Introduction
Colorectal cancer (CRC) is considered to be one of
the most common causes of cancer-associated mortality worldwide
(1,2). Although therapeutic strategies,
including surgical resection, chemotherapy and radiotherapy, have
greatly improved the outcome of patients with CRC, the treatment of
patients with CRC requires further improvement (3). Therefore, more efforts should be made
in investigating the molecular mechanisms involved in the
development of CRC, which might provide novel targets and
therapeutic methods for the treatment of CRC.
MicroRNAs (miRNAs/miRs) are defined as a category of
endogenous single-stranded, non-coding RNAs that are 21-23
nucleotides in length (4-6).
miRNAs act as key negative regulators of gene expression, via
binding to the 3'-untranslated regions (3'-UTRs) of target mRNAs,
which consequently inhibit the translation or induce the
degradation of mRNAs (7).
Increasing evidence has indicated that miRNAs play essential roles
in diverse cellular processes, including cell proliferation,
differentiation and apoptosis (8).
The vital function of miRNAs in the progression of human cancer was
also demonstrated by recent studies (9-12).
miRNAs modulate the progression of cancer by acting as oncogenes or
tumor suppressors (9,12-16).
Recently, miR-26b was found to improve the sensitivity of CRC cells
to 5-flurouracil and inhibited the growth of CRC cells (17). Wang et al (18) reported that miR-410 promoted the
malignancy of CRC and could be used as a potential biomarker in the
progression of CRC. Inhibiting the expression of miR-30d promoted
the cell proliferation and tumor growth of CRC by targeting G
protein subunit α13(19).
miR-296-5p was recently reported as a tumor suppressor in non-small
cell lung cancer by directly targeting polo-like kinase 1 (PLK1)
(20). Similarly, miR-296-5p
suppressed the proliferation of prostate cancer cells, implicating
the potential application of miR-296-5p in the prognosis of
prostate cancer (21). Although
previous reports have demonstrated the tumor suppressive role of
miR-296-5p in cancer, the function and molecular mechanism of
miR-296-5p in CRC remains largely unknown.
The present study aimed to investigate the possible
role of miR-296-5p in the progression of CRC and characterize the
potential underlying molecular mechanism.
Materials and methods
Tissue samples and cell lines
A cohort of 40 CRC tissues and corresponding
adjacent normal tissues (5 cm from the tumor margin and
histologically confirmed) were collected from CRC patients (female:
male=1.86:1; age range, 39-72 years; mean age, 61.4 years) at
Zhongshan Hospital (Xiamen, China), via surgical resection between
May 2012 and September 2014. None of these patients received
chemotherapy, radiotherapy and immunotherapy prior to the tissue
collection. Tissues were stored at -80˚C before further
experiments. Tumors sample were staged according to the National
Comprehensive Cancer Network guidelines (22). Informed consent was obtained from
all patients. This study was approved by the Institutional Ethics
Committee of Zhongshan Hospital, Xiamen University.
The CRC cell lines (HCT116, HT-29, LOVO) and human
normal colorectal epithelial cell (FHC) were purchased from the
American Type Culture Collection. The cells were cultured in DMEM
(Thermo Fisher Scientific, Inc.), supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin, at 37˚C with 5% CO2.
Reagents
The antibodies used in this study included:
Anti-HMGA1 (cat. no. 7777; Cell Signaling Technology, Inc.),
anti-GAPDH (cat. no. G8795; Sigma-Aldrich; Merck KGaA) and
HRP-conjugated secondary antibody (cat. no. 7047; Cell Signaling
Technology, Inc.).
Cell transfection
The miR-296-5p mimics (5'-AGGGCCCCCCCUCAAUCCUGU),
control miRNA (5'-GGUUCGUACGUACACUGUUCA), miR-296-5p antagomir
(5'-UCCCGGGGGGGAGUUAGGACA-3') and the negative control miRNA
(5'-CGGUACGAUCGCGGC GGGAUAUC-3') were synthesized by Shanghai
GenePharma Co., Ltd. Both the negative control miRNAs were
non-targeting sequences. To construct Flag-tagged HMGA1, the
full-length of HMGA1 cDNA was obtained by reverse transcription
from RNA samples extracted form HCT116 cells and ligated into the
c-Flag pcDNA3 vector (cat. no. 20011; Addgene, Inc.) at the EcoRI
and XhoI sites. A total of 20 nM miRNAs and/or 0.5 µg of Flag-HMGA1
or Flag-vector were transfected into HCT116 and HT-29 cells, seeded
at the density of 10,000 cells/well using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Following transfection for 48 h, the
cells were harvested for the subsequent experiments.
Reverse transcription-quantitative
(RT-q)PCR
The extraction of RNA from CRC tissues and cell
lines was performed using the TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). RNA was reverse transcribed into cDNA
using the miScript RT kit (Takara Biotechnology Co., Ltd.),
according to the manufacturer's protocols using the temperature
protocol of 25˚C for 5 min, 46˚C for 20 min and 95˚C for 1 min.
qPCR was performed to determine the expression of miR-296-5p or
HMGA1, using SYBR Green mix (Bio-Rad Laboratories, Inc.) on the
Applied Biosystems 7500 Sequence Detection System (Applied
Biosystems; Thermo Fisher Scientific, Inc). The primers used in
this study were as follows: miR-296-5p forward,
5'-GTATCCAGTGCAGGGTCCGA-3'; miR-296-5p reverse,
5'-CGACGAGGGCCCCCCCT-3'; U6 RNA forward, 5'-CGAGCACAGAATCGCTTCA-3';
U6 RNA reverse, 5'-CTCGCTTCGGCAGCACATAT-3'; HMGA1 forward,
5'-CAACTCCGGGGAGGAAACCA-3'; HMGA1 reverse,
5'-AGGACTCCTGGGAGATGC-3'; GAPDH forward,
5'-GCCTTCTCCATGGTGGTGAA-3'; and GAPDH reverse,
5'-GGTCGGTGTGAACGGATTTG-3'. The PCR conditions were as follows:
95˚C for 10 min, followed by 40 cycles of 95˚C for 10 sec and 58˚C
for 60 sec. The relative expression of miR-296-5p and HMGA1 was
calculated using the 2-ΔΔCq method (23) and was normalized to the expression
of U6 RNA or GAPDH, respectively.
