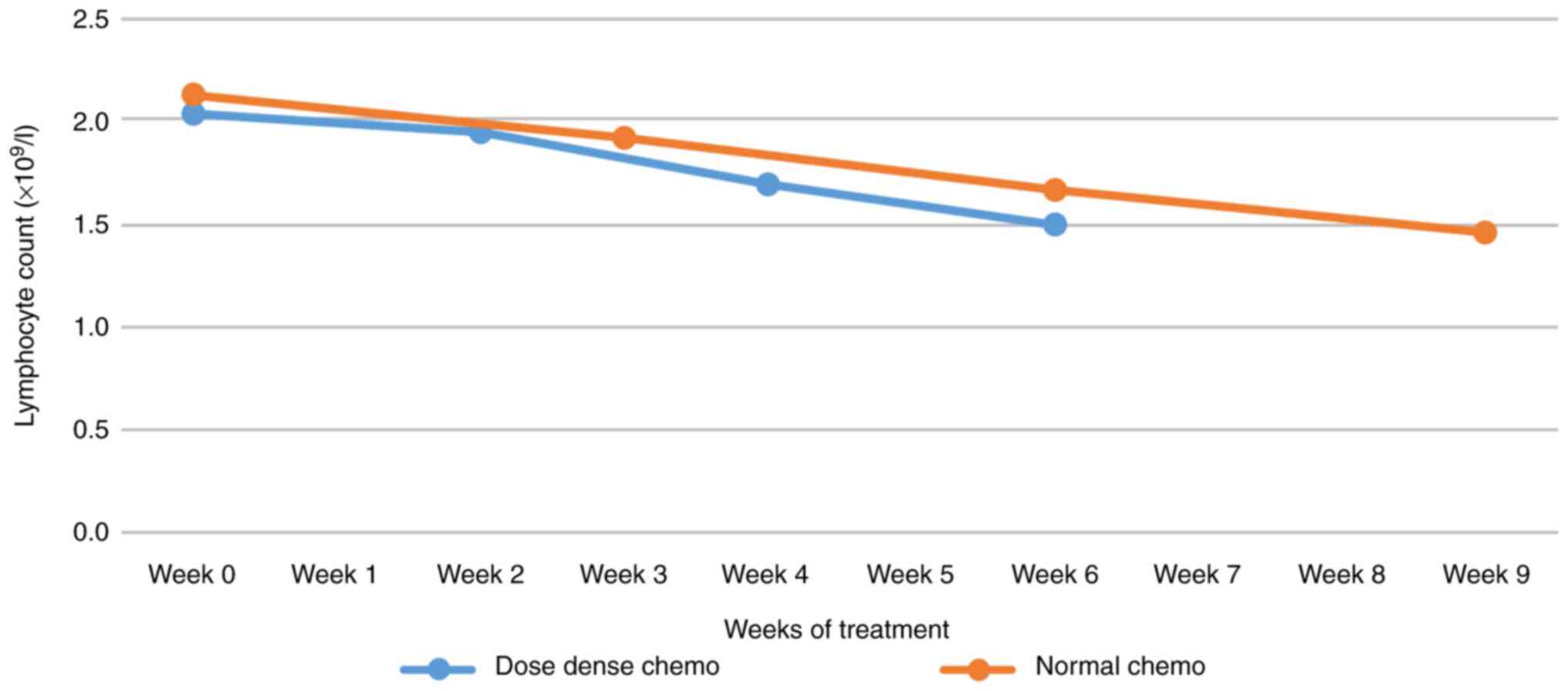
Lymphocyte count
Regarding the average lymphocyte count variation
between dose-dense and regular regimens, the decrease in dose-dense
was slightly steeper without necessarily being deeper (Fig. 7).
