Introduction
Acute pancreatitis (AP) is a common clinical
condition characterized by pancreatic edema and inflammation
(1) that leads to poor outcomes,
such as organ failure and necrosis (2). Pancreatic inflammation and acinar cell
death (including apoptosis) are the major pathophysiological
features of AP (3-5).
Caerulein, a cholecystokinin analog, causes intra-acinar activation
of trypsinogen in the pancreas, which can lead to AP-like symptoms
(6). The downregulation of Bcl-2
and the upregulation of Bax and activated caspase 3/9 are
associated with caerulein-induced apoptosis of acinar cells in AP
(7-9).
Hence, elucidating the mechanism of caerulein-induced pancreatic
acinar cell injury is crucial to identify therapeutic targets for
AP.
MicroRNAs (miRs) are single-stranded non-coding RNAs
that serve essential roles in inflammatory diseases, including AP
(10). For example, Fu et al
(11) reported that, by targeting
tumor necrosis factor (TNF) receptor 1A, miR-29 was upregulated and
subsequently promoted AR42J cell apoptosis, which were used as the
cellular model of AP. Furthermore, Zhang et al (12) demonstrated that miR-551b-5p promoted
the inflammatory response and AP progression. miR-9 has been
reported to act as an oncogene or tumor inhibitor in various types
of tumor, such as synovial sarcoma and pancreatic cancer (13,14).
Bone marrow-derived mesenchymal stem cells have been demonstrated
to upregulate miR-9 to inhibit the inflammatory response and
necroptosis in AP rats (15).
Furthermore, a previous study hypothesized that miR-9 may be
associated with the nuclear factor κB (NF-κB) pathway and
p50(16). These results suggested
that miR-9 may exert a therapeutic effect in AP. However, its
mechanism of action remains unclear and further research into the
role of miR-9 is required to elucidate the pathway of AP
pathogenesis.
Growth factors, including vascular endothelial
growth factor, transforming growth factor and fibroblast growth
factor (FGF), have been implicated in AP pathogenesis and
pancreatic carcinoma (17). FGF10
is a member of the FGF family, which is associated with the
development of the pancreas (18).
Activation of NF-κB signaling is a critical event in AP development
(19) and miR-9 and FGF10 have been
reported to be essential mediators of this pathway in human disease
(20-23).
The aim of the present study was to explore the
function of miR-9 on the inflammatory response and apoptosis in
caerulein-treated AR42J cells, and to analyze the interaction
between miR-9 and the FGF10/NF-κB pathway.
Materials and methods
AP cell model and cell
transfection
Rat pancreatic acinar AR42J cells (American Type
Culture Collection) were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) at 37˚C and 5% CO2.
AR42J cells were then incubated with 10 nM caerulein
(Sigma-Aldrich, Merck KGaA) at 37˚C for 0, 2, 4, 6 or 8 h to
establish an in vitro model of AP. An untreated group was
regarded as the control.
miR-9 mimic (miR-9; 5'-UCUUUGGUUAUCUAGCUGUAUGA-3'),
mimic negative control (miR-NC; 5'-CGAUCGCAUCAGCAUCGAUUGC-3'),
miR-9 inhibitor (anti-miR-9; 5'-UCAUACAGCUAGAUAACCAAAGA-3'),
inhibitor negative control (anti-miR-NC;
5'-CAGUACUUUUGUGUAGUACAA-3'), small interfering RNA (siRNA) against
FGF10 (si-FGF10; 5'-UGUUGUAUCCAUUUUCCUCUA-3'), siRNA negative
control (si-NC; 5'-AAGACAUUGUGUGUCCGCCTT-3'), pcDNA-based FGF10
overexpression vector (pc-FGF10) and pcDNA negative control (pc-NC)
were generated by Shanghai GenePharma, Co., Ltd. Vectors (1 µg) and
miRNA or siRNA oligos (20 nM) were then transfected into AR42J
cells using Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Following 24 h incubation at 37˚C, cells were harvested for
caerulein treatment. A group that did not undergo transfection was
regarded as the control.
ELISA
AR42J cells were seeded into 24-well plates
(4x104 per well) in sextuplicate and subjected to the
treatment with 10 nM caerulein at 37˚C for 0, 2, 4, 6 or 8 h. Cell
culture supernatant was collected after centrifugation at 1,000 x g
at room temperature for 10 min. The concentration of TNF-α, IL-1β
and IL-6 inflammatory cytokines were measured using specific TNF-α
(cat. no. BMS622), IL-1β (cat. no. BMS630) and IL-6 (cat. no.
BMS625) rat ELISA kits (Thermo Fisher Scientific, Inc.), according
to the manufacturer's protocol. The optical density was determined
at a wavelength of 450 nm using an iMark microplate reader (Bio-Rad
Laboratories, Inc.). The concentrations were determined from the
standard curve.
Cell apoptosis
Flow cytometry was performed using an Annexin
V-FITC/PI apoptosis detection kit (Beyotime Institute of
Biotechnology), according to the manufacturer's protocol.
Transfected or non-transfected AR42J cells (1x105
cells/well) were seeded into 6-well plates in triplicate overnight
at 37˚C. Following 10 nM caerulein incubation at 37˚C for 0, 2, 4,
6 or 8 h cells were collected and incubated with 10 µl Annexin
V-FITC and PI solution in the dark at room temperature for 10 min.
Apoptotic cells were analyzed using a FACSCalibur flow cytometer
(Becton Dickinson) with CellQuest Pro 3.0 software (Becton
Dickinson). The apoptotic rate was displayed as the percentage of
cells exhibiting positive Annexin V-FITC and positive/negative
PI.
Western blotting
AR42J cells were harvested and lysed in RIPA lysis
buffer (Beyotime Institute of Biotechnology) and protein samples
were quantified using a BCA protein assay kit (Beyotime Institute
of Biotechnology) followed by denaturation in a boiling water bath
for 10 min. Total protein (20 µg/lane) was separated by 10%
SDS-PAGE and transferred onto nitrocellulose membranes (EMD
Millipore) with Tris-Glycine transfer buffer (Novex; Thermo Fisher
Scientific, Inc.). Membranes were incubated with 5% non-fat milk at
room temperature for 1 h to block non-specific binding sites,
followed by incubation with primary antibodies overnight at 4˚C and
secondary antibodies at room temperature for 2 h. Primary
antibodies anti-Bcl-2 (cat. no. ab196495; 1:1,000; Abcam), anti-Bax
(cat. no. ab53154; 1:1,000; Abcam), anti-pro-caspase 3 and
anti-cleaved (cl) caspase 3 (cat. no. 14220; 1:1,000; Cell
Signaling Technology, Inc.), anti-pro-caspase 9 and anti-cl caspase
9 (cat. no. 9508; 1:1,000; Cell Signaling Technology, Inc.),
anti-FGF10 (cat. no. ab227102; 1:3,000; Abcam) anti-NF-kappa-B
inhibitor alpha (IKBα; cat. no. ab7217; 1:2,000; Abcam), anti-NFKB1
(subunit p50; cat. no. ab32360; 1:5,000; Abcam), anti-p-p65 (cat.
no. ab86299; 1:2,000; Abcam), anti-total-p65 (cat. no. ab16502;
1:1,000; Abcam), anti-GAPDH (cat. no. ab181603; 1:10,000; Abcam)
and horseradish peroxidase-conjugated IgG secondary antibodies
(cat. nos. ab205718 and ab205719; 1:10,000; Abcam). Protein signals
were developed and visualized utilizing enhanced chemiluminescence
Western Blotting Substrate reagent (Pierce; Thermo Fisher
Scientific, Inc.). Relative protein level was normalized to GAPDH
following densitometry analysis using Image Lab 3.0 software
(Bio-Rad Laboratories, Inc.). All experiments were performed in
triplicate.
