Introduction
Stroke has become the main clinical type of
cerebrovascular disease, which is a type of disorder of blood
circulation in brain tissues (1).
Pathologically, stroke can be divided into ischemic stroke and
hemorrhagic stroke (2). More than
80% of the global burden of stroke is attributed to ischemic stroke
(3). Ischemic strokes often present
with high rates of incidence, recurrence, disability and mortality
for patients (4). In 2008, an
epidemiological survey indicated that strokes, with an incidence of
136.64 per 100,000 individuals, had replaced cancer as the leading
cause of mortality in China (5). At
present, intravenous thrombolytic therapy is the main clinical
treatment for ischemic stroke (1,6), and
there is still a lack of effective drugs to protect neurons from
death. Therefore, there is a need for multi-target and improved
therapeutic drugs, which is why the beneficial effects of
Traditional Chinese Medicine (TCM) is worth investigating (7).
Danshen and Honghua (Danhong) are classic
blood-activating drugs often used for promoting blood circulation
and believed to remove blood stasis in TCM. They have a long
history in the treatment of cardiovascular and cerebrovascular
diseases in traditional clinical trials (8-11).
With the progress of modern research and separation technology, it
has been revealed that the primary effective ingredients in Danshen
are tanshinol, salvianolic acid A and salvianolic acid B. These
water-soluble molecules were indicated to exhibit a variety of
favorable effects, including neuroprotective activity,
antioxidation, regenerative effects and responses similar to those
of an antidepressant (12-14).
Hydroxysafflor yellow A is the main bioactive component in Honghua,
which could protect against ischemic stroke by promoting the
dilation of cerebral vessels to improve cerebrovascular
permeability (15-17).
In addition, these four molecules displayed protective and
regulatory effects on disturbed metabolism and the regulation of
neuroinflammatory responses (17-22).
Collectively, the four effective ingredients of
Danhong were indicated to attenuate cerebral ischemic injury in
vitro (23). In the present
study, the orthogonal compatibility of the four effective
ingredients of Danhong (tanshinol, salvianolic acid A, salvianolic
acid B and hydroxysafflor yellow A) were examined to explore the
protective effect of Danhong on cerebral ischemia-reperfusion (IR)
injury in rats. The current study aimed to provide novel insights
and guidance for the clinical and experimental treatment of
ischemic cerebrovascular disease.
Materials and methods
Animals
Healthy adult male Sprague-Dawley rats (total, 216;
weighing 260-300 g) with clean grade were purchased from Zhejiang
Laboratory Animal Center. Animal license number was SCXK (Zhejiang)
2014-0001. The temperature of the animal room was controlled at
25±1˚C, and air humidity was 60-65%. The rats were placed in a
12:12 h light/dark cycle with access to food and water ad
libitum. The rats were euthanized via cervical dislocation
under pentobarbital sodium anesthesia [1% in normal saline (NS); 35
mg/kg; intraperitoneally administered].
Chemicals and reagents
Danhong injection was supplied by Shandong Buchang
Pharmaceuticals Co., Ltd. 2,3,5-triphenyltetrazolium chloride (TTC)
and H&E were obtained from ShangHai SSS Reagent Co., Ltd.
Xylene was purchased from Huadong Medicine Co., Ltd. Rat Bcl-2
(cat. no. MB-7297B) and Bax ELISA kits (cat. no. MB-6629A) were
obtained from Shanghai YuanYe Biotechnology Co., Ltd. DAB
chromogenic kit (cat. no. ZLI-9018) was obtained from Beijing
Zhongshan Jinqiao Biotechnology Co., Ltd. TRIzol®
reagent (cat. no. 15596-026) and caspase-3 antibody (1:100
dilution; cat. no. 43-7800) were purchased from Thermo Fisher
Scientific, Inc. Tanshinol (purity >98%; cat. no. 76822-21-4;
batch. no. SZ201707038), salvianolic acid A (purity >98%; cat.
no. 96574-01-5; batch. no. SZ201706001), salvianolic acid B (purity
>98%; cat. no. 121521-90-2; batch. no. SZ201706003) and
hydroxysafflor yellow A (purity >98%; cat. no. 78281-02-4;
batch. no. Z201702005) were obtained from Nanjing Shizhou
Biotechnology Co., Ltd.
Instruments
The instruments used in the present study were as
follows: OHAUS AR153CN electronic balance (OHAUS Instruments
Shanghai Co., Ltd.), analytical balance (Mettler Toledo), Pall
Cascada Bio Mk2 Water Filtration system (Pall Life Sciences),
fluorescence quantitative PCR instrument (Bio-Rad Laboratories,
Inc.), ZH-003 stainless steel brain matrices (Anhui Zhenghua
Biological Equipment Co., Ltd.), Rotary Microtome Microm HM 340E
(Thermo Fisher Scientific, Inc.) and Leica DM LB2 microscope camera
(Leica Microsystems GmbH).
Transient focal cerebral ischemia
model
The experimental procedure was developed and
performed after certain adjustments to the method by Longa et
al (24). The rats were
anesthetized intraperitoneally with 1% pentobarbital sodium (35
mg/kg). Their body temperature was kept constant at 37˚C. The rats
were immobilized in the supine position and sanitized with alcohol
before the skin was removed. A median longitudinal incision was
made on the neck. The superficial fascia was cut from the bilateral
submandibular glands to expose one side of the mastoid muscle. The
muscle gap was bluntly separated between the right
sternocleidomastoid muscle and the sternohyoid muscle to expose the
right side. This allowed for visualization of three major blood
vessels: The right common carotid artery (CCA), external carotid
artery (ECA) and internal carotid artery (ICA). The root of the ECA
and the proximal end of the CCA were ligated and the ICA was
clamped with an arterial clip. Subsequently, a nylon wire with a
smooth rounded tip (diameter, 0.28-mm; Beijing Cinontech Co., Ltd.)
was inserted from CCA into ICA gently, and the arterial clip was
removed. The insertion depth was stopped at the origin of the
middle cerebral artery (18-20 mm), and the ischemic time was
recorded. After ischemia for 1 h, the wire was gently withdrawn for
reperfusion, and rats were euthanized 3 days after. The incision
was sutured layer by layer and disinfected. The rats were returned
to their cages and kept in the lateral position after the
operation, and their body temperature was maintained at 37˚C.