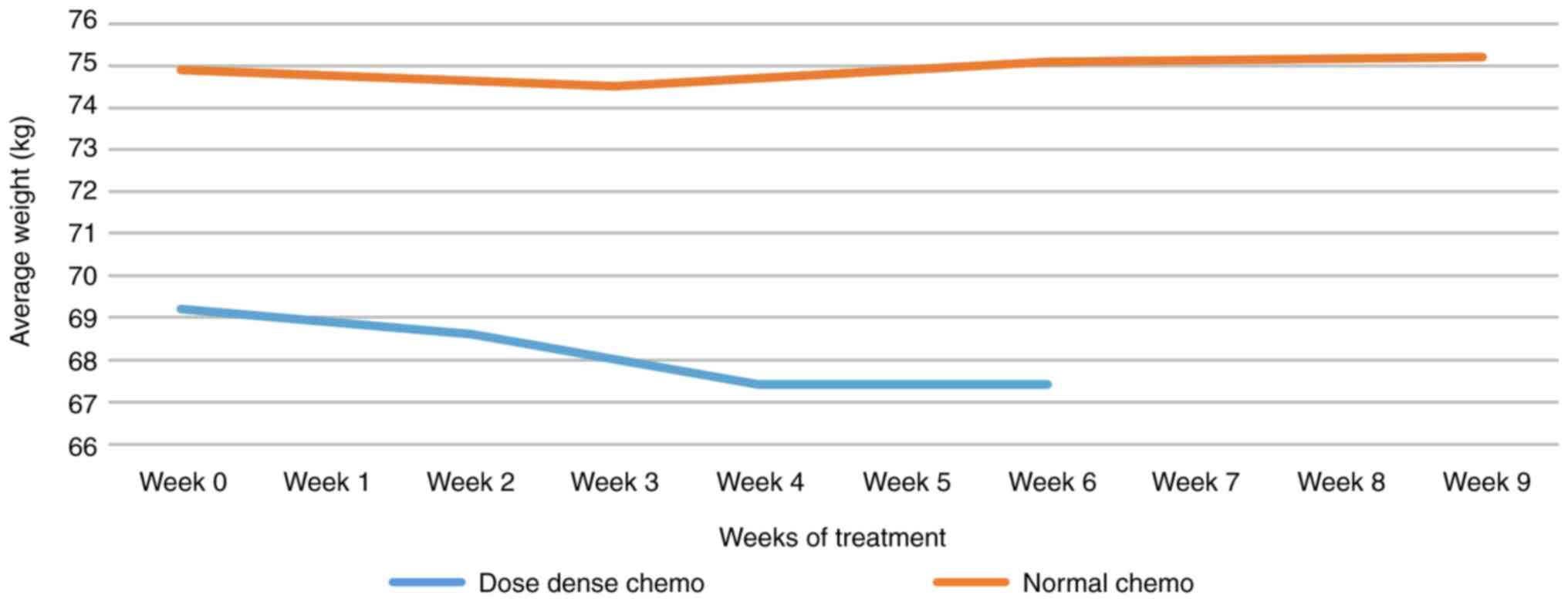
Average weight
Average weight variation in dose-dense vs. regular
chemotherapy: patients with a dose-dense started from lower average
weight and lost an average of 1.8 kg. Note the decreased cap after
C2. For standard chemotherapy, the weight variation was
insignificant (Fig. 8).
