Introduction
Hemodialysis is the primary renal replacement
therapy for patients with end-stage renal disease (ESRD), and is
designed to perform some of the functions lost as a result of
chronic renal failure (CRF), such as clearing metabolic wastes and
regulating the balance of water, electrolytes and the acid-base
balance, which increases the survival of patients and improves the
quality of life (1). Arteriovenous
fistula (AVF) is the preferred mode of access for hemodialysis. The
artery near the wrist of the forearm and the adjacent vein are
sutured to allow arterial blood flow in the vein following
anastomosis, which forms the AVF (2), the ‘lifeline’ for patients with
hemodialysis. However, the patency rate of AVF after 1 year is 70%,
and 48% after 4 years (3). The
failure of AVF can be attributed to venous stenosis, intimal
hyperplasia, technical problems and inflow issues (4-6).
The primary cause of AVF stenosis is venous stenosis
caused by intimal hyperplasia at the anastomosis site of AVF
(7). Previous studies have reported
that intimal hyperplasia is predominantly caused by the viability
and migration of vascular smooth muscle cells (VSMCs) (8,9).
Extensive neointimal hyperplasia composed of VSMCs has been
observed at the anastomosis site of AVF (10). Thus, inhibiting the viability and
migration of VSMCs may be an effective intervention of AVF
stenosis. It has been confirmed that activation of the PI3K/Akt
signaling pathway promotes aberrant viability and migration of
VSMCs (11,12). Park et al (13) demonstrated that inhibiting the
PI3K/Akt signaling pathway disrupts the viability of rat aortic
VSMCs. Conversely, activation of the PI3K/Akt signaling pathway
induces the viability and migration of VSMCs (14). Therefore, therapies targeting the
PI3K/Akt signaling pathway may be promising in inhibiting AVF
stenosis.
Hydroxysafflor Yellow A (HSYA) is a water-soluble
chalcone glycoside extracted from Carthami Flos, the flower of
safflower (Carthamus tinctorius L.), which is the
primary active ingredient in the pharmacological action of Carthami
Flos (15). HSYA exerts several
pharmacological effects, such as cardiovascular effects (16), neuroprotective effects (17), antitumor effects (18) and endothelium cell protection
(19). Jiang et al (20) reported that HSYA suppresses the
viability, migration and invasion of lipopolysaccharide-induced
non-small cell lung cancer cells by suppressing the PI3K/AKT/mTOR
signaling pathway. Previous studies have demonstrated that HSYA
inhibits the viability and migration of VSMCs by regulating Akt
signaling (21,22). However, whether HSYA can modulate
AVF stenosis in patients with CRF via inhibiting VSMC viability and
migration remains largely unknown.
In the present study, VSMCs were induced using serum
from CRF rats, and the aim was to assess the effects of HSYA on the
viability and migration of VSMCs, as well as uncover the potential
mechanisms.
Materials and methods
Animal model of CRF
Animal experiments were performed as previously
described (23). A total of 40 male
Wistar rats (age, 6-7 weeks; weight, 160-180 g) were obtained from
Nanjing Jiancheng Bioengineering Institute, and maintained in a 12
h light/dark cycle, with 50-60% humidity at 22-26˚C. All rats were
provided ad libitum access to a standard diet and water.
Rats were randomly divided into two groups; a control (n=20) and
CRF (n=20) groups after 1 week of adaptive feeding. Adenine (2.5 g;
Sigma-Aldrich; Merck KGaA) was added to 100 ml normal saline to
prepare a 2.5% adenine suspension. Rats in the CRF group received
250 mg/kg adenine once a day via oral gavage for a total of 14
days, and adenine was administrated every other day for the next 14
days. Rats in the control group received the same amount of normal
saline. All rats were fasted for 12 h prior to the last
administration. Rats were anesthetized with 2% sodium pentobarbital
(50 mg/kg) 1 h after the final administration, and cervical
dislocation of the spine was immediately performed following
collection of 4-6 ml blood from the abdominal aorta. All animal
experiments were approved by the Experimental Animal Center of
Lianyungang Hospital of Traditional Chinese Medicine (Lianyungang,
China; approval. no. IACUC-20200312-07).
Serum parameters
All experiments were performed as previously
described (23-26).
Blood samples were centrifuged at 1,000 x g for 10 min to collect
serum. The concentrations of serum creatinine (SCr) and blood urea
nitrogen (BUN) were measured using commercial kits (cat. nos.
C011-2-1 and C013-2-1, respectively; Nanjing Jiancheng
Bioengineering Institute), and measured using a biochemical
autoanalyzer (ROCHE Modular P800; Roche Diagnostics GmbH).
Cell culture and treatment
Human umbilical vein smooth muscle cells (HUVSMCs;
cat. no. CP-H084) were purchased from Procell Life Science &
Technology Co., Ltd., with the approval of Ethics Committee of
Lianyungang Hospital of Traditional Chinese Medicine (Lianyungang,
China. Approval no. IACUC-20200611-03), and maintained in DMEM
supplemented with 10% FBS (both purchased from Gibco; Thermo Fisher
Scientific, Inc.), at 37˚C with 5% CO2.