Groups and treatment
Sprague-Dawley rats were randomly divided in one of
12 groups: Sham operation (sham), IR untreated model (IRU), Danhong
injection group (DHI) and orthogonal groups [L9
(34)]. The nine different combinations of the four key
ingredients of Danhong were prepared according to orthogonal
experimental design (25,26), which is a design method to study
multi-factors and multi-levels. The orthogonal design is presented
in Table I. For example, Group 1 is
made of four components at dose 1 (15 mg/kg tanshinol, 2.5 mg/kg
salvianolic acid A, 8 mg/kg salvianolic acid B and 2 mg/kg
hydroxysafflor yellow A). The doses of the four individual
components were all within the safe range according to previous
pharmacological research and related literature (16-22).
 | Table IDoses of nine compatibility groups of
four effective ingredients according to L9
(34). |
Table I
Doses of nine compatibility groups of
four effective ingredients according to L9
(34).
| | Dose (mg/kg) |
|---|
| Group | A | B | C | D |
|---|
| 1 | 15 | 2.5 | 8 | 2 |
| 2 | 15 | 5 | 16 | 4 |
| 3 | 15 | 10 | 24 | 8 |
| 4 | 30 | 2.5 | 16 | 8 |
| 5 | 30 | 5 | 24 | 2 |
| 6 | 30 | 10 | 8 | 4 |
| 7 | 60 | 2.5 | 24 | 4 |
| 8 | 60 | 5 | 8 | 8 |
| 9 | 60 | 10 | 16 | 2 |
Each group contained 18 rats (six rats were used for
TTC staining, H&E staining and immunohistochemistry and PCR,
respectively). Firstly, the drug was dissolved in physiological
saline. Subsequently, the orthogonal group dose was administered to
the tail vein of the L9 (34) groups directly
at 0 h after reperfusion. Sham and IRU groups were administered an
equal amount of physiological saline. The positive control group
was administered Danhong injection (2 ml/kg) (27-29).
Neurological assessments
Assessments of neurological function were performed
following reperfusion in accordance with previously described
methods (24). Neurological
function was assessed using the modified five-point scale scoring
system ranging from 0 to 4, with higher scores being indicative of
a more severe neurological impairment. Rats with scores of 1-4
following MCAO were used for analysis.
Measurement of infarct volume
Rats were euthanized under anesthesia on the
3rd day after surgery for TTC staining. The rat brains
were cut into small sections (2.0 mm), immersed in 2% TTC at 37˚C
for 30 min. Areas of red staining indicated normal brain tissue,
and pale gray areas represented infarcted tissue. Image-Pro Plus
v6.0 software (Media Cybernetics, Inc.) was used to calculate the
infarct volumes. The following formula was used to calculate
cerebral infarction rate: Infarct Rate=Infarct Volume/Whole Brain
Volume x100%.
H&E staining
A total of 3 days after cerebral IR, the rats were
anesthetized with 35 mg/kg pentobarbital sodium and then fixed with
200 ml 4% paraformaldehyde via perfusion of the heart until the
right atrial appendage produced clear liquid. The rats were
decapitated, and the brains were fixed in 4% paraformaldehyde (Ph
7.4) for 24 h at 4˚C. After gradient elution (100 and 95% ethanol
for 5 min, respectively), brain tissues were embedded in paraffin
and serially sliced (3-4 µm). Subsequently, the slices were
immersed in hematoxylin for 5 min and eosin for 2 min at room
temperature. The results of H&E staining were observed
under a light microscope (magnification, x100).
Measurement of Bcl-2 and Bax levels in
serum
At day 3 after MCAO, the rats were deeply
anesthetized with 35 mg/kg pentobarbital sodium. A total of ~6 ml
blood was drawn from the abdominal aorta and subsequently
centrifuged at 1500 x g for 15 min at 4˚C. The levels of Bcl-2 and
Bax in the serum were measured via ELISA using commercially
available kits according to the manufacturer's instructions.
Immunohistochemistry
After fixation, embedding and routine paraffin
sectioning of 3-4-µm as aforementioned, the experiment followed the
procedure of DAKO En Vision™ two-step immunohistochemistry kit
(cat. no. K5007; Hangzhou Xincheng Biotech Co., Ltd.) (30). Under a light microscope
(magnification, x200), the positive cell status of
immunohistochemistry was shown as yellow or yellow brown in the
cytoplasm. The staining result was determined based on
immunoreactivity score (31) by
multiplying the intensity of staining (0=not stained; 1=low
intensity; 2=moderate intensity; 3=high intensity) and the
percentage of immune positive cells (0=not stained; 1=1-10%;
2=11-50%; 3=51-80%; 4≥80%).
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Frozen brain tissue was placed in a centrifuge tube
and the RNA from the right hippocampus of each group of rats was
extracted with TRIzol® reagent. RNA concentration and
purity were determined using a NanoDrop 2000 spectrometer (Thermo
Fisher Scientific, Inc.). The extracted RNA was then reverse
transcribed into cDNA using a ThermoScript RT-PCR system (cat. no.
11146016; Toyobo Life Science) according to the manufacturer's
instructions. The target genes and GAPDH internal reference gene
(Sangon Biotech Co., Ltd.) were amplified by an Applied Biosystems
7500 Fast RT-PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The reaction conditions were as follows: 94˚C
for 3 min, followed by 95˚C for 10 sec, 58˚C for 30 sec and 72˚C
for 15 sec for a total of 40 cycles. After the reaction was
completed, melting curve analysis was performed to identify the
specificity of the PCR reaction product. The relative expression of
each target gene normalized to GAPDH was analyzed using the
2-ΔΔCq method (32). The
primer sequences are listed in Table
II.
 | Table IIPrimer sequences of selected genes
designed for reverse transcription-quantitative PCR. |
Table II
Primer sequences of selected genes
designed for reverse transcription-quantitative PCR.
| Gene | Forward primer
(5'-3') | Reverse primer
(5'-3') |
|---|
| Apaf-1 |
TGGATGAAGCCATGTCCATA |
TCCCAGAGAACACACAGCAC |
| Cytochrome
c |
AAGACTGGACCAAACCTCCA |
CTCCATCAGGGTATCCTCTCC |
| Caspase-9 |
GCCTCATCATCAACAACGTG |
CTTCACCTCCACCATGAAGC |
| Caspase-3 |
CTGGACTGCGGTATTGAG |
GGGTGCGGTAGAGTAAGC |
| p53 |
GCTGAGTATCTGGACGACA |
CAGGCACAAACACGAACC |
| GAPDH |
GGAAATCGTGCGTGACATTA |
AGGAAGGAAGGCTGGAAGAG |
Statistical data analysis
All statistical analyses were performed using SPSS
v25.0 software (IBM Corp.), and one-way ANOVA followed by Tukey's
post hoc test or Kruskal-Wallis followed by Dunn's post hoc test
was used. Data are presented as the mean ± standard deviation or
the median (interquartile range) for normally or nonnormally
distributed parameters, respectively. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of compatibility groups of
four effective ingredients on neurological deficits in rats with
cerebral IR injury
The neurological deficit of the IRU group was more
severe (P<0.01) than that of the sham group. Compared with the
IRU group, the DHI group indicated a significant improvement in the
symptoms of neurological deficit (P<0.05). In addition, all
orthogonal compatibility groups were indicated to exhibit an
improvement in the symptoms of neurological deficit to different
degrees compared with IRU group. Specifically, the symptoms of
neurological deficit in the orthogonal groups 4 and 6 were more
similar to those in DHI group, and had an improved neurological
score compared with the other orthogonal groups. These results are
presented in Table III.
