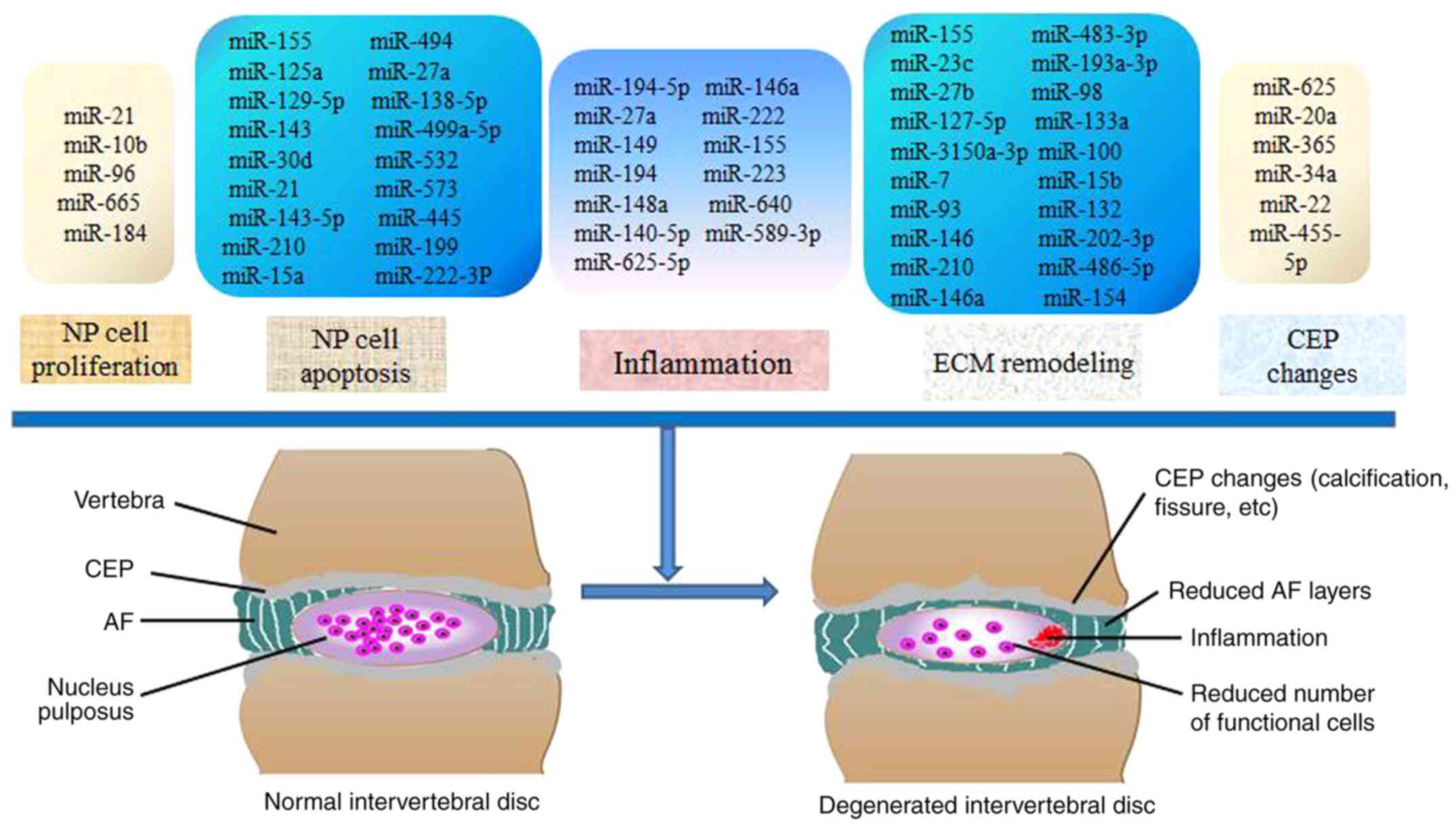
Intervertebral disc (IVD) degeneration (IDD) is one
of the main causes of low back pain (1-3).
Low back pain is common, with ~80% of the population experiencing
low back pain at different time points during their lifetime
(4,5), and ~40% of all cases are caused by IDD
(6). Low back pain caused by IDD
costs ~70 billion euros annually worldwide (7). IDD not only affects the quality of
life of the patients, but also poses a major socioeconomic burden.
However, there is currently no effective and reliable treatment for
IDD, mainly due to its unknown pathogenesis. Reversing or delaying
the progression of IDD is particularly important for restoring the
original physiological structure and function of the spine.
IDD has been widely considered as the result of
‘wear and tear’ due to aging and mechanical strain (8), but these factors have limited impact
on the IVDs (9), and several
studies have found that genetic factors account for 74% of all
cases of IDD (10). MicroRNAs
(miRNAs/miRs) are small endogenous non-coding RNAs that regulate
gene expression at the post-transcriptional level (11), participating in a number of
processes, including cell proliferation, differentiation and
apoptosis, among others (12,13).
miRNAs have also been closely linked to the process of disc
degeneration, with several advances in related research (14-17).