Targets prediction
The potential targets of miR-296-5p were predicted
using the miRDB (http://mirdb.org/) (24) and TargetScan databases (release 7.2;
http://www.targetscan.org/vert_72/).
Western blot analysis
Protein was extracted by lysing the CRC cells using
NP-40 buffer (Beyotime Institute of Biotechnology) containing
protease inhibitors (Roche Diagnostics). Protein concentration was
quantified using the bicinchoninic acid method (Beyotime Institute
of Biotechnology). A total of 20 µg protein was separated by
SDS-PAGE on a 15% gel, and transferred onto a PVDF membrane (EMD
Millipore). After blocking with 5% non-fat milk at room temperature
(RT) for 1 h, the membrane was incubated with anti-HMGA1 (1:1,000),
anti-GAPDH (1:3,000) or anti-Flag (1:3,000; cat. no. F7425;
Sigma-Aldrich; Merck KGaA) primary antibodies overnight at 4˚C.
After washing twice with PBS, the membrane was incubated with
horseradish peroxidase (HRP)-conjugated secondary antibody
(1:10,000) for 1 h at RT. The blot signals were visualized with
enhanced chemiluminescence chromogenic substrate (EMD Millipore),
according to the manufacturer's instructions. The expression of
GAPDH was determined as the loading control. The protein bands were
analyzed with Image J analysis software (v.1.52n; National
Institutes of Health). Western blot analysis was performed three
times independently.
Ultrasound-mediated microbubble
destruction
The doxorubicin (Dox)-liposome-microbubble complex
(DLMC) was prepared as previously described (19). To induce the uptake of Dox, CRC
cells were seeded in a 24-well plate and incubated overnight at
37˚C. Cells were subsequently incubated with DLMC containing 10
µg/ml Dox, and ultrasound (US) radiation was applied for 15 sec by
moving a 20 mm US probe E1609 (Valpey Fisher, Inc.) over the cell
culture plate with the following parameters: 1 MHz, 20% duty cycle,
US intensity of 1.65 W/cm2 and US peak intensity of 0.35
MPa, as described previously (25).
The cells were then rinsed with serum-free DMEM to remove the
uninternalized Dox. Cells with DLMC containing 10 µg/ml of Dox
without US treatment were investigated as the mock. Cells were then
cultured with fresh medium overnight at 37˚C and subjected to
subsequent experiments.
Cell Counting Kit-8 (CCK-8) assay
Both HCT116 and HT-29 cells were plated in the
96-well plate at the density of 1,000 cells per well. Following
culture for 24 h, the cells were transfected with miR-296-5p mimics
or control miRNA using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). After 24 h, 10 µl CCK-8 solution (Dojindo
Molecular Technologies, Inc.) was added into the medium and
incubated at 37˚C for an additional 3 h, according to the
manufacturer's protocol. The absorbance of each well at 450 nm was
measured using a microplate reader (Bio-Rad Laboratories, Inc.).
The experiments were performed in triplicate.
Luciferase reporter assay
The 3'-UTR of HMGA1 containing the putative binding
sites of miR-296-5p was amplified from human genomic DNA and
constructed into the pGL3 luciferase reporter vector (Promega
Corporation). A total of 0.5 µg luciferase vector encoding the
wild-type or mutant 3'-UTR, and miR-296 mimics or control miRNA
were co-transfected into the CRC cells (density, ~10,000
cells/well) with Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). After transfection for 48 h, the cells
were harvested and the luciferase activity was determined using the
Dual-Luciferase Reporter Assay System (Promega Corporation). The
activity of Renilla luciferase was detected for normalization.
Cell apoptosis analysis
Both HCT116 and HT-20 cells were seeded on a 6-well
plate and transfected with miR-296-5p mimics or control miRNA.
After transfection for 48 h, the percentage of cell apoptosis was
evaluated using the Annexin-V FITC Apoptosis Detection kit
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. Cell apoptosis was determined using a
flow cytometer (BD Biosciences). Data analysis was performed using
ModFit software (v.3.3; BD Biosciences).
Cell cycle analysis
Cells were cultured with serum-free medium for 24 h
and then transfected with miR-296-5p mimics or control miRNA for 48
h with medium containing 10% FBS. The transfected cells were
harvested and washed twice with pre-cooled PBS and fixed with 70%
ethanol overnight at 4˚C. After washing with PBS, the cells were
stained with 100 µg/ml propidium iodide and 50 µg/ml RNase for 30
min at RT in the dark. The cell cycle was detected with a flow
cytometer (BD Biosciences). The profile was analyzed using ModFit
software (v.3.3; BD Biosciences).
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed with the GraphPad Prism software (v.5.0; GraphPad
Software, Inc.). The differences between two groups were analyzed
using the Student's t-test; the differences between normal colon
and colon cancer tissues were analyzed by paired t-test. The
comparisons between three or more groups were assessed using the
one-way analysis of variance, followed by Tukey's test. The
correlation between the expression of miR-296-5p and HMGA1 was
determined by the Spearman test. The association between the level
of miR-296-5p and the clinical features of patients with CRC was
analyzed using the χ2 test. P<0.05 was considered to
indicate a statistically significance difference.