Reverse transcription quantitative PCR
(RT-qPCR)
Total RNA was extracted from AR42J cells following
incubation with TRIzol® reagent (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. RNA (1
µg) was reverse transcribed (denaturation at 65˚C for 15 min,
followed by reaction at 25˚C for 10 min and 42˚C for 60 min, and
denaturation at 99˚C for 5 min) using a first-strand cDNA kit
(Sigma-Aldrich, Merck KGaA) according to the manufacturer's
protocol. RT-qPCR was performed using cDNA, specific primers and
SYBR™ Green (Takara Bio, Inc.) on a 7500 RT-q PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions were 95˚C for 5 min and 40 cycles, 95˚C for 15 sec and
60˚C for 1 min, followed by 72˚C for 5 min. The following pairs of
rat RNA primers were used: FGF10 forward, 5'-AAGAACGGCAAGGTCAGC-3'
and reverse, 5'-GAGGAAGTGAGCGGAGGTG-3'; GAPDH forward,
5'-GACATGCCGCCTGGAGAAAC-3' and reverse, 5'-AGCCCAGGATGCCCTTTAGT-3';
miR-9 forward, 5'-GCCCGCTCTTTGGTTATCTAG-3' and reverse,
5'-CCAGTGCAGGGTCCGAGGT-3'; and U6 forward, 5'-CTCGCTTCGGCAGCACA-3'
and reverse, 5'-AACGCTTCACGAATTTGCGT-3'. FGF10 and miR-9
expressions were normalized to U6 or GAPDH, respectively and
calculated according to the 2-ΔΔCq method (24). All experiments were performed in
triplicate.
Bioinformatics analysis and luciferase
reporter assay
Bioinformatics analysis using TargetScan 7.2
(targetscan.org/vert_72) indicated there
were binding sites between miR-9 and FGF10. FGF10 3' untranslated
(UTR) sequences containing miR-9 binding sites (ACCAAAG) were
amplified and cloned into pmirGLO vectors (Promega Corporation) to
generate wild type luciferase (wt-FGF10) and mutant (mut-FGF10)
vectors by mutating the seed sites to UGGUUUC using a Fast
Site-Directed Mutagenesis kit (Tiangen Biotech Co., Ltd.) according
to the manufacturer's protocol.
Luciferase reporter assay was performed in AR42J
cells after co-transfection with wt-FGF10 or mut-FGF10 and miR-NC,
miR-9 mimic, anti-miR-NC or anti-miR-9 using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.).
Cells were harvested 24 h post-transfection and luciferase activity
was measured using a Dual-Glo luciferase assay system (Promega
Corporation) by normalizing to Renilla luciferase. All experiments
were performed in triplicate.
RNA immunoprecipitation (RIP)
A magna RNA immunoprecipitation kit (EMD Millipore)
was used on AR42J cells for RIP according to the manufacturer's
protocol. Cells were lysed with RIP lysis buffer containing
proteinase and RNase inhibitors (provided in the kit) and incubated
with magnetic beads pre-coated with anti-argonaute-2 (Ago2)
antibodies (cat. no. ab32381; Abcam) for 6 h at 4˚C. Immunoglobulin
G (IgG; cat. no. AP112; Sigma-Aldrich; Merck KGaA) and cell lysates
were used as controls. The beads were then washed with RIP washing
buffer and the immunoprecipitate was digested with proteinase K
(provided in the kit). FGF10 and miR-9 RNA levels were detected via
RT-qPCR as describe above. All experiments were performed in
triplicate.
RNA pull-down assay
An RNA pull-down assay was performed using an
RNA-Protein pull-down kit (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Biotinylated FGF10
(bio-FGF10-wt), mutant FGF10 (bio-FGF10-mut) and negative control
(bio-NC) probes were designed by Guangzhou RiboBio Co., Ltd. and
conjugated with streptavidin magnetic beads (Thermo Fisher
Scientific, Inc.). AR42J cells were lysed using RIP lysis buffer,
and incubated with probe-coated beads for 2 h at 4˚C. Beads were
then washed with washing buffer (provided in the kit), and the
biotin-coupled RNA complex was pulled down using elution buffer by
vortexing. Enriched miR-9 levels were analyzed via RT-qPCR as
described above. All experiments were performed in triplicate.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software (version 7; GraphPad Software, Inc.). Data are
presented as the mean ± standard deviation. Student's t-test or
one-way ANOVA followed by Tukey's post hoc test was performed to
compare differences between groups, as applicable. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-9 expression is reduced in
caerulein-treated AR42J cells
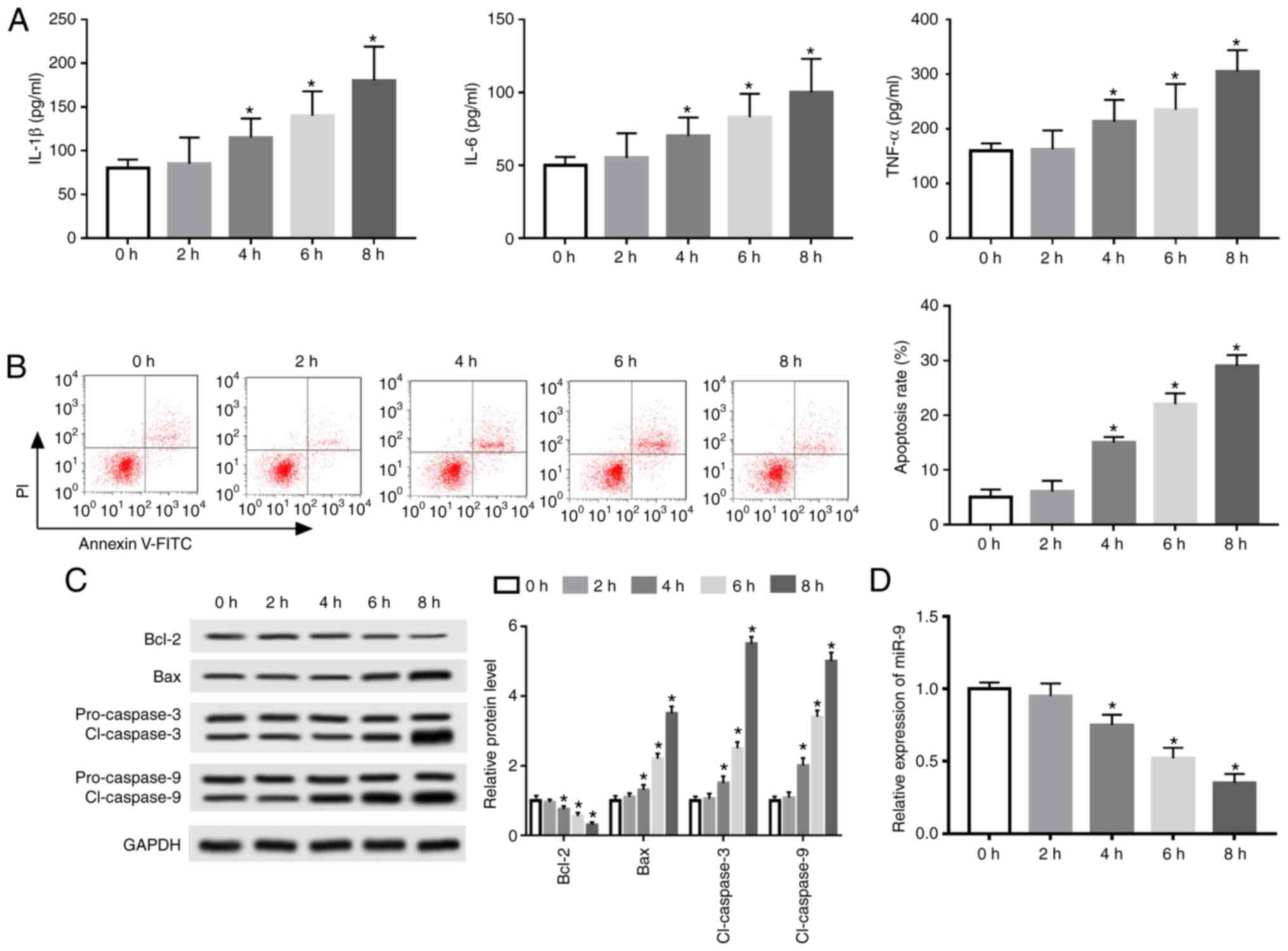
An AP model was established using caerulein-treated
AR42J cells to investigate the potential role of miR-9 in AP
progression. IL-1β, IL-6 and TNF-α levels were elevated in a
time-dependent manner following treatment for 0-8 h (Fig. 1A). Furthermore, flow cytometry data
revealed that caerulein treatment induced apoptosis in a
time-dependent manner (Fig. 1B).