 | Table IIIEffects of compatibility groups of
four effective ingredients on neurological deficit in rats with
cerebral IR injury. |
Table III
Effects of compatibility groups of
four effective ingredients on neurological deficit in rats with
cerebral IR injury.
| Group | Neurological
score |
|---|
| 1 | 2 (2-2.25) |
| 2 | 2 (1.75-2) |
| 3 | 2 (2-2) |
| 4 | 2 (1-2) |
| 5 | 2 (1.75-2.25) |
| 6 | 2 (1-2) |
| 7 | 2 (2-2.25) |
| 8 | 2 (2-2.25) |
| 9 | 2 (1.75-2) |
| Sham | 0 |
| IRU | 3
(2.75-3)a |
| DHI | 1.5
(1-2)b |
Effects of compatibility groups of
four effective ingredients on cerebral infarct volume in rats with
cerebral IR injury
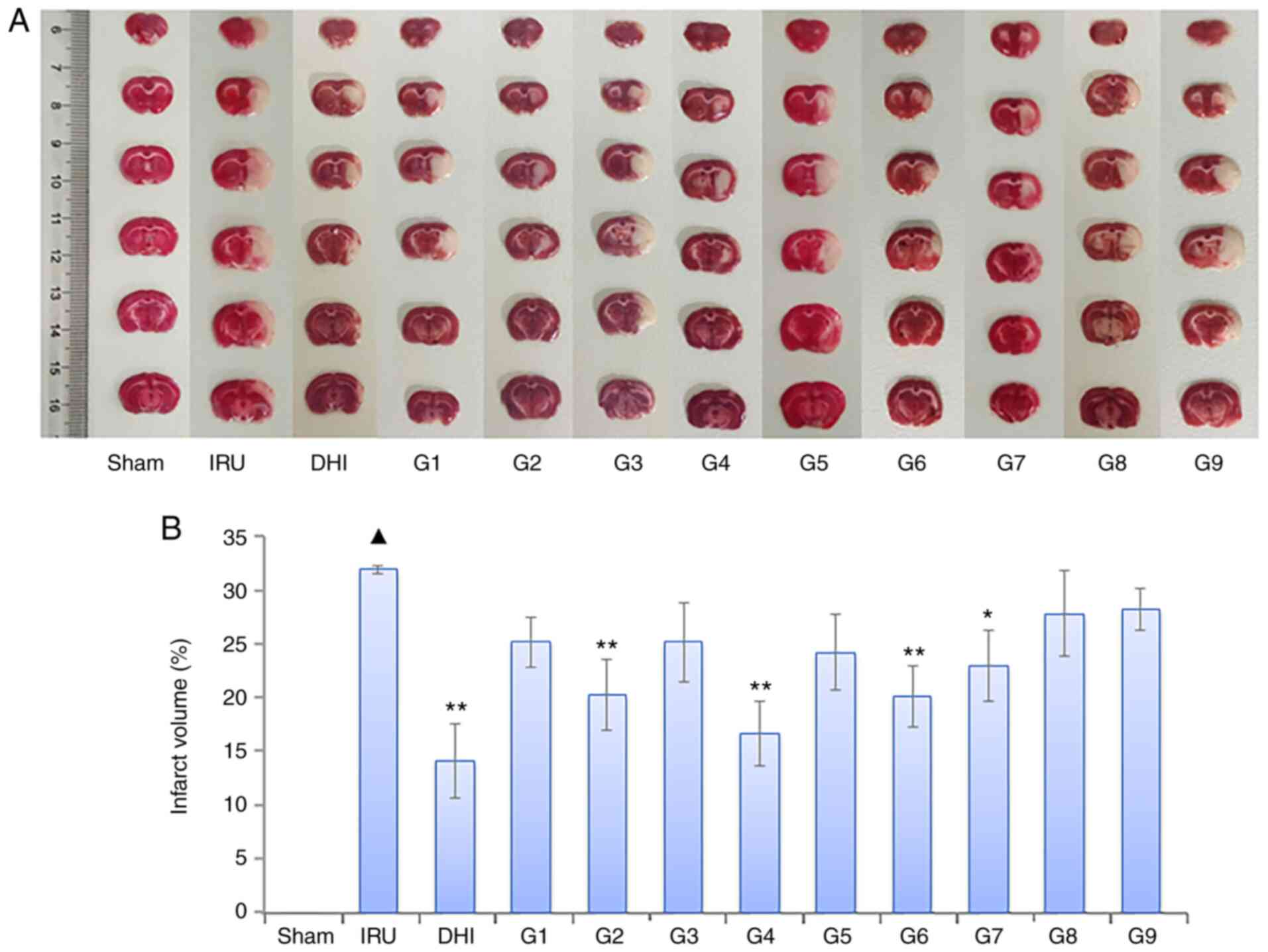
Following TTC staining, the brain sections of the
sham operation group appeared red. The cerebral infarct area of the
IRU group was more pronounced (P<0.01) than that of the sham
group. Compared with the IRU group, the infarct volume of the DHI
group was observed to be significantly reduced (P<0.01). In
addition, the cerebral infarct volume of each drug group decreased
to different degrees. The cerebral infarct volume in the orthogonal
compatibility groups 2, 4, 6 and 7 was significantly decreased
compared with the sham group (P<0.01 or P<0.05). These
results are presented in Fig.
1.