Results
Expression of miR-296-5p is
downregulated in CRC
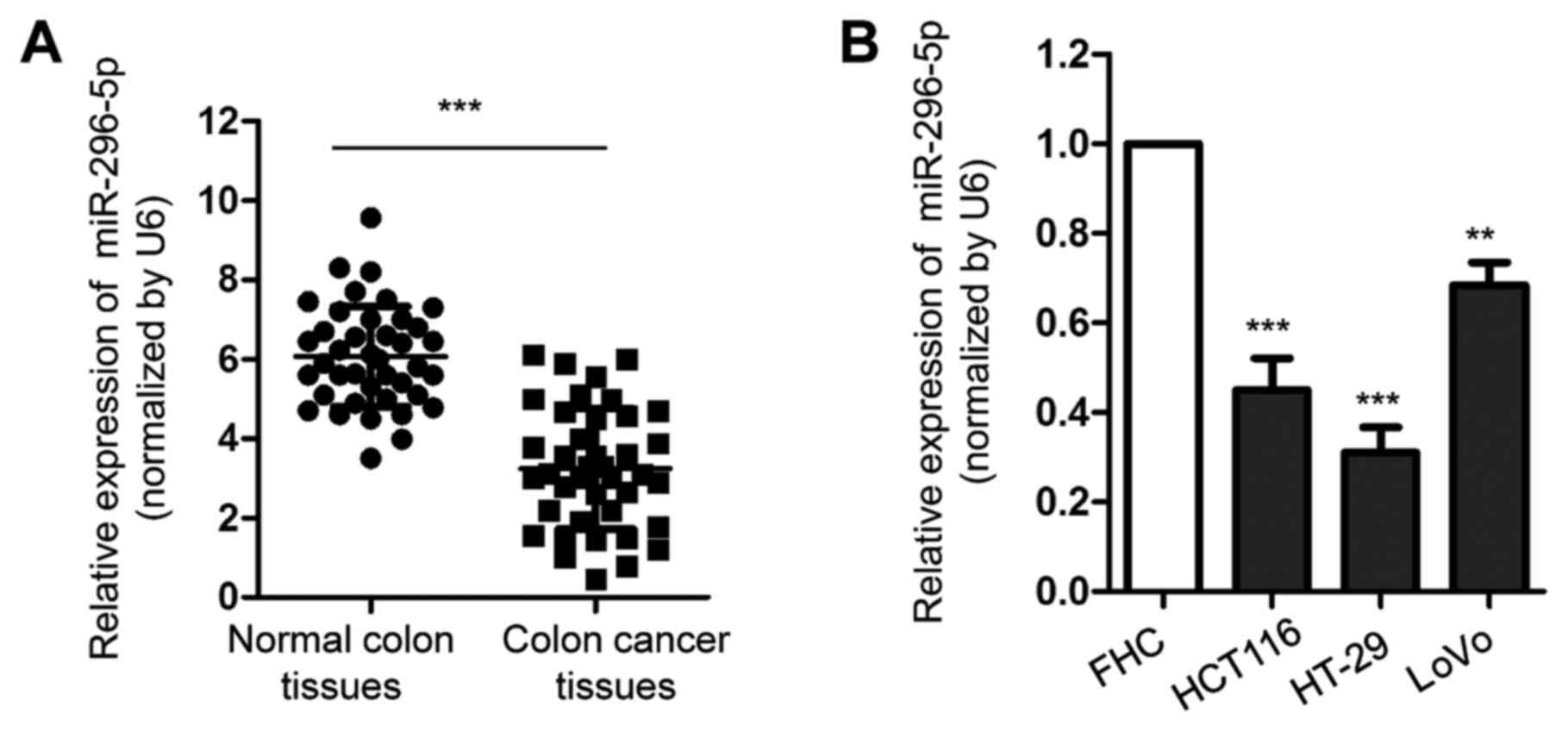
To evaluate the potential involvement of miR-296-5p
in CRC, the expression of miR-296-5p in CRC tissues and matched
adjacent normal tissues was detected by RT-qPCR. The results showed
that the expression of miR-296-5p was significantly decreased in
CRC tissues compared with adjacent non-tumor tissues (Fig. 1A). Consistently, the downregulation
of miR-296-5p was also observed in CRC cell lines, including
HCT116, HT-29 and LOVO, compared with the expression of miR-296-5p
in normal FHC cells (Fig. 1B). To
further characterize the association between the expression of
miR-296-5p and the prognosis of patients with CRC, all 40 patients
enrolled in the present study were divided into a miR-296-5p high
and low group, according to the mean value (3.85) of miR-296-5p
expression. The results showed that low expression of miR-296-5p
was significantly associated with a higher Tumor-Node-Metastasis
stage, lymph node metastasis and tumor size, suggesting the
potential clinical significance of miR-296-5p in CRC (Table I). The decreased expression of
miR-296-5p in CRC cells compared with non-cancerous colon tissues
and cell lines suggested the potential role of miR-296-5p in
CRC.
 | Table IAssociation between the expression of
miR-296-5p and the clinicopathological characteristics of patients
with colorectal carcinoma. |
Table I
Association between the expression of
miR-296-5p and the clinicopathological characteristics of patients
with colorectal carcinoma.
| Clinical
characteristics | Cases, n | Low, n | High, n | P-value |
|---|
| Age, years | | | | 0.345 |
|
≤60 | 14 | 10 | 4 | |
|
>60 | 26 | 18 | 8 | |
| Sex | | | | 0.176 |
|
Male | 19 | 15 | 4 | |
|
Female | 21 | 13 | 8 | |
| Tumor size, cm | | | | 0.004 |
|
≥5 | 24 | 19 | 5 | |
|
<5 | 16 | 9 | 7 | |
| TNM stage | | | | <0.001 |