Western blotting also demonstrated that exposure to caerulein
decreased Bcl-2 expression and increased Bax and cl-caspase 3 and 9
expression time-dependently (Fig.
1C). The results indicated that caerulein induced an
inflammatory response and apoptosis in caerulein-treated AR42J
cells.
Additionally, miR-9 expression was measured in
caerulein-induced AR42J cells. The results revealed that miR-9
expression was significantly decreased in a time-dependent manner
(Fig. 1D). The optimum time of
caerulein exposure was determined to be 8 h.
miR-9 decreases the inflammatory
response and apoptosis in caerulein-treated AR42J cells
AR42J cells were transfected with miR-NC, miR-9
mimic, anti-miR-NC or anti-miR-9 and treated for 8 h to investigate
the effect of miR-9 on caerulein-induced injury. miR-9 expression
was upregulated 4.2-fold in the miR-9 mimic group, while
anti-miR-9-transfected cells demonstrated a 68% reduction in miR-9
expression compared with corresponding controls (Fig. 2A). Furthermore, miR-9 overexpression
significantly inhibited IL-1β, IL-6 and TNF-α expression, while
miR-9 knockdown exerted the opposite effect (Fig. 2B). Additionally, overexpression of
miR-9 significantly decreased caerulein-induced apoptosis, whereas
miR-9 knockdown significantly promoted cell apoptosis (Fig. 2C). The results also demonstrated
that miR-9 overexpression upregulated Bcl-2 and downregulated Bax
and cl-caspases 3 and 9, while miR-9 knockdown exerted the opposite
effect (Fig. 2D).
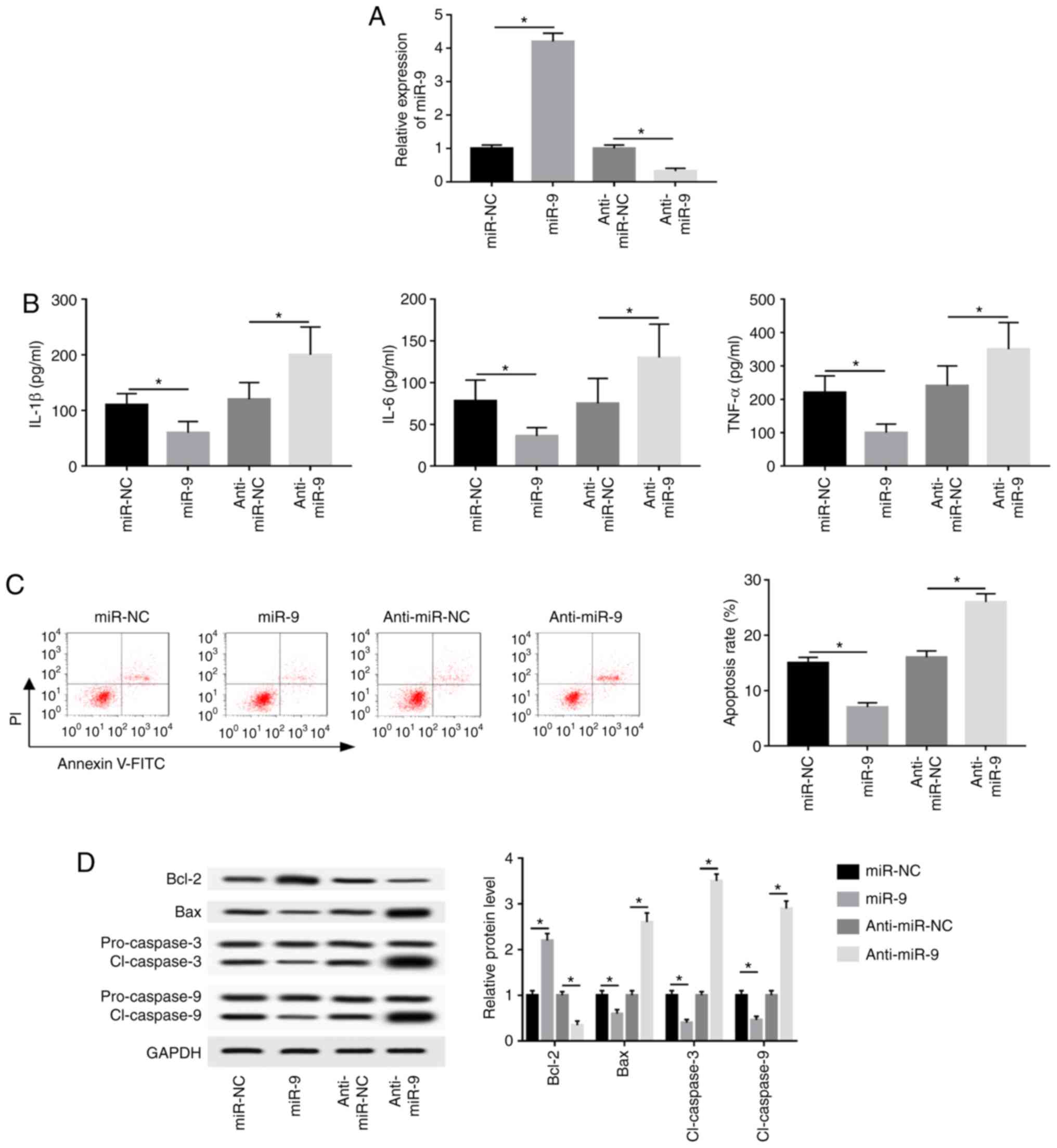 | Figure 2miR-9 inhibits caerulein-induced
inflammatory response and apoptosis in AR42J cells. AR42J cells
were transfected with miR-NC, miR-9, anti-miR-NC or anti-miR-9
prior to caerulein treatment. (A) miR-9 expression, (B)
inflammatory cytokine levels (C) apoptotic rate and (D)
apoptotic-related protein levels were detected in the treated cells
by reverse transcription quantitative PCR, ELISA, flow cytometry
and western blotting, respectively. Data are presented as the mean
± SD. *P<0.05, as indicated. miR, microRNA; miR-NC,
mimic negative control; miR-9, mir-9 mimic; anti-miR-NC, inhibitor
negative control; anti-miR-9, miR-9 inhibitor; IL, interleukin;
TNF, tumor necrosis factor; PI, propidium iodide; FITC, fluorescein
isothiocyanate; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2 associated X;
cl-caspase, cleaved caspase. |
FGF10 is a target of miR9
A potential miR-9 target was investigated via
bioinformatics analysis using TargetScan to elucidate the mechanism
by which miR-9 regulates AP progression. Predicted binding sites
between miR-9 and FGF10 at position 509-515 of the 3'UTR were
exhibited and luciferase reporter vectors containing wt or mut
miR-9 seeding sites were generated (Fig. 3A). The results demonstrated that
miR-9 overexpression resulted in a 55% reduction in luciferase
activity (Fig. 3B). Additionally,
miR-9 knockdown significantly increased luciferase activity in the
wt-FGF10 group, but remained unaffected in the mut-FGF10 group
(Fig. 3C). Furthermore, miR-9 and
FGF10 were enriched in the same complex by protein Ago2 RIP
compared with the IgG RIP group (Fig.