Effects of compatibility groups of
four effective ingredients on pathological alterations of brain
tissue in rats with cerebral IR injury
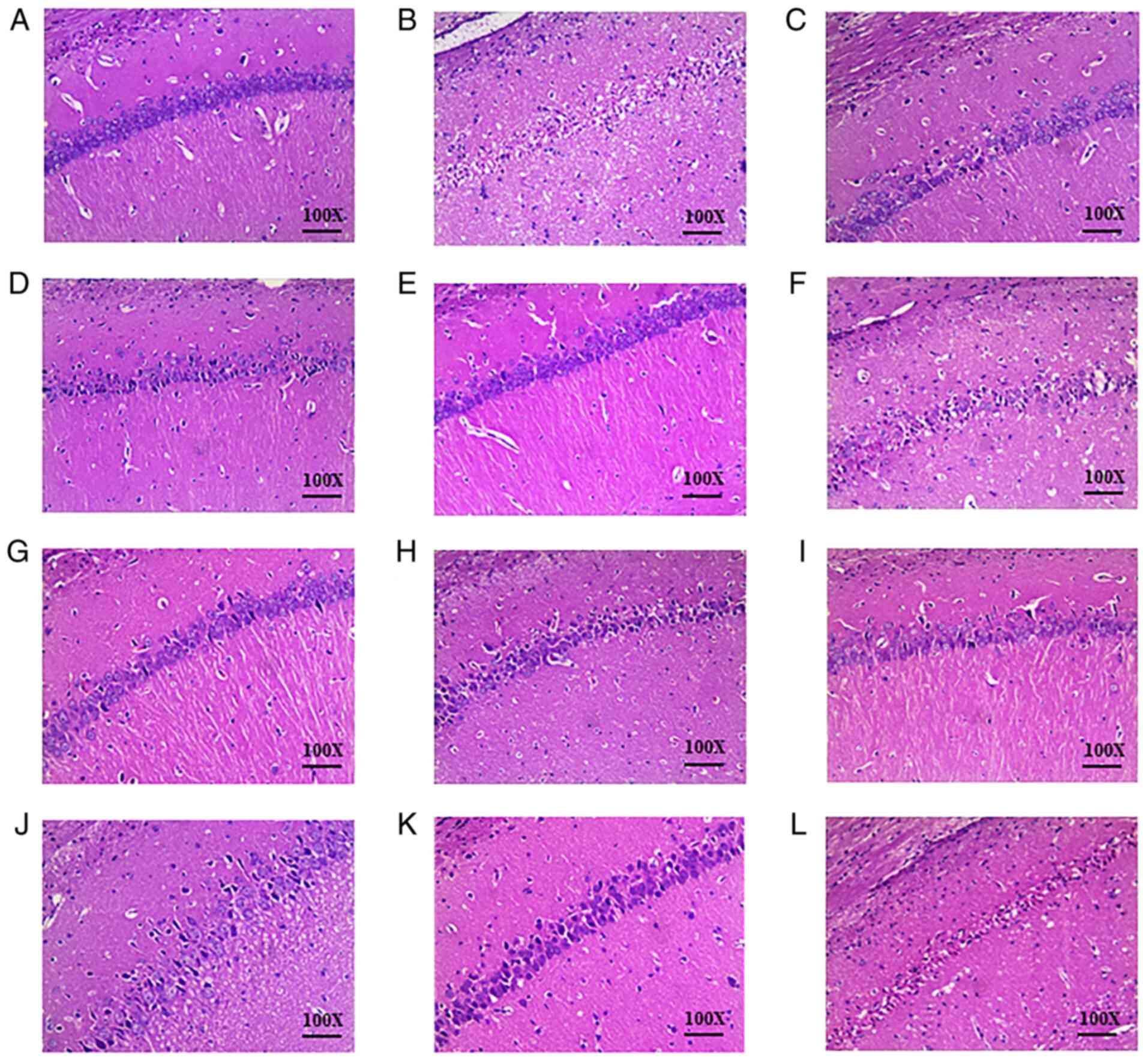
There was no evident pathological damage in the
brain tissue of the sham group (Fig.
2A). The structure was normal and clear: The arrangement of
cells were tight and uniform, the nucleus was intact and the
intercellular space was normal without edema. Typical necrotic foci
were observed in the brain tissue of the IRU group (Fig. 2B). Cell edema was visible, the
number of cells was reduced, and the arrangement of cells was
sparse and disordered. In addition, the boundaries between cells
were blurred, the nuclei were atrophied, and a triangular dense
nucleus was visible. Compared with the IRU group, brain tissue
damage was markedly improved in the DHI group (Fig. 2C). The DHI group presented an
increased number of normal neurons and only partial edema
degeneration. The orthogonal compatibility of Danshen and Honghua
was observed to be most effective in the reduction of pathological
tissue damage in groups 2 and 4. These results are presented in
Fig. 2.
Effects of compatibility groups of
four effective ingredients on the expression levels of Bcl-2 and
Bax in the serum of rats with cerebral IR injury
The serum ratio of Bcl-2/Bax in the IRU group was
significantly lower (P<0.01) than the serum ratio of the sham
group. When compared with the IRU group, the DHI group and the
orthogonal administration groups (groups 2, 3, 4, 5, 6 and 8)
indicated a significant increase in the Bcl-2/Bax ratio (P<0.01
or P<0.05). In addition, there was no significant difference
observed among orthogonal groups (groups 2, 3, 4, 5, 6 and 8) and
the DHI group (P>0.05). However, orthogonal groups 1, 7 and 9
showed statistical difference compared with DHI group (P<0.01 or
P<0.05). The result of group 4 was the closest to that of DHI
group, which indicates that group 4 and the DHI group exhibited
similar efficacy. These results are presented in Table IV.
 | Table IVEffects of compatibility groups of
four effective ingredients on the secretion of Bcl-2 and Bax in the
serum of rats with cerebral IR injury. |
Table IV
Effects of compatibility groups of
four effective ingredients on the secretion of Bcl-2 and Bax in the
serum of rats with cerebral IR injury.
| Group |
Bcl-2/ng·ml-1 |
Bax/ng·ml-1 | Bcl-2/Bax |
|---|
| 1 | 97.58±9.30 |
6.52±1.02a |
14.97±1.43b |
| 2 | 109.08±11.86 |
5.04±0.79c |
21.64±2.35c |
| 3 | 102.15±11.25 |
5.64±0.72c |
18.11±1.99d |
| 4 |
117.33±16.11c |
4.53±0.52c |
25.90±3.56c |
| 5 | 101.08±11.88 |
5.84±0.93c |
17.31±2.03d |
| 6 | 107.88±11.06 |
5.09±0.60c |
21.19±2.17c |
| 7 | 98.12±11.32 |
6.18±0.84b |
15.88±1.83b |
| 8 | 102.56±11.70 |
5.69±0.66c |
18.02±2.06d |
| 9 | 99.76±12.82 |
7.51±1.05a |
13.28±1.71a |
| Sham | 149.38±26.59 | 3.48±0.18 | 42.93±7.64 |
| IRU |
82.17±18.34e |
7.67±0.75e |
10.71±2.39e |
| DHI | 121.