|
I + II | 15 | 7 | 8 | |
|
III +
IV | 25 | 21 | 4 | |
| Metastasis | | | | <0.001 |
|
Yes | 27 | 22 | 5 | |
|
No | 13 | 6 | 7 | |
Overexpression of miR-296-5p inhibits
proliferation and induces apoptosis in CRC cells
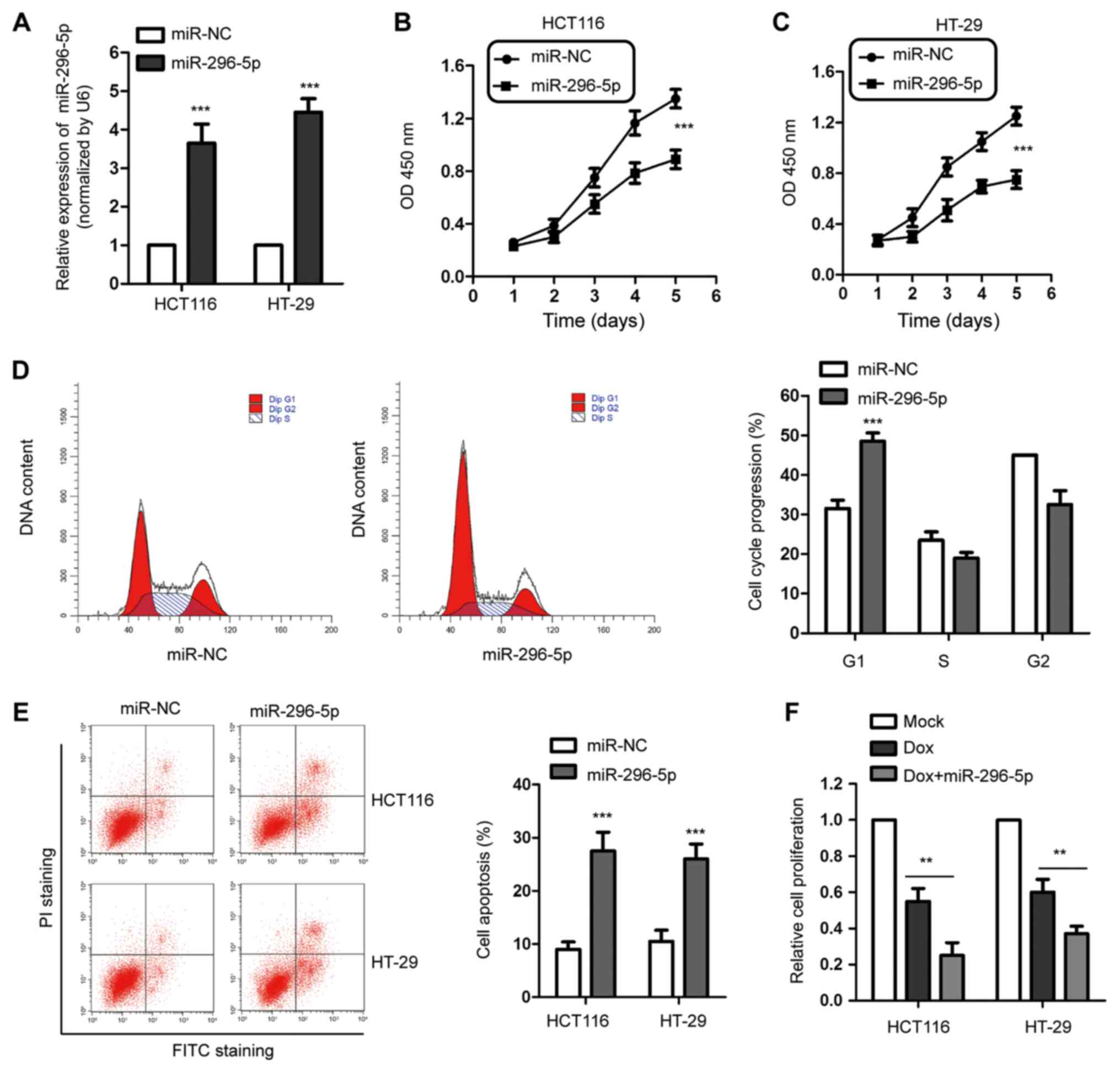
In order to explore the regulatory role of
miR-296-5p in CRC progression, HCT116 and HT-29 cells were
transfected with miR-296-5p mimics or control miRNA. As presented
in Fig. 2A, compared with the
control cells, the expression of miR-296-5p was significantly
increased in both HCT116 and HT-29 cells following the transfection
with miR-296-5p mimics. The CCK-8 assay was performed to determine
the effect of miR-296-5p on the proliferation of CRC cells. The
data showed that the overexpression of miR-296-5p significantly
inhibited the proliferation of both HCT116 and HT-29 cells
(Fig. 2B and C). Additionally, the flow cytometry
indicated that the overexpression of miR-296-5p led to G1-phase
cell cycle arrest in HCT116 cells (Fig.
2D). In accordance, the apoptosis of CRC cells was also
significantly upregulated following the overexpression of
miR-296-5p (Fig. 2E).
To further validate the suppressive function of
miR-296-5p in CRC, HCT116 and HT-29 cells were treated with Dox via
US-mediated microbubble destruction. The results showed that high
expression of miR-296-5p and treatment with Dox synergistically
inhibited the proliferation of CRC cells (Fig. 2F). These findings suggested the
potential tumor suppressive function of miR-296-5p in modulating
the growth of CRC cells.
HMGA1 is a target of miR-296-5p in CRC
cells
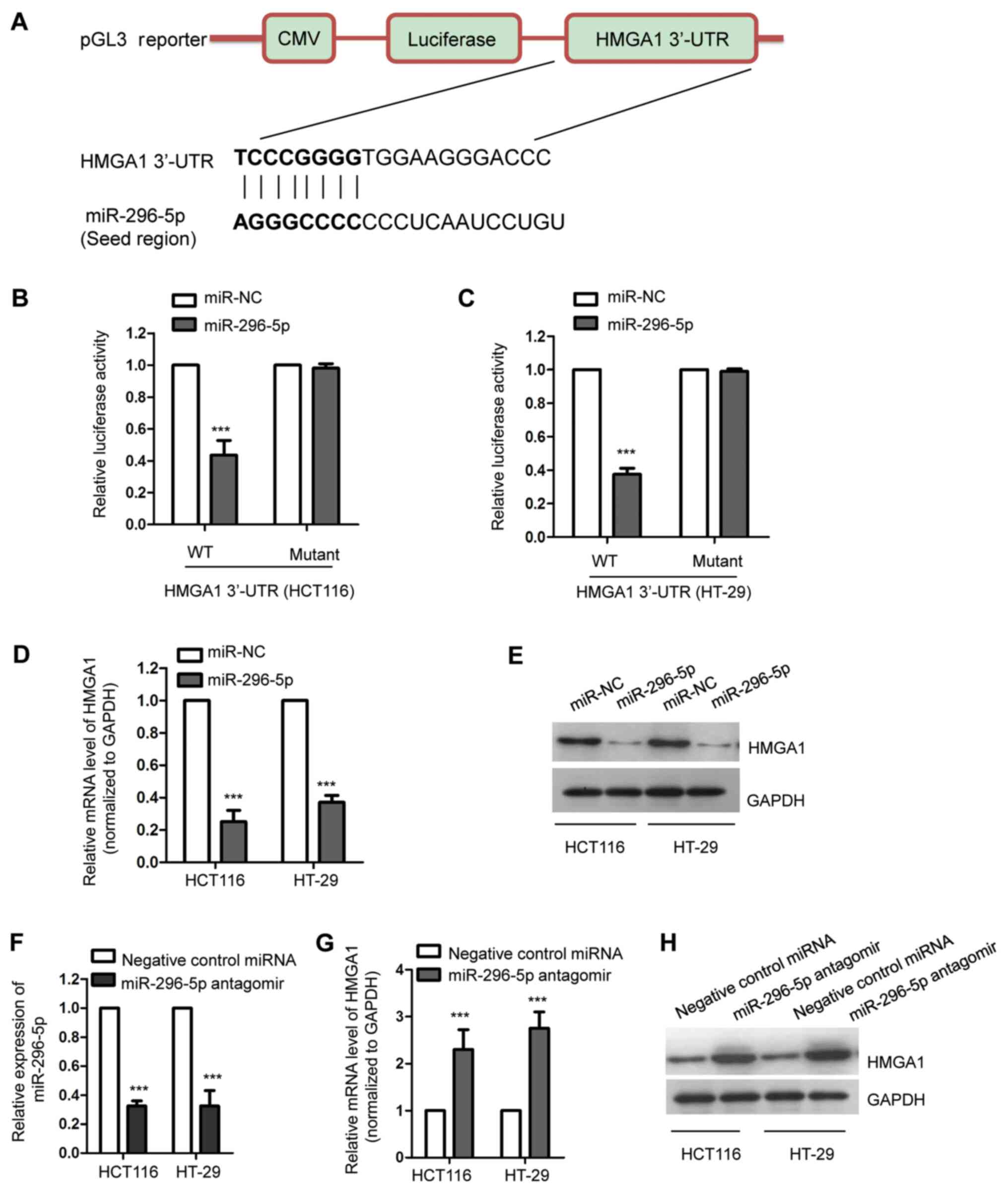
To further investigate the functional mechanism of
miR-296-5p in CRC, the potential targets of miR-296-5p were
predicted using the miRDB and TargetScan databases. Following the
search, HMGA1 was identified as a possible target of miR-296-5p.