3D). RNA pull-down assay demonstrated that biotinylated FGF10
induced higher miR-9 expression compared with the negative control
group, and that the binding was abolished in the bio-FGF10-mut
group by mutating its binding sites (Fig. 3E). Additionally, the effect of miR-9
on FGF10 expression was analyzed by overexpressing or knocking down
miR-9. The results of western blotting demonstrated that FGF10
expression was decreased by 70% when miR-9 was overexpressed and
enhanced 2.8-fold by miR-9 knockdown (Fig. 3F).
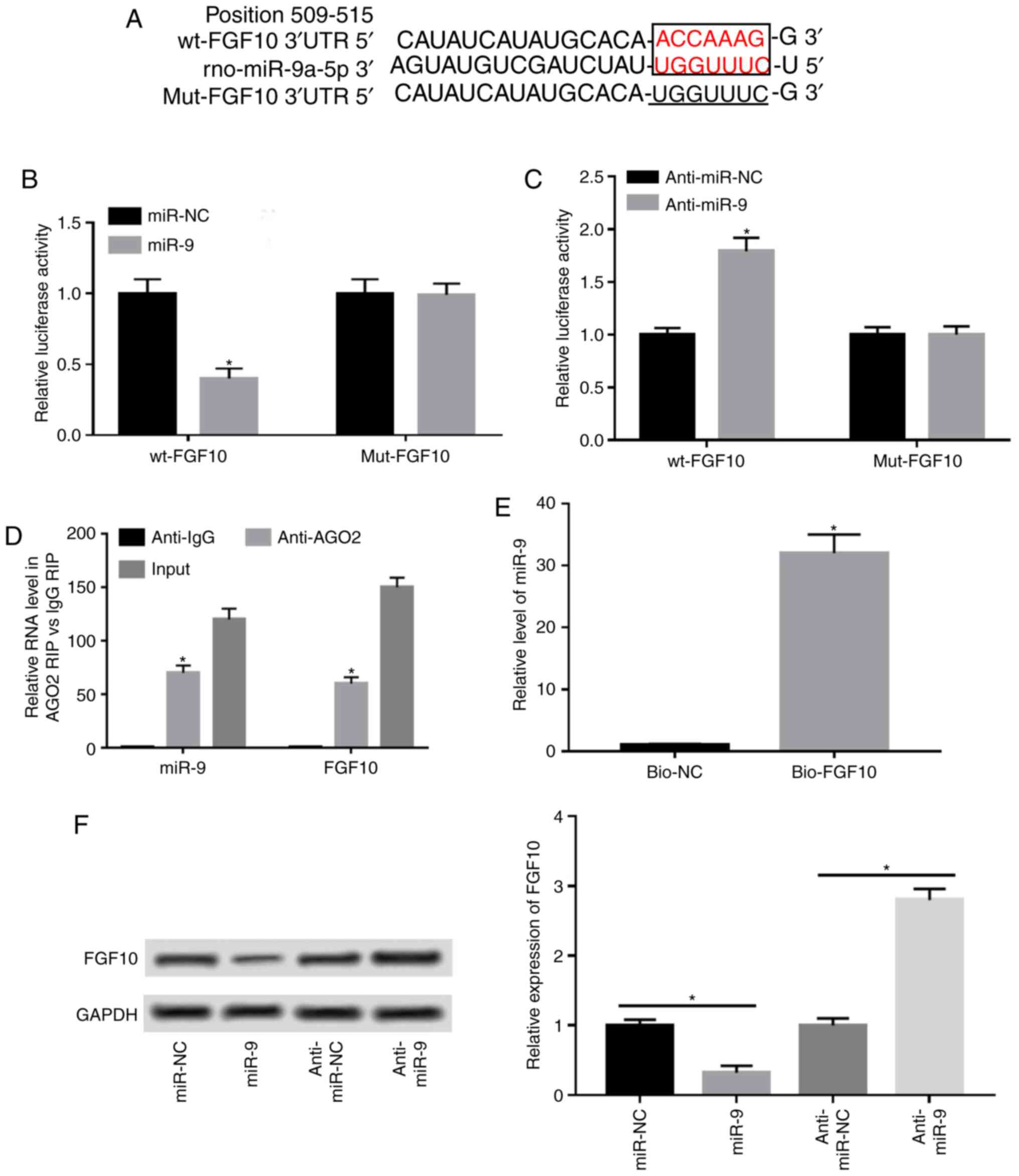 | Figure 3FGF10 is a target of miR-9 in
caurulein-treated AR42J cells. (A) Predicted binding sites of miR-9
and FGF10 generated by TargetScan. Binding sites are presented in
red and mutant sites are underlined. Luciferase reporter assay was
performed in cells co-transfected with (B) wt-FGF10 or mut-FGF10
and miR-NC, miR-9, (C) anti-miR-NC or anti-miR-9. (D) Ago2 RIP
assay was performed and miR-9 and FGF10 levels were measured via
RT-qPCR. (E) RNA pull-down assay was performed in AR42J cells and
miR-9 expression was detected via RT-qPCR. (F) FGF10 expression was
detected in cells transfected with miR-NC, miR-9 mimic, anti-miR-NC
or anti-miR-9 via western blotting. Data are presented as the mean
± standard deviation. *P<0.05 as indicated. FGF10,
fibroblast growth factor 10; wt, wild type; mut, mutant; miR-NC,
mimic negative control; miR-9, miR-9 mimic; anti-miR-NC, inhibitor
negative control; anti-miR-9, miR-9 inhibitor; Ago2, protein
argonaute-2; RIP, RNA immunoprecipitation; RT-qPCR, reverse
transcription quantitative PCR; IgG, immunoglobulin G; bio-NC,
negative control; bio-FGF10-wt, biotinylated FGF10; bio-FGF10-mut,
biotinylated mutant FGF10; input, cell lystates as the positive
control. |
FGF10 promotes inflammatory response
and apoptosis in caerulein-treated AR42J cells
FGF10 expression was progressively upregulated
following treatment in a time-dependent manner (Fig. 4A). Cells were transfected with
pc-NC, pc-FGF10, si-NC or si-FGF10 to investigate the role of FGF10
in caerulein-induced injury. FGF10 expression was upregulated
4.2-fold by pc-FGF10 and reduced by 67% following si-FGF10
treatment, as demonstrated via western blotting (Fig. 4B). IL-1β, IL-6 and TNF-α expression
was significantly increased by FGF10 overexpression and inhibited
by FGF10 interference (Fig. 4C).
Additionally, flow cytometry and western blotting data revealed
that FGF10 overexpression significantly increased caerulein-induced
apoptosis, increased apoptotic-associated protein expression (Bax,
cl-caspases 3 and 9) and reduced antiapoptotic protein levels
(Bcl-2). FGF10 knockdown demonstrated opposite results (Fig. 4D-F).