21±17.79c |
4.49±0.39c |
27.00±3.96c |
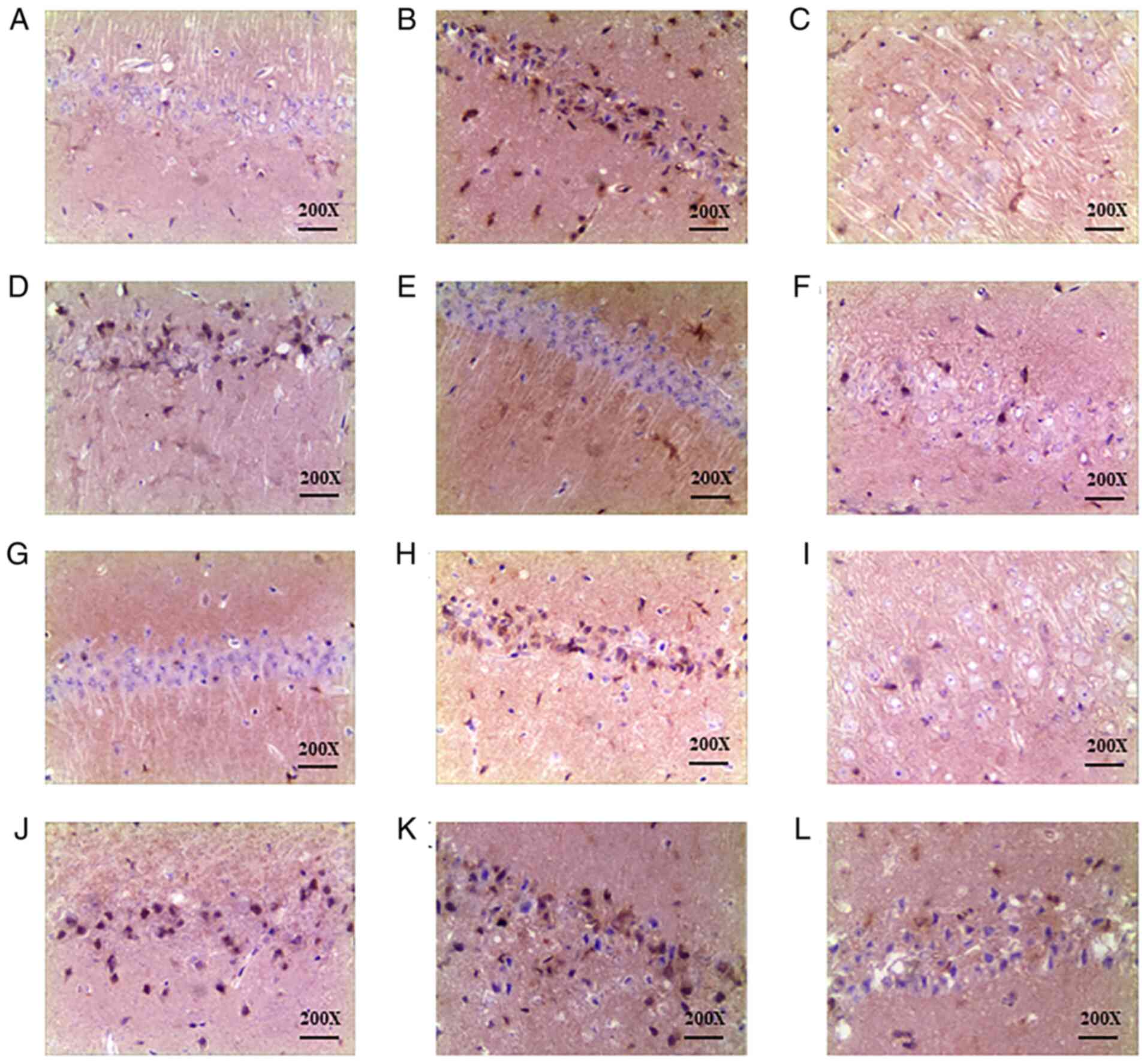
Effect of DHI and compatibility groups
of four effective ingredients on caspase-3 expression in the CA1
area of the hippocampus as detected by immunohistochemistry
Rats in the sham-operated group (Fig. 3A) exhibited low numbers of yellow
brown caspase-3 positive cells in the CA1 area of the hippocampus.
When compared with the sham group, the IRU group (Fig. 3B) presented increased cytoplasmic
staining of caspase-3 in the hippocampal CA1 region (P<0.01).
Orthogonal group 4 was observed to exhibit significantly reduced
expression of caspase-3 protein (P<0.05) when compared with the
IRU group. The results are reflected in Fig. 3 and Table V.
 | Table VEffect of compatibility groups of
four effective ingredients on caspase-3 protein expression in rats
after cerebral IR injury. |
Table V
Effect of compatibility groups of
four effective ingredients on caspase-3 protein expression in rats
after cerebral IR injury.
| Group | Caspase-3 |
|---|
| 1 | 6 (4.75-6.25) |
| 2 | 4 (2.75-5) |
| 3 | 4 (3.75-5) |
| 4 | 3.5
(2.75-4)a |
| 5 | 4.5 (3.5-6) |
| 6 | 4.5
(3.75-5.25) |
| 7 | 5 (4.75-6) |
| 8 | 5 (3.75-6) |
| 9 | 5.5 (3.75-7) |
| Sham | 2 (1.75-2) |
| IRU | 7
(5.5-8)b |
| DHI | 4 (2.75-5) |
Expression levels of cytochrome c,
apoptotic peptidase activating factor 1 (apaf-1), caspase-9,
caspase-3 and p53 mRNA
RT-qPCR results indicated that the expression level
of cytochrome c, apaf-1, caspase-9, caspase-3 and p53 mRNA
in the IRU group was significantly higher than that of the sham
group (P<0.01). Compared with the IRU group, the orthogonal
compatibility groups were indicated to exhibit decreased expression
level of cytochrome c, apaf-1, caspase-9, caspase-3 and p53
mRNA genes. In groups 2, 4 and 6, the expression level of
cytochrome c, apaf-1, caspase-9, caspase-3 and p53 mRNA was
significantly decreased (P<0.01 or P<0.05). When
compared with the DHI group, the expression levels of cytochrome
c in groups 1, 7 and 8, apaf-1 in groups 7 and 9, caspase-9
in groups 7 and 8, caspase-3 in groups 5, 7 and 9 exhibited
significant differences (P<0.01 or P<0.05). This indicated
that the efficacy of the orthogonal compatibility groups 2, 4 and 6
and the DHI group was similar. These results are presented in
Table VI.
 | Table VIEffect of compatibility groups of
four effective ingredients on the expression levels of cytochrome
c, apaf-1, caspase-9, caspase-3 and p53 in rats after
cerebral IR injury. |
Table VI
Effect of compatibility groups of
four effective ingredients on the expression levels of cytochrome
c, apaf-1, caspase-9, caspase-3 and p53 in rats after
cerebral IR injury.
| Group | Cytochrome
c | Apaf-1 | Caspase-9 | Caspase-3 | p53 |
|---|
| 1 |
3.64±0.86a | 2.69±0.60 | 2.43±0.67 | 2.69±0.68 | 2.61±0.54 |
| 2 |
2.32±0.57b |
2.25±0.51c |
1.98±0.54b |
1.73±0.36b |
2.14±0.40b |
| 3 | 3.18±0.61 | 2.76±0.72 | 2.64±0.76 | 2.51±0.57 | 2.55±1.05 |
| 4 |
2.02±0.56b |
1.86±0.57b |
2.01±0.48b |
1.90±0.53b |
2.32±0.71c |
| 5 |
2.31±0.59b | 2.65±0.65 | 2.61±0.72 |
2.84±0.39d | 2.85±1.05 |
| 6 |
2.46±0.63b |
1.95±0.49b |
1.91±0.56b |
1.92±0.70b |
2.41±1.14c |
| 7 |
3.31±0.74d |
3.08±0.66d |
2.88±0.40d |
2.84±0.44d |
2.26±1.26c |
| 8 |
3.70±0.67a | 2.94±0.91 |
2.96±0.66d | 2.78±0.79 | 2.65±1.02 |
| 9 |
2.74±0.61b |
3.09±0.78d | 2.69±0.73 |
2.96±0.70d | 2.96±0.99 |
| Sham | 1.01±0.18 | 1.02±0.21 | 1.01±0.18 | 1.01±0.18 | 1.02±0.26 |
| IRU |
4.45±0.86e |
3.65±0.79e |
3.43±0.85e |
3.43±0.85e |
4.22±0.78e |
| DHI |
1.75±0.51b |
1.75±0.51b |
1.69±0.27b |
1.69±0.27b |
2.11±0.73b |
Discussion
Known as one of the top four life-threatening
diseases (33), strokes are
frequent in clinic patients (34).