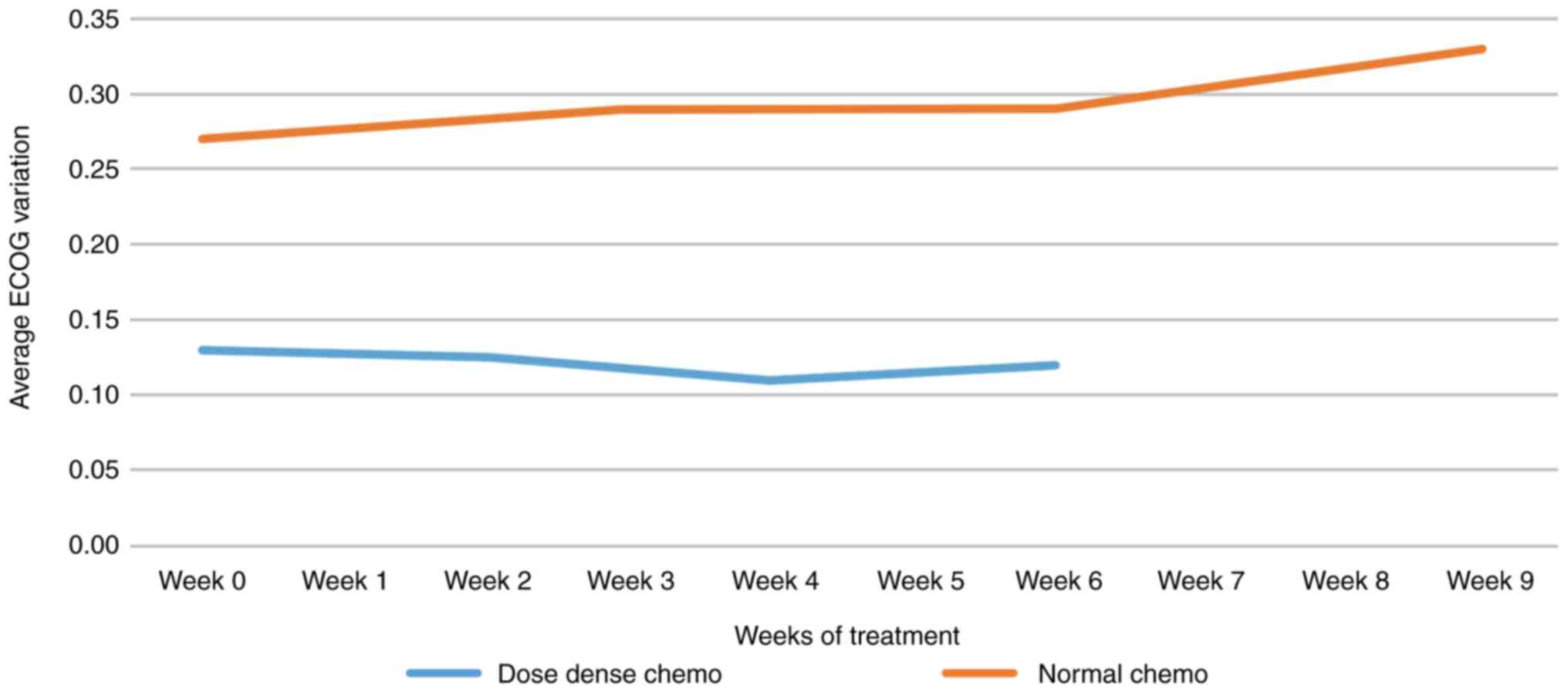
ECOG performance
Regarding the average ECOG performance status
variation in dose-dense vs. regular AC chemotherapy, it should be
noted that the average ECOG performance status decreased during
dose-dense regimens (subjective assessment, also related to the
observer's expectations). In regular chemotherapy, there was a
degradation of ECOG performance status (Fig. 9).
Discussion
The present study is retrospective and is based on a
group of 168 patients diagnosed with non-metastatic breast cancer.
The aim was to examine the feasibility and toxicity of dose-dense
neoadjuvant chemotherapy.
The hematological profiles of the patients were
analyzed. Both patients receiving dense-dose chemotherapy and those
receiving standard chemotherapy had similar hematologic profiles
when initiating chemotherapy. Following the analysis performed on
Hb values during the 9 weeks of treatment, we found that although
it started from an average Hb value equal for both types of
chemotherapy, there was a faster and more pronounced decrease in
the case of dense-dose chemotherapy. The average erythrocyte volume
also changed, signaling a decrease until the 4th week after which
the slopes of both types of chemotherapy become ascending, in the
case of dose-dense chemotherapy, the slope is steep, and so the
increase is more sustained. Unlike the MCV values, the number of
erythrocytes shows a continuous decrease, with a steeper slope for
dose-dense chemotherapy. RDW-SD is a size that describes the width
of the red blood cell volume distribution curve (measured at 20% of
its height). It indicates how much these cells differ in size and
volume, quantifying the difference between a small blood cell and a
large one. The average value ranges between 46 and 47. Values
>47 observed during standard chemotherapy reflect anisocytosis
caused by the production of macrocytes in the hematogenous bone
marrow as an effect of chemotherapy. During the use of dose-dense
regimens, there is a tendency towards neutrophilia, probably in the
context of constant use of G-CSF. In standard chemotherapy, a
plateau of the ANC is obtained, possibly in the context of the
progressive use of G-CSF. Dose-dense chemotherapy leads to a 15%
decrease in platelet count (possibly in the context of using
granulocyte growth factors), while regular chemotherapy shows an
exhaustible tendency to thrombocytosis. Regarding the number of
leucocytes, progressive leucopenia was observed, more accentuated
in dose-dense chemotherapy. Overall, as seen in other studies,
dose-dense regimen associated hematologic toxicity has been more
pronounced. However, hematologic toxicity did not affect the
treatment protocol, further confirming the safety of dose-dense
administration, with prophylactic measures (14-17).
The weight loss aspect was also taken into account,
so that the average weight of patients treated with the dose-dense
regimen is lower than the average weight of regular chemotherapy.
The medium-weight loss was ~1.8 kg over 9 weeks. Note the constant
maintenance of weight after C3. During regular chemotherapy, weight
variations were insignificant.
It should be noted that the average of ECOG
performance status decreases during dose-dense (subjective
assessment, also related to the observer's expectations). ECOG
performance status degradation is noted in standard
chemotherapy.
As a conclusion, a retrospective study of 168
patients with non-metastatic breast cancer showed a good safety
profile when administering dose-dense chemotherapy regimens in
neoadjuvant settings. It should be noted that the mean age of
patients on dose-dense chemotherapy regimens was lower than in the
group of patients on normal-dose chemotherapy regimens. ECOG
performance status was similar in the two groups of patients at the
initiation of chemotherapy. The evolution of the monitored
parameters recommends that the dose-dense and regular AC followed
by T can be given with manageable toxicity. It was shown that the
group under 60 years of age, despite the hematological toxicity, is
suitable for dose-dense chemotherapy and the toxicity is
manageable. Further studies are needed in order to define the
optimal regimen and the patient population that will receive the
most significant benefit from the dose-dense strategy.
Acknowledgements
Professional editing, linguistic and technical
assistance performed by Irina Radu, Individual Service Provider,
certified translator in Medicine and Pharmacy (certificate
credentials: Series E no. 0048).
Funding
Funding: No funding was received.
Availability of data and materials
The data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
DP, CO and SS organized the study, analyzed and
interpreted the study data and wrote the manuscript. AN, MM, HTS,
AT and SS analyzed the data and helped draft the output and
critically reviewed the manuscript; SN interpreted the data and
critically reviewed the manuscript for intellectual content. All
the authors have read and approved the final version of the
manuscript for publication.
Ethics approval and consent to
participate
All patients gave their informed consent for the
procedure. The study protocol was conducted according to the
principles of the Declaration of Helsinki after the approval of our
institution's Ethical Committee. All patients provided written
informed consent for the study participation and data
collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Romania: Cluj Regional Cancer Registry,
Timisoara Regional Cancer Registry. http://ghdx.healthdata.org/organizations/timisoara-regional-cancer-registry-romania.