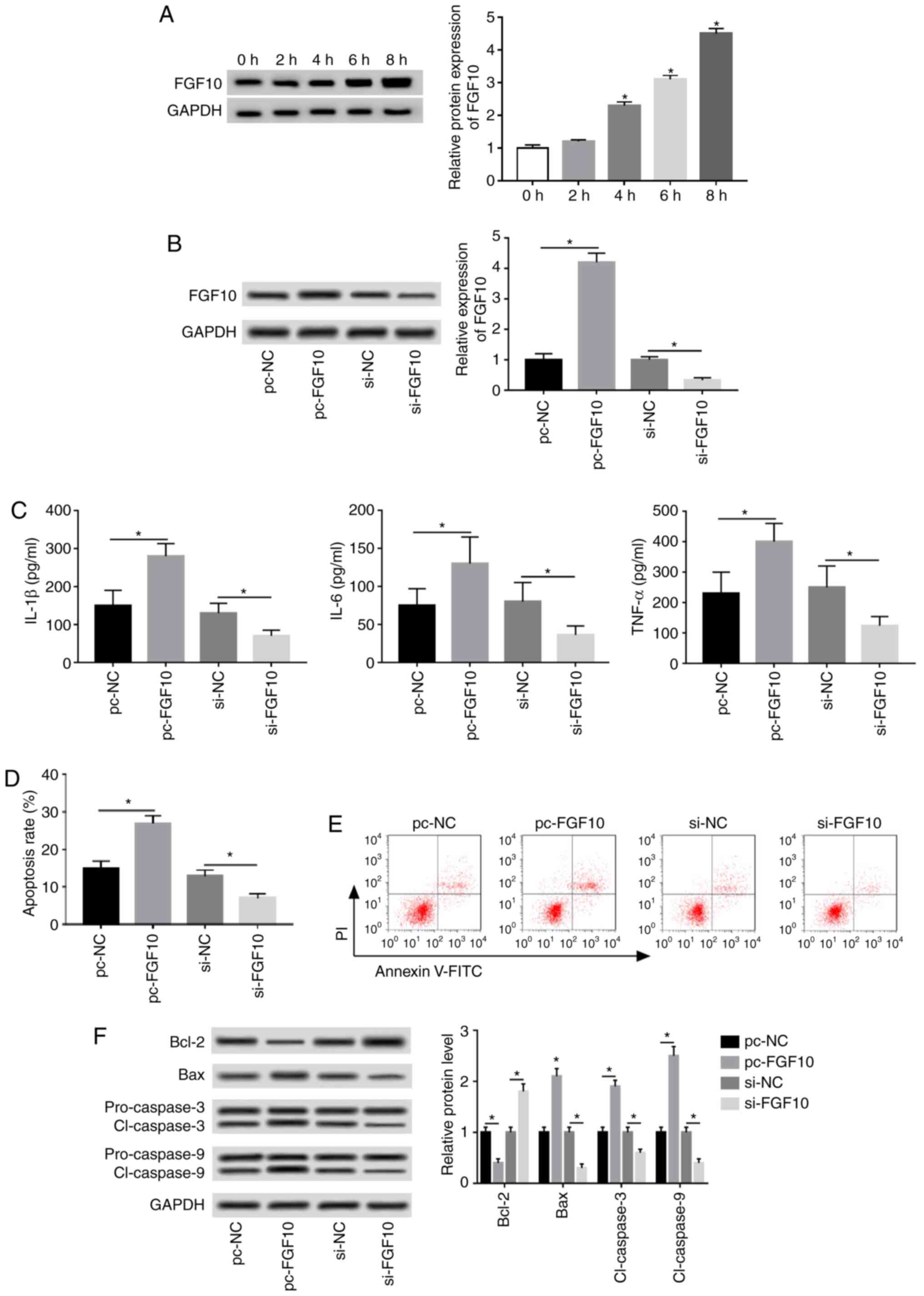 | Figure 4FGF10 promotes caerulein-induced
inflammatory response and apoptosis in AR42J cells. (A) FGF10
expression was measured following treatment at different time
points via western blotting. Cells were transfected with pc-NC,
pc-FGF10, si-NC or si-FGF10 and (B) FGF10 expression, (C)
inflammatory cytokine levels, (D) apoptotic rate, (E) cell
apoptosis and (F) apoptotic-associated protein expression were
detected via western blotting, ELISA and flow cytometry. Data are
presented as the mean ± standard deviation. *P<0.05
as indicated. FGF10, fibroblast growth factor 10; pc-NC, pcDNA
empty vector; pc-FGF10, pcDNA-based FGF10 overexpression vector;
si-NC, siRNA negative control; si-FGF10, small interfering RNA
against FGF10; IL, interleukin; TNF, tumor necrosis factor; Bcl-2,
B-cell lymphoma 2; Bax, Bcl-2 associated X; cl-caspase, cleaved
caspase. |
miR-9 regulates the inflammatory
response and apoptosis by targeting FGF10 in caerulein-treated
AR42J cells
Cells were co-transfected with miR-9 mimic and pc-NC
or pc-FGF10, anti-miR-9 and si-NC, or si-FGF10 prior to caerulein
treatment to investigate whether FGF10 was involved in
miR-9-mediated AP progression in vitro. FGF10 expression was
rescued by pc-FGF10 and decreased by si-FGF10 in the presence of
the miR-9 mimic or anti-miR-9 (Fig.
5A). FGF10 restoration caused the miR-9-induced downregulation
of inflammatory cytokines to be reversed, and silence of FGF10
attenuated knockdown of miR-9-induced the secretion of IL-1β, IL-6
and TNF-α (Fig. 5B-D). Furthermore,
FGF10 alleviated miR-9-mediated apoptosis inhibition and FGF10
interference counteracted the effect of miR-9 knockdown on cell
apoptosis (Fig. 5E and F).
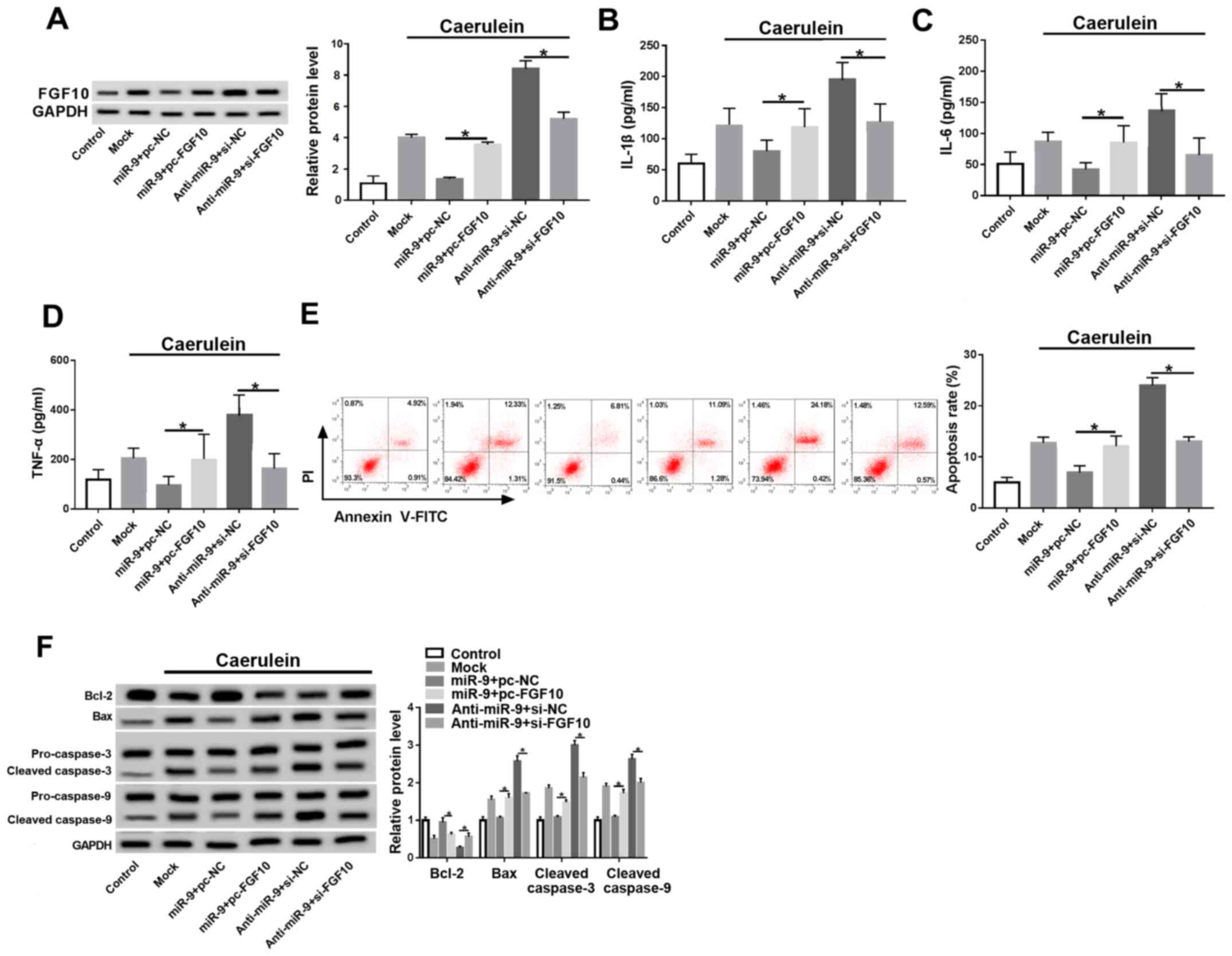 | Figure 5FGF10 reverses the miR-9-mediated
inflammatory response and apoptosis in caerulein-treated AR42J
cells. (A) FGF10 expression, inflammatory cytokine (B) IL-1β, (C)
IL-6 and (D) TNF-α levels, (E) apoptotic rate and (F)
apoptotic-associated protein expressions were detected in cells
transfected with miR-9 and pc-NC or pc-FGF10, anti-miR-9 and si-NC