Ischemic strokes are common, accounting for ~87% of all strokes
worldwide (2,35). The incidence of ischemic strokes is
higher than that of other types of stroke, which could pose a
serious threat to human health (1).
Consequently, the prevention and treatment of ischemic stroke and
cerebrovascular disease has become a priority throughout the world
(36). It is also of great clinical
significance and social value to explore the effective treatment
methods for patients with ischemic stroke (37).
The compatibility law is one of the core issues in
the study of prescription science. It requires a higher level of
understanding and generalization of prescription compatibility
methods (36,38). This law was a helpful and
significant guide for writing clinical prescriptions and further
developing the theory of prescription science (37). The study of the compatibility of
prescription drugs has been considered important by ancient and
modern doctors (38).
Drug pairs are a commonly used compatibility form of
TCM clinical prescriptions (38).
Drug pairs follow the theory of TCM, including four odors and five
flavors, ascents and descents, channel tropism, toxicity and side
effects and the principle of complementary or opposite combination
(39). A drug pair has the
characteristics of a simple structure and clear compatibility
effect (8). This theory is the
culmination of accumulated clinical medication experience by
physicians of past dynasties (40).
The present study on the main effective ingredients of Danshen and
Honghua as effective prescriptions will help clarify the mechanism
of action of these drugs and reveal their useful characteristics
(41).
The pathophysiological process of cerebral IR injury
is a complex cascade reaction (2,42). The
pathogenesis involves a variety of dysregulations, including
excitatory amino acid toxicity, intracellular calcium overload,
excessive formation of oxygen free radicals, cascade free radical
chain reactions, inflammatory reactions, mitochondrial dysfunction
and apoptosis (42-45).
These events can ultimately cause irreversible brain injury
(46). A notable cause of IR injury
is the increased apoptosis of local neurons after the initial
cerebral ischemia (44).
The regulation of factors and signal transduction
pathways involved in neuronal apoptosis reduces the degree of brain
injury during ischemia and prevents further development of
apoptosis (44,45). This encourages the possibility of a
breakthrough in the treatment of cerebrovascular diseases. The
caspase family serves an important role in the apoptotic process of
neurons (47,48). This family of proteins represents
the common pathway for the final implementation of apoptosis
(49).
Cerebral ischemia and hypoxia can initiate a series
of pathological changes within cells (42). One important response is the opening
of a permeability transition pore that activates the endogenous
apoptotic pathway (50). The
precursors of caspase-9, procaspase-9 and cytochrome c are
then released from the mitochondria to form apoptotic bodies with
apaf-1 (47,51). These apoptotic bodies activate
caspase-9 and downstream caspase-3, which causes apoptosis
(50,51).
The damage of the mitochondrial membrane is also
closely associated with Bcl-2 family members, including Bcl-2, Bax
and Bad (52). These proteins are
involved in the regulation of apoptosis (53). Bcl-2 and Bax are a group of channel
proteins, which can affect the state of cells by regulating the
permeability of the mitochondrial membrane. Specifically, Bax can
regulate the permeability of the mitochondrial extracorporeal
membrane, causing increased release of cytochrome c from the
mitochondria and promotion of apoptosis (54,55).
Bcl-2 inhibits the activation of the caspase family and halts
apoptosis by preventing the formation of the Bax channel (56-58).
The p53 protein is a key molecule in promoting neuronal apoptosis
(59), which can upregulate Bax and
downregulate Bcl-2 (51,54). It can also cause a caspase family
cascade reaction and promote cell apoptosis (47,60).
The present study has several limitations. Firstly,
the experimental period in the present research was 3 days as a
result of the small treatment time. Therefore, the efficacy of drug
treatment for 1, 5 and 7 days was not examined. Secondly, as
oxidative stress and mitochondrial dysfunction are upstream factors
leading to apoptosis, a further study could evaluate the
comprehensive and in-depth effect of these pathways regulated by
the combination of Danshen and Honghua after cerebral IR
injury.
The present study indicated that the expression
levels of apoptosis-related factors, such as cytochrome c,
apaf-1, caspase-9, caspase-3 and p53, were significantly increased
after cerebral IR injury. In addition, the damage of hippocampal
cells was improved to varying degrees after drug treatment. These
findings suggested that the combination of Danshen and Honghua
exhibited a protective effect on rats after cerebral IR injury. In
addition, orthogonal group 4 (30 mg/kg tanshinol; 2.5 mg/kg
salvianolic acid A; 16 mg/kg salvianolic acid B; and 8 mg/kg
hydroxysafflor yellow A) exhibited a significant inhibition of
apoptosis. These drugs may function by inhibiting key targets
upstream of caspase-3 to prevent apoptosis. Ultimately, the
effective and compatible ingredients of Danshen and Honghua were
revealed to exhibit a significant protective effect on cerebral IR
injury in rats.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by National Key R&D
Projects of China (grant. nos. 2019YFC1708600 and 2019YFC1708604),
Zhejiang Provincial Natural Science Foundation of China (grant. no.
LQ19H270001), National Natural Science Foundation of China (grant.
no. 81874366), Key Laboratory of TCM Encephalopathy of Zhejiang
Province (grant. no. 2020E10012) and Open Foundation of Scientific
Research of Zhejiang Chinese Medical University (no.
ZYX2018009).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY and HZ conceived the idea and designed the study.
HW and LC performed the experiments. ZD and ZL established the
cerebral IR model in rats. ZL wrote the manuscript. YY and HW
participated in the data acquisition and statistical analysis, and
YY revised the manuscript. All authors have read and approved the
final manuscript. JY and HZ confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Animal welfare and experiments were strictly in
accordance with the Regulation for the Administration of Affairs
Concerning Experimental Animals (State Science and Technology
Commission, 1988) and approved by the Institutional Animal Care and
Use Committee of Zhejiang Laboratory Animal Center (Hangzhou,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gao L, Song Z, Mi J, Hou P, Xie C, Shi J,
Li Y and Manaenko A: The effects and underlying mechanisms of cell
therapy on blood-brain barrier integrity after ischemic stroke.
Curr Neuropharmacol. 18:1213–1226. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ajoolabady A, Wang S, Kroemer G, Penninger
JM, Uversky VN, Pratico D, Henninger N, Reiter RJ, Bruno A,
Joshipura K, et al: Targeting autophagy in ischemic stroke: From
molecular mechanisms to clinical therapeutics. Pharmacol Ther.
3(107848)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shekhar S, Liu Y, Wang S, Zhang H, Fang X,
Zhang J, Fan L, Zheng B, Roman RJ, Wang Z, et al: Novel mechanistic
insights and potential therapeutic impact of trpc6 in neurovascular
coupling and ischemic stroke. Int J Mol Sci.