The rat whole blood was collected from the control
(control serum) and CRF (CRF serum) groups into coagulation tubes
and allowed to clot on ice for 50 min. Subsequently, centrifugation
at 1,000 x g for 15 min was used for separating serum. Rat serum
was maintained in DMEM at 37˚C for 48 h at dilutions of 2.5:100,
5:100 or 10:100. HUVSMCs were pretreated with 1, 5 or 25 µM HSYA
(27-30)
(purity >98%; Beijing Solarbio Science & Technology Co.,
Ltd.) prior to stimulation of rat serum. AMG 511 (5 nM;
MedChemExpress) was used to inhibit PI3K.
Cell viability assay
A Cell Counting Kit-8 (CCK-8) assay was performed to
assess the effect of rat serum and/or HSYA on cell viability.
HUVSMCs were seeded in 96-well plates at a density of
5x103 cells/well and treated with rat serum and/or HSYA
at 37˚C for 48 h. Cells were subsequently incubated with 10 µl
CCK-8 reagent (cat. no. C0037; Beyotime Institute of Biotechnology)
for 1 h at 37˚C. Cell viability was analyzed at a wavelength of 450
nm, using a microplate spectrophotometer (BioTek Instruments,
Inc.).
Wound healing assay
HUVSMCs were seeded into 6-well plates at a density
of 1x106 cells/well. Sterile 200 µl pipette tips were
used to scratch the cell monolayers. Cell medium was replaced with
fresh serum-free DMEM. The migratory ability of cells characterized
by wound width was observed at 0 and 48 h under a light microscope
(Olympus Corporation; magnification, x100).
Apoptosis analysis
HUVSMCs (5x105) were collected by
centrifugation at 37˚C and 300 x g for 3 min and re-suspended in
200 µl Annexin V binding buffer (cat. no. C1062M; Beyotime
Institute of Biotechnology). After resuspension, cells were
incubated with 10 µl PI (cat. no. C1062M; Beyotime Institute of
Biotechnology) for 15 min at room temperature in the dark.
Apoptotic cells were subsequently analyzed using a flow cytometer
(Beckman Coulter, Inc.).
Measurement of nitric oxide (NO)
HUVSMCs were seeded in 12-well plates at a density
of 2x105 cells/well and treated with rat serum and/or