The aim of the present review was to focus on the progress of the
research on the role of miRNAs in nucleus pulposus (NP) cell (NPC)
proliferation and apoptosis, inflammation, extracellular matrix
(ECM) remodeling and cartilaginous endplate (CEP) changes, and to
discuss the pathogenic mechanisms and potential therapeutic
prospects of miRNAs in the treatment of IDD.
The molecular pathological mechanism of IDD remains
largely unclear. However, previous studies have shown that IDD is
closely associated with apoptosis, cell proliferation, ECM
degradation and inflammation (18,19).
There is increasing evidence that miRNAs are involved in several
aspects of cellular function, such as proliferation, apoptosis and
inflammation, thereby regulating a series of pathophysiological
changes that affect a number of processes. Several studies have
reported significant changes in miRNA expression in degenerated IVD
tissue (20-28),
a number of which may be involved in the pathological process of
IDD.
Young and healthy human IVDs contain two types of
cells: Notochordal cells, which are vacuolated cells originating
from the embryonic notochord; and NPCs. NPCs are less dense in
healthy human IVDs and have specific distribution areas (29,30).
One characteristic of IVD degeneration is the appearance of
clusters of cells, particularly in damaged areas (31). The appearance of cell clusters is
considered to be the result of abnormal proliferation of NPCs,
which is closely associated with IVD degeneration (32). Proliferation is the basic life
activity of cells and is affected by a number of factors. miRNAs
regulate a variety of physiological activities and pathological
processes, including cell proliferation, at the
post-transcriptional level (12,33).
miR-21 is one of the most extensively investigated
miRNAs. It is expressed in a variety of tissue types (34-36)
and is involved in the regulation of cell proliferation (37). The expression of miR-21 in
degenerated NP tissue is significantly higher compared with that in
healthy NP tissue and is closely associated with the degree of IVD
degeneration (14). Moreover,
bioinformatics target prediction has indicated that PTEN may be the
target of miR-21, that miR-21 inhibits PTEN expression by directly
targeting its 3'-untranslated region, and this inhibition is
eliminated by miR-21 binding site mutation (14). In addition, miR-21
overexpression-mediated cell proliferation and increased cyclin D1
expression were almost completely blocked by the Akt inhibitor,
Ly294002(14). In conclusion, the
abnormal upregulation of miR-21 in IVDs may target PTEN, which is
involved in the abnormal proliferation of NPCs through derepressing
the Akt pathway (14). This
suggests that the miR-21 and PTEN/Akt pathways may be potential
targets for inhibiting the abnormal proliferation of NPCs. In
addition, miR-21 inhibitors can inhibit the expression of
hypoxia-inducible factor-1α and VEGF in the annulus fibrosus (AF)
and NP, and inhibit NPC apoptosis (15). Furthermore, miR-21 may promote the
proliferation of NPCs via targeting programmed cell death
4(16). Upregulation of miR-21 also
increases the expression of MMP-2 and MMP-9 mRNA (16). Proteins in the MMP family are
classified into three categories based on the degradation
substrate: Collagenases (for example, MMP-1 and MMP-13),
gelatinases (for example, MMP-2 and MMP-9), which act on denatured
collagen and collagen types IV and V, and stromelysins (for
example, MMP-3) (17). Therefore,
miR-21 not only regulates the number of NPCs, but also regulates in
the expression of MMP.
As another potentially important miRNA, miR-10b has
not only been found to be expressed in a variety of tissue types,
but also has several functions (38), and its abnormal expression is
closely associated with the occurrence of malignant tumors
dominated by uncontrollable cell proliferation (39). Compared with the NP tissue of
patients with idiopathic scoliosis, miR-10b expression in
degenerated NP tissues is significantly increased and is closely
associated with the degree of disc degeneration (40). In vitro, miR-10b
overexpression was shown to stimulate NPC proliferation and inhibit
the translation of the homeobox D10 (HOXD10) gene, whereas restored
HOXD10 expression reversed the pro-mitotic effect of miR-10b
(40). miR-10b-mediated
downregulation of HOXD10 expression resulted in increased Ras
homolog gene family member C (RhoC) expression and Akt
phosphorylation. By downregulating RhoC or inhibiting Akt, the
effects of miR-10b on NPC proliferation were eliminated (40). This suggests that abnormal
upregulation of miR-10b in IDD may result in abnormal proliferation
of NPCs by targeting HOXD10 to inhibit the RhoC/Akt pathway.