The putative complementary sequence of miR-296-5p at the 3'-UTR of
HMGA1 is presented in Fig. 3A. To
further confirm this, the luciferase reporter assay was performed
by co-transfecting luciferase vectors harboring the wild-type or
mutant 3'-UTR of HMGA1, and miR-296-5p mimics or control miRNA,
into both HCT116 and HT-29 cells. The overexpression of miR-296-5p
significantly decreased the luciferase activity of the wild-type,
but not the mutant, 3'-UTR of HMGA1 (Fig. 3B and C). To determine whether the binding of
miR-296-5p with the 3'-UTR of HMGA1 affects the mRNA stability of
HMGA1, RT-qPCR was performed following the transfection of HCT116
and HT-29 cells with miR-296-5p mimics or control miRNA. The
results showed that the overexpression of miR-296-5p significantly
decreased the mRNA levels of HMGA1 in CRC cells (Fig. 3D). Consistently, the protein
abundance of HMGA1 was also decreased in both HCT116 and HT-29
cells overexpressing miR-296-5p (Fig.
3E). To further validate the suppressive effect of miR-296-5p
on the expression of HMGA1, miR-296-5p was downregulated by
transfecting CRC cells with miR-296-5p antagomir. The transfection
efficiency of miR-29605p was validated via RT-qPCR, with the GFP as
the transfection control (Fig. 3F).
In accordance, the downregulation of miR-296-5p resulted in
significantly increased mRNA and protein levels of HMGA1 (Fig. 3G and H). Overall, these results demonstrated
that miR-296-5p targeted HMGA1 and negatively modulated the
expression levels of HMGA1 in CRC cells.
Overexpression of HMGA1 attenuates the
suppressive role of miR-296-5p on the proliferation of CRC
cells
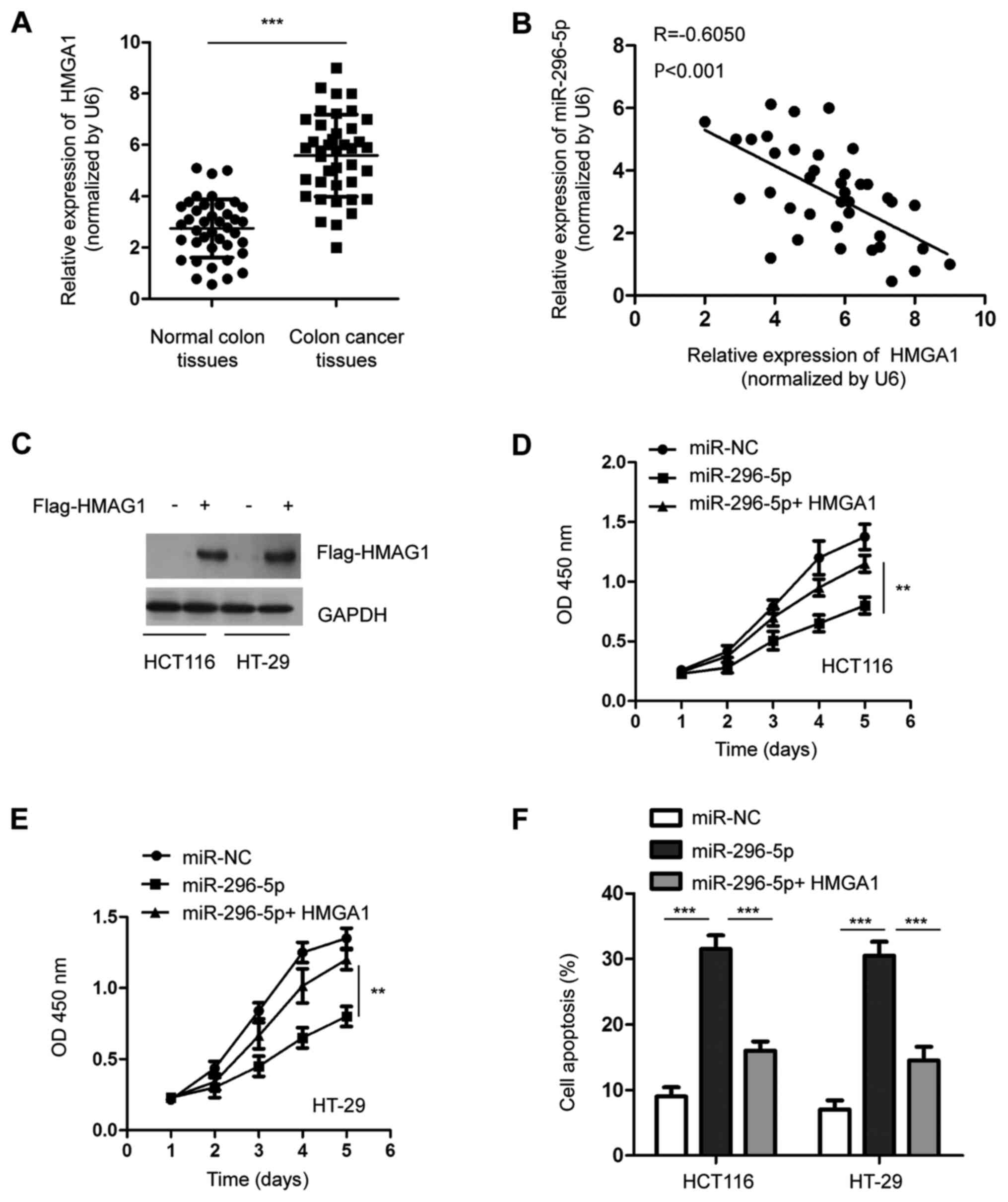
The expression of HMGA1 in CRC tissues and matched
adjacent normal tissues was detected via RT-qPCR. The data showed
that the mRNA level of HMGA1 was significantly overexpressed in CRC
tissues compared with the adjacent normal tissues (Fig. 4A). As HMGA1 was identified as a
target of miR-296-5p, the correlation between the expression of
miR-296-5p and HMGA1 in CRC tissues was determined by Spearman
analysis. As presented in Fig. 4B,
a significantly negative correlation was observed between the level
of miR-296-5p and HMGA1 in CRCC tissues.