or si-FGF10 by ELISA, flow cytometry and western blotting. Data are
presented as the mean ± standard deviation. *P<0.05
as indicated. FGF10, fibroblast growth factor 10; miR, microRNA;
miR-9, miR-9 mimic; pc-NC, pcDNA empty vector; pc-FGF10,
pcDNA-based FGF10 overexpression vector; anti-miR, miR-9 inhibitor;
si-NC, siRNA negative control; si-FGF10, siRNA against FGF10; Mock,
non-transfected group; IL, interleukin; TNF, tumor necrosis factor;
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2 associated X; cl-caspase,
cleaved caspase. |
miR-9 repressed the NF-κB pathway by
targeting FGF10 in caerulein-treated AR42J cells
Cells were transfected with miR-NC, miR-9 mimic,
miR-9 mimic and pc-NC or pc-FGF10, anti-miR-NC, anti-miR-9,
anti-miR-9 and si-NC or si-FGF10 prior to caerulein treatment.
Levels of proteins involved in the NF-κB pathway were subsequently
measured. Caerulein treatment led to reduction of IKBα and an
increase in NFKB1 (subunit p50) and phosphorylated p65, indicating
that caerulein induced NF-κB pathway activation. Additionally,
miR-9 overexpression suppressed the activation of the NF-κB
pathway, which was mitigated by FGF10 restoration (Fig. 6A). Furthermore, miR-9 knockdown
aggravated caerulein-induced pathway activation and this effect was
abolished by FGF10 silencing (Fig.
6B). Collectively, miR-9 mitigated the inflammatory response
and cell apoptosis in caerulein-induced AP cells, possibly by
targeting FGF10 and regulating the NF-κB pathway.
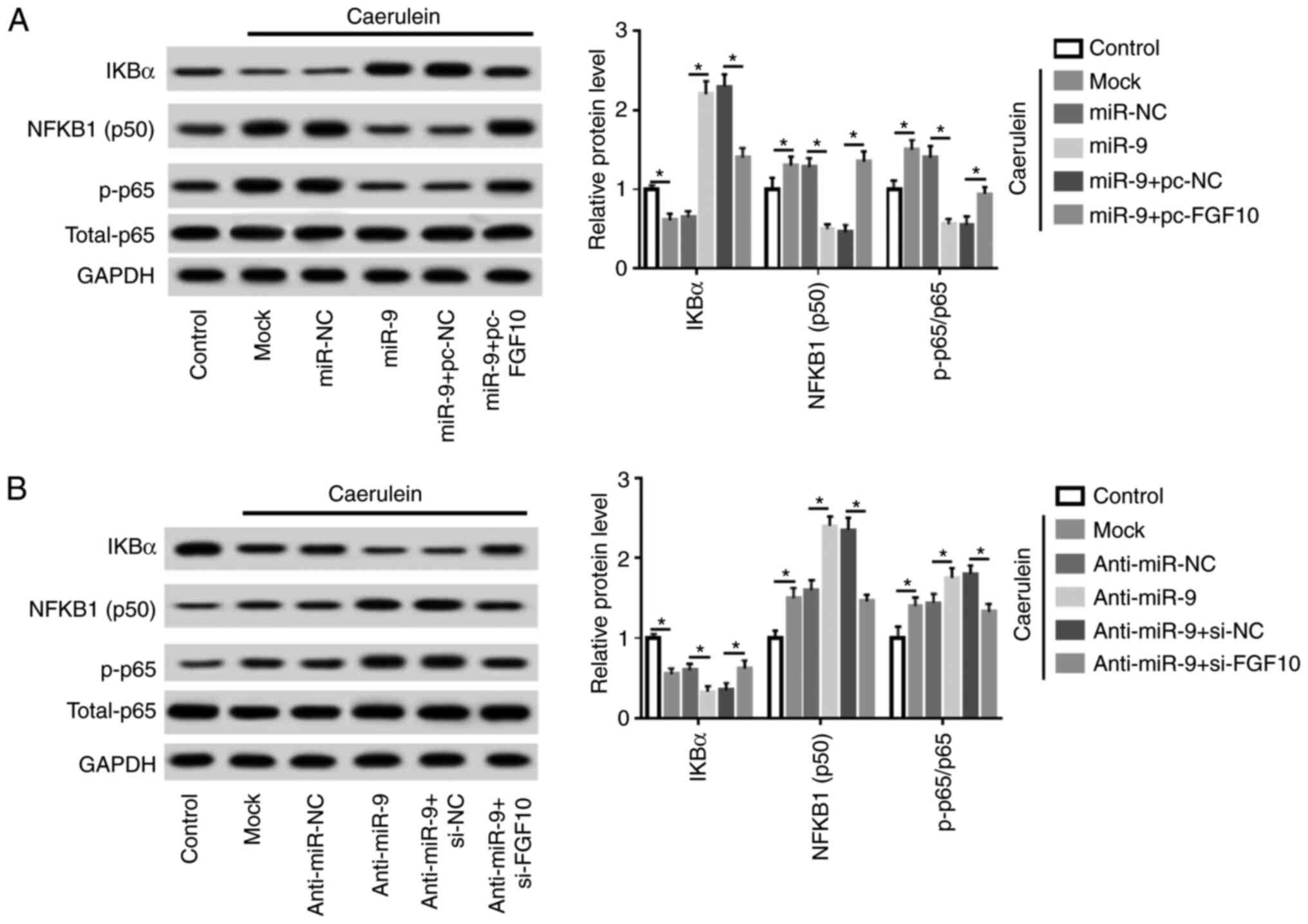 | Figure 6miR-9 inhibits the NF-κB pathway
through FGF10 in caerulein-treated AR42J cells. (A) IKBα, NFKB1
(p50) and p-p65 expression was measured in AR42J cells transfected
with miR-NC, miR-9 mimic, miR-9 mimic and pc-NC or pc-FGF10 after
treatment of caerulein by western blotting. (B) IKBα, NF-κB subunit
1 (subunit p50) and phosphorylated p65 expression was measured in
AR42J cells transfected with anti-miR-NC, anti-miR-9, anti-miR-9 +
si-NC or si-FGF10 following caerulein treatment by western
blotting. Data are presented as the mean ± standard deviation.