miR-96 was found to be upregulated in human
degenerated NP tissue and was positively correlated with the degree
of IDD (41). Overexpression of
miR-96 may promote NPC proliferation by targeting AT-rich
interaction domain 2 to activate the Akt signaling pathway
(41). miR-665 is similar to miR-9
in that its expression increases with the aggravation of disc
degeneration. The increased expression of miR-665 not only promotes
NPC proliferation, but also reduces aggrecan and type II collagen
expression and increases MMP-3 and MMP-13 expression by inhibiting
the expression of growth differentiation factor 5 in NPCs (42). miR-125b-1-3p regulates the cell
cycle proteins cyclin D1 and B1 by targeting teashirt zinc finger
homeobox 3, which may be involved in the regulation of NPC
proliferation (43). In addition,
miR-184 was elevated in degenerative NP tissues and promoted
abnormal proliferation of NPCs via the growth arrest-specific 1/Akt
pathway (44). The abnormal
proliferation of NPCs is one of the early changes observed in IDD,
and some miRNAs are involved in this important pathological change
(Table I). Therefore, these miRNAs
are expected to represent targets for the early detection and
prevention of IDD.
Apoptosis, or programmed cell death, is an important
factor in IDD. NPCs are the main source of ECM in IVD tissue. When
NPCs become apoptotic, the amount of ECM is reduced, and the water
in the IVD tissue cannot be retained, resulting in the loss of
biomechanical properties (45). NPC
apoptosis is considered to be an important mechanism involved in
IDD, and some miRNAs have been reported to be involved in the
regulation of apoptosis in NPCs (Table
II) (46-64).
A growing body of literature suggests that miR-494
plays an important role in the regulation of apoptosis in NPCs. It
has been found that miR-494 is upregulated in degenerated human IVD
tissue, and inhibition of miR-494 may protect NPCs from
TNF-α-induced apoptosis by targeting JunD and cytochrome c
(46). In addition, miR-494
promotes the expression of ECM resolution factors, such as MMPs and
a disintegrin and metalloproteinase with thrombospondin motifs
(ADAMTS), by directly targeting sex-determining region Y-box (SOX)9
and reducing the expression of type II collagen and aggrecan, which
mediates the apoptosis of degenerated human NPCs (47). Some scholars have observed elevated
miR-494 expression levels in IDD rats, whereas miR-494 inhibitors
reduced caspase-3 and Bax expression, and increased
neurooncological ventral antigen 1 (NOVA1) and Bcl-2 expression
(48). NOVA1 was identified as a
target gene of miR-494 by a dual-luciferase reporter assay
(48). miR-494 is widely involved
in the apoptosis of NPCs and plays an important role in the
progression of IDD. Therefore, it may represent a promising
potential target for IDD treatment.
Recent research has found decreased expression of
miR-129-5p in human IDD, whereas NPCs treated with miR-129-5p
mimics or bone morphogenic protein 2 (BMP-2) siRNA exhibited
improved survival and inhibition of apoptosis (49). Therefore, the abnormal expression of
miR-129-5p may serve a role in IDD by targeting BMP-2. Similar to
miR-129-5p, miR-499a-5p was also found to be significantly
downregulated in human degenerated NP tissue. miR-499a-5p knockout
promoted NPC apoptosis, stimulated caspase activation, enhanced
MMP-3 and MMP-13 expression, and decreased aggrecan and type II
collagen expression (50). In
addition, overexpression of miR-499a-5p alleviated the apoptosis of
TNF-α-treated NPCs and the imbalance of ECM anabolism and
catabolism; however, the abnormal expression of SOX4 weakened the
negative effect of miR-499a-5p on NPC apoptosis and the positive
effect on ECM synthesis (50). This
indicates that the effects of miR-499a-5p may be mediated by
targeting SOX4. NPCs are the main functional cells of the IVD. The
ECM produced by NPCs is the structural basis of the biomechanical
properties of the IVD.
Consequently, the abnormal proliferation of NPCs is
an early pathological change of IDD (44) and may be helpful for the early
identification of IDD. Whether the function of NPCs after early
abnormal proliferation is the same as those of the parental NPCs
needs further study. The ECM in IVD tissue is mainly derived from
NPCs, and the apoptosis of NPCs will accelerate the degeneration of
the IVD. Further understanding the association between miRNAs and
NPC apoptosis in the process of IDD may provide new approaches to
delaying or reversing IDD.
Inflammation is considered to be an important
mechanism in the IDD process. The concentrations of nitric oxide,
prostaglandin E2, IL-1β, IL-6 and TNF-α in degenerated IVDs were
found to be higher compared with those in normal IVDs (64-66).
Furthermore, IL-1β and TNF-α mediate catabolism and anti-anabolism
within the NP, which are largely involved in the establishment and
progression of IDD (67,68). In recent years, numerous studies
(69-83)
have revealed that miRNAs may be involved in IDD through the
regulation of inflammation (Table
III).
miR-146a has been reported to inhibit the mRNA
expression of IL-1-mediated inflammatory genes and catabolic
proteinases, as well as the protein level of IL-1-mediated MMPs and
aggrecanases (69). In 2017, Lv
et al (70) demonstrated
that the expression of miR-146a in peripheral blood mononuclear
cells of patients with IDD was significantly downregulated. In
addition, they found that overexpression of miR-146a could
significantly downregulate the levels of pro-inflammatory cytokines
(IL-1β, IL-6 and TNF-α) in lipopolysaccharide-stimulated NPCs, and
confirmed that these effects depend on the TNF receptor
associated-associated factor 6/NF-κB pathway.
miR-194-5p was also found to be significantly
downregulated in patients with IDD by miRNA-based microarray
analysis (71).