To further confirm the function of HMGA1 in the
tumor suppressive role of miR-296-5, the expression of HMGA1 was
restored through transfecting Flag-tagged HMGA1 into both HCT116
and HT-29 cells (Fig. 4C). The
CCK-8 assay showed that the transfection of HMGA1 significantly
reversed the inhibitory effect of miR-296-5p on the proliferation
of both HCT116 and HT-29 cells (Fig.
4D and E). Moreover, the
overexpression of HMGA1 eliminated the miR-296-5p-induced apoptosis
of CRC cells (Fig. 4F). These
results indicated the essential role of HMGA1 in mediating the
suppressive function of miR-296-5p in CRC.
Discussion
Accumulating evidence suggests the critical roles of
miRNAs in the initiation and progression of cancer by modulating
the expression of target genes (13-15).
The aberrant expression of miR-296-5p has been found in a number of
different types of cancer in humans, including non-small cell lung
cancer, prostate cancer and hepatocellular carcinoma (20,21,26).
The downregulation of miR-296-5p is associated with a poor
prognosis in patients with cancer (27). In the present study, it was found
that the expression of miR-296-5p was significantly decreased in
CRC tissues and cell lines, suggesting the potential involvement of
miR-296-5p in CRC.
The tumor suppressive function of miR-296-5p has
been established in several types of cancer. For example,
miR-296-5p is prominently downregulated in hepatocellular carcinoma
(HCC) and inhibits the epithelial-to-mesenchymal transition of HCC
(26). The decreased expression of
miR-296-5p is significantly associated with a favorable prognosis
in patients with HCC (26). The
inhibitory effect of miR-296-5p on the growth of cancer cells has
also been observed in non-small cell lung cancer, by targeting
PLK1(20). Additionally, miR-296-5p
plays a tumor-suppressive role in prostate cancer by downregulating
peptidyl-prolyl cis-trans isomerase NIMA-interacting 1, indicating
its potential application in the prognosis of prostate cancer
(21). Studies have also
demonstrated the promoting effect of miR-296-5p on the development
of cancer (28,29). For example, miR-296-5p enhances the
invasiveness of glioblastoma by suppressing the expression of nerve
growth factor receptor and caspase-8(29). Similarly, miR-296-5p enhances the
cell proliferation of gastric cancer (28). In the present study, miR-296-5p was
downregulated in CRC tissues compared with matched normal tissues.
The overexpression of miR-296-5p significantly inhibited the
proliferation and induced apoptosis of CRC cells. These results
indicated the tumor suppressive function of miR-296-5p in the
progression of CRC.
As an important transcription factor, HMGA1 is
involved in regulating autophagy and cell invasion, which
contributes to cancer progression (30,31).
Moreover, HMGA1 was found to enhance tumorigenesis by conferring
resistance to chemotherapies, including trabectedin, temozolomide,
paclitaxel, doxorubicin and antineoplastic drugs (32-35).
These findings suggested the potential oncogenic function of HMGA1
in cancer progression. Notably, HMGA1 has been identified as the
target of several miRNAs, including miR-26a, miR-195, miR-625 and
miR-142-3p (36-39).
miR-26a targets and decreases the expression of HMGA1 and inhibits
the migration of osteosarcoma cells (40). HMGA1 has also been identified as a
target of miR-214, which suppresses the proliferation, migration
and invasion of cervical and colorectal cancer cells (41). Additionally, decreased expression of
miR-195 promotes the progression of prostate cancer cells by
targeting HMGA1(37). In the
present study, HMGA1 was predicted as one of the targets of
miR-296-5p. miR-296-5p bound the 3'-UTR of HMGA1 and inhibited the
expression of HMGA1 in CRC cells. A higher abundance of HMGA1 was
observed in CRC tissues, which was inversely correlated with the
expression of miR-296-5p. The restoration of HMGA1 reversed the
inhibitory effect of miR-296-5p on the proliferation of CRC cells.
These results suggest the important role of miR-296-5p/HMGA1 axis
in regulating the progression of CRC.
Notably, a recent study showed that miR-296-5p
inhibits glioblastoma cell stemness by targeting HMGA1 and
Sox2(42). The overexpression of
Sox2 has been found in a variety of cancer types and is associated
with increased cancer aggressiveness, resistance to chemoradiation
therapy and a decreased survival rate (43). Since HMGA1 was identified to be a
target of miR-296-5p, it would be interesting to further detect the
effect of miR-296-5p on the expression of Sox2 in CRC.