*P<0.05 as indicated. IKBα, NF-kappa-B inhibitor
alpha; NF-κB, nuclear factor kappa-light-chain-enhancer of
activated B cells; FGF10, fibroblast growth factor 10; p-p65,
p-p65; total-p65, p65 in nucleus; anti-miR-NC, inhibitor negative
control; pc-NC, pcDNA empty vector; pc-FGF10; pcDNA-based FGF10
overexpression vector; mock, non-transfected group. |
Discussion
Caerulein-treated AR42J cells were used in a model
of AP in vitro as previously described (25-27).
In the current study, AR42J cells were incubated with 10 nM
caerulein for 0, 2, 4, 6 or 8 h. The results demonstrated that
caerulein triggered the inflammatory response and apoptosis. The
present study also investigated the role of miR-9 in
caerulein-induced injury and demonstrated an association between
miR-9 and FGF10 in vitro.
In the present study, miR-9 levels were decreased in
caerulein-treated AR42J cells, suggesting that miR-9 may serve a
protective role in AP development. These results are consistent
with those of previous studies (16,28).
However, Lu et al (29)
reported that miR-9 was highly expressed in the serum of patients
with AP and that this expression could be used as a critical
diagnostic and prognostic marker of AP. The current study
hypothesized that this result may have been caused by the different
microenvironment observed in serum and cells.
Inflammatory response and acinar cell apoptosis are
the main features of AP (26,30). A
recent study reported that miR-9 inhibited apoptosis and the
inflammatory response in human umbilical vascular endothelial cells
(31). The current study revealed
that miR-9 suppressed caerulein-induced inflammatory response by
decreasing IL-1β, IL-6 and TNF-α expression and regulating Bcl-2
family proteins and caspases in AR42J cells to repress apoptosis,
thereby exhibiting a potential therapeutic role of miR-9 in AP.
Functional miRs regulate mRNA expression by
targeting the 3'-UTR (32). In the
current study, luciferase, RIP and RNA pull-down assays revealed
that miR-9 could bind to FGF10, indicating that FGF10 served as a
functional target of miR-9. FGF2 has been reported to exhibit high
expressions in AP and to stimulate the inflammatory response
(33,34). FGF10, a high-affinity ligand of
FGF2, was expressed in AP tissues, suggesting that FGF10 may
contribute to AP development as it was not expressed in normal
pancreas tissue (35). In the
current study, gain- and loss-of-function experiments demonstrated
that FGF10 promoted inflammatory cytokine secretion and apoptosis
in caerulein-treated cells, indicating that FGF10 may facilitate AP
progression. Furthermore, overexpression or knockdown of FGF10
attenuated the effect of miR-9 on caerulein-induced AP progression,
revealing that miR-9 may attenuate AP-like injury by targeting
FGF10.
Previous studies have reported that FGF10 is a vital
regulator of the NF-κB-dependent inflammatory response (22,23).
IKBα is a major inhibitor of NF-κB and may remove NF-κB complexes
in nuclei (36). NF-κB is comprised
of p50 and p65 subunits and is activated by p65, which may promote
the secretion of the inflammatory cytokines IL-1β, IL-6 and TNF-α
(37,38). Furthermore, the NF-κB pathway has
been associated with cell apoptosis in AP (39,40).
The results of the current study demonstrated that caerulein led to
the activation of NF-κB signaling in AR42J cells by decreasing IKBα
and increasing p50 and p65, indicating that NF-κB pathway
activation was involved in AP progression. Inhibition of NF-κB
signaling has been regarded as a key avenue for therapeutics of AP
(41-43).
Additionally, the results suggested that miR-9 inhibited the
caerulein-induced activation of the NF-κB pathway, which has also
been reported in previous work (16). The results of the current study
revealed that this effect was associated with FGF10. Data indicated
that miR-9 may target FGF10 to block the NF-κB pathway, leading to
the inhibition of the inflammatory response and apoptosis in
caerulein-treated cells. However, the current study only reported
in vitro results. Further research is required to
investigate the role of miR-9 in vivo to fully elucidate AP
pathogenesis.
In conclusion, miR-9 expression was decreased in a
caerulein-induced cellular model of AP. miR-9 attenuated the
caerulein-induced inflammatory response and cell apoptosis,
possibly by targeting FGF10 and regulating the NF-κB pathway. The
current study elucidated a novel mechanism of AP pathogenesis and
hypothesized a novel target for AP treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS and CL conceived and designed the present study.
YS, CX and GY performed the experiments. YS and CX confirm the
authenticity of all the raw data. YS and CL analyzed the data. YS
and CL wrote the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Forsmark CE, Vege SS and Wilcox CM: Acute
pancreatitis. N Engl J Med. 375:1972–1981. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Petrov MS, Shanbhag S, Chakraborty M,
Phillips AR and Windsor JA: Organ failure and infection of
pancreatic necrosis as determinants of mortality in patients with
acute pancreatitis. Gastroenterology. 139:813–820. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang G, Qu FZ, Li L, Lv JC and Sun B:
Necroptosis: A potential, promising target and switch in acute
pancreatitis. Apoptosis. 21:121–129. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tan JH, Cao RC, Zhou L, Zhou ZT, Chen HJ,
Xu J, Chen XM, Jin YC, Lin JY, Qi ZC, et al: EMC6 regulates acinar
apoptosis via APAF1 in acute and chronic pancreatitis. Cell Death
Dis. 11(966)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bansod S and Godugu C: Nimbolide
ameliorates pancreatic inflammation and apoptosis by modulating
NF-κB/SIRT1 and apoptosis signaling in acute pancreatitis model.
Int Immunopharmacol. 90(107246)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jeong YK and Kim H: A mini-review on the
effect of docosahexaenoic acid (DHA) on cerulein-induced and
hypertriglyceridemic acute pancreatitis. Int J Mol Sci.
18(2239)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cai Y, Shen Y, Xu G, Tao R, Yuan W, Huang
Z and Zhang D: TRAM1 protects AR42J cells from caerulein-induced
acute pancreatitis through ER stress-apoptosis pathway. In Vitro
Cell Dev Biol Anim. 52:530–536. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cui L, Liu R, Li C, Yu X, Liu X, Hou F,
Chi C, Yin C and Wang C: Angiotensin-(1-7) attenuates
caerulein-induced pancreatic acinar cell apoptosis. Mol Med Rep.
16:3455–3460. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cao S, Bian Y, Zhou X, Yuan Q, Wei S, Xue
L, Yang F, Dong QQ, Wang WJ, Zheng B, et al: A small-molecule
activator of mitochondrial aldehyde dehydrogenase 2 reduces the
severity of cerulein-induced acute pancreatitis. Biochem Biophys
Res Commun. 522:518–524. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xiang H, Tao X, Xia S, Qu J, Song H, Liu J
and Shang D: Targeting microRNA function in acute pancreatitis.
Front Physiol. 8(726)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fu Q, Qin T, Chen L, Liu CJ, Zhang X, Wang
YZ, Hu MX, Chu HY and Zhang HW: miR-29a up-regulation in AR42J
cells contributes to apoptosis via targeting TNFRSF1A gene. World J
Gastroenterol. 22:4881–4890. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang Y, Yan L and Han W: Elevated level
of miR-551b-5p is associated with inflammation and disease
progression in patients with severe acute pancreatitis. Ther Apher
Dial. 22:649–655. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xu XZ, Li XA, Luo Y, Liu JF, Wu HW and
Huang G: MiR-9 promotes synovial sarcoma cell migration and
invasion by directly targeting CDH1. Int J Biochem Cell Biol.
112:61–71. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang J, Wang B, Ren H and Chen W: miR-9-5p
inhibits pancreatic cancer cell proliferation, invasion and
glutamine metabolism by targeting GOT1. Biochem Biophys Res Commun.
509:241–248. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Song G, Ma Z, Liu D, Qian D, Zhou J, Meng
H, Zhou B and Song Z: Bone marrow-derived mesenchymal stem cells
attenuate severe acute pancreatitis via regulation of microRNA-9 to
inhibit necroptosis in rats. Life Sci. 223:9–21. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Qian D, Wei G, Xu C, He Z, Hua J, Li J, Hu
Q, Lin S, Gong J, Meng H, et al: Bone marrow-derived mesenchymal
stem cells (BMSCs) repair acute necrotized pancreatitis by
secreting microRNA-9 to target the NF-κB1/p50 gene in rats. Sci
Rep. 7(581)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nandy D and Mukhopadhyay D: Growth factor
mediated signaling in pancreatic pathogenesis. Cancers (Basel).
3:841–871. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ndlovu R, Deng LC, Wu J, Li XK and Zhang
JS: Fibroblast growth factor 10 in pancreas development and
pancreatic cancer. Front Genet. 9(482)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jakkampudi A, Jangala R, Reddy BR, Mitnala
S, Nageshwar Reddy D and Talukdar R: NF-κB in acute pancreatitis:
Mechanisms and therapeutic potential. Pancreatology. 16:477–488.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu W, Wang X, Zheng Y, Shang G, Huang J,
Tao J and Chen L: Electroacupuncture inhibits inflammatory injury
by targeting the miR-9-mediated NF-κB signaling pathway following
ischemic stroke. Mol Med Rep. 13:1618–1626. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gu R, Liu N, Luo S, Huang W, Zha Z and
Yang J: MicroRNA-9 regulates the development of knee osteoarthritis
through the NF-kappaB1 pathway in chondrocytes. Medicine
(Baltimore). 95(e4315)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li YH, Fu HL, Tian ML, Wang YQ, Chen W,
Cai LL, Zhou XH and Yuan HB: Neuron-derived FGF10 ameliorates
cerebral ischemia injury via inhibiting NF-κB-dependent
neuroinflammation and activating PI3K/Akt survival signaling
pathway in mice. Sci Rep. 6(19869)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen J, Wang Z, Zheng Z, Chen Y, Khor S,
Shi K, He Z, Wang Q, Zhao Y, Zhang H, et al: Neuron and
microglia/macrophage-derived FGF10 activate neuronal FGFR2/PI3K/Akt
signaling and inhibit microglia/macrophages TLR4/NF-κB-dependent
neuroinflammation to improve functional recovery after spinal cord
injury. Cell Death Dis. 8(e3090)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang Y, Wang G, Cui L, Liu R, Xiao H and
Yin C: Angiotensin 1-7 ameliorates caerulein-induced inflammation
in pancreatic acinar cells by downregulating Toll-like receptor
4/nuclear factor-κB expression. Mol Med Rep. 17:3511–3518.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhao D, Ge H, Ma B, Xue D, Zhang W, Li Z
and Sun H: The interaction between ANXA2 and lncRNA Fendrr promotes
cell apoptosis in caerulein-induced acute pancreatitis. J Cell
Biochem. 120:8160–8168. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jaworek J, Szklarczyk J, Kot M, Góralska
M, Jaworek A, Bonior J, Leja-Szpak A, Nawrot-Porąbka K,
Link-Lenczowski P, Ceranowicz P, et al: Chemerin alleviates acute
pancreatitis in the rat thorough modulation of NF-κB signal.
Pancreatology. 19:401–408. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Qian D, Song G, Ma Z, Wang G, Jin L, Hu M,
Song Z and Wang X: MicroRNA-9 modified bone marrow-derived
mesenchymal stem cells (BMSCs) repair severe acute pancreatitis
(SAP) via inducing angiogenesis in rats. Stem Cell Res Ther.
9(282)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lu P, Wang F, Wu J, Wang C, Yan J, Li ZL,
Song JX and Wang JJ: Elevated serum miR-7, miR-9, miR-122, and
miR-141 are noninvasive biomarkers of acute pancreatitis. Dis
Markers. 2017(7293459)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lesina M, Wörmann SM, Neuhöfer P, Song L
and Algül H: Interleukin-6 in inflammatory and malignant diseases
of the pancreas. Semin Immunol. 26:80–87. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yi J and Gao ZF: MicroRNA-9-5p promotes
angiogenesis but inhibits apoptosis and inflammation of high
glucose-induced injury in human umbilical vascular endothelial
cells by targeting CXCR4. Int J Biol Macromol. 130:1–9.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yang Y, Huang Q, Luo C, Wen Y, Liu R, Sun
H and Tang L: MicroRNAs in acute pancreatitis: From pathogenesis to
novel diagnosis and therapy. J Cell Physiol. 235:1948–1961.
2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Warzecha Z, Dembinski A, Ceranowicz P,
Dembinski M, Kownacki P, Konturek SJ, Tomaszewska R, Stachura J,
Hładki W and Pawlik WW: Immunohistochemical expression of FGF-2,
PDGF-A, VEGF and TGF beta RII in the pancreas in the course of
ischemia/reperfusion-induced acute pancreatitis. J Physiol
Pharmacol. 55:791–810. 2004.PubMed/NCBI
|
|
34
|
Andoh A, Bamba S, Fujino S, Inatomi O,
Zhang Z, Kim S, Takayanagi A, Shimizu N and Fujiyama Y: Fibroblast
growth factor-2 stimulates interleukin-6 secretion in human
pancreatic periacinar myofibroblasts. Pancreas. 29:278–283.
2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ishiwata T, Naito Z, Lu YP, Kawahara K,
Fujii T, Kawamoto Y, Teduka K and Sugisaki Y: Differential
distribution of fibroblast growth factor (FGF)-7 and FGF-10 in
L-arginine-induced acute pancreatitis. Exp Mol Pathol. 73:181–190.
2002.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhou P, Hua F, Wang X and Huang JL:
Therapeutic potential of IKK-β inhibitors from natural phenolics
for inflammation in cardiovascular diseases. Inflammopharmacology.
28:19–37. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Perkins ND: The diverse and complex roles
of NF-κB subunits in cancer. Nat Rev Cancer. 12:121–132.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
38
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer.
12(86)2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Liu Z, Liu J, Zhao K, Shi Q, Zuo T, Wang G
and Wang W: Role of daphnetin in rat severe acute pancreatitis
through the regulation of TLR4/NF-[Formula: see text]B signaling
pathway activation. Am J Chin Med. 44:149–163. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhao S, Yang J, Liu T, Zeng J, Mi L and
Xiang K: Dexamethasone inhibits NF-κBp65 and HMGB1 expression in
the pancreas of rats with severe acute pancreatitis. Mol Med Rep.
18:5345–5352. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jo IJ, Bae GS, Choi SB, Kim DG, Shin JY,
Seo SH, Choi MO, Kim TH, Song HJ and Park SJ: Fisetin attenuates
cerulein-induced acute pancreatitis through down regulation of JNK
and NF-κB signaling pathways. Eur J Pharmacol. 737:149–158.
2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Jiang CY and Wang W: Resistin aggravates
the expression of proinflammatory cytokines in ceruleinstimulated
AR42J pancreatic acinar cells. Mol Med Rep. 15:502–506.
2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhu M, Xu Y, Zhang W, Gu T and Wang D:
Inhibition of PAK1 alleviates cerulein-induced acute pancreatitis
via p38 and NF-κB pathways. Biosci Rep.
39(BSR20182221)2019.PubMed/NCBI View Article : Google Scholar
|