Inhibition/overexpression of miR-194-5p led to
inhibition/overexpression of cullin family (CUL) gene 4A (CUL4A)
and CUL4B (71). Furthermore, IL-6
and TNF-α inhibitors in NPCs and AF cells reduced the expression of
CUL4A and CUL4B (71). Similar to
miR-194-5p, miR-149 was significantly reduced in
lipopolysaccharide-induced NPCs (71). Overexpression of miR-149 reversed
the expression of aggrecan and collagen II, and alleviated the
increase in MMP-3, ADAMTS4 and inflammatory cytokines by targeting
myeloid differentiation factor 88(72). In vitro, miR-222
mimics/inhibitors were able to promote/inhibit NPC apoptosis,
respectively (74). Moreover,
transfection of miR-222 mimics/inhibitors could significantly
increase/reduce the production of TNF-α, IL-1β, and IL-6 and
inhibit/enhance the expression of collagen II and aggrecan,
respectively (73).
Therefore, miRNAs directly or indirectly affect the
ECM, NPCs and AF cells of the IVD through the regulation of
inflammatory factors, subsequently influencing the process of IDD.
Taking specific miRNAs as the entry point to control the
progression of inflammation in the process of IDD may be an
important approach to delaying the progression of IDD.
In human IVDs, the ECM is mainly composed of
proteoglycans and type II collagen, which not only retain water,
but also help maintain osmotic pressure, thus conferring unique
biomechanical properties (84). The
balance of ECM catabolism and anabolism is the basis of the
biomechanical function of the IVD. An important feature of IDD is
that ECM catabolism is greater than its anabolism (85). Recently, a number of studies
demonstrated that miRNAs may be involved in the regulation of the
ECM in IDD by regulating key molecules (for example, MMPs, collagen
II and ADAMTS) that affect anabolic and catabolic processes
(25,86-103)
(Table IV).
In degenerated disc tissue, inhibition of miR-155
was shown to reduce the expression of collagen II and
glycosaminoglycans by targeting ERK1/2(86). A previous study using an IDD mouse
model demonstrated that upregulation of miR-155 upregulated the
expression of aggrecan and collagen type II, and downregulated
MMP-16(87), suggesting that
miR-155 serves an important regulatory role in ECM anabolism and
catabolism.
MMPs are classified into three categories based on
the degradation substrate: Collagenases, gelatinases, or
stromelysins (37). MMPs are key
molecules regulating ECM catabolism. Several miRNAs targeting the
MMP family participate in the regulation of ECM metabolism
(18-20,88,89).
These findings indicate that the involvement of miRNAs in ECM
catabolism may be closely associated with the regulation of MMP
expression. The effects of MMPs on ECM catabolism and anabolism are
important. Therefore, by regulating MMPs, these miRNAs may
represent important biological molecules in the alleviation, or
even the reversal of ECM loss.
Reversing or alleviating ECM loss is crucial for
IDD. Aucubin, a compound found in traditional Chinese medicines,
was reported to play a key role in the regulation of the ECM
(90). In IDD, the increased
release of pro-inflammatory factors by NPCs can cause the
degradation of the ECM. However, aucubin can alleviate the
degradation of ECM mediated by IL-1β or TNF-α by regulating the
miR-140/cAMP responsive element binding protein 1 axis (90). In addition, Bu Shen Hu Xue Fang
(BSHXF), a traditional Chinese medicine, is composed of six herbs
(Cortex Eucommiae ulmoides, Fructus Psoraleae,
Achyranthes bidentata, Salvia miltiorrhiza, Radix
clematidis and Chaenomeles speciosa). Through the
regulation of the Wnt signaling pathway by miR-483-3p and miR-23c,
BSHXF was shown to affect ECM synthesis and NPC proliferation
(91). Thus, traditional Chinese
medicine may also be of value in the study of IDD.
The CEP is located between the vertebral endplate
and the NP, and is mainly composed of hyaline cartilage cells and
chondrocytes, and the ECM they produce. CEP degradation is
accompanied by a loss of nutrients in the ECM, which is a major
cause for the development of IDD (104). CEP serves as a mechanical shock
absorber and is also an important channel for the free transmission
of nutrients and metabolites between the avascular NP and the
vertebral body (105). It also
serves as a barrier and part of the body's defense against toxic
and harmful substances, such as inflammatory factors, MMPs and
immune molecules, entering the NP (106,107). The occurrence of IDD may be
associated with CEP degeneration, dysfunction, and calcification
(108,109). Some miRNAs (110-114)
have been found to be involved in the changes of the CEP in the
progress of IDD (Table V).