Additionally, HMGA1 has been reported to bind the AT-rich regions
of DNA and to regulate the expression of cyclin D/E, which
modulates cell cycle progression (44). A recent study demonstrated that
HMGA1 activates the STAT3/cyclo-oxygenase 2 pathway and promotes
the malignant behaviors of cancer (45). All these findings suggest the
possible mechanism of HMGA1 in regulating the survival of CRC and
merit further investigation. Recently, a nano/technological
platform was designed to evaluate the expression of HMGA1b in the
peripheral blood of patients with cancer (46). Thus, the influence of miR-296-5p on
the secretion of HMGA1 in CRC cells should be determined in future
studies.
In conclusion, the present study demonstrated the
tumor suppressive function of miR-296-5p in CRC, at least in part
by negatively modulating the expression of HMGA1. These results
indicated the potential clinical significance of miR-296-5p in the
diagnosis and prognosis of CRC.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Natural Science
Foundation of Fujian Province (grant no. 2015J01531), Overseas
Students in Science and Technology Activities, Ministry of Human
Resources and Social Security of the People's Republic of China
(grant no. 2015), and Fujian Health and Family Planning Commission
for Young and Middle-aged Talents Training Project (grant no.
2016-ZQN-87). Joint Health Fund Project of Fujian Science and
Technology Department (grant no. 2018J01396) and Youth Foundation
Project of Fujian Provincial Health Department (grant no.
2014-2-69).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GY, SY and YZ designed the study. GY and SY
performed most of the experiments and analyzed the data. SL
collected the clinical samples and cell lines. WT constructed the
luciferase reporter vector. XY performed the bioinformatics
analysis. JJW analyzed the data, finished the revision and proof of
the manuscript and provided funding support. YZ wrote the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Zhongshan Hospital, Xiamen University (approval no. 2015032013;
Xiamen, China). Consent for participation was obtained from all the
patients.
Patient consent for publication
Not applicable.
Competing interests
These authors declare that they have no competing
interests.
References
|
1
|
Sabit H, Cevik E and Tombuloglu H:
Colorectal cancer: The epigenetic role of microbiome. World J Clin
Cases. 7:3683–3697. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fakih MG: Metastatic colorectal cancer:
Current state and future directions. J Clin Oncol. 33:1809–1824.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Simon K: Colorectal cancer development and
advances in screening. Clin Interv Aging. 11:967–976.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mohr AM and Mott JL: Overview of microRNA
biology. Semin Liver Dis. 35:3–11. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fabian MR, Sonenberg N and Filipowicz W:
Regulation of mRNA translation and stability by microRNAs. Annu Rev
Biochem. 79:351–379. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Valenti MT, Dalle Carbonare L and Mottes
M: Role of microRNAs in progenitor cell commitment and osteogenic
differentiation in health and disease (Review). Int J Mol Med.
41:2441–2449. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gentilin E, Degli Uberti E and Zatelli MC:
Strategies to use microRNAs as therapeutic targets. Best Pract Res
Clin Endocrinol Metab. 30:629–639. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Momtazi AA, Shahabipour F, Khatibi S,
Johnston TP, Pirro M and Sahebkar A: Curcumin as a microRNA
regulator in cancer: A review. Rev Physiol Biochem Pharmacol.
171:1–38. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 9(852)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Asadzadeh Z, Mansoori B, Mohammadi A,
Aghajani M, Haji-Asgarzadeh K, Safarzadeh E, Mokhtarzadeh A, Duijf
PHG and Baradaran B: microRNAs in cancer stem cells: Biology,
pathways, and therapeutic opportunities. J Cell Physiol.
234:10002–10017. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kwak PB, Iwasaki S and Tomari Y: The
microRNA pathway and cancer. Cancer Sci. 101:2309–2315.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Farazi TA, Spitzer JI, Morozov P and
Tuschl T: miRNAs in human cancer. J Pathol. 223:102–115.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Qu H, Xu W, Huang Y and Yang S:
Circulating miRNAs: Promising biomarkers of human cancer. Asian Pac
J Cancer Prev. 12:1117–1125. 2011.PubMed/NCBI
|
|
16
|
Hosseinahli N, Aghapour M, Duijf PHG and
Baradaran B: Treating cancer with microRNA replacement therapy: A
literature review. J Cell Physiol. 233:5574–5588. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang B, Lu FY, Shi RH, Feng YD, Zhao XD,
Lu ZP, Xiao L, Zhou GQ, Qiu JM and Cheng CE: MiR-26b regulates
5-FU-resistance in human colorectal cancer via down-regulation of
Pgp. Am J Cancer Res. 8:2518–2527. 2018.PubMed/NCBI
|
|
18
|
Wang W, He Y, Rui J and Xu MQ: miR-410
acts as an oncogene in colorectal cancer cells by targeting
dickkopf-related protein 1 via the Wnt/β-catenin signaling pathway.
Oncol Lett. 17:807–814. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Muhammad S, Tang Q, Wei L, Zhang Q, Wang
G, Muhammad BU, Kaur K, Kamchedalova T, Gang Z, Jiang Z, et al:
miRNA-30d serves a critical function in colorectal cancer
initiation, progression and invasion via directly targeting the
GNA13 gene. Exp Ther Med. 17:260–272. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xu C, Li S, Chen T, Hu H, Ding C, Xu Z,
Chen J, Liu Z, Lei Z, Zhang HT, et al: miR-296-5p suppresses cell
viability by directly targeting PLK1 in non-small cell lung cancer.
Oncol Rep. 35:497–503. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lee KH, Lin FC, Hsu TI, Lin JT, Guo JH,
Tsai CH, Lee YC, Lee YC, Chen CL, Hsiao M, et al: MicroRNA-296-5p
(miR-296-5p) functions as a tumor suppressor in prostate cancer by
directly targeting Pin1. Biochim Biophys Acta. 1843:2055–2066.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Benson AB III, Venook AP, Al-Hawary MM,
Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D,
Engstrom PF, et al: NCCN Guidelines Insights: Colon Cancer, Version
2.2018. J Natl Compr Canc Netw. 16:359–369. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wong N and Wang X: miRDB: An online
resource for microRNA target prediction and functional annotations.
