Introduction
Melanoma is one of the most aggressive skin cancers
that annually claims over 20,000 lives in Europe. The eastern half
of European countries report low incidence rates but have a high
case fatality, increasing mortality, mostly due to a late diagnosis
(1). Several studies report a
possible linear progression from common to atypical nevi that
eventually progress to melanoma (2). Moreover, there are two types of
melanoma: De novo (DNM) and nevus-associated melanoma
(3,4).
Dermoscopy is a non-invasive method of examination
that can be used for early melanoma diagnosis and can help
differentiate between benign and malignant tumors (5). Still, it is recommended to be used as
an adjuvant tool for clinical skin examination (5,6). The
‘Chaos and Clues’ algorithm is practical and easy to use (7); to date, there are few reports that use
this algorithm to distinguish atypical nevi from melanoma.
Atypical nevi are considered cutaneous lesions that
identify individuals who are at increased risk of developing
melanoma (8). They can have certain
dermoscopic features regarding pattern, colors, and clues; their
pattern can be typical (reticular/reticular with dots or clods),
occasional (structureless hyperpigmented areas in the center and
reticular at the periphery), or a combination of reticular lines
with/without clods with a structureless skin-colored area (9). The standard colors of Clark nevi are a
uniform light-brown or various shades of brown with
hyperpigmentation (9). Polychromy
can occur with multiple shades of brown or eccentric
hyperpigmentation. The specific clues of atypical nevi that can
help differentiate them from an early stage melanoma are the
presence of reticular lines, usually thin, regular dots and clods,
that can appear peripherally in an early phase of growth. Usually,
in atypical nevi, the vessels are monomorphic compared to melanoma,
where the vessels are irregular and polymorphous (9). In comparison to nevi, melanoma can
have a chaotic dermoscopic appearance, but according to several
studies, a melanoma in its early stages can be challenging to
differentiate from a Clark nevus (10).
Several algorithms can aid in the dermoscopic
differentiation between an atypical nevus and an early melanoma,
such as the ‘ABCD rule’, the Menzies method, the 7-point checklist,
the 3-point checklist, ‘Chaos and clues’, and CASH (color,
architecture, symmetry, homogeneity) (11,12).
Each algorithm is unique, with a different sensitivity and
specificity in the diagnosis of melanocytic lesions. A study
conducted by Carrera et al demonstrated that the Menzies
method was the most sensitive for melanoma diagnosis (95.1%) but
had the lowest specificity (24.8%), while the ABCD rule algorithm
had the highest specificity (59.4%) (11).
This study aimed to diagnose and differentiate
atypical nevi from early melanomas using specific clinical and
dermoscopic criteria, including the ‘Chaos and clues’ algorithm
introduced by Rosendahl et al (7).
Materials and methods
We present an observational, retrospective study of
103 melanocytic lesions dermatologically monitored between 2017 and
2019 at the Clinical Hospital and private Dermatology offices of
Sibiu and Oradea County. The lesions were examined clinically,
dermoscopically, and histopathologically. The data collected were
related to the assessed clinical and dermoscopic features of the
lesions, which were examined and revised by three evaluators. The
dimensions of the tumors were measured in millimeters (mm), and the
dermoscopic images were evaluated for the presence/absence of chaos
and any of Rosendahl's et al (7) clues for malignancy. The colors of the
lesions and clinical criteria were also recorded. As this was an
observational study, it was exempted from the Ethics Committee of
Sibiu's County Clinical Hospital review (Sibiu, Romania).
Statistical analysis
Data were collected and tabulated on Microsoft Excel
spreadsheets for statistical analysis [calculation of the
prevalence of the variables (%), the median size of the lesions,
and the number of colors]. The variables are expressed in numbers
and percentages to simplify the statistical process.