Chondrocytes are an important part of the CEP.
However, it was demonstrated that upregulation of miR-34a
expression in human degenerated chondrocytes caused Fas-mediated
CEP chondrocyte apoptosis (110).
Similar to miR-34a, downregulation of miR-625 also caused
Fas-mediated cervical CEP chondrocyte apoptosis (111). These findings may indicate that
the effects of miRNAs on chondrocyte apoptosis in the CEP may be
mediated by Fas. Further research may elucidate the role of miRNAs
in the apoptosis of CEP chondrocytes.
Interestingly, estrogen (17β-estradiol, E2) could
inhibit apoptosis of CEP cells and restore cell viability and cell
cycle progression in the G0/G1 phase (112). The luciferase assay demonstrated
that estrogen receptor α (ERα) was a target of miR-221(112). This indicates that miR-221 may
affect the protective effect of estrogen on degenerating CEP cells
through targeting ERα. However, there are few studies on estrogen
in IDD, and its specific effect and mechanism require further
research.
The CEP is an important load-bearing structure, and
long-term mechanical loads are considered to be one of the causes
of IDD (113). miR-365 is a
mechanically sensitive miRNA, which directly targets histone
deacetylase 4 to regulate the degeneration of human chondrocytes
(114). In addition, the
TGF-β/SMAD signaling pathway inhibits the intermittent cyclic
mechanical tension-mediated degeneration of CEP chondrocytes by
regulating the miR-455-5p/Runt-related transcription factor 2
(Runx2) axis (115). In addition,
it was previously demonstrated that miR-20a/ankylosis protein
homolog regulates stiff matrix-promoted CEP calcification (116). Chondrocyte degeneration and
calcification affect the function of the CEP, which is an important
factor in CEP degeneration.
The AF consists of bundles of fibers arranged in a
crisscross pattern and is an important structure surrounding the
NP. AF cells may be a source of pluripotent stem cells with the
potential to differentiate into adipocytes, chondrocytes, neurons,
osteoblasts and endothelial cells (117). The normal AF and degenerated AF
cells were found to undergo osteogenic differentiation, as shown by
mineralization of cultured cells and increased mRNA expression of
BMP2, Runx2, alkaline phosphatase and osteocalcin (118). However, the osteogenic
differentiation potential of degenerated AF cells is higher than
that of normal AF cells, which may be associated with the
regulation of the BMP/SMAD pathway by miR-221(118). AF is an important structure of the
IVD. However, the association between pathological changes in the
AF and miRNAs in IDD remains elusive.
Early treatment intervention usually improves the
outcome of most diseases; however, early diagnosis is a
prerequisite for early intervention. There are currently no early
diagnostic methods for IDD, and imaging findings are often lacking.
Therefore, it is necessary to explore and develop laboratory
diagnostic methods for early diagnosis of IDD. The expression level
of miR-26a-5p in the serum of mice with IVD degeneration was found
to be consistently higher compared with that of young pre-injury
samples or a normal control group without IVD degeneration
(119). This indicates that
miR-26a-5p is a potential molecule for IDD diagnosis. In addition,
the expression of miR-146a in peripheral blood mononuclear cells
from patients with IDD was significantly lower compared with that
of healthy controls (70). Some
scholars recently demonstrated that several miRNAs (for example,
miR-199a-5p, miR-574-3p, miR-551a and miR-640) may be candidate
markers for predicting IDD (120).
The clinical correlation between these miRNAs and IDD suggests that
miRNAs may be useful as early diagnostic markers of IDD.
IDD is currently a common disease. However, there is
yet no optimal treatment for IDD, and the main reason is that its
pathogenesis is unknown. miRNAs participate in the various
pathological processes implicated in IDD (Fig. 1). Significant progress has been made
in the study of miRNAs affecting the development of IDD, revealing
the association between genetic susceptibility and exposure to risk
factors, and improving our understanding of the pathogenesis of
IDD. Taken together, the findings of the currently available
studies highlight miRNAs as a promising research direction for IDD.
Further study on the association between miRNAs and IDD may reveal
new diagnostic markers and therapeutic targets for IDD.
Not applicable.
Funding: The present study was supported by the National Natural
Science Foundation of China, Regional fund (grant no.
82060409).
Not applicable.
QY and SG conceived this review article. FY, JW, ZC,
YY and WZ searched the literature and collected the
articles/published data, for inclusion and interpretation in this
review. All the authors were involved in the writing of the
manuscript. All the authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Park EH, Moon SW, Suh HR, Hochman S, Lee
MG, Kim YI, Jang IT and Han HC: Disc degeneration induces a
mechano-sensitization of disc afferent nerve fibers that associates
with low back pain. Osteoarthritis Cartilage. 27:1608–1617.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lv B, Yuan J, Ding H, Wan B, Jiang Q, Luo
Y, Xu T, Ji P, Zhao Y, Wang L, et al: Relationship between endplate
defects, modic change, disc degeneration, and facet joint
degeneration in patients with low back pain. Biomed Res Int.