Nucleic Acids Res. 43 (D1):D146–D152. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Deng Z, Yan F, Jin Q, Wu J, Liu X and
Zheng H: Reversal of multidrug resistance phenotype in human breast
cancer cells using doxorubicin-liposome-microbubble complexes
assisted by ultrasound. J Control Release. 174:109–116.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shi DM, Li LX, Bian XY, Shi XJ, Lu LL,
Zhou HX, Pan TJ, Zhou J, Fan J and Wu WZ: miR-296-5p suppresses EMT
of hepatocellular carcinoma via attenuating NRG1/ERBB2/ERBB3
signaling. J Exp Clin Cancer Res. 37(294)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Maia D, de Carvalho AC, Horst MA, Carvalho
AL, Scapulatempo-Neto C and Vettore AL: Expression of miR-296-5p as
predictive marker for radiotherapy resistance in early-stage
laryngeal carcinoma. J Transl Med. 13(262)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li T, Lu YY, Zhao XD, Guo HQ, Liu CH, Li
H, Zhou L, Han YN, Wu KC, Nie YZ, et al: MicroRNA-296-5p increases
proliferation in gastric cancer through repression of
Caudal-related homeobox 1. Oncogene. 33:783–793. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lee H, Shin CH, Kim HR, Choi KH and Kim
HH: MicroRNA-296-5p promotes invasiveness through downregulation of
nerve growth factor receptor and Caspase-8. Mol Cells. 40:254–261.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Conte A, Paladino S, Bianco G, Fasano D,
Gerlini R, Tornincasa M, Renna M, Fusco A, Tramontano D and
Pierantoni GM: High mobility group A1 protein modulates autophagy
in cancer cells. Cell Death Differ. 24:1948–1962. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu L, Zhang S, Hu L, Liu L, Guo W and
Zhang J: HMGA1 participates in MHCC97H cell proliferation and
invasion through the ILK/Akt/GSK3β signaling pathway. Mol Med Rep.
16:9287–9294. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Colamaio M, Tosti N, Puca F, Mari A,
Gattordo R, Kuzay Y, Federico A, Pepe A, Sarnataro D, Ragozzino E,
et al: HMGA1 silencing reduces stemness and temozolomide resistance
in glioblastoma stem cells. Expert Opin Ther Targets. 20:1169–1179.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kim DK, Seo EJ, Choi EJ, Lee SI, Kwon YW,
Jang IH, Kim SC, Kim KH, Suh DS, Seong-Jang K, et al: Crucial role
of HMGA1 in the self-renewal and drug resistance of ovarian cancer
stem cells. Exp Mol Med. 48(e255)2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Quintavalle C, Burmeister K, Piscuoglio S,
Quagliata L, Karamitopoulou E, Sepe R, Fusco A, Terracciano LM,
Andersen JB, Pallante P, et al: High mobility group A1 enhances
tumorigenicity of human cholangiocarcinoma and confers resistance
to therapy. Mol Carcinog. 56:2146–2157. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
Loria R, Laquintana V, Bon G, Trisciuoglio
D, Frapolli R, Covello R, Amoreo CA, Ferraresi V, Zoccali C,
Novello M, et al: HMGA1/E2F1 axis and NFkB pathways regulate LPS
progression and trabectedin resistance. Oncogene. 37:5926–5938.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lin Y, Chen H, Hu Z, Mao Y, Xu X, Zhu Y,
Xu X, Wu J, Li S, Mao Q, et al: miR-26a inhibits proliferation and
motility in bladder cancer by targeting HMGA1. FEBS Lett.
587:2467–2473. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang X, Tao T, Liu C, Guan H, Huang Y, Xu
B and Chen M: Downregulation of miR-195 promotes prostate cancer
progression by targeting HMGA1. Oncol Rep. 36:376–382.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhou WB, Zhong CN, Luo XP, Zhang YY, Zhang
GY, Zhou DX and Liu LP: miR-625 suppresses cell proliferation and
migration by targeting HMGA1 in breast cancer. Biochem Biophys Res
Commun. 470:838–844. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jia XP, Meng LL, Fang JC, Wang HW, Chen J,
Zhou J, Wang CN and Jiang WF: Aberrant expression of miR-142-3p and
its target gene HMGA1 and FZD7 in breast cancer and its clinical
significance. Clin Lab. 64:915–921. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liu J, Mi B, Wang Y, Shi C, Mi X, Lu Y and
Yu P: miR-26a suppresses osteosarcoma migration and invasion by
directly targeting HMGA1. Oncol Lett. 15:8303–8310. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chandrasekaran KS, Sathyanarayanan A and
Karunagaran D: MicroRNA-214 suppresses growth, migration and
invasion through a novel target, high mobility group AT-hook 1, in
human cervical and colorectal cancer cells. Br J Cancer.
115:741–751. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lopez-Bertoni H, Lal B, Michelson N,
Guerrero-Cázares H, Quiñones-Hinojosa A, Li Y and Laterra J:
Epigenetic modulation of a miR-296-5p:HMGA1 axis regulates Sox2
expression and glioblastoma stem cells. Oncogene. 35:4903–4913.
2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chaudhary S, Islam Z, Mishra V, Rawat S,
Ashraf GM and Kolatkar PR: Sox2: A Regulatory Factor in
Tumorigenesis and Metastasis. Curr Protein Pept Sci. 20:495–504.
2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xi Y, Li YS and Tang HB: High mobility
group A1 protein acts as a new target of Notch1 signaling and
regulates cell proliferation in T leukemia cells. Mol Cell Biochem.
374:173–180. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Belton A, Xian L, Huso T, Koo M, Luo LZ,
Turkson J, Page BD, Gunning PT, Liu G, Huso DL, et al: STAT3
inhibitor has potent antitumor activity in B-lineage acute
lymphoblastic leukemia cells overexpressing the high mobility group
A1 (HMGA1)-STAT3 pathway. Leuk Lymphoma. 57:2681–2684.
2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Capo A, Sepe R, Pellino G, Milone M,
Malapelle U, Pellecchia S, Pepe F, Cacciola NA, Manigrasso M,
Bruzzaniti S, et al: Setting up and exploitation of a
nano/technological platform for the evaluation of HMGA1b protein in
peripheral blood of cancer patients. Nanomedicine (Lond).
15:231–242. 2019.PubMed/NCBI View Article : Google Scholar
|