Results
The selected tumors were examined clinically,
through dermoscopy, and a part of them were confirmed
histopathologically. Out of 103 lesions, only 45.63% (47 lesions)
were excised and had a histopathological exam, partly because most
patients refused to have an interventional treatment and preferred
to be followed-up at 3-6 months. Among the excised lesions, 70.21%
(33 lesions) were atypical nevi, 14.89% (7 lesions) melanomas, and
14.89% (7 lesions) common nevi. Regarding the pathologically
confirmed melanomas, the clinical and dermoscopic examinations were
in accordance with the pathology reports. The majority of the
melanoma subtypes was lentigo maligna melanoma (LMM) with a median
Breslow index (BI) of 1.28 mm, followed by superficial spreading
melanoma (SSM) with an IB of 0.5-1 mm. Two achromic melanomas
(ungual and SSM) were also observed. A percentage of 48.54% of the
selected lesions belonged to patients with atypical mole syndrome
(AMS). Clinically, the ‘ABCD rule’ (A-asymmetry, B-border, C-color,
D-diameter) was used to assess the most frequent criteria found in
the histologically confirmed benign and malignant tumors. We
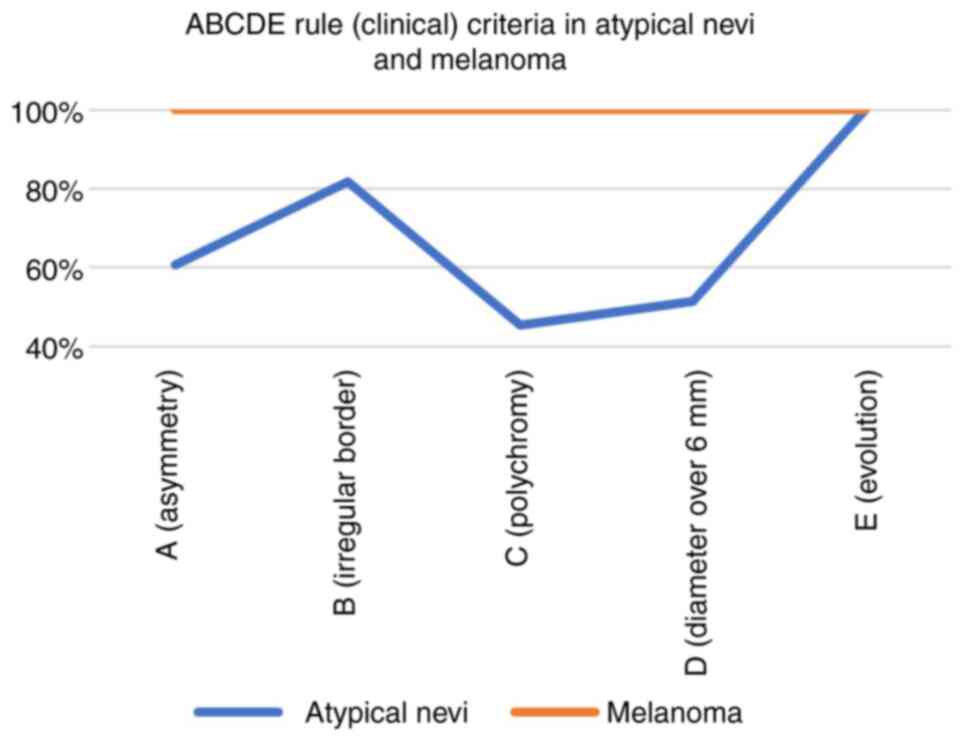
obtained the following: The ‘E’ (100.00%) and ‘B’ (81.81%) were the
most frequently encountered criteria in the population with
atypical nevi (Fig. 1). All the
ABCDE criteria were present in the melanoma tumors (100.00%); the
criteria ‘A’, ‘B’, and ‘C’ were the most frequently encountered in
the biopsied common nevi, with a percentage of 85.71%, which
clinically justified the decision to biopsy them.
The tumors were also assessed using the ‘Chaos and
clues’ algorithm and specific dermoscopy criteria to differentiate
benign from malignant lesions and observe the most specific clues
for atypical nevi and melanoma.
First, the lesions were assessed by the ‘chaos’
(asymmetry of pattern or color) criterion, and out of 103 examined
tumors, a percentage of 42.71% had a chaotic appearance and were
analyzed further to see which clues of malignancy were the most
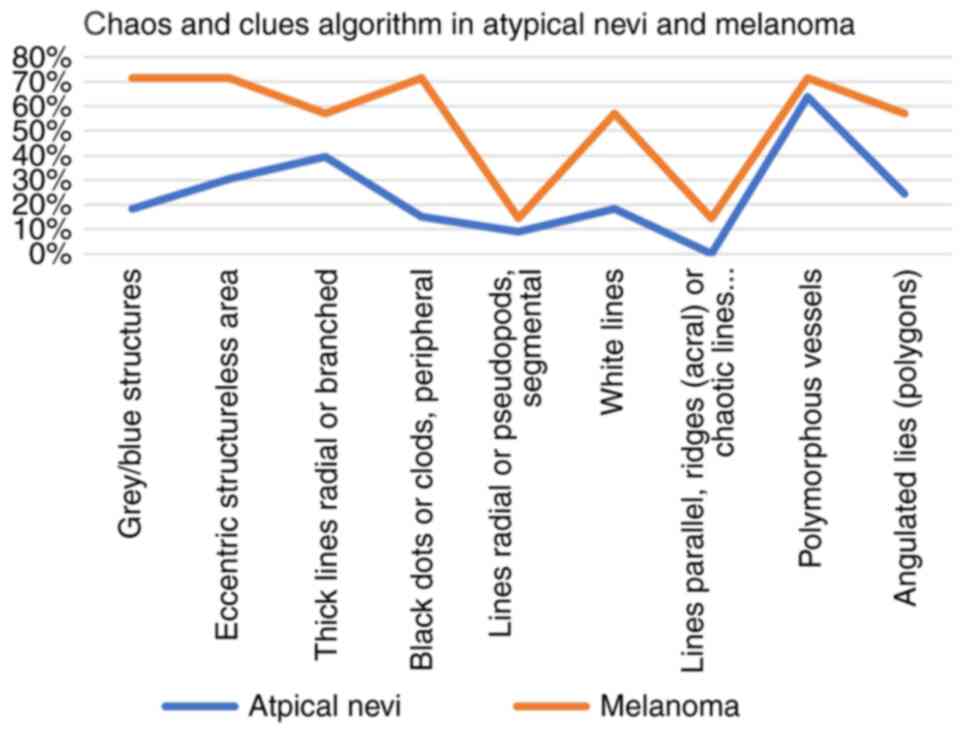
detected. A percentage of 66.66% of the atypical nevi had a chaotic
appearance, with a median of 2.21 out of 9 clues. The most
encountered clues were polymorphous vessels (63.63%) and
reticular/branched thick lines (39.39%), while less frequent were
the radial lines/pseudopods, 9.09% (Fig. 2). Parallel lines, ridges (acral), or
chaotic lines (nails) were not present in the atypical nevi
population.
All melanoma tumors presented chaos, with a median
of 4.85 out of 9 clues.
The most specific clues for melanoma were
polymorphous vessels, grey/blue structures, eccentric structureless
area, peripheral black dots/clods (71.42%), followed by white
lines, angulated lines, thick reticular/branched lines (57.14%)
(Fig. 3).
All atypical nevi had more than one color (median of
3.27 colors), and the most prevalent colors were: Light brown
(100.00%), dark brown (96.96%), and black (57.57%). The studied
dysplastic nevi had a median size of 6.46 mm. All melanomas had
more than one color with a median of 5.71 colors; the most
encountered colors were light and dark brown (85.71%), followed by
black, white, and grey (71.42%). The median size of the melanoma
tumors was 16.42 mm, most of them having a size >10 mm (85.71%)
(Table I).
 | Table ISize and colors of atypical nevi and
melanoma. |
Table I
Size and colors of atypical nevi and
melanoma.
| Atypical nevi
features % | Melanoma features
% |
|---|
| Colors | Colors |
|
Blue
3.03% |
Blue
57.14% |
|
Light brown
100.00% |
Light brown
85.71% |
|
Dark brown
96.96% |
Dark brown
85.71% |
|
Black
57.57% |
Black
71.42% |
|
White
12.12% |
White
71.42% |
|
Grey
24.24% |
Grey
71.42% |
|
Red
24.24% |
Red
42.85% |
|
Purple
0.00% |
Purple
42.85% |
|
Yellow
9.09% |
Yellow
28.57% |
|
Orange
0.00% |
Orange
14.28% |
|
Median no.
of colors 3.27 |
Median no.
of colors 5.71 |
| Size | Size |
|
<5
mm=39.39% |
<5
mm=0.00% |
|
5-10
mm=42.42% |
5-10
mm=14.28% |
|
>10
mm=18.18% |
>10
mm=85.71% |
|
Median size
of 6.46 mm |
Median size
of 16.42 mm |
| Total no. of
lesions=33 | Total no. of
lesions=7 |
Discussion
Dermoscopy is a helpful method of examination that
can improve the early diagnosis of melanoma compared to clinical
examination. The ‘Chaos and clues’ algorithm was created to be
applied to melanocytic lesions, and it is used to detect
malignancy. According to Rosendahl et al, this algorithm has
a sensitivity of 90.60% and a specificity of 62.70% for malignancy
diagnosis (7). However, the lesions
should first be examined through dermoscopy for chaos (asymmetry of
pattern or color).
When chaos is encountered, the clinician should
search for at least one clue of malignancy: Grey/blue structures,
eccentric structureless area, thick reticular/branched lines,
peripheral black dots/clods, segmental pseudopods/radial lines,
white lines, parallel lines, ridges (acral) or chaotic lines
(nails), polymorphous vessels, angulated lines (polygons). If at
least one clue of malignancy is present, the lesion is indicated
for excision. All malignant tumors examined by dermoscopy presented
‘chaos’, similar to Ramji et al (13).
The most specific melanoma criteria of the present
study included polymorphous vessels, grey/blue structures,
eccentric structureless areas, peripheric black dots/clods
(71.42%), followed by white lines, thick reticular/branched lines,
and angulated lines (57.14%). In atypical nevi, white lines were
uncommon, similar to a study by Verzi et al, which reported
that white streaks are highly unusual and more specific to
melanomas; 31 out of 144 melanomas presented white lines (22%)
(14).
According to a study by Marghoob et al, white
lines (structures) have a specificity of 80.6% for melanoma (2.5 to
9.7 OR) (15). Recently, the
angulated lines clue was added to the original ‘Chaos and clues’
algorithm by Jaimes and coworkers, as they consider that it is a
specific feature of flat melanomas on chronic-sun damaged skin
(16). In the present study,
angulated lines were present in 57.14% of the melanoma population
and 24.24% of the atypical nevi, which demonstrates their
specificity for malignant tumors. Carrera et al report that
structureless areas were detected in 47.6% of the nevi examined,
while in our study, we observed this criterion (eccentric
structureless areas) in 30.30% of the atypical nevi, in 50.00% of
the common nevi, and 71.42% of the melanoma tumors (11). A study by Lallas et al
suggested that irregular hyperpigmented areas represent melanoma
indicators, compared to atypical nevi (17); the same was demonstrated in our
study regarding the eccentrical structureless areas.
According to Rezze et al, the ‘ABCDE rule’
can be useful in the clinical diagnosis of atypical nevi (18). Clinically, our study showed that all
the biopsied atypical nevi had the ‘E’ (evolution) criterion of the
‘ABCDE rule’, which outlines the importance of periodic clinical
and dermoscopic examination of these lesions since many patients
can be unaware of changes in their nevi. In a study by Rivers et
al, 13 out of 16 patients were unaware of any change in their
cutaneous lesions (8).
The color and size analyses showed that melanomas
present more colors (median of 3.27 colors in NA vs. 5.71 in MM)
and are more prominent (median of 6.46 mm in NA vs. 16.42 mm in MM)
than atypical nevi. All melanomas had more than one color, and the
most encountered colors were light and dark brown (85.71%),
followed by white, black, and grey (71.42%), similar to the study
by Ramji et al in which the most frequent colors were light
and dark brown (100 and 98%) and grey (75%) (13).
Several risk factors can drive a common nevus to
malignancy since there seems to be a linear progression from
atypical nevi to melanoma (2). The
most important risk factors are UV radiation and genetics.
According to a study by Fechete et al, melanoma patients are
more likely to have fair skin, freckles, a large number of nevi
(over 20), atypical nevi, frequently or partially outdoor
occupations, had over three sunburns during early life, or had used
sun beds (19). In our study,
48.54% of the patients had multiple nevi (atypical mole
syndrome).
According to different studies, most melanomas
develop de novo (DNM), while nevus-associated melanomas
(NAM) are less frequent (3,4). Vezzoni et al report two types
of NAM: Melanoma that develops in the center of a mole (probably a
congenital nevus) and melanoma that arises next to the mole
(dysplastic nevus) (20). In our
study, three melanomas had eccentrical black pigmentation (possible
melanomas developed from atypical nevi), and only one had central
pigmentation (possible melanoma developed from a congenital nevus).
According to Haenssle et al, patients with multiple nevi and
without previous melanomas or atypical mole syndrome had a higher
frequency of NAM (21). Alendar and
Kittler (22) and Lin et al
(23) report that survival does not
differ significantly between patients with NAM and patients with
DNM). On the contrary, Cymerman et al suggest that patients
with DNM have a more unsatisfactory outcome than NAM patients
(24).
Our study’s limitations are the relatively low
number of the examined tumors and the subjectivity of the clinical
and dermoscopic assessment of the melanocytic lesions, which is why
some results may differ from other studies. The ‘Chaos and clues’
algorithm did not necessarily help distinguish between benign and
malignant tumors but helped demonstrate that nevi and melanoma have
similar characteristics. Possibly, confocal laser scanning
microscopy (CLSM) would have aided in establishing a prompt
diagnosis, as it is known to differentiate between different skin
tumors (25), but unfortunately, we
do not have such equipment in our daily practice.
In conclusion, all suspicious pigmented lesions with
a recent change in history should be monitored carefully through
clinical and dermoscopic examination. Most atypical nevi can mimic
an early melanoma; that is why a highly atypical pigmented lesion
should be excised after a thorough clinical and dermoscopic
examination. Early detection of melanoma is critical in individuals
with atypical nevi because they have an increased risk of
developing melanoma compared to the general population (8). The presence of chaos/malignancy clues
should lead to the excision of the assessed lesion to exclude
malignancy (13). Certain authors
report a linear progression of common nevi to atypical nevi that
may transform into melanoma in time (2). A malignant transformation of a nevus
cannot be predicted, but periodical dermoscopic follow-up can help
differentiate a nevus from an early melanoma (26). It is often difficult to
differentiate between atypical nevi and early melanoma through the
usual examination methods (dermoscopy, histology). In this case,
modern techniques of molecular biology or CLSM can be used to
distinguish benign from malignant tumors (melanoma). Moreover,
investigating the molecular biology of melanoma or using the CLSM
could help in finding the best therapeutic approach and to possibly
identify new therapies (25,27).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used or analyzed during the present
study are available from the corresponding author on reasonable
request revision of the manuscript for important intellectual
content.
Authors' contributions
CRJM was responsible for the manuscript design,
conception, drafting, analysis, and interpretation of the data. SF
contributed to the manuscript drafting, data acquisition, and
critical revision of the manuscript. MR contributed to the
manuscript's conception and design, analysis, acquisition, and
interpretation of the data, manuscript drafting, and critical
revision of the manuscript for important intellectual content. All
authors contributed significantly to this publication and read and
approved the final manuscript.
Ethics approval and consent to
participate
As this was an observational study, it was exempted
from the Ethics Committee of Sibiu's Emergency Clinical County
Hospital review (Sibiu, Romania).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Forsea AM: Melanoma epidemiology and early
detection in europe: Diversity and disparities. Dermatol Pract
Concept. 10(e2020033)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shain AH and Bastian BC: From melanocytes
to melanomas. Nat Rev Cancer. 16:345–358. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pampena R, Kyrgidis A, Lallas A,
Moscarella E, Argenziano G and Longo C: A meta-analysis of
nevus-associated melanoma: Prevalence and practical implications. J
Am Acad Dermatol. 77:938–945.e4. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pandeya N, Kvaskoff M, Olsen CM, Green AC,
Perry S, Baxter C, Davis MB, Mortimore R, Westacott L, Wood D, et
al: Factors related to nevus-associated cutaneous melanoma: A
case-case study. J Invest Dermatol. 138:1816–1824. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gniadecki R and Mourad A: Differentiating
malignant melanoma from other lesions using dermoscopy. Can Fam
Physician. 65:412–414. 2019.PubMed/NCBI
|
|
6
|
Rotaru M, Jitian CR and Iancu GM: A
10-year retrospective study of melanoma stage at diagnosis in the
academic emergency hospital of Sibiu county. Oncol Lett.
17:4145–4148. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rosendahl C, Cameron A, McColl I and
Wilkinson D: Dermatoscopy in routine practice-’chaos and clues’.
Aust Fam Physician. 41:482–487. 2012.PubMed/NCBI
|
|
8
|
Rivers JK, Kopf AW, Vinokur AF, Rigel DS,
Friedman RJ, Heilman ER and Levenstein M: Clinical characteristics
of malignant melanomas developing in persons with dysplastic nevi.
Cancer. 65:1232–1236. 1990.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kittler H, Rosendahl C, Cameron A and
Tschandl P: Dermatoscopy pattern analysis of pigmented and
non-pigmented lesions. 2nd edition. Facultas Verlags, 2016.
|
|
10
|
Rose SE, Argenziano G and Marghoob AA:
Melanomas difficult to diagnose via dermoscopy. G Ital Dermatol
Venereol. 145:111–126. 2010.PubMed/NCBI
|
|
11
|
Carrera C, Marchetti MA, Dusza SW,
Argenziano G, Braun RP, Halpern AC, Jaimes N, Kittler HJ, Malvehy
J, Menzies SW, et al: Validity and reliability of dermoscopic
criteria used to differentiate nevi from melanoma: A web-based
international dermoscopy society study. JAMA Dermatol. 152:798–806.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Weber P, Tschandl P, Sinz C and Kittler H:
Dermatoscopy of neoplastic skin lesions: Recent advances, updates
and revisions. Curr Treat Options Oncol. 19(56)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ramji R, Valdes-Gonzalez G, Oakley A and
Rademaker M: Dermoscopic ‘Chaos and Clues’ in the diagnosis of
melanoma in situ. Australas J Dermatol. 59:201–205. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Verzi AE, Quan VL, Walton KE, Martini MC,
Marghoob AA, Garfield EM, Kong BY, Isales MC, VandenBoom T, Zhang
B, et al: The diagnostic value and histologic correlate of distinct
patterns of shiny white streaks for the diagnosis of melanoma: A
retrospective, case-control study. J Am Acad Dermatol. 78:913–919.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Marghoob NG, Liopyris K and Jaimes N:
Dermoscopy: A review of the structures that facilitate melanoma
detection. J Am Osteopath Assoc. 119:380–390. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jaimes N, Marghoob AA, Rabinovitz H, Braun
RP, Cameron A, Rosendahl C, Canning G and Keir J: Clinical and
dermoscopic characteristics of melanomas on nonfacial chronically
sun-damaged skin. J Am Acad Dermatol. 72:1027–1035. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lallas A, Longo C, Manfredini M, Benati E,
Babino G, Chinazzo C, Apalla Z, Papageorgiou C, Moscarella E,
Kyrgidis A and Argenziano G: Accuracy of dermoscopic criteria for
the diagnosis of melanoma in situ. JAMA Dermatol. 154:414–419.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rezze GG, Leon A and Duprat J: Dysplastic
nevus (atypical nevus). An Bras Dermatol. 85:863–871.
2010.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
19
|
Fechete O, Ungureanu L, Șenilă S,
Vornicescu D, Dănescu S, Vasilovici A, Candrea E, Vesa ȘC and
Cosgarea R: Risk factors for melanoma and skin health behaviour: An
analysis on Romanian melanoma patients. Oncol Lett. 17:4139–4144.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vezzoni R, Conforti C, Vichi S, Giuffrida
R, Retrosi C, Magaton-Rizzi G, Di Meo N, Pizzichetta MA and
Zalaudek I: Is there more than one road to nevus-associated
melanoma? Dermatol Pract Concept. 10(e2020028)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Haenssle HA, Mograby N, Ngassa A, Buhl T,
Emmert S, Schön MP, Rosenberger A and Bertsch HP: Association of
patient risk factors and frequency of nevus-associated cutaneous
melanomas. JAMA Dermatol. 152:291–298. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Alendar T and Kittler H: Morphologic
characteristics of nevi associated with melanoma: A clinical,
dermatoscopic and histopathologic analysis. Dermatol Pract Concept.
8:104–108. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lin WM, Luo S, Muzikansky A, Lobo AZ,
Tanabe KK, Sober AJ, Cosimi AB, Tsao H and Duncan LM: Outcome of
patients with de novo versus nevus-associated melanoma. J Am Acad
Dermatol. 72:54–58. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cymerman RM, Shao Y, Wang K, Zhang Y,
Murzaku EC, Penn LA, Osman I and Polsky D: De novo vs
nevus-associated melanomas: Differences in associations with
prognostic indicators and survival. J Natl Cancer Inst.
108(djw121)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ilie MA, Caruntu C, Lupu M, Lixandru D,
Tampa M, Georgescu SR, Bastian A, Constantin C, Neagu M, Zurac SA
and Boda D: Current and future applications of confocal laser
scanning microscopy imaging in skin oncology. Oncol Lett.
17:4102–4111. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rotaru M, Nati AE, Avrămoiu I, Grosu F and
Mălăescu GD: Digital dermoscopic follow-up of 1544 melanocytic
nevi. Rom J Morphol Embryol. 56:1467–1472. 2015.PubMed/NCBI
|
|
27
|
Surcel M, Căruntu C, Tampa M, Matei C,
Pițuru S, Georgescu SR, Constantin C, Zurac C and Neagu M:
Adrenergic modulation of melanoma cells proliferation. Farmacia.
66:820–825. 2018.
|