HSYA. HUVSMCs were subsequently lysed using Lysis Buffer (cat. no.
S3090; Beyotime Institute of Biotechnology). The Nitrate/Nitrite
assay kit (cat. no. S0023; Beyotime Institute of Biotechnology) was
used to determine NO concentration in HUVSMCs, according to the
manufacturer's protocol. NO concentration was measured at a
wavelength of 540 nm, using a microplate spectrophotometer (BioTek
Instruments, Inc.).
Western blotting
Total protein was extracted from HUVSMCs using RIPA
lysis buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology). Cell supernatants were collected by centrifugation
at 12,000 x g for 15 min at 4˚C. Total protein was quantified using
the BCA protein assay kit (cat. no. P0012s; Beyotime Institute of
Biotechnology) and 20 µg protein/lane was separated by 12%
SDS-PAGE. The separated proteins were subsequently transferred to
PVDF membranes (EMD Millipore) and blocked with 5% skimmed milk for
2 h at room temperature. The membranes were incubated with primary
antibodies against PI3K (1:1,000), Akt (1:1,000), phosphorylated
(p)-Akt (cat. no. Ser473, 1:1,000), endothelial NO synthase (eNOS;
1:1,000), p-eNOS (1:1,000) and GAPDH (1:10,000) overnight at 4˚C
(all purchased from Affinity Biosciences). Following the primary
incubation, membranes were incubated with goat anti-rabbit/mouse
IgG (H+L) HRP-conjugated secondary antibodies (1:10,000; Affinity
Biosciences) for 2 h at room temperature. Protein bands were
semi-quantified using Image Lab version 4.1 software (Bio-Rad
Laboratories, Inc.).
Statistical analysis
All experiments were performed in triplicate and
data are presented as the mean ± standard deviation. A Student's
t-test was used to compare differences between two groups, and a
one-way ANOVA followed by a Tukey's post hoc test was used to
compare differences between multiple groups in GraphPad Prism
version 5.0 (GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Serum from CRF rats promotes the
viability and migration of HUVSMCs
Rat serum from the control and CRF groups was
collected to assess changes in the parameters of renal function. As
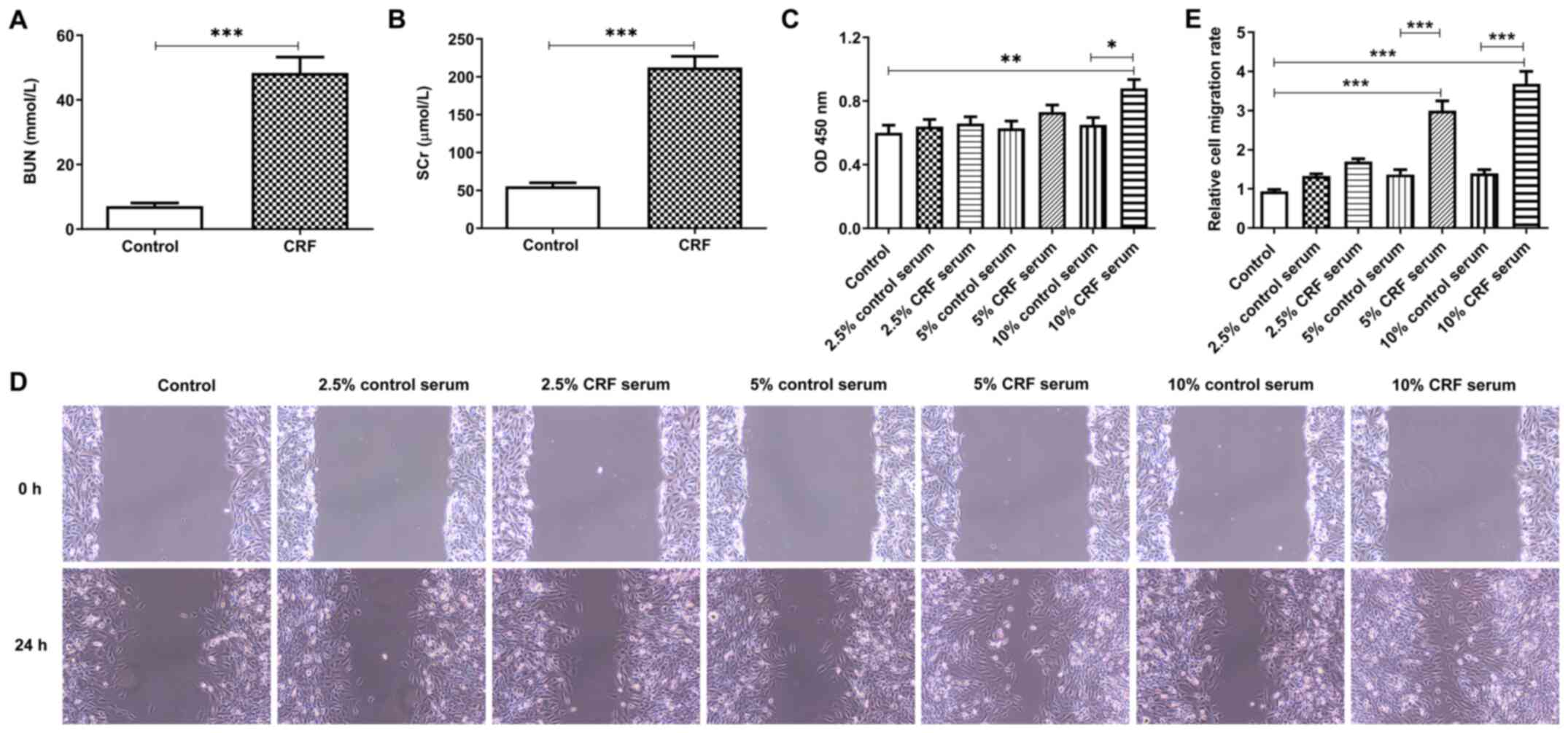
presented in Fig. 1A, the BUN
concentration was significantly higher in the CRF group compared
with the control group. Similarly, SCr concentration was higher in
the CRF group compared with the control group (Fig. 1B). These results suggest that the
renal failure model was established successfully (24-26).
HUVSMCs were treated with rat serum to assess the
effect of CRF serum on HUVSMCs. The results demonstrated that 10%
CRF serum significantly promoted cell viability compared with 10%
control serum, whereas low concentrations of CRF serum (2.5 and
5.0%) slightly enhanced cell viability (Fig. 1C). In addition, the migratory
ability of HUVSMCs was inhibited following stimulation of CRF serum
compared with the same concentration of control serum. Furthermore,
the results of the wound healing assay demonstrated that CRF serum
promoted cell migration in a concentration-dependent manner,
whereas control serum had little effect on cell migration (Fig. 1D and E). Collectively, these results suggest
that serum from CRF rats promotes the viability and migration of
HUVSMCs.
Serum from CRF rats promotes the
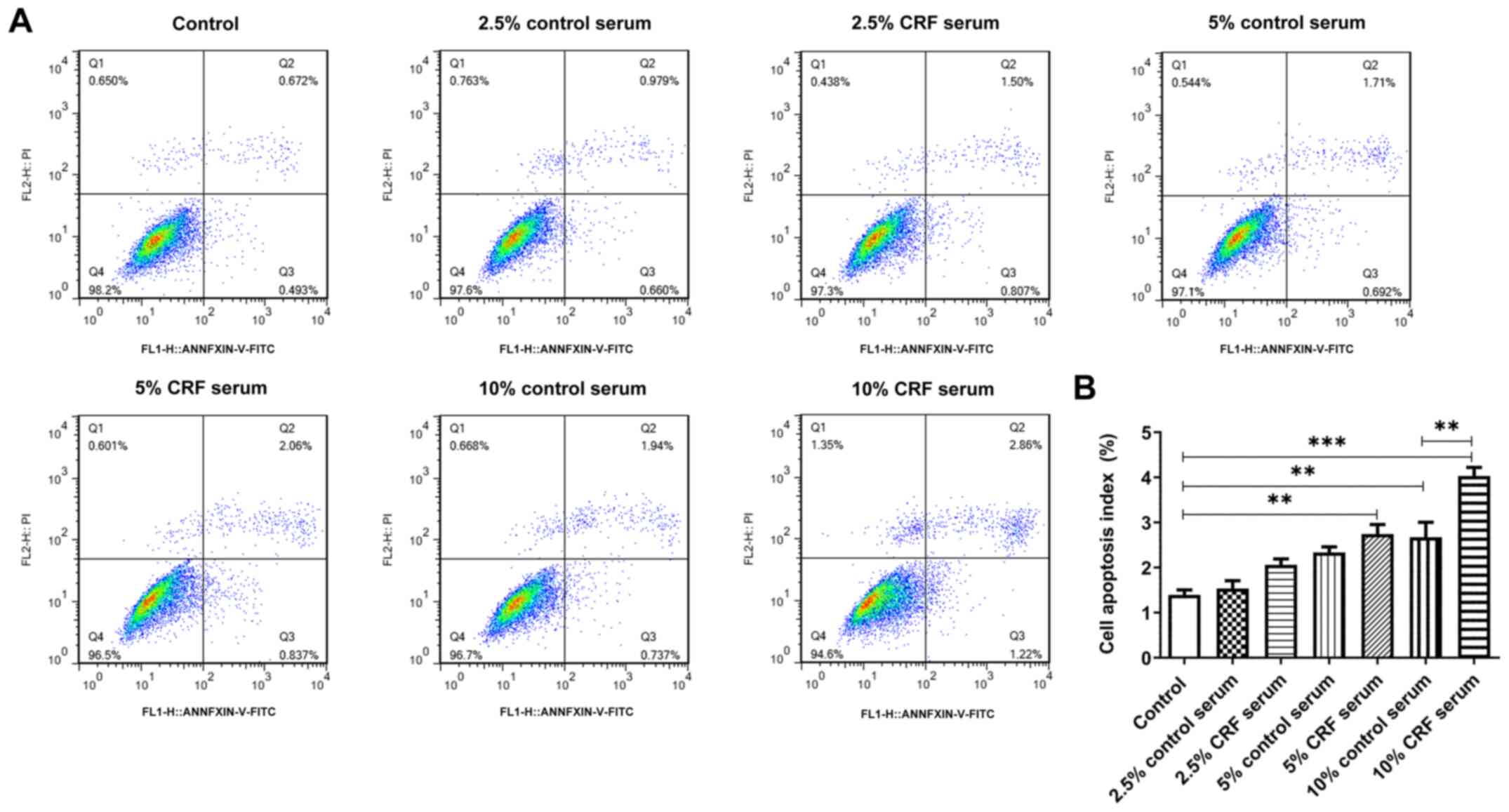
apoptosis of HUVSMCs
The results demonstrated that the apoptotic rate
increased following treatment of HUVSMCs with rat serum. Notably,
10% CRF serum markedly promoted cell apoptosis compared with 10%
control serum (Fig. 2A). This may
be explained by the presence of toxic substances in the serum of
rats with CRF, which affects cell survival and promotes
apoptosis.
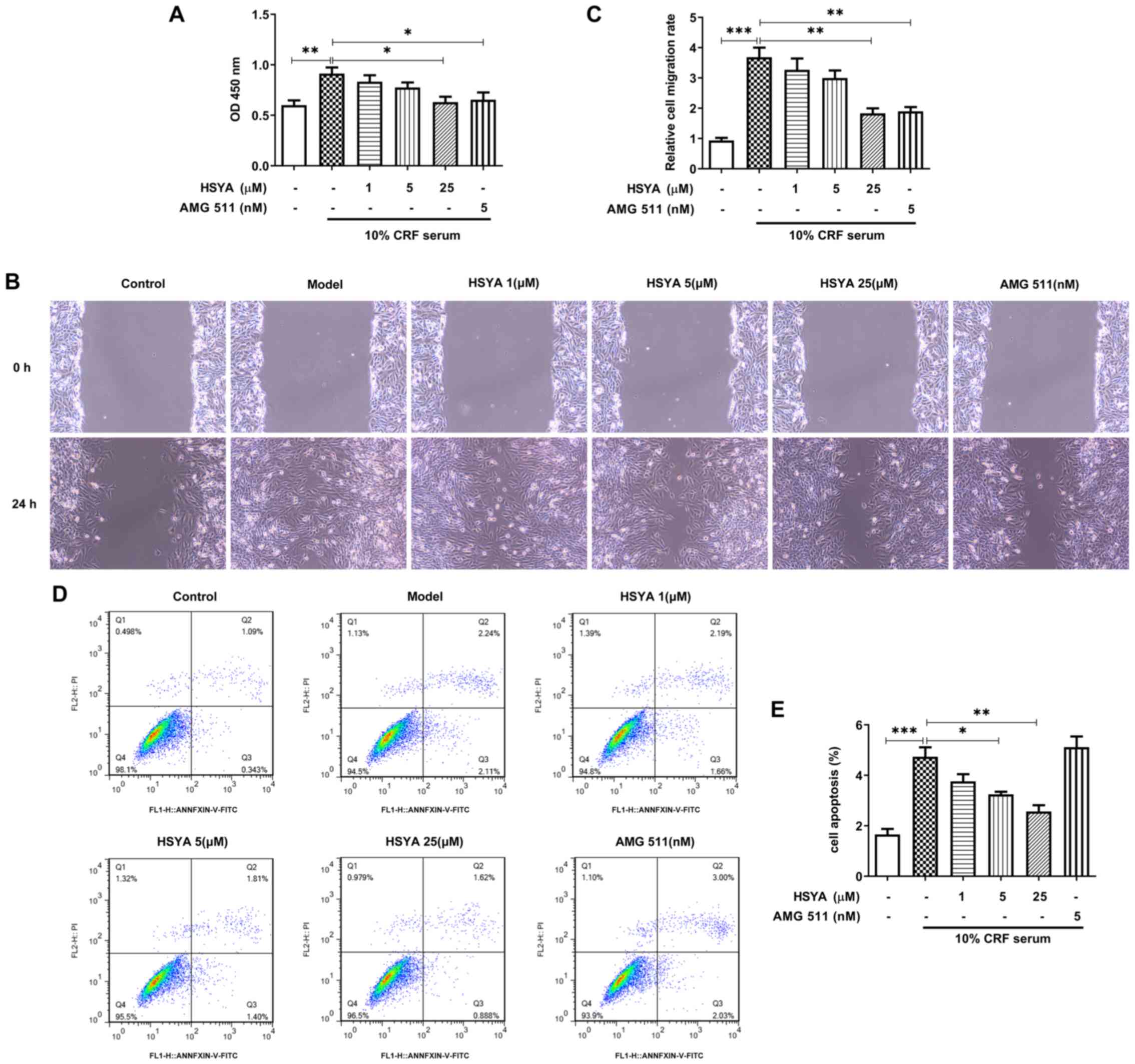
HSYA inhibits cell viability,
migration and apoptosis of HUVSMCs
HUVSMCs were treated with HSYA or AMG 511 in the
presence of 10% CRF serum. As presented in Fig. 3A, HSYA inhibited cell viability
induced by 10% CRF serum in a concentration-dependent manner.
Similarly, AMG 511 suppressed the viability of HUVSMCs. Notably,
pretreatment with HSYA or AMG 511 alleviated 10% CRF serum-induced
cell migration (Fig. 3B),
suggesting that HSYA reverses the effect of CRF serum on cell
migration. In addition, HSYA partially alleviated 10% CRF
serum-induced apoptosis, the effects of which were reversed
following pretreatment with AMG 511 (Fig. 3C). Taken together, these results
suggest that HSYA counteracts the effects of CRF serum on the
viability, migration and apoptosis of HUVSMCs.
HSYA inactivates the PI3K/Akt
signaling pathway and promotes NO production
NO inhibits neointimal hyperplasia (31); thus, the NO concentration in HUVSMCs
was detected. The NO concentration was notably decreased in the
presence of 10% CRF serum compared with the control group; however,
treatment with HSYA elevated NO levels in a concentration-dependent
manner. Similarly, treatment with AMG 511 promoted NO production
(Fig. 4A).
 | Figure 4HSYA inactivates PI3K/Akt signaling
and enhances NO production. Solutions of 1, 5, 25 µM HSYA or 5 nM
AMG 511 were prepared to treat HUVSMCs 30 min prior to stimulation
with 10% CRF serum. (A) Production of NO in each group was examined
using a Nitrate/Nitrite assay kit. (B) Protein levels of PI3K, Akt,
p-Akt, eNOS and p-eNOS were estimated via western blotting.
*P<0.05, **P<0.01 and
***P<0.001. HSYA, Hydroxysafflor yellow A; CRF,
chronic renal failure; HUVSMCs, human umbilical vein smooth muscle
cells; p-, phospho-; NO, nitric oxide; eNOS, endothelial NO
synthase. |
Given that HSYA exhibited similar inhibitory effects
to AMG 511 on the viability and migration of HUVSMCs, it was next
investigated whether HSYA can exert these effects by inhibiting the
PI3K/Akt pathway. As presented in Fig.
4B, 10% CRF serum upregulated PI3K expression and
phosphorylation of Akt, suggesting that serum from CRF rats can
activate PI3K/Akt signaling in HUVSMCs. Notably, the expression
levels of PI3K and p-Akt decreased following treatment with AMG
511. In addition, treatment with HSYA markedly decreased the
protein expression levels of PI3K and p-Akt. Treatment with both
HSYA and AMG 511 upregulated p-eNOS expression, respectively, which
explains the increase in NO production (Fig. 4A).
Discussion
AVF stenosis caused by neointimal hyperplasia is
frequently observed in patients (32), and can lead to the morbidity of
patients with ESRD (33,34). However, the molecular mechanism
underlying neointima formation in AVF remains unclear. It is
well-known that injury induced pathological viability and migration
of VSMCs is a major cause of neointima formation (35,36).
In the present study, CRF rats were established and the serum from
rats were prepared to stimulate HUVSMCs to determine whether HSYA
could inhibit neointimal hyperplasia in vitro.
High concentrations of CRF serum significantly
promoted the viability and migration of HUVSMCs, which is
consistent with the pathological condition of AVF stenosis in
patients with ESRD (37,38). However, high concentrations of CRF
serum also increase cell apoptosis, which may be due to toxicants
in the serum that disrupt cell survival. Activation of the PI3K/Akt
signaling pathway is closely associated with aberrant viability and
migration of VSMCs (11,12,39).
In addition, activation of the PI3K/Akt signaling pathway has been
observed in mice with renal dysfunction caused by
ischemia/reperfusion-injury (40,41).
Previous studies have reported that the PI3K/Akt signaling pathway
is activated in kidney injury induced by cisplatin (42) and kidneys of rats with unilateral
ureteral obstruction (43).
HSYA is an active ingredient isolated from
Carthami Flos (15). It has
been reported that HSYA inhibits platelet derived growth factor
BB-induced activation of Akt signaling, which in-turn disrupts the
viability and migration of VSMCs (21). In addition, Yang et al
(22) demonstrated that HSYA
suppresses the viability and migration of
lipopolysaccharide-induced VSMCs by inhibiting the Toll-like
receptor 4/Rac1/Akt pathway. Thus, it was hypothesized that HSYA
exerts antiproliferative and anti-migratory effects on HUVSMCs via
the PI3K/Akt signaling pathway.
The results of the present study demonstrated that
HSYA suppressed CRF serum-induced cell viability and migration,
suggesting its role in HUVSMCs-mediated intimal hyperplasia. AMG
511, which is a potent and selective PI3K inhibitor that decreases
the phosphorylation of Akt, exhibited similar effects to HSYA on
the viability and migration of HUVSMCs. Notably, HSYA decreased
cell apoptosis induced by CRF serum, whereas AMG 511 had little
effect on the apoptosis of HUVSMCs. This may be explained by the
hypothesis that HSYA ameliorates the toxic effects of toxicants in
CRF serum on HUVSMCs.
In the present study, NO production and p-eNOS
expression decreased in CRF serum-induced HUVSMCs, whereas
treatment with HSYA and AMG 511 enhanced the levels of NO and
p-eNOS, respectively. Previous studies have demonstrated that
NO-based therapies can decrease neointimal hyperplasia (44,45).
NO production is regulated by NO synthases (NOSs), including eNOS.
eNOS activity is mainly regulated through phosphorylation, which is
primarily regulated by the PI3K/Akt/eNOS pathway. It has been
reported that activation of PI3K/AKT/eNOS pathways serves an
important role in regulating cell migration, migration,
angiogenesis and apoptosis (46).
The results of the present study showed that HSYA may rescue NO
production in CRF serum-treated cells via inhibiting PI3K/Akt
activation.
To further investigate whether HSYA can regulate the
PI3K/Akt signaling pathway, the protein expression levels of PI3K
and p-Akt were detected. The results demonstrated that HSYA
affected PI3K expression and Akt phosphorylation. In addition, HSYA
inhibited the viability and migration of HUVSMCs by regulating
PI3K/Akt signaling, which suggests that HSYA may mediate intimal
hyperplasia-induced AVF stenosis. However, given that the present
study only focused on HSYA-mediated viability and migration of
HUVSMCs, further studies are required to confirm the effects of
HSYA in AVF stenosis in vivo. In addition, other alternative
assays will be utilized to enrich the experimental content and
further validate these findings. Finally, prospective studies
should focus on investigating the involvement of other pathways on
the effects of HSYA on AVF stenosis.
Taken together, the current study for the first time
demonstrated the inhibitory effects of HSYA on HUVSMC viability and
migration, as well as showing the underlying mechanism involved
regulation of the PI3K/Akt signaling pathway. The results provide
primary evidence for the therapeutic application of HSYA in intimal
hyperplasia-induced AVF stenosis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Six One Project
of Top-notch Talent item for High-level Health Talents in Jiangsu
Province in 2017 (grant. no. LGY 2017064) and the Fifth Phase of
the 333 Project for Scientific Research Project of Jiangsu Province
in 2020 (grant. no. BRA2020259).
Availability of data and materials
All data generated or analysed during the present
study are included in this published article.
Authors' contributions
BC, CH, LW and QW conceived and designed the present
study. CH and LW, performed the experiments and acquired the data.
QW and YY analyzed the data. BC drafted the initial manuscript,
including the figures. All authors have read and approved the final
manuscript. BC and CH confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
All animal experiments were approved by the
Experimental Animal Center of Lianyungang Hospital of Traditional
Chinese Medicine (Lianyungang, China. Approval. no.
IACUC-20200312-07). The use of Human umbilical vein smooth muscle
cells (cat. no. CP-H084; Procell Life Science & Technology Co.,
Ltd.) was approved by the Ethics Committee of Lianyungang Hospital
of Traditional Chinese Medicine (Lianyungang, China. Approval. no.
IACUC-20200611-03).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mallick NP and Gokal R: Haemodialysis.
Lancet (London, England). 353:737–742. 1999.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Radosa CG, Radosa JC, Weiss N, Schmidt C,
Werth S, Hofmockel T, Plodeck V, Gatzweiler C, Laniado M and
Hoffmann RT: Endovascular creation of an arteriovenous fistula
(endoAVF) for hemodialysis access: First results. Cardiovasc
Intervent Radiol. 40:1545–1551. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gh K, Mhs M, Ravari H, Daliri M, Hoseini L
and Nateghi M: Primary patency rate of native AV fistula: Long term
follow up. Int J Clin Exp Med. 5:173–178. 2012.PubMed/NCBI
|
|
4
|
Dixon BS: Why don't fistulas mature?
Kidney Int. 70:1413–1422. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sullivan KL, Besarab A, Bonn J, Shapiro
MJ, Gardiner GA Jr and Moritz MJ: Hemodynamics of failing dialysis
grafts. Radiology. 186:867–872. 1993.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bashar K, Conlon PJ, Kheirelseid EA,
Aherne T, Walsh SR and Leahy A: Arteriovenous fistula in dialysis
patients: Factors implicated in early and late AVF maturation
failure. Surgeon. 14:294–300. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Langer S, Kokozidou M, Heiss C, Kranz J,
Kessler T, Paulus N, Krüger T, Jacobs MJ, Lente C and Koeppel TA:
Chronic kidney disease aggravates arteriovenous fistula damage in
rats. Kidney Int. 78:1312–1321. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Low EL, Baker AH and Bradshaw AC: TGFβ,
smooth muscle cells and coronary artery disease: A review. Cell
Signal. 53:90–101. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yue Y, Ma K, Li Z and Wang Z: Angiotensin
II type 1 receptor-associated protein regulates carotid intimal
hyperplasia through controlling apoptosis of vascular smooth muscle
cells. Biochem Biophys Res Commun. 495:2030–2037. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhao J, Jourd'heuil FL, Xue M, Conti D,
Lopez-Soler RI, Ginnan R, Asif A, Singer HA, Jourd'heuil D and Long
X: Dual function for mature vascular smooth muscle cells during
arteriovenous fistula remodeling. J Am Heart Assoc.
30(e004891)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu C, Su T, Li F, Li L, Qin X, Pan W,
Feng F, Chen F, Liao D and Chen L: PI3K/Akt signaling transduction
pathway is involved in rat vascular smooth muscle cell viability
induced by apelin-13. Acta Biochim Biophys Sin (Shanghai).
42:396–402. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gerthoffer WT: Mechanisms of vascular
smooth muscle cell migration. Circ Res. 100:607–621.
2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Park ES, Kang SI, Yoo KD, Lee MY, Yoo HS,
Hong JT, Shin HS, Kim B and Yun YP: Camptothecin inhibits
platelet-derived growth factor-BB-induced viability of rat aortic
vascular smooth muscle cells through inhibition of PI3K/Akt
signaling pathway. Exp Cell Res. 319:982–991. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fang H, Yang S, Luo Y, Zhang C, Rao Y, Liu
R, Feng Y and Yu J: Notoginsenoside R1 inhibits vascular smooth
muscle cell viability, migration and neointimal hyperplasia through
PI3K/Akt signaling. Sci Rep. 8(7595)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ao H, Feng W and Peng C: Hydroxysafflor
yellow A: A promising therapeutic agent for a broad spectrum of
diseases. Evid Based Complement Alternat Med.
2018(8259280)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bai Y, Lu P, Han C, Yu C, Chen M, He F, Yi
D and Wu L: Hydroxysafflor yellow A (HSYA) from flowers of
carthamus tinctorius L. and its vasodilatation effects on pulmonary
artery. Molecules. 17:14918–14927. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen L, Xiang Y, Kong L, Zhang X, Sun B,
Wei X and Liu H: Hydroxysafflor yellow A protects against cerebral
ischemia-reperfusion injury by anti-apoptotic effect through
PI3K/Akt/GSK3β pathway in rat. Neurochem Res. 38:2268–2275.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xiao J, Lv Y, Jin F, Liu Y, Ma Y, Xiong Y,
Liu L, Zhang S, Sun Y, Tipoe GL, et al: LncRNA HANR promotes
tumorigenesis and increase of chemoresistance in hepatocellular
carcinoma. Cell Physiol Biochem. 43:1926–1938. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ye F, Wang J, Meng W, Qian J and Jin M:
Proteomic investigation of effects of hydroxysafflor yellow A in
oxidized low-density lipoprotein-induced endothelial injury. Sci
Rep. 7(17981)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jiang M, Zhou LY, Xu N and An Q:
Hydroxysafflor yellow A inhibited lipopolysaccharide-induced
non-small cell lung cancer cell proliferation, migration, and
invasion by suppressing the PI3K/AKT/mTOR and ERK/MAPK signaling
pathways. Thorac Cancer. 10:1319–1333. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Song Y, Long L, Zhang N and Liu Y:
Inhibitory effects of hydroxysafflor yellow A on PDGF-BB-induced
viability and migration of vascular smooth muscle cells via
mediating akt signaling. Mol Med Rep. 10:1555–1560. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yang G, Zhou X, Chen T, Deng Y, Yu D, Pan
S and Song Y: Hydroxysafflor yellow A inhibits
lipopolysaccharide-induced viability and migration of vascular
smooth muscle cells via toll-like receptor-4 pathway. Int J Clin
Exp Med. 8:5295–5302. 2015.PubMed/NCBI
|
|
23
|
Chen J, Shi W, Xu Y, Zhang H and Chen B:
Hirudin prevents vascular endothelial cell apoptosis and
permeability enhancement induced by the serum from rat with chronic
renal failure through inhibiting RhoA/ROCK signaling pathway. Drug
Dev Res 20: doi:10.1002, 2020.
|
|
24
|
Zhang G, Cui G, Tong S and Cao Q:
Salvianolic acid A alleviates the renal damage in rats with chronic
renal failure1. Acta Cir Bras. 34(e201900204)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xue L, Pan Z, Yin Q, Zhang P, Zhang J and
Qi W: Liraglutide promotes autophagy by regulating the AMPK/mTOR
pathway in a rat remnant kidney model of chronic renal failure. Int
Urol Nephrol. 51:2305–2313. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ngai HH, Sit WH and Wan JM: The
nephroprotective effects of the herbal medicine preparation, WH30+,
on the chemical-induced acute and chronic renal failure in rats. Am
J Chin Med. 33:491–500. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen S, Ma J, Zhu H, Deng S, Gu M and Qu
S: Hydroxysafflor yellow A attenuates high glucose-induced human
umbilical vein endothelial cell dysfunction. Hum Exp Toxicol.
38:685–693. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sun L, Xu YW, Han J, Xiao C, Cao SS, Liang
H and Cheng Y: Hydroxysafflor yellow A shows protection against
PPAR γ inactivation in nitrosative neurons. Oxid Med Cell Longev.
2018(9101740)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen Z, Liu L, Liu Y, Wang S, Zhang S,
Dong R, Xu M, Ma Y, Wang J, Zhang Q and Wei P: Hydroxysafflor
yellow A induces autophagy in human liver cancer cells by
regulating beclin 1 and ERK expression. Exp Ther Med. 19:2989–2996.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Guo X, Zheng M, Pan R, Zang B, Gao J, Ma H
and Jin M: Hydroxysafflor yellow A (HSYA) targets the
platelet-activating factor (PAF) receptor and inhibits human
bronchial smooth muscle activation induced by PAF. Food Funct.
10:4661–4673. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bahnson ES, Koo N, Cantu-Medellin N, Tsui
AY, Havelka GE, Vercammen JM, Jiang Q, Kelley EE and Kibbe MR:
Nitric oxide inhibits neointimal hyperplasia following vascular
injury via differential, cell-specific modulation of SOD-1 in the
arterial wall. Nitric Oxide. 44:8–17. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rothuizen TC, Wong C, Quax PH, van
Zonneveld AJ, Rabelink TJ and Rotmans JI: Arteriovenous access
failure: More than just intimal hyperplasia? Nephrol Dial
Transplant. 28:1085–1092. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rotmans JI, Pasterkamp G, Verhagen HJ,
Pattynama PM, Blankestijn PJ and Stroes ES: Hemodialysis access
graft failure: Time to revisit an unmet clinical need? J Nephrol.
18:9–20. 2005.PubMed/NCBI
|
|
34
|
Kokubo T, Ishikawa N, Uchida H, Chasnoff
SE, Xie X, Mathew S, Hruska KA and Choi ET: CKD accelerates
development of neointimal hyperplasia in arteriovenous fistulas. J
Am Soc Nephrol. 20:1236–1245. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Marx SO, Totary-Jain H and Marks AR:
Vascular smooth muscle cell viability in restenosis. Circ
Cardiovasc Interv. 4:104–111. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Rzucidlo EM, Martin KA and Powell RJ:
Regulation of vascular smooth muscle cell differentiation. J Vas
Surg. 45 (Suppl A):A25–A32. 2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Dember LM, Beck GJ, Allon M, Delmez JA,
Dixon BS, Greenberg A, Himmelfarb J, Vazquez MA, Gassman JJ, Greene
T, et al: Effect of clopidogrel on early failure of arteriovenous
fistulas for hemodialysis: A randomized controlled trial. JAMA.
299:2164–2171. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Al-Jaishi AA, Oliver MJ, Thomas SM, Lok
CE, Zhang JC, Garg AX, Kosa SD, Quinn RR and Moist LM: Patency
rates of the arteriovenous fistula for hemodialysis: A systematic
review and meta-analysis. Am J Kidney Dis. 63:464–478.
2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tang F, Liu M, Zeng O, Tan W, Long J, Liu
S, Yang J and Chu C: Gefitinib-coated balloon inhibits the
excessive hyperplasia of intima after vascular injuries through
PI3K/AKT pathway. Technol Health Care. 27:331–343. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhu J, Chen X, Wang H and Yan Q: Catalpol
protects mice against renal ischemia/reperfusion injury via
suppressing PI3K/Akt-eNOS signaling and inflammation. Int J Clin
Exp Med. 8:2038–2044. 2015.PubMed/NCBI
|
|
41
|
Hu S, Zhang Y, Zhang M, Guo Y, Yang P,
Zhang S, Simsekyilmaz S, Xu JF, Li J, Xiang X, et al: Aloperine
protects mice against ischemia-reperfusion (IR)-induced renal
injury by regulating PI3K/AKT/mTOR signaling and AP-1 activity. Mol
Med. 21:912–923. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Potočnjak I and Domitrović R: Carvacrol
attenuates acute kidney injury induced by cisplatin through
suppression of ERK and PI3K/Akt activation. Food Chem Tox.
98:251–261. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ma SK, Joo SY, Kim CS, Choi JS, Bae EH,
Lee J and Kim SW: Increased hosphorylation of PI3K/Akt/mTOR in the
obstructed kidney of rats with unilateral ureteral obstruction.
Chonnam Med J. 49:108–112. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ahanchi SS, Tsihlis ND and Kibbe MR: The
role of nitric oxide in the pathophysiology of intimal hyperplasia.
J Vasc Surg. 45 Suppl A:A64–A73. 2007.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hogg ME, Varu VN, Vavra AK, Popowich DA,
Banerjee MN, Martinez J, Jiang Q, Saavedra JE, Keefer LK and Kibbe
MR: Effect of nitric oxide on neointimal hyperplasia based on sex
and hormone status. Free Rad Biol Med. 50:1065–1074.
2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chen R, Chen T, Wang T, Dai X, Meng K,
Zhang S, Jiang D, Wang Y, Zhou K, Geng T, et al: Tongmai Yangxin
pill reduces myocardial no-reflow by regulating apoptosis and
activating PI3K/Akt/eNOS pathway. J Ethnopharmacol.
261(113069)2020.PubMed/NCBI View Article : Google Scholar
|