22(2074)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Radu RA, Terecoasă EO, Băjenaru OA and Tiu
C: Etiologic classification of ischemic stroke: Where do we stand?
Clin Neurol Neurosurg. 159:93–106. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Writing Group Members. Mozaffarian D,
Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de
Ferranti S, Després JP, et al: Heart disease and stroke
statistics-2016 update: A report from the American heart
association. Circulation. 26:e38–e360. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Orellana-Urzúa S, Rojas I, Líbano L and
Rodrigo R: Pathophysiology of ischemic stroke: Role of oxidative
stress. Curr Pharm Des. 26:4246–4260. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang Z, Wan H, Tong X, He Y, Yang J, Zhang
L, Shao C, Ding Z, Wan H and Li C: An integrative strategy for
discovery of functional compound combination from traditional
Chinese medicine: Danhong injection as a model. Biomed
Pharmacother. 138(111451)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cui Y, Liu X, Li X and Yang H: In-Depth
proteomic analysis of the hippocampus in a rat model after cerebral
ischaemic injury and repair by danhong injection (DHI). Int J Mol
Sci. 18(1335)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gu S, Ma Y, Ge K, Nie R, Wu E and Li Y:
Danshen-Honghua ameliorates stress-induced menopausal depression in
rats. Neural Plast. 2018:1–5. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cheng Q, Pu ZJ, Zhou GS, Wang J, Zhu ZH,
Yue SJ, Li JP, Shang LL, Tang YP, Shi XQ, et al: Comparative
analysis of main bio-active components in the herb pair
danshen-honghua and its single herbs by ultra-high performance
liquid chromatography coupled to triple quadrupole tandem mass
spectrometry. J Sep Sci. 40:3392–3401. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang YL, Zhang Q, Yin SJ, Cai L, Yang YX,
Liu WJ, Hu YJ, Chen H and Yang FQ: Screening of blood-activating
active components from Danshen-Honghua herbal pair by
spectrum-effect relationship analysis. Phytomedicine. 54:149–158.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Meng X, Jiang J, Pan H, Wu S, Wang S, Lou
Y and Fan G: Preclinical absorption, distribution, metabolism, and
excretion of sodium danshensu, one of the main water-soluble
ingredients in salvia miltiorrhiza, in rats. Front Pharmacol.
10(554)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chien MY, Chuang CH, Chern CM, Liou KT,
Liu DZ, Hou YC and Shen YC: Salvianolic acid A alleviates ischemic
brain injury through the inhibition of inflammation and apoptosis
and the promotion of neurogenesis in mice. Free Radic Biol Med.
99:508–519. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhao R, Liu X, Zhang L, Yang H and Zhang
Q: Current progress of research on neurodegenerative diseases of
salvianolic acid B. Oxid Med Cell Longev.
24(3281260)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lu Y, Yanhong D, Zheng Z, He W, Xia M,
Zhang Q and Cao X: Hydroxysafflor yellow A (HSYA) improves learning
and memory in cerebral ischemia reperfusion-injured rats via
recovering synaptic plasticity in the hippocampus. Front Cell
Neurosci. 12(371)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sun Y, Xu DP, Qin Z, Wang PY, Hu BH, Yu
JG, Zhao Y, Cai B, Chen YL, Lu M, et al: Protective cerebrovascular
effects of hydroxysafflor yellow A (HSYA) on ischemic stroke. Eur J
Pharmacol. 818:604–609. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xu H, Liu T, Wang W, Su N, Yang L, Yang Z,
Dou F, Cui J, Fei F, Ma J, et al: Proteomic analysis of
hydroxysafflor yellow A against cerebral ischemia/reperfusion
injury in rats. Rejuvenation Res. 22:503–512. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yang Y, Wang L, Wu Y, Su D, Wang N, Wang
J, Shi C, Lv L and Zhang S: Tanshinol suppresses inflammatory
factors in a rat model of vascular dementia and protects
LPS-treated neurons via the MST1-FOXO3 signaling pathway. Brain
Res. 1646:304–314. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wei ZZ, Chen D, Liu LP, Gu X, Zhong W,
Zhang YB, Wang Y, Yu SP and Wei L: Enhanced neurogenesis and
collaterogenesis by sodium danshensu treatment after focal cerebral
ischemia in mice. Cell Transplant. 4:622–636. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang W, Song JK, Zhang X, Zhou QM, He GR,
Xu XN, Rong Y, Zhou WX and Du GH: Salvianolic acid A attenuates
ischemia reperfusion induced rat brain damage by protecting the
blood brain barrier through MMP-9 inhibition and anti-inflammation.
Chin J Nat Med. 16:184–193. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Feng SQ, Aa N, Geng JL, Huang JQ, Sun RB,
Ge C, Yang ZJ, Wang LS, Aa JY and Wang FJ: Pharmacokinetic and
metabolomic analyses of the neuroprotective effects of salvianolic
acid A in a rat ischemic stroke model. Acta Pharmacol Sinica.
11:1435–1444. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ling C, Liang J, Zhang C, Li R, Mou Q, Qin
J, Li X and Wang J: Synergistic effects of salvianolic acid B and
puerarin on cerebral ischemia reperfusion injury. Molecules.
3(564)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yu L, Wan H, Jin W, Yang J, Li C, Dai L,
Ge L, Zhou H, Wan H and He Y: Protective effects of effective
ingredients of danshen (Radix Salviae Miltiorrhizae) and
honghua (Flos Carthami) compatibility after rat hippocampal
neurons induced by hypoxia injury. J Tradit Chin Med. 38:685–697.
2018.PubMed/NCBI
|
|
24
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lu Z, Cao H, Liu D, Zheng Y, Tian C, Liu
S, Quan J, Shi L, Liu J and Yu L: Optimal combination of
anti-inflammatory components from Chinese medicinal formula
Liang-Ge-San. J Ethnopharmacol. 269(113747)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shao J, Liu Z, Wang L, Song Z, Chang H,
Han N and Yin J: Screening of the optimized prescription from
suqingwan in terms of its therapeutic effect on DSS-induced
ulcerative colitis by its regulation of inflammatory and oxidative
mediators. J Ethnopharmacol. 18:54–62. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Alawieh A, Zhao J and Feng W: Factors
affecting post-stroke motor recovery: Implications on neurotherapy
after brain injury. Behav Brain Res. 340:94–101. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yasar U: Two-Sided action of danshen on
cytoprotective endogenous substances, epoxyeicosatrienoic acids.
Chem Biol Interact. 291(152)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zou JB, Zhang XF, Wang J, Wang F, Cheng
JX, Yang FY, Song X, Wang Y, Liang YL and Shi YJ: The therapeutic
efficacy of danhong injection combined with percutaneous coronary
intervention in acute coronary syndrome: A systematic review and
meta-analysis. Front Pharmacol. 9(550)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Simani L, Naderi N, Khodagholi F, Mehrpour
M and Nasoohi S: Association of long-term atorvastatin with
escalated stroke-induced neuroinflammation in rats. J Mol Neurosci.
61:32–41. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Beilner D, Kuhn C, Kost BP, Vilsmaier T,
Vattai A, Kaltofen T, Mahner S, Schmoeckel E, Dannecker C,
Jückstock J, et al: Nuclear receptor corepressor (NCoR) is a
positive prognosticator for cervical cancer. Arch Gynecol Obstet.
16(1007)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta DeltaC(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jin Y, Pu ZJ, Tang YP, Shang EX, Shi XQ,
Juan S, Pang H and Duan J: Effect of promoting blood circulation of
herb pair containing Angelicae Sinensis Radix and CarthamSi Flos.
Chinese Traditional and Herbal Drugs. 48:2087–2092. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Vavers E, Zvejniece L, Svalbe B, Volska K,
Makarova E, Liepinsh E, Rizhanova K, Liepins V and Dambrova M: The
neuroprotective effects of R-phenibut after focal cerebral
ischemia. Pharmacol Res. 113:796–801. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Katan M and Luft A: Global burden of
stroke. Semin Neurol. 38:208–211. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Culman J, Nguyen-Ngoc M, Glatz T, Gohlke
P, Herdegen T and Zhao Y: Treatment of rats with pioglitazone in
the reperfusion phase of focal cerebral ischemia: A preclinical
stroke trial. Exp Neurol. 238:243–253. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Decano JL, Viereck JC, Mckee AC, Hamilton
JA, Ruiz-Opazo N and Herrera VLM: Early-Life sodium exposure
unmasks susceptibility to stroke in hyperlipidemic, hypertensive
heterozygous Tg25 rats transgenic for human cholesteryl ester
transfer protein. Circulation. 119:1501–1509. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang Y, Yang H, Chen L, Jafari M and Tang
J: Network-Based modeling of herb combinations in traditional
Chinese medicine. Brief Bioinform. 8(1093)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhong LY, Cui MN, Yang M and Gong QF:
Modern researches on effect of processing of Chinese herb medicine
on Chinese medical properties. Zhongguo Zhong Yao Za Zhi.
44:5109–5113. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
40
|
Guo Zl, Zhu Y, Su Xt, Liu J, Yang Qx, Nan
Jy, Zhao Bc, Zhang Yy, Yu Yn, Li B, et al: DanHong injection
dose-dependently varies amino acid metabolites and metabolic
pathways in the treatment of rats with cerebral ischemia. Acta
Pharmacol Sin. 36:748–757. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Fei YX, Wang SQ, Yang LJ, Qiu YY, Li YZ,
Liu WY, Xi T, Fang WR and Li YM: Salvia miltiorrhiza bunge
(Danshen) extract attenuates permanent cerebral ischemia through
inhibiting platelet activation in rats. J Ethnopharmacol.
207:57–66. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yang JL, Mukda S and Chen SD: Diverse
roles of mitochondria in ischemic stroke. Redox Biol. 16:263–275.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ouyang YB and Giffard RG: Cellular
neuroprotective mechanisms in cerebral ischemia: Bcl-2 family
proteins and protection of mitochondrial function. Cell Calcium.
36:303–311. 2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Seth L: Apoptosis and brain ischaemia.
Prog Neuropsychopharmacol Biol Psychiatry. 27:267–282.
2003.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gupta S, Sharma U, Jagannathan NR and
Gupta YK: Neuroprotective effect of lercanidipine in middle
cerebral artery occlusion model of stroke in rats. Exp Neurol.
288:25–37. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wang Y, Zhan G, Cai Z, Jiao B, Zhao Y, Li
S and Luo A: Vagus nerve stimulation in brain diseases: Therapeutic
applications and biological mechanisms. Neurosci Biobehav Rev.
21:37–53. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Mouw G, Zechel JL, Zhou Y, Lust WD, Selman
WR and Ratcheson RA: Caspase-9 inhibition after focal cerebral
ischemia improves outcome following reversible focal ischemia.
Metab Brain Dis. 17:143–151. 2002.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Marcel V, Fernandes K, Terrier O, Lane DP
and Bourdon JC: Modulation of p53β and p53γ expression by
regulating the alternative splicing of TP53 gene modifies cellular
response. Cell Death Differ. 21:1377–1387. 2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Li L, Su Z, Zou Z, Tan H, Cai D, Su L and
Gu Z: Ser46 phosphorylation of p53 is an essential event in
prolyl-isomerase pin1-mediated p53-independent apoptosis in
response to heat stress. Cell Death Dis. 4(96)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Bai X, Tan TY, Li YX, Li Y, Chen YF, Ma R,
Wang SY, Li Q and Liu ZQ: The protective effect of cordyceps
sinensis extract on cerebral ischemic injury via modulating the
mitochondrial respiratory chain and inhibiting the mitochondrial
apoptotic pathway. Biomed Pharmacother. 124(109834)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Hong B, Van Den Heuvel AP, Prabhu VV,
Zhang S and El-Deiry WS: Targeting tumor suppressor p53 for cancer
therapy: Strategies, challenges and opportunities. Curr Drug
Targets. 15:80–89. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Birkinshaw RW and Czabotar PE: The BCL-2
family of proteins and mitochondrial outer membrane
permeabilisation. Semin Cell Dev Biol. 72:152–162. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Cheng CY, Kao ST and Lee YC: Ferulic acid
exerts anti-apoptotic effects against ischemic injury by activating
HSP70/Bcl-2- and HSP70/autophagy-mediated signaling after permanent
focal cerebral ischemia in rats. Am J Chin Med. 47:39–61.
2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Enomoto A, Yamada J, Morita A and Miyagawa
K: Bisdemethoxycurcumin enhances X-ray-induced apoptosis possibly
through p53/Bcl-2 pathway. Mutat Res. 815:1–5. 2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Jiang J, Dai J and Cui H: Vitexin reverses
the autophagy dysfunction to attenuate MCAO-induced cerebral
ischemic stroke via mTOR/Ulk1 pathway. Biomed Pharmacother.
99:583–590. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Anilkumar U and Prehn JH: Anti-Apoptotic
BCL-2 family proteins in acute neural injury. Front Cell Neurosci.
8(281)2014.PubMed/NCBI View Article : Google Scholar
|
|
58
|
D'Orsi B, Matekya J and Prehn JHM: Control
of mitochondrial physiology and cell death by the Bcl-2 family
proteins bax and bok. Neurochem Int. 109:162–170. 2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Lim Y, Dorstyn L and Kumar S: The
p53-caspase-2 axis in the cell cycle and DNA damage response. Exp
Mol Med. 53:517–527. 2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zhang L, Zhao H, Zhang X, Chen L, Zhao X,
Bai X and Zhang J: Nobiletin protects against cerebral ischemia via
activating the p-Akt, p-CREB, BDNF and Bcl-2 pathway and
ameliorating BBB permeability in rat. Brain Res Bull. 96:45–53.
2013.PubMed/NCBI View Article : Google Scholar
|