Accessed: 29 Sept., 2020.
|
|
3
|
DeSantis CE, Ma J, Gaudet MM, Newman LA,
Miller KD, Sauer AG, Jemal A and Siegel RL: Breast cancer
statistics, 2019. CA Cancer J Clin. 69:438–451. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ward EM, Sherman RL, Henley SJ, Jemal A,
Siegel DA, Feuer EJ, Firth AU, Kohler BA, Scott S, Ma J, et al:
Annual Report to the nation on the status of cancer, featuring
cancer in men and women age 20-49 years. J Natl Cancer Inst.
111:1279–1297. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Merlo DF, Ceppi M, Filiberti R, Bocchini
V, Znahor A, Gamulin M, Primic-Žakelj M, Bruzzi P, Bouchardy C and
Fucci A: Breast cancer incidence trends in European women aged
20-39 years at diagnosis. Breast Cancer Res Treat. 134:363–370.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bodmer A, Feller A, Bordoni A, Bouchardy
C, Dehler S, Ess S, Levi F, Konzelmann I, Rapiti E, Steiner A, et
al: Breast cancer in younger women in Switzerland 1996-2009: A
longitudinal population-based study. Breast. 24:112–117.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Guo F, Kuo YF, Shih YCT, Giordano SH and
Berenson AB: Trends in breast cancer mortality by stage at
diagnosis among young women in the United States. Cancer.
124:3500–3509. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Brenner DR, Ruan Y, Shaw E, O'Sullivan D,
Poirier AE, Heer E, Villeneuve PJ, Walter SD, Friedenreich CM,
Smith L and De P: Age-Standardized cancer-incidence trends in
Canada, 1971-2015. Can Med Assoc J. 191:E1262–E1273.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Leclère B, Molinié F, Trétarre B, Stracci
F, Daubisse-Marliac L and Colonna M: GRELL Working Group. Trends in
the incidence of breast cancer among women under 40 in seven
European countries: A GRELL cooperative study. Cancer Epidemiol.
37:544–549. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Baeyens-Fernández JA, Molina-Portillo E,
Pollán M, Rodriguez-Barranco M, Del Moral R, Arribas-Mir L,
Sánchez-Cantalejo Ramírez E and Sánchez MJ: Trends in incidence,
mortality and survival in women with breast cancer from 1985 to
2012 in Granada, Spain: A population-based study. BMC Cancer.
18(781)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ruggeri M, Pagan E, Bagnardi V, Bianco N,
Gallerani E, Buser K, Giordano M, Gianni L, Rabaglio M, Freschi A,
et al: Fertility concerns, preservation strategies and quality of
life in young women with breast cancer: Baseline results from an
ongoing prospective cohort study in selected European centers.
Breast. 47:85–92. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ganz PA, Greendale GA, Petersen L, Kahn B
and Bower JE: Breast cancer in younger women: Reproductive and late
health effects of treatment. J Clin Oncol. 21:4184–4193.
2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG). Increasing the dose intensity of
chemotherapy by more frequent administration or sequential
scheduling. A patient-level meta-analysis of 37 298 women with
early breast cancer in 26 randomised trials. Lancet. 393:1440–1452.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Papakonstantinou A, Matikas A, Bengtsson
NO, Malmström P, Hedayati E, Steger G, Untch M, Hübbert L,
Johansson H, Hellström M, et al: Efficacy and safety of tailored
and dose-dense adjuvant chemotherapy and trastuzumab for resected
HER2-positive breast cancer: Results from the phase 3 PANTHER
trial. Cancer. 15:1175–1182. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhou W, Chen S, Xu F and Zeng X: Survival
benefit of pure dose-dense chemotherapy in breast cancer: A
meta-analysis of randomized controlled trials. World J Surg Oncol.
16(144)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cocciolone V, Cannita K, Tessitore A,
Mastroiaco V, Rinaldi L, Paradisi S, Irelli A, Baldi PL, Sidoni T,
Ricevuto E, et al: Neoadjuvant chemotherapy in breast cancer: A
dose-dense schedule in real life and putative role of PIK3CA
mutations. Oncotarget. 9:27380–27396. 2018.PubMed/NCBI View Article : Google Scholar
|