2019(9369853)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Brinjikji W, Diehn FE, Jarvik JG, Carr CM,
Kallmes DF, Murad MH and Luetmer PH: MRI findings of disc
degeneration are more prevalent in adults with low back pain than
in asymptomatic controls: A systematic review and meta-analysis.
AJNR Am J Neuroradiol. 36:2394–2399. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Borenstein D: Mechanical low back pain-a
rheumatologist's view. Nat Rev Rheumatol. 9:643–653.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
GBD 2016 DALYs and HALE Collaborators.
Global, regional, and national disability-adjusted life-years
(DALYs) for 333 diseases and injuries and healthy life expectancy
(HALE) for 195 countries and territories, 1990-2016: A systematic
analysis for the global burden of disease study 2016. Lancet.
390:1260–1344. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cheung KM, Karppinen J, Chan D, Ho DW,
Song YQ, Sham P, Cheah KS, Leong JC and Luk KD: Prevalence and
pattern of lumbar magnetic resonance imaging changes in a
population study of one thousand forty-three individuals. Spine
(Phila Pa 1976). 34:934–940. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
van Uden S, Silva-Correia J, Oliveira JM
and Reis RL: Current strategies for treatment of intervertebral
disc degeneration: Substitution and regeneration possibilities.
Biomater Res. 21(22)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Battié MC, Videman T, Kaprio J, Gibbons
LE, Gill K, Manninen H, Saarela J and Peltonen L: The twin spine
study: Contributions to a changing view of disc degeneration. Spine
J. 9:47–59. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Frymoyer JW: Lumbar disk disease:
Epidemiology. Instr Course Lect. 41:217–223. 1992.PubMed/NCBI
|
|
10
|
MacGregor AJ, Andrew T, Sambrook PN and
Spector TD: Structural, psychological, and genetic influences on
low back and neck pain: A study of adult female twins. Arthritis
Rheum. 51:160–167. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li Z, Lei H, Luo M, Wang Y, Dong L, Ma Y,
Liu C, Song W, Wang F, Zhang J, et al: DNA methylation
downregulated mir-10b acts as a tumor suppressor in gastric cancer.
Gastric Cancer. 18:43–54. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zheng F, Liao YJ, Cai MY, Liu YH, Liu TH,
Chen SP, Bian XW, Guan XY, Lin MC, Zeng YX, et al: The putative
tumour suppressor microRNA-124 modulates hepatocellular carcinoma
cell aggressiveness by repressing ROCK2 and EZH2. Gut. 61:278–289.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu H, Huang X, Liu X, Xiao S, Zhang Y,
Xiang T, Shen X, Wang G and Sheng B: miR-21 promotes human nucleus
pulposus cell proliferation through PTEN/AKT signaling. Int J Mol
Sci. 15:4007–4018. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sheng X, Guo Q, Yu J and Xu Y:
Experimental research on the effect of microRNA-21 inhibitor on a
rat model of intervertebral disc degeneration. Exp Ther Med.
16:67–72. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen B, Huang SG, Ju L, Li M, Nie FF,
Zhang Y, Zhang YH, Chen X and Gao F: Effect of microRNA-21 on the
proliferation of human degenerated nucleus pulposus by targeting
programmed cell death 4. Braz J Med Biol Res.
49(e5020)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Millward-Sadler SJ, Costello PW, Freemont
AJ and Hoyland JA: Regulation of catabolic gene expression in
normal and degenerate human intervertebral disc cells: Implications
for the pathogenesis of intervertebral disc degeneration. Arthritis
Res Ther. 11(R65)2009.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Battié MC, Videman T and Parent E: Lumbar
disc degeneration: Epidemiology and genetic influences. Spine
(Phila Pa 1976). 29:2679–2690. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cheng X, Zhang L, Zhang K, Zhang G, Hu Y,
Sun X, Zhao C, Li H, Li YM and Zhao J: Circular RNA VMA21 protects
against intervertebral disc degeneration through targeting miR-200c
and X linked inhibitor-of-apoptosis protein. Ann Rheum Dis.
77:770–779. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Feng C, Liu M, Fan X, Yang M, Liu H and
Zhou Y: Intermittent cyclic mechanical tension altered the microRNA
expression profile of human cartilage endplate chondrocytes. Mol
Med Rep. 17:5238–5246. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cheng X, Zhang G, Zhang L, Hu Y, Zhang K,
Sun X, Zhao C, Li H, Li YM and Zhao J: Mesenchymal stem cells
deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus
cell apoptosis and reduce intervertebral disc degeneration. J Cell
Mol Med. 22:261–276. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xu YQ, Zhang ZH, Zheng YF and Feng SQ:
Dysregulated miR-133a mediates loss of type II collagen by directly
targeting matrix metalloproteinase 9 (MMP9) in human intervertebral
disc degeneration. Spine (Phila Pa 1976). 41:E717–E724.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li HR, Cui Q, Dong ZY, Zhang JH, Li HQ and
Zhao L: Downregulation of miR-27b is involved in loss of type II
collagen by directly targeting matrix metalloproteinase 13 (MMP13)
in human intervertebral disc degeneration. Spine (Phila Pa 1976).
41:E116–E123. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ji ML, Zhang XJ, Shi PL, Lu J, Wang SZ,
Chang Q, Chen H and Wang C: Downregulation of microRNA-193a-3p is
involved in invertebral disc degeneration by targeting MMP14. J Mol
Med (Berl). 94:457–468. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ji ML, Lu J, Shi PL, Zhang XJ, Wang SZ,
Chang Q, Chen H and Wang C: Dysregulated miR-98 contributes to
extracellular matrix degradation by targeting IL-6/STAT3 signaling
pathway in human intervertebral disc degeneration. J Bone Miner
Res. 31:900–909. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhao B, Yu Q, Li H, Guo X and He X:
Characterization of microRNA expression profiles in patients with
intervertebral disc degeneration. Int J Mol Med. 33:43–50.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ohrt-Nissen S, Døssing KB, Rossing M,
Lajer C, Vikeså J, Nielsen FC, Friis-Hansen L and Dahl B:
Characterization of miRNA expression in human degenerative lumbar
disks. Connect Tissue Res. 54:197–203. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang HQ, Yu XD, Liu ZH, Cheng X, Samartzis
D, Jia LT, Wu SX, Huang J, Chen J and Luo ZJ: Deregulated miR-155
promotes Fas-mediated apoptosis in human intervertebral disc
degeneration by targeting FADD and caspase-3. J Pathol.
225:232–242. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fontana G, See E and Pandit A: Current
trends in biologics delivery to restore intervertebral disc
anabolism. Adv Drug Deliv Rev. 84:146–158. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Maroudas A, Stockwell RA, Nachemson A and
Urban J: Factors involved in the nutrition of the human lumbar
intervertebral disc: Cellularity and diffusion of glucose in vitro.
J Anat. 120:113–130. 1975.PubMed/NCBI
|
|
31
|
Mern DS, Beierfuß A, Thomé C and Hegewald
AA: Enhancing human nucleus pulposus cells for biological treatment
approaches of degenerative intervertebral disc diseases: A
systematic review. J Tissue Eng Regen Med. 8:925–936.
2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Johnson WE, Eisenstein SM and Roberts S:
Cell cluster formation in degenerate lumbar intervertebral discs is
associated with increased disc cell proliferation. Connect Tissue
Res. 42:197–207. 2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Araldi E, Chamorro-Jorganes A, van
Solingen C, Fernandez-Hernando C and Suarez Y: Therapeutic
potential of modulating microRNAs in atherosclerotic vascular
disease. Curr Vasc Pharmacol. 13:291–304. 2015.PubMed/NCBI
|
|
34
|
Toiyama Y, Takahashi M, Hur K, Nagasaka T,
Tanaka K, Inoue Y, Kusunoki M, Boland CR and Goel A: Serum miR-21
as a diagnostic and prognostic biomarker in colorectal cancer. J
Natl Cancer Inst. 105:849–859. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vicinus B, Rubie C, Stegmaier N, Frick VO,
Kölsch K, Kauffels A, Ghadjar P, Wagner M and Glanemann M: miR-21
and its target gene CCL20 are both highly overexpressed in the
microenvironment of colorectal tumors: Significance of their
regulation. Oncol Rep. 30:1285–1292. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang P, Zhuang L, Zhang J, Fan J, Luo J,
Chen H, Wang K, Liu L, Chen Z and Meng Z: The serum miR-21 level
serves as a predictor for the chemosensitivity of advanced
pancreatic cancer, and miR-21 expression confers chemoresistance by
targeting FasL. Mol Oncol. 7:334–345. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang YX, Yue Z, Wang PY, Li YJ, Xin JX,
Pang M, Zheng QY and Xie SY: Cisplatin upregulates MSH2 expression
by reducing miR-21 to inhibit A549 cell growth. Biomed
Pharmacother. 67:97–102. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhao FL, Hu GD, Wang XF, Zhang XH, Zhang
YK and Yu ZS: Serum overexpression of microRNA-10b in patients with
bone metastatic primary breast cancer. J Int Med Res. 40:859–866.
2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yu X, Li Z, Shen J, Wu WK, Liang J, Weng X
and Qiu G: MicroRNA-10b promotes nucleus pulposus cell
proliferation through RhoC-Akt pathway by targeting HOXD10 in
intervetebral disc degeneration. PLoS One. 8(e83080)2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tao B, Yi J, Huang C, Xu W, Qin C, Chen L,
Chen J, Gao Y and Wang R: microRNA-96 regulates the proliferation
of nucleus pulposus cells by targeting ARID2/AKT signaling. Mol Med
Rep. 16:7553–7560. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tan H, Zhao L, Song R, Liu Y and Wang L:
microRNA-665 promotes the proliferation and matrix degradation of
nucleus pulposus through targeting GDF5 in intervertebral disc
degeneration. J Cell Biochem. 119:7218–7225. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Meng X, Zhu Y, Tao L, Zhao S and Qiu S:
MicroRNA-125b-1-3p mediates intervertebral disc degeneration in
rats by targeting teashirt zinc finger homeobox 3. Exp Ther Med.
15:2627–2633. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Li W, Wang P, Zhang Z, Wang W, Liu Y and
Qi Q: MiR-184 regulates proliferation in nucleus pulposus cells by
targeting GAS1. World Neurosurg. 97:710–715.e1. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Heuer F, Schmidt H and Wilke HJ: The
relation between intervertebral disc bulging and annular fiber
associated strains for simple and complex loading. J Biomech.
41:1086–1094. 2008.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wang T, Li P, Ma X, Tian P, Han C, Zang J,
Kong J and Yan H: MicroRNA-494 inhibition protects nucleus pulposus
cells from TNF-α-induced apoptosis by targeting JunD. Biochimie.
115:1–7. 2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kang L, Yang C, Song Y, Zhao K, Liu W, Hua
W, Wang K, Tu J, Li S, Yin H and Zhang Y: MicroRNA-494 promotes
apoptosis and extracellular matrix degradation in degenerative
human nucleus pulposus cells. Oncotarget. 8:27868–27881.
2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Li L, Zhang L and Zhang Y: Roles of
miR-494 in intervertebral disk degeneration and the related
mechanism. World Neurosurg, Dec 30, 2018 (Online ahead of
print).
|
|
49
|
Yang W and Sun P: Downregulation of
microRNA-129-5p increases the risk of intervertebral disc
degeneration by promoting the apoptosis of nucleus pulposus cells
via targeting BMP2. J Cell Biochem. 120:19684–19690.
2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sun JC, Zheng B, Sun RX, Meng YK, Wang SM,
Yang HS, Chen Y, Shi JG and Guo YF: MiR-499a-5p suppresses
apoptosis of human nucleus pulposus cells and degradation of their
extracellular matrix by targeting SOX4. Biomed Pharmacother.
113(108652)2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yang Q, Guo XP, Cheng YL and Wang Y:
MicroRNA-143-5p targeting eEF2 gene mediates intervertebral disc
degeneration through the AMPK signaling pathway. Arthritis Res
Ther. 21(97)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Liu P, Chang F, Zhang T, Gao G, Yu C, Ding
SQ, Zuo GL and Huang XH: Downregulation of microRNA-125a is
involved in intervertebral disc degeneration by targeting
pro-apoptotic Bcl-2 antagonist killer 1. Iran J Basic Med Sci.
20:1260–1267. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ma JF, Zang LN, Xi YM, Yang WJ and Zou D:
MiR-125a Rs12976445 polymorphism is associated with the apoptosis
status of nucleus pulposus cells and the risk of intervertebral
disc degeneration. Cell Physiol Biochem. 38:295–305.
2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhao K, Zhang Y, Kang L, Song Y, Wang K,
Li S, Wu X, Hua W, Shao Z, Yang S and Yang C: Epigenetic silencing
of miRNA-143 regulates apoptosis by targeting BCL2 in human
intervertebral disc degeneration. Gene. 628:259–266.
2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Lv J, Li S, Wan T, Yang Y, Cheng Y and Xue
R: Inhibition of microRNA-30d attenuates the apoptosis and
extracellular matrix degradation of degenerative human nucleus
pulposus cells by up-regulating SOX9. Chem Biol Interact.
296:89–97. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Liu G, Cao P, Chen H, Yuan W, Wang J and
Tang X: MiR-27a regulates apoptosis in nucleus pulposus cells by
targeting PI3K. PLoS One. 8(e75251)2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wang B, Wang D, Yan T and Yuan H:
MiR-138-5p promotes TNF-α-induced apoptosis in human intervertebral
disc degeneration by targeting SIRT1 through PTEN/PI3K/Akt
signaling. Exp Cell Res. 345:199–205. 2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Sun Z, Jian Y, Fu H and Li B: MiR-532
downregulation of the Wnt/β-catenin signaling via targeting Bcl-9
and induced human intervertebral disc nucleus pulposus cells
apoptosis. J Pharmacol Sci. 138:263–270. 2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Wang R, Wen B and Sun D: miR-573 regulates
cell proliferation and apoptosis by targeting Bax in nucleus
pulposus cells. Cell Mol Biol Lett. 24(2)2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Liu ZQ, