1. Introduction
Ginseng, a perennial plant belonging to genus
Panax, has been widely used in traditional herbal medicine
in east Asia and North America for millennia to reinforce immunity,
provide nutrition and reduce fatigue (1,2).
Ginsenosides are unique triterpenoid saponins predominantly
extracted from Panax ginseng C.A. Meyer that act as the main
bioactive constituents of ginseng (3,4). To
date, >100 ginsenosides have been extracted from the roots,
leaves, stems, fruits and flower heads of ginseng (3). All ginsenosides share a common
four-ring hydrophobic structure, but differ in the number and types
of glycosyl (5). Ginsenosides are
classified into 20(S)-protopanaxadiol, 20(S)-protopanaxatriol
saponins and oleanoic acid ginsenosides (6,7). The
variability of attached positions, inner and outer residues, and
types of sugar moieties may be associated with the specific
pharmacological activities of different ginsenosides (4). Glycosylated major ginsenosides, such
as Rb1, Rb2, Rc, Rd, Re and Rg1, constitute >80% of the total
ginsenosides in various parts of ginseng (8). Deglycosylated minor ginsenosides,
which have fewer sugar moieties attached on aglycon, are absent or
present in smaller amounts in wild ginseng (9,10).
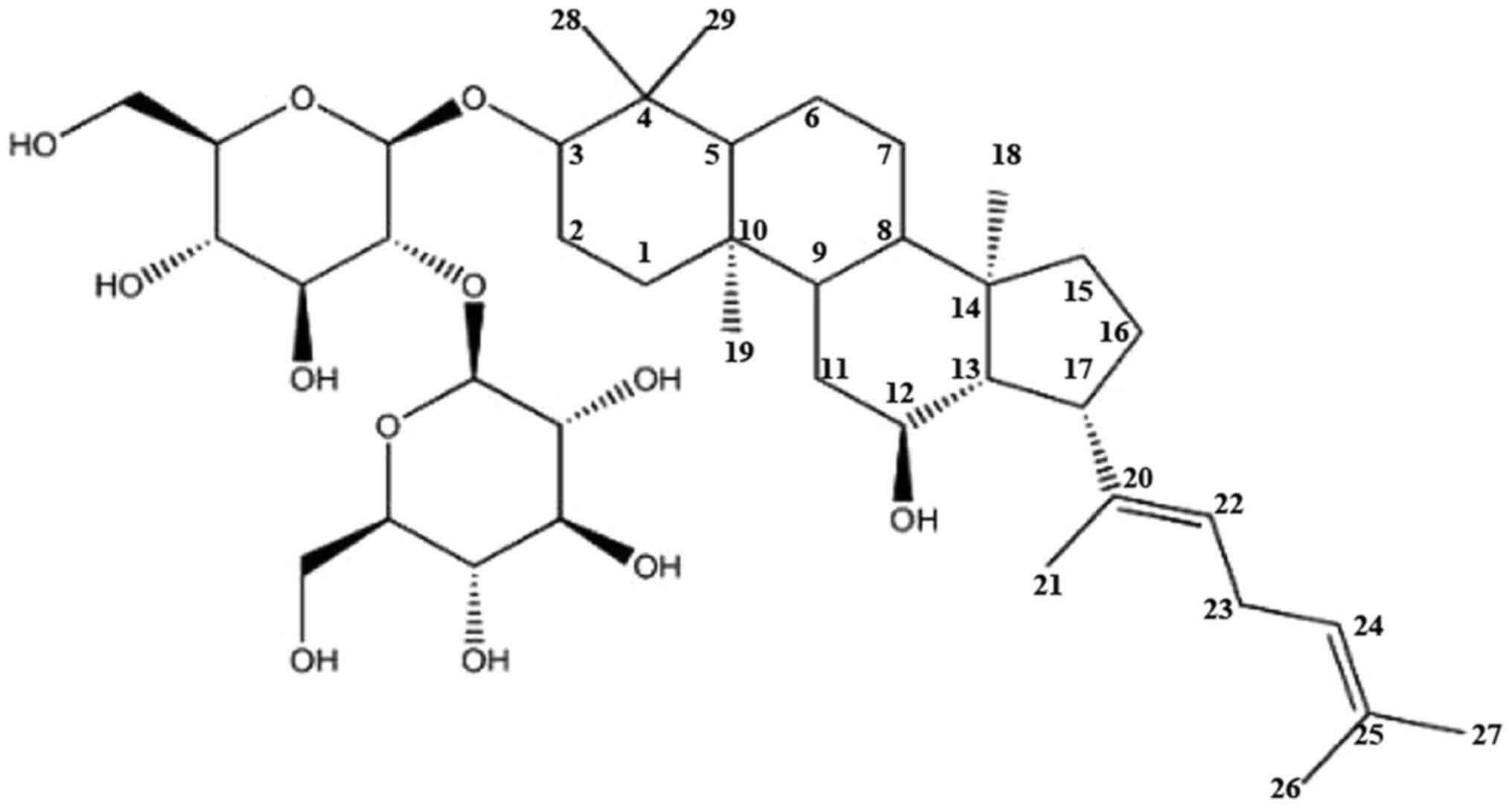
Ginsenoside Rg5 (Rg5) is a minor ginsenoside
synthesized during ginseng steaming treatment; the structural
formula is displayed in Fig. 1
(11). It is obtained by the
deglycosylation of ginsenoside Rb1 and dehydration of carbon at
position 20 of ginsenoside Rg3, and exhibits superior
pharmaceutical effect compared with major ginsenosides (12,13).
In previous studies, Rg5 was found to exert multiple
pharmacological effects, such as antitumor, anti-inflammatory,
antidiabetic, anti-osteoarthritis (OA), neuroprotective and
cardioprotective properties (14-20).
With high safety and various biological functions, Rg5 has the
potential to act as a potential therapeutic candidate for diverse
diseases. The present article reviewed the Rg5 literature and
summarized its pharmacological activities.
2. Pharmacological activities of Rg5
Anticancer effects
Cancer is a group of life-threatening diseases that
are characterized by abnormal proliferation of cells with potential
to invade and spread to surrounding and distant tissues.
Conventional therapies for cancer include surgical resection,
radiotherapy and chemotherapy (21,22).
Chemotherapy serves an important role in the treatment of malignant
tumors (23). However, the severe
side effects of traditional chemotherapy, such as myelosuppression
and immune suppression, hinder the therapeutic effects (24). The use of ginsenosides as
alternative antitumor agents has gained increasing attention
(25,26). Several studies have reported the
antitumor effects on human gastric and breast cancer of Rg5, which
are mainly associated with promoting apoptosis, autophagy and cell
cycle arrest (15-18).
Breast cancer is a major health risk for the adult
female population. The mechanism underlying the effects of Rg5 on
breast cancer has been investigated in vivo and in
vitro. Kim and Kim (15)
demonstrated that Rg5 promoted breast cancer cell apoptosis via
downregulation of the Bax/Bcl-2 pathway. It was also demonstrated
that Rg5 inhibited breast cancer cell proliferation by arresting
the cell cycle at the G0/G1 phase by promoting the expression of
p53, p21WAF1/CIP1 and p15INK4B and inhibiting
the expression levels of cyclin D1, cyclin E2 and CDK4(15). Moreover, it was discovered that Rg5
exhibited improved pro-apoptotic effects on human breast cancer
cell lines compared with ginsenoside Rg3(15). Zou and Liu (16) reported that Rg5 exhibited
antiproliferative effects against breast cancer cells by activating
the AMPK pathway. More recently, Liu et al (14) discovered that Rg5 induced breast
cancer cell apoptosis and autophagy by inhibiting the PI3K/Akt/mTOR
signaling pathway. In addition, Kim et al (13) demonstrated that Rg5 inhibited the
growth of tumors in breast cancer mouse models by promoting tumor
cell autophagy and apoptosis without damaging the normal functions
of major organs and immune cells, which indicated that Rg5 exerts
effects against breast cancer in vivo (17).
The effects and associated mechanisms underlying Rg5
in the treatment of digestive system cancer have also been
reported. Liu and Fan (18)
investigated the anticancer activity of ginsenoside in human
gastric cancer cell lines and suggested that Rg5 inhibited cell
proliferation by inducing G2/M phase arrest, apoptosis and
autophagy by activating reactive oxygen species (ROS)-mediated MAPK
pathways. Moreover, in a human xenograft nude mouse model, Rg5
displayed significant effects against gastric cancer with few side
effects (18). Zhang et al
(27) revealed that Rg5 suppressed
proliferation and promoted the apoptosis of human esophageal cancer
cells, which was associated with inhibition of the PI3K/Akt
signaling pathway. Wang et al (28) demonstrated that Rg5 bound to annexin
A2, and inhibited the interaction between annexin A2 and NF-κB p50
subunit, which resulted in the promotion of caspase activation and
NF-κB activity in human hepatoma HepG2 cells. Lee et al
(29) reported that Rg5 arrested
the cell cycle of human hepatoma SK-HEP-1 cells at the G1/S
transition phase through cyclin E-dependent protein kinase activity
by increasing the expression of p21Cip/WAF1 and reducing
cyclin E.
Additionally, Rg5 promotes apoptosis in
retinoblastoma cells by inhibiting the Akt signaling pathway and
thereby downregulating Bcl-2 expression (30). Rg5 also promotes human cervical
cancer cell apoptosis in concentration- and time-dependent manners
by inducing DNA double-strand breaks and fragments (11). Rg5 has also been demonstrated to
effectively overcome ATP binding cassette subfamily B member 1
(ABCB1)-mediated drug resistance by inhibiting ABCB1 transporter
and blocking the activation of Akt/nuclear-related factor 2
pathways without affecting the expression of ABCB1 transporter
(31). Therefore, Rg5 may serve as
a chemosensitizer for reversing multidrug resistance.
With the development of nanomedicine, the antitumor
effects of Rg5 through biocompatible nanoscale drug delivery
systems has also been investigated (32). Dong et al (32) used folic acid (FA)-modified bovine
serum albumin (BSA) nanoparticles (FA-Rg5-BSA NPs) as carriers to
entrap Rg5. It was discovered that the FA-Rg5-BSA NPs exhibited
superior anticancer activity compared with Rg5 in MCF-7 cells with
low cytotoxicity to L929 cells. The FA-Rg5-BSA NPs facilitated
cellular uptake and induced apoptosis in MCF-7 cells. Furthermore,
an in vivo antitumor study demonstrated that FA-Rg5-BSA NPs
were more effective in reducing tumor growth than Rg5 and Rg5-BSA
NPs in an MCF-7 xenograft mouse model. This in vivo
real-time bioimaging study demonstrated that the FA-Rg5-BSA NPs had
an advanced ability to accumulate in tumors. These results
indicated that FA-Rg5-BSA NPs has the potential to serve as an
attractive therapeutic strategy for the management of cancer
(32).
Anti-inflammatory effects
Inflammation is defined as a defensive mechanism
characterized by the release of proinflammatory cytokines and the
transmigration of inflammatory cells to protect human bodies from
harmful stimuli (19,20). Excessive or abnormal
inflammatory responses have the potential to lead to various
diseases, such as organ failure, central nerve system injury,
tissue damage and even death (33).
Thus, regulating the expression of inflammatory mediators should be
beneficial for the treatment of inflammation-related diseases
(13,34). A number of studies have reported
that Rg5 exerted anti-inflammatory effects in inflammatory
responses by inhibiting the production of proinflammatory
cytokines, such as TNF-α and IL-1β by interfering NF-κB or the
NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3)
inflammasome pathways (35-40).
It has been demonstrated that Rg5 exhibited
protective effects on major organs through its anti-inflammatory
mechanisms. Kim et al (35)
suggested that Rg5 ameliorates lung inflammation in mice by
blocking the binding of lipopolysaccharide (LPS) to Toll-like
receptor (TLR)-4 on macrophages, which are associated with
inhibition of NF-κB activation. Park et al (36) demonstrated that Rg5 exerted
protective effects against cisplatin-induced renal damage by
attenuating JNK/p53/caspase-3 cascade-mediated inflammation. Li
et al (37) demonstrated
that Rg5 attenuated renal dysfunction by reducing the expression of
inflammatory mediators, including NF-κB, p65 and cyclooxygenase-2
(COX-2). Similarly, Lee (38)
reported that Rg5 also decreased the expression of NF-κB, inducible
nitric oxide (NO) synthase (iNOS) and COX-2 in HepG2 cells treated
with TNF-α and thereby acted as a potential anti-inflammatory agent
against hepatitis. The anti-inflammatory activity of Rg5 was
increased when compared with ginsenoside Rb1, Rd and Rg3 and this
increased bioactivity of Rg5 was hypothesized to be due to the
higher lipophilicity compared with Rb1, Rd and Rg3(38). Rg5 also ameliorated acetaminophen
(APAP)-induced liver injury by suppressing APAP-induced expression
of the inflammatory cytokines, TNF-α and IL-1β (39). Moreover, Rg5 demonstrated protective
effects in high-fat diet/streptozotocin-induced diabetic
nephropathy mice and improved renal injury by attenuating oxidative
stress and inflammatory states by suppressing ROS-mediated
activation of NLRP3 inflammasome, p38 MAPK and NF-κB signaling
pathways in the kidneys of diabetic nephropathy mice (40).
Sepsis is a systemic inflammatory response syndrome
caused by the body's response to infection (41). High mobility group box 1 (HMGB1) is
regarded as a crucial mediator of sepsis (42). The suppression of HMGB1-induced
inflammatory reactions and maintenance of endothelial integrity
have served as promising therapeutic strategies for the treatment
of sepsis (43). Kim et al
(44) demonstrated that Rg5
suppressed the release of HMGB1 in LPS-activated human umbilical
vein endothelial cells (HUVECs). Moreover, Rg5 inhibited the
adhesion and migration of leukocytes toward HUVECs. The
aforementioned study indicated that Rg5 may be a potential
therapeutic option for the treatment of severe vascular
inflammatory diseases, such as sepsis and septic shock.
Rg5 has also demonstrated anti-inflammatory
properties in dermal diseases (45,46).
Shin et al (45)
demonstrated the inhibitory effects of Rg5 and its metabolite
ginsenoside Rh3 in oxazolone-induced mouse ear contact dermatitis
by inhibiting the expression of COX-2, TNF-α and IL-1β produced by
macrophage cells and IFN-γ produced by Thl cells. Ahn et al
(46) discovered that Rg5 had
anti-inflammatory effects in two atopic dermatitis-related cell
lines. LPS-induced production of NO and ROS was downregulated by
Rg5 in RAW264.7 cells, indicating that Rg5 has the ability to
improve chronic inflammatory skin disease by blocking the
NF-κB/p38/MAPK/STAT1 signaling pathways.
Anti-inflammatory effects of Rg5 in the central
neural system have also been demonstrated. Chu et al
(47) reported that Rg5
significantly suppressed the expression of pro-inflammation-related
cytokines, including IL-1β, TNF-α, COX-2 and iNOS, and thereby
attenuated neuroinflammatory responses in STZ-induced
memory-impaired rats. Rg5 was also revealed to relieve cerebral
ischemic injury by decreasing NF-κB transcriptional activity and
the expression of proinflammatory cytokines, such as IL-1β, TNF-α
and IL-6, by activating TLR4/MyD88 and sirtuin 1 signaling
pathways, contributing to reductions in cerebral ischemic injury
(48). In addition, Lee et
al (49) demonstrated that Rg5
suppressed neuroinflammation induced by LPS in BV2 microglial cells
by inhibiting the MAPK and PI3K/Akt pathways.
Neuroprotective effects
Neurodegenerative diseases have become another
category of health-threatening diseases (50). Rg5 exerts beneficial effects on
nervous system diseases, such as Alzheimer's disease (AD) and
Huntington's disease (HD) (51-53).
AD is a multifactorial neurodegenerative disease featuring
extracellular β-amyloid (Aβ) plaques and intracellular
neurofibrillary tangles in the brain (51). Inhibition of cAMP response
element-binding protein (CREB) and brain-derived neurotrophic
factor (BDNF) has the potential to lead to memory deficits in
patients with AD (52,53). Kim et al (54) revealed that Rg5 significantly
reversed memory deficits induced by acetylcholinesterase using the
passive avoidance, Y-maze and Morris water maze tasks in mice. The
research revealed that treatment with Rg5 ameliorated the reduction
of BDNF expression and CREB phosphorylation induced by scopolamine
(54). Chu et al (47) demonstrated that Rg5 improves
cognitive dysfunction in streptozotocin-induced AD rats by
modulating the cholinergic system, decreasing Aβ deposition and
promoting the expression levels of neurotrophic factors BDNF and
insulin-like growth factor 1 (IGF-1). Moreover, Rg5 has significant
ameliorative effects on STZ-induced neuroinflammatory responses
(47). Choi et al (55) revealed that Rg5 suppressed thermal
stress-induced cell cycle arrest at G1/S phase by activating p21
and poly(ADP-ribose) polymerase cleavage. CREB and BDNF were also
increased by Rg5 in thermal stress-exposed HT22 cells (55). HD is an autosomal-dominant
neurogenic disorder that leads to progressive nerve cell damage in
the brain. Wu et al (56)
demonstrated Rg5 attenuates neuronal apoptosis by inhibiting
glutamate-induced increases of Ca2+ concentrations in
cultured medium spiny neurons, which indicated Rg5 has the
potential to be useful for HD therapy. Wu et al (56) further discovered that the inhibitory
activity of Rg5 on glutamate-induced Ca2+ responses was
similar to ginsenoside Rc and far greater than ginsenoside Re.
In addition, an in vivo study revealed that
Rg5 may regulate nerve transmission by affecting neurotransmitter
and neuroregulatory receptors (57). Glutamate (Glu) is known as a major
excitatory neurotransmitter, whereas γ-aminobutyric acid (GABA) is
well-known as a major inhibitory neurotransmitter in the CNS
(58,59). Shao et al (57) suggested that Rg5 downregulates the
GABA/Glu ratio, and augments the expression of GABAA and
GABAB receptors. Serotonin (5-HT) is a neurotransmitter
involved in sleep-wake cycle regulation (60). Shao et al (57) demonstrated that 5-hydroxytryptophan,
together with a precursor of 5-HT, promotes the sleep effects of
Rg5 in mice and Rg5 augments the expression of 5-HT1A. The results
indicated that Rg5 exhibited hypotensive and sedative activities by
modulating GABA and serotonin signaling in the nervous system, thus
ameliorating sleep in mice models (57).
Cardioprotective effects
Studies into the therapeutic effects of Rg5 on
cardiovascular diseases have also been reported. Cho et al
(61) demonstrated that Rg5
regulates neovascularization and vasorelaxation by activating IGF-1
receptor (IGF-1R). The angiogenic activity of Rg5 is highly
associated with a specific increase in IGF-1R phosphorylation and
the subsequent activation of multiple angiogenic signals (61). Furthermore, the vasodilative
activity of Rg5 is mediated by the endothelial NOS/NO/cGMP pathway
(61). These findings offer a
mechanistic explanation of the beneficial effects of Rg5 on
neovascularization and endothelial function under pathological
conditions. Yang et al (62)
reported that Rg5 increased cardiomyocyte resistance to ischemic
injury by regulating the translocation of two important enzymes,
hexokinase-II (HK-II) and dynamin-related protein 1 (Drp1). Drp1
and HK-II exert opposite effects on mitochondrial function in
cardiomyocytes by competing for binding to mitochondria (62). Rg5 protects mitochondrial
morphological and functional integrity by suppressing Drp1
activation and increasing HK-II binding to cardiomyocyte
mitochondria through Akt activation (62). These results provide a rationale for
utilizing Rg5 for treating cardiovascular diseases.
Anti-OA and anti-osteoporosis (OP)
effects
OA and OP are common bone diseases in middle-aged
and elderly populations. OA involves a series of complicated
processes characterized by the destruction of chondrocytes and
remodeling of subchondral bones, resulting in progressive
joint degeneration (63). The
regulation of inflammatory cytokine networks by ginsenosides has
attracted increased attention for the treatment of OA (64,65).
Zhang (66) revealed that Rg5
prevented articular cartilage degradation and inhibited synovium
disintegration in OA rat models. The level of OA-related enzyme
metalloproteinase-13 decreased to 45% compared with controls;
tissue inhibitors of metalloproteinase-1 increased by 67% after
treatment with Rg5. The levels of inflammatory mediators, such as
IL-1β, TNF-α, NO and iNOS, decreased by 67, 54, 32 and 49%,
respectively, after 1 month of treatment with Rg5(66). The expression of bone morphogenetic
protein-2 (BMP-2) and TGF-β1 increased to 67 and 52%, respectively,
after treatment with Rg5. Therefore, Zhang (66) considered Rg5 useful for OA
therapy.
OP systemically decreases bone mass and strength,
and is characterized by the disturbance of osteoblast activity
(67). Siddiqi et al
(68) demonstrated that Rg5/Rk1
stimulates osteoblast cell growth and promotes the expression of
osteoblastic markers, such as alkaline phosphatase activity and
type I collagen content, BMP-2 and calcium deposition in
dose-dependent manners. Moreover, Rg5/Rk1 also stimulates the mRNA
expression of Runt-related transcription factor 2 (Runx2) and
osteocalcin (68). These results
indicate that Rg5/Rk1 has the potential to prevent OP by
stimulating osteoblast proliferation and differentiation via the
BMP-2/Runx2 signaling pathway.
Antidiabetic and anti-obesity
effects
The antidiabetic effect of Rg5 can be attributed to
the amelioration of insulin resistance and reduction of glucagon
response (67-72).
A high level of insulin is required to regulate blood glucose under
insulin resistance conditions. During endoplasmic reticulum stress,
the Rk1/Rg5 ginsenoside complex was found to improve insulin
sensitivity and increase glucose uptake to exert protective effects
in 3T3-L1 cells through CHOP-mediated glucose transporter 4
translocation (69). Furthermore,
Xiao et al (71) discovered
that Rg5 inhibited succinate-associated lipolysis by reducing
cellular energy charge and effectively prevented insulin resistance
in muscle by reducing lipid deposits. The inhibitory effects of Rg5
in hepatic glucagon response have also been demonstrated (70). Xiao et al (70) revealed that Rg5 decreased succinate
accumulation by suppressing hepatic fatty acid oxidation and cAMP
accumulation by blocking succinate/hypoxia-inducible factor-1α
expression, leading to an attenuated hepatic glucagon response.
Ginsenosides have also been widely reported to have
an anti-obesity effect (73-76)
and the anti-obesity effect of Rg5 has been reported in
vitro. Yesmin Simu et al (77) demonstrated that Rg5/Rk1 inhibited
lipid droplet accumulation and decreased triglyceride content in
3T3-L1 adipocyte cells. The expression levels of STAT3, peroxisome
proliferator-activated receptor (PPAR)γ, CCAAT/enhancer-binding
protein (CEBP)α and adaptor protein complex were also reduced in
dose-dependent manners after treatment with Rg5/Rk1. Furthermore,
Yesmin Simu et al (77)
reported no significant cytotoxicity effects on 3T3-L1 cells up to
100 µg/ml. Their results indicated that Rg5 may have therapeutic
potential for treating obesity via the STAT3/PPARγ/CEBPα signaling
pathway.
3. Conclusion
The current review summarized the pharmacological
effects of Rg5. In general, Rg5 has substantial potential activity
for use as a broad-spectrum anticancer and anti-inflammatory drug.
Rg5 has been reported to exert several positive effects on the
nervous system, which potentiate the clinical applications of Rg5
in the treatment of neurodegenerative diseases. Additional studies
have investigated other pharmacological properties, such as
cardioprotective, anti-OA, anti-OP, antidiabetic and anti-obesity
effects. The biological activities of Rg5 have been widely
investigated and the mechanisms underlying the actions of Rg5 based
on the existing studies are summarized in Table I and Fig. 2. These optimized therapies should
also be evaluated for their efficacies in vivo. It may be
possible to develop novel Rg5 analogues with improved efficacy,
pharmacokinetics and bioavailability profiles. Evidence of Rg5
efficacy has yet to be demonstrated in humans. There is a
significant need to perform larger cohort clinical studies to
confirm Rg5 efficacy for improved applications in the clinic. With
this considered, further investigations in clinical trials are
highly recommended to provide more reliable evidence for the
clinical efficacy of Rg5.
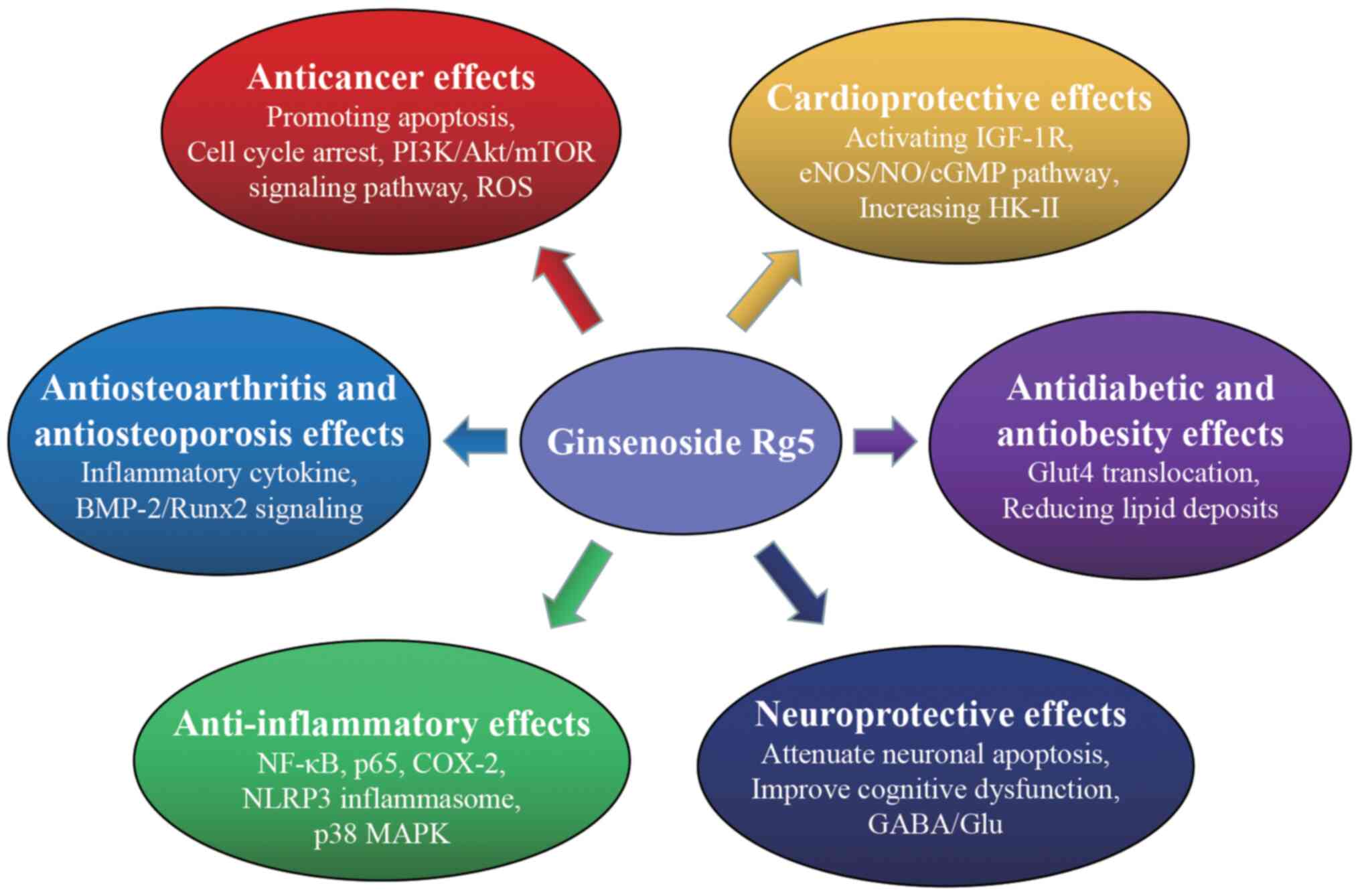 | Figure 2Potential mechanisms of
pharmacological activities of ginsenoside Rg5. ROS, reactive oxygen
species; IGF-1R, insulin-like growth factor; COX-2,
cyclooxygenase-2; BMP-2, bone morphogenetic protein 2; Runx2,
runt-related transcription factor 2; Glut4, glucose transporter 4;
NO, nitric oxide; eNOS, endothelial NO synthase; HK-II,
hexokinase-II; GABA, γ-aminobutyric acid; Glu, glutamate. |
 | Table ISummary of the pharmacological
activities of Rg5. |
Table I
Summary of the pharmacological
activities of Rg5.
| A,
Anti-inflammation |
|---|
| First author,
year | Model | Effects | (Refs.) |
|---|
| Zhu et al,
2020 | DN mice | Rg5 attenuates
oxidative stress and inflammatory states in HFD/STZ-induced DN mice
by inactivating p38 MAPK and NF-κB signaling pathways | (40) |
| Kim et al,
2019 | HUVECs | Rg5/Rk1 reduces the
secretion of HMGB1, and the adhesion and migration of leukocytes
toward HUVECs | (44) |
| Kim et al,
2019 | Male C57BL/6
mice | Rg5 reduces
CLP-induced mortality and pulmonary injury | (44) |
| Yang et al,
2017 | I/R rats | Rg5 reduces TNF-α,
IL-6, and IL-1β tissue levels in I/R rats | (62) |
| Wang et al,
2018 | Male ICR mice | Rg5 protects
against oxidative/nitrative stress injury, inflammation and
apoptosis in APAP-induced hepatotoxicity | (28) |
| Li et al,
2016 | Male ICR mice | Rg5 attenuates
oxidative stress, suppresses inflammation and inhibits apoptosis in
cisplatin-treated kidney cells | (37) |
| Ahn et al,
2016 | HaCaT cells | Rg5/Rk1 suppresses
NF-κB/p38 MAPK/STAT1 signaling | (46) |
| Ahn et al,
2016 | RAW264.7 cells | Rg5/Rk1 suppresses
NF-κB/p38 MAPK/STAT1 signaling | (46) |
| Park et al,
2015 | LLC-PK1 cells | Rg5 ameliorates
renal cell damage by inhibiting inflammation and preventing
apoptosis | (36) |
| Lee et al,
2013 | BV2 microglial
cells | Rg5 exhibits
anti-inflammatory effects in LPS-stimulated microglia | (49) |
| Kim et al,
2017 | Male C57BL/6
mice | Rg5 ameliorates
lung inflammation via downregulation of NF-κB activation by
inhibiting binding of LPS to TLR4 on macrophages | (13) |
| Shin et al,
2006 | Female ICR
mice | Rg5 improves
chronic dermatitis or psoriasis in oxazolone-induced ICR mice via
downregulation of IL-lβ, TNF-α and IFN-γ production | (45) |
| Chu et al,
2014 | Male Wistar
rats | Rg5 induces
amelioration of STZ-induced neuroinflammatory responses | (47) |
| B,
Neuroprotection |
| First author,
year | Model | Effects | (Refs.) |
| Shao et al,
2018 | Male Kunming mice,
male Wistar rats | Rg5 exerts sedative
and hypnotic effects by affecting GABA and serotonin signaling | (57) |
| Choi et al,
1994 | HT22 cells | Rg5 inhibits
thermal stress-induced apoptosis in HT22 cells | (55) |
| Chu et al,
2014 | Wistar rats | Rg5 alleviates
cognitive dysfunction in STZ-induced AD rats by regulating
cholinergic signaling, attenuating Aβ deposition and increasing
neurotrophic factor expression | (47) |
| Kim et al,
2013 | Male ICR mice | Rg5/Rh3 protects
against memory deficits by inhibiting AChE activity, and increasing
BDNF expression and CREB activation | (54) |
| Wu et al,
2009 | YAC128 mice | Rg5 protects
striatal neurons via inhibition of Ca2+ signaling | (56) |
| C,
Cardioprotection |
| First author,
year | Model | Effects | (Refs.) |
| Yang et al,
2017 | Male ICR mice | Rg5 protects
mitochondrial morphological and functional integrity by regulating
HK-II and Drp1 translocation via Akt activation | (62) |
| Cho et al,
2015 | HUVECs | Rg5 promotes
angiogenesis and vasorelaxation by activating signal transduction
pathways downstream of IGF-1R | (61) |
| Cho et al,
2015 | C57BL/6J mice | Rg5 promotes
angiogenesis and vasorelaxation by activating signal transduction
pathways downstream of IGF-1R | (61) |
| D,
Anti-osteoarthritis/anti-osteoporosis |
| First author,
year | Model | Effects | (Refs.) |
| Zhang, 2017 | Male Wistar
rats | Rg5 prevents
destruction of articular cartilage via inhibition of chondrocyte
apoptosis and matrix damage in osteoarthritis rats | (66) |
| Siddiqi et
al, 2014 | MC3T3-E1 | Rg5/Rk1 promotes
the function of MC3T3-E1 cells via BMP-2/Runx2 signaling | (68) |
| E,
Antidiabetes/anti-obesity |
| First author,
year | Model | Effects | (Refs.) |
| Ponnuraj et
al, 2014 | 3T3-L1 cells | Rg5/Rk1 ameliorates
insulin sensitivity in 3T3-L1 cells via CHOP signaling | (69) |
| Xiao et al,
2017 | 3T3-L1 cells | Rg5 inhibits
succinate-associated lipolysis via reducing cellular energy charge,
and effectively prevented insulin resistance by reducing lipid
deposits | (70) |
| Xiao et al,
2017 | Male ICR mice | Rg5 inhibits
succinate-associated lipolysis and prevents insulin resistance by
reducing lipid deposition | (70) |
| Xiao et al,
2017 | Male C57BL/6J
mice | Rg5 reduces
succinate accumulation and inhibits hepatic cAMP accumulation | (70) |
| Yesmin Simu et
al, 2017 | 3T3-L1 cells | Rg5/Rk1 exhibits
anti-adipogenic activity via downregulation of STAT3/PPARγ/CEBPα
signaling | (77) |
Acknowledgements
Not applicable.
Funding
Funding: This study was supported in part by the National
Natural Science Foundation of China (grant nos. 81970529 and
81700018), the Jilin Provincial Natural Science Foundation of China
(grant no. 20180101135JC), the Jilin Provincial Finance Foundation
(grant no. 2018SCZWSZX-043), Jilin Provincial Health Commission
Foundation (grant no. 2018Q020), the Interdisciplinary Chemistry
and Medicine Foundation of Jilin University (grant no.
JDYYJCHX004), the Norman Bethune Program of Jilin University (grant
no. 2019007) and the Foundation for The Excellent Youth Scholars of
Jilin University.
Availability of data and materials
Not applicable.
Authors' contributions
MYL and FL wrote the manuscript. YLG and JNY
collected the references and produced the figure. HJL designed,
interpreted and funded the study and revised the manuscript. WQY
and JGL revised the manuscript. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
De Souza LR, Jenkins AL, Sievenpiper JL,
Jovanovski E, Rahelić D and Vuksan V: Korean red ginseng (Panax
ginseng C.A. Meyer) root fractions: Differential effects on
postprandial glycemia in healthy individuals. J Ethnopharmacol.
137:245–250. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhou QL, Zhu DN, Yang YF, Xu W and Yang
XW: Simultaneous quantification of twenty-one ginsenosides and
their three aglycones in rat plasma by a developed UFLC-MS/MS
assay: Application to a pharmacokinetic study of red ginseng. J
Pharm Biomed Anal. 137:1–12. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Christensen LP: Ginsenosides chemistry,
biosynthesis, analysis, and potential health effects. Adv Food Nutr
Res. 55:1–99. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shi W, Wang Y, Li J, Zhang H and Ding L:
Investigation of ginsenosides in different parts and ages of
Panax ginseng. Food Chem. 102:664–668. 2007.
|
|
5
|
Chen RJ, Chung TY, Li FY, Lin NH and Tzen
JTC: Effect of sugar positions in ginsenosides and their inhibitory
potency on Na+/K+-ATPase activity. Acta Pharmacol Sin. 30:61–69.
2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shin BK, Kwon SW and Park JH: Chemical
diversity of ginseng saponins from Panax ginseng. J Ginseng
Res. 39:287–298. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shin KC and Oh DK: Classification of
glycosidases that hydrolyze the specific positions and types of
sugar moieties in ginsenosides. Crit Rev Biotechnol. 36:1036–1049.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jin S, Jeon JH, Lee S, Kang WY, Seong SJ,
Yoon YR, Choi MK and Song IS: Detection of 13 ginsenosides (Rb1,
Rb2, Rc, Rd, Re, Rf, Rg1, Rg3, Rh2, F1, compound K,
20(S)-protopanaxadiol, and 20(S)-protopanaxatriol) in human plasma
and application of the analytical method to human pharmacokinetic
studies following two week-repeated administration of red ginseng
extract. Molecules. 24(2618)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cui CH, Jeon BM, Fu Y, Im WT and Kim SC:
High-density immobilization of a ginsenoside-transforming
β-glucosidase for enhanced food-grade production of minor
ginsenosides. Appl Microbiol Biotechnol. 103:7003–7015.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Noh KH and Oh DK: Production of the rare
ginsenosides compound K, compound Y, and compound Mc by a
thermostable beta-glycosidase from sulfolobus acidocaldarius. Biol
Pharm Bull. 32:1830–1835. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liang LD, He T, Du TW, Fan YG, Chen DS and
Wang Y: Ginsenoside-Rg5 induces apoptosis and DNA damage in human
cervical cancer cells. Mol Med Rep. 11:940–946. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Qi LW, Wang CZ and Yuan CS: Ginsenosides
from American ginseng: Chemical and pharmacological diversity.
Phytochemistry. 72:689–699. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim JH, Yi YS, Kim MY and Cho JY: Role of
ginsenosides, the main active components of Panax ginseng,
in inflammatory responses and diseases. J Ginseng Res. 41:435–443.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu Y and Fan D: The preparation of
ginsenoside Rg5, its antitumor activity against breast cancer cells
and its targeting of PI3K. Nutrients. 12(246)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim SJ and Kim AK: Anti-breast cancer
activity of fine black ginseng (Panax ginseng Meyer) and
ginsenoside Rg5. J Ginseng Res. 39:125–134. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zou Y and Liu P: Ginsenoside-Rg5 inhibits
proliferation of the breast carcinoma cells through promotion of
the proteins involved in AMP kinase pathway. Int J Clin Exp Med.
9:17664–17669. 2016.
|
|
17
|
Liu Y and Fan D: Ginsenoside Rg5 induces
apoptosis and autophagy via the inhibition of the PI3K/Akt pathway
against breast cancer in a mouse model. Food Funct. 9:5513–5527.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu Y and Fan D: Ginsenoside Rg5 induces
G2/M phase arrest, apoptosis and autophagy via regulating
ROS-mediated MAPK pathways against human gastric cancer. Biochem
Pharmacol. 168:285–304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jin HS, Suh HW, Kim SJ and Jo EK:
Mitochondrial control of innate immunity and inflammation. Immune
Netw. 17:77–88. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Medzhitov R: Origin and physiological
roles of inflammation. Nature. 454:428–435. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Harrison DJ, Geller DS, Gill JD, Lewis VO
and Gorlick R: Current and future therapeutic approaches for
osteosarcoma. Expert Rev Anticancer Ther. 18:39–50. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Akram M, Iqbal M, Daniyal M and Khan AU:
Awareness and current knowledge of breast cancer. Biol Res.
50(33)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sun M, Ye Y, Xiao L, Duan X, Zhang Y and
Zhang H: Anticancer effects of ginsenoside Rg3 (Review). Int J Mol
Med. 39:507–518. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Baldo BA and Pham NH: Adverse reactions to
targeted and non-targeted chemotherapeutic drugs with emphasis on
hypersensitivity responses and the invasive metastatic switch.
Cancer Metastasis Rev. 32:723–761. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Majeed F, Malik FZ, Ahmed Z, Afreen A,
Afzal MN and Khalid N: Ginseng phytochemicals as therapeutics in
oncology: Recent perspectives. Biomed Pharmacother. 100:52–63.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nakhjavani M, Hardingham JE, Palethorpe
HM, Tomita Y, Smith E, Price TJ and Townsend AR: Ginsenoside Rg3:
Potential molecular targets and therapeutic indication in
metastatic breast cancer. Medicines (Basel). 6(17)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang D, Wang A, Feng J, Zhang Q, Liu L
and Ren H: Ginsenoside Rg5 induces apoptosis in human esophageal
cancer cells through the phosphoinositide-3 kinase/protein kinase B
signaling pathway. Mol Med Rep. 19:4019–4026. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang YS, Li H, Li Y, Zhu H and Jin YH:
Identification of natural compounds targeting annexin A2 with an
anti-cancer effect. Protein Cell. 9:568–579. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lee KH, Lee YH, Kim SI, Park JH and Lee
SK: Ginsenoside-Rg5 suppresses cyclin E-dependent protein kinase
activity via up-regulating p21Cip/WAF1 and down-regulating cyclin E
in SK-HEP-1 cells. Anticancer Res. 17:1067–1072. 1997.PubMed/NCBI
|
|
30
|
Cui Y, Su Y, Deng L and Wang W:
Ginsenoside-Rg5 inhibits retinoblastoma proliferation and induces
apoptosis through suppressing BCL2 expression. Chemotherapy.
63:293–300. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Feng SL, Luo HB, Cai L, Zhang J, Wang D,
Chen YJ, Zhan HX, Jiang ZH and Xie Y: Ginsenoside Rg5 overcomes
chemotherapeutic multidrug resistance mediated by ABCB1
transporter: In vitro and in vivo study. J Ginseng Res. 44:247–257.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Dong Y, Fu R, Yang J, Ma P, Liang L, Mi Y
and Fan D: Folic acid-modified ginsenoside Rg5-loaded bovine serum
albumin nanoparticles for targeted cancer therapy in vitro and in
vivo. Int J Nanomedicine. 14:6971–6988. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bortolotti P, Faure E and Kipnis E:
Inflammasomes in tissue damages and immune disorders after trauma.
Front Immunol. 9(1900)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yi YS: Roles of ginsenosides in
inflammasome activation. J Ginseng Res. 43:172–178. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kim TW, Joh EH, Kim B and Kim DH:
Ginsenoside Rg5 ameliorates lung inflammation in mice by inhibiting
the binding of LPS to toll-like receptor-4 on macrophages. Int
Immunopharmacol. 12:110–116. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Park JY, Choi P, Kim T, Ko H, Kim HK, Kang
KS and Ham J: Protective effects of processed ginseng and its
active ginsenosides on cisplatin-induced nephrotoxicity: In vitro
and in vivo studies. J Agric Food Chem. 63:5964–5969.
2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Li W, Yan M, Liu Y, Liu Z, Wang Z, Chen C,
Zhang J and Sun YS: Ginsenoside Rg5 ameliorates cisplatin-induced
nephrotoxicity in mice through inhibition of inflammation,
oxidative stress, and apoptosis. Nutrients. 8(566)2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lee SM: Anti-inflammatory effects of
ginsenosides Rg5, Rz1, and Rk1: Inhibition of TNF-α-induced NF-κB,
COX-2, and iNOS transcriptional expression. Phytother Res.
28:1893–1896. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang Z, Hu JN, Yan MH, Xing JJ, Liu WC and
Li W: Caspase-mediated anti-apoptotic effect of ginsenoside Rg5, a
main rare ginsenoside, on acetaminophen-induced hepatotoxicity in
mice. J Agric Food Chem. 65:9226–9236. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhu Y, Zhu C, Yang H, Deng J and Fan D:
Protective effect of ginsenoside Rg5 against kidney injury via
inhibition of NLRP3 inflammasome activation and the MAPK signaling
pathway in high-fat diet/streptozotocin-induced diabetic mice.
Pharmacol Res. 155(104746)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cecconi M, Evans L, Levy M and Rhodes A:
Sepsis and septic shock. Lancet. 392:75–87. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Andersson U, Wang H, Palmblad K, Aveberger
AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M,
Yang H and Tracey KJ: High mobility group 1 protein (HMG-1)
stimulates proinflammatory cytokine synthesis in human monocytes. J
Exp Med. 192:565–570. 2000.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Bae JS: Role of high mobility group box 1
in inflammatory disease: Focus on sepsis. Arch Pharm Res.
35:1511–1523. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kim JE, Lee W, Yang S, Cho SH, Baek MC,
Song GY and Bae JS: Suppressive effects of rare ginsenosides, Rk1
and Rg5, on HMGB1-mediated septic responses. Food Chem Toxicol.
124:45–53. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Shin YW, Bae EA and Kim DH: Inhibitory
effect of ginsenoside Rg5 and its metabolite ginsenoside Rh3 in an
oxazolone-induced mouse chronic dermatitis model. Arch Pharm Res.
29:685–690. 2006.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ahn S, Siddiqi MH, Aceituno VC, Simu SY,
Zhang J, Jimenez Perez ZE, Kim YJ and Yang DC: Ginsenoside Rg5:Rk1
attenuates TNF-α/IFN-γ-induced production of thymus- and
activation-regulated chemokine (TARC/CCL17) and LPS-induced NO
production via downregulation of NF-κB/p38 MAPK/STAT1 signaling in
human keratinocytes and macrophages. In Vitro Cell Dev Biol Anim.
52:287–295. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chu S, Gu J, Feng L, Liu J, Zhang M, Jia
X, Liu M and Yang D: Ginsenoside Rg5 improves cognitive dysfunction
and beta-amyloid deposition in STZ-induced memory impaired rats via
attenuating neuroinflammatory responses. Int Immunopharmacol.
19:317–326. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Cheng Z, Zhang M, Ling C, Zhu Y, Ren H,
Hong C, Qin J, Liu T and Wang J: Neuroprotective effects of
ginsenosides against cerebral ischemia. Molecules.
24(1102)2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lee YY, Park JS, Jung JS, Kim DH and Kim
HS: Anti-inflammatory effect of ginsenoside Rg5 in
lipopolysaccharide-stimulated BV2 microglial cells. Int J Mol Sci.
14:9820–9833. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Liu FF, Zhang Z, Chen W, Gu HY and Yan QJ:
Regulatory mechanism of microRNA-377 on CDH13 expression in the
cell model of Alzheimer's disease. Eur Rev Med Pharmacol Sci.
22:2801–2808. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Bolognin S, Blanchard J, Wang X,
Basurto-Islas G, Tung YC, Kohlbrenner E, Grundke-Iqbal I and Iqbal
K: An experimental rat model of sporadic Alzheimer's disease and
rescue of cognitive impairment with a neurotrophic peptide. Acta
Neuropathol. 123:133–151. 2012.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Tao X, Finkbeiner S, Arnold D, Shaywitz A
and Greenberg M: Ca2+ influx regulates BDNF
transcription by a CREB family transcription factor-dependent
mechanism. Neuron. 20:709–726. 1998.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Bourtchuladze R, Frenguelli B, Blendy J,
Cioffi D, Schutz G and Silva A: Deficient long-term memory in mice
with a targeted mutation of the cAMP-responsive element-binding
protein. Cell. 79:59–68. 1994.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Kim EJ, Jung IH, Van Le TK, Jeong JJ, Kim
NJ and Kim DH: Ginsenosides Rg5 and Rh3 protect scopolamine-induced
memory deficits in mice. J Ethnopharmacol. 146:294–299.
2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Choi SY, Kim KJ, Song JH and Lee BY:
Ginsenoside Rg5 prevents apoptosis by modulating
heme-oxygenase-1/nuclear factor E2-related factor 2 signaling and
alters the expression of cognitive impairment-associated genes in
thermal stress-exposed HT22 cells. J Ginseng Res. 42:225–228.
2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wu J, Jeong HK, Bulin SE, Kwon SW, Park JH
and Bezprozvanny I: Ginsenosides protect striatal neurons in a
cellular model of Huntington's disease. J Neurosci Res.
87:1904–1912. 2009.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Shao J, Zheng X, Qu L, Zhang H, Yuan H,
Hui J, Mi Y, Ma P and Fan D: Ginsenoside Rg5/Rk1 ameliorated sleep
via regulating the GABAergic/serotoninergic signaling pathway in a
rodent model. Food Funct. 11:1245–1257. 2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Yuan CS, Attele AS, Wu JA and Liu D:
Modulation of American ginseng on brainstem GABAergic effects in
rats. J Ethnopharmacol. 62:215–222. 1998.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Sattler R and Tymianski M: Molecular
mechanisms of glutamate receptor-mediated excitotoxic neuronal cell
death. Mol Neurobiol. 24:107–129. 2001.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Yuan Q, Joiner WJ and Sehgal A: A
sleep-promoting role for the Drosophila serotonin receptor 1A. Curr
Biol. 16:1051–1062. 2006.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Cho YL, Hur SM, Kim JY, Kim JH, Lee DK,
Choe J, Won MH, Ha KS, Jeoung D, Han S, et al: Specific activation
of insulin-like growth factor-1 receptor by ginsenoside Rg5
promotes angiogenesis and vasorelaxation. J Biol Chem. 290:467–477.
2015.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Yang YL, Li J, Liu K, Zhang L, Liu Q, Liu
B and Qi LW: Ginsenoside Rg5 increases cardiomyocyte resistance to
ischemic injury through regulation of mitochondrial hexokinase-II
and dynamin-related protein 1. Cell Death Dis.
8(e2625)2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Wojdasiewicz P, Poniatowski Ł and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014(561459)2014.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Cheng W, Jing J, Wang Z, Wu D and Huang Y:
Chondroprotective effects of ginsenoside Rg1 in human
osteoarthritis chondrocytes and a rat model of anterior cruciate
ligament transection. Nutrients. 9(263)2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Chen Y, Lin S, Sun Y, Pan X, Xiao L, Zou
L, Ho KW and Li G: Translational potential of ginsenoside Rb1 in
managing progression of osteoarthritis. J Orthop Translat. 6:27–33.
2016.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Zhang P: Ginsenoside-Rg5 treatment
inhibits apoptosis of chondrocytes and degradation of cartilage
matrix in a rat model of osteoarthritis. Oncol Rep. 37:1497–1502.
2017.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Wang T, Liu Q, Tjhioe W, Zhao J, Lu A,
Zhang G, Tan RX, Zhou M, Xu J and Feng HT: Therapeutic potential
and outlook of alternative medicine for osteoporosis. Current Drug
Targets. 18:1051–1068. 2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Siddiqi MH, Siddiqi MZ, Ahn S, Kang S, Kim
YJ, Veerappan K, Yang DU and Yang DC: Stimulative effect of
ginsenosides Rg5:Rk1 on murine osteoblastic MC3T3-E1 cells.
Phytother Res. 28:1447–1455. 2014.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Ponnuraj SP, Siraj F, Kang S, Noh HY, Min
JW, Kim YJ and Yang DC: Amelioration of insulin resistance by Rk1 +
Rg5 complex under endoplasmic reticulum stress conditions.
Pharmacognosy Res. 6:292–296. 2014.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Xiao N, Lou MD, Lu YT, Yang LL, Liu Q, Liu
B, Qi LW and Li P: Ginsenoside Rg5 attenuates hepatic glucagon
response via suppression of succinate-associated HIF-1α induction
in HFD-fed mice. Diabetologia. 60:1084–1093. 2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Xiao N, Yang LL, Yang YL, Liu LW, Li J,
Liu B, Liu K, Qi LW and Li P: Ginsenoside Rg5 inhibits
succinate-associated lipolysis in adipose tissue and prevents
muscle insulin resistance. Front Pharmacol. 8(43)2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Bai L, Gao J, Wei F, Zhao J, Wang D and
Wei J: Therapeutic potential of ginsenosides as an adjuvant
treatment for diabetes. Front Pharmacol. 9(423)2018.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Park HJ, Kim JH and Shim I: Anti-obesity
effects of ginsenosides in high-fat diet-fed rats. Chin J Integr
Med. 25:895–901. 2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Liu H, Liu M, Jin Z, Yaqoob S, Zheng M,
Cai D, Liu J and Guo S: Ginsenoside Rg2 inhibits adipogenesis in
3T3-L1 preadipocytes and suppresses obesity in
high-fat-diet-induced obese mice through the AMPK pathway. Food
Funct. 10:3603–3614. 2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Liu H, Wang J, Liu M, Zhao H, Yaqoob S,
Zheng M, Cai D and Liu J: Antiobesity effects of ginsenoside Rg1 on
3T3-L1 preadipocytes and high fat diet-induced obese mice mediated
by AMPK. Nutrients. 10(830)2018.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Zhang L, Zhang L, Wang X and Si H:
Anti-adipogenic effects and mechanisms of ginsenoside Rg3 in
pre-adipocytes and obese mice. Front Pharmacol.
8(113)2017.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Yesmin Simu S, Ahn S, Castro-Aceituno V
and Yang DC: Ginsenoside Rg5: Rk1 exerts an anti-obesity effect on
3T3-L1 Cell Line by the downregulation of PPARγ and CEBPα. Iran J
Biotechnol. 15:252–259. 2017.PubMed/NCBI View Article : Google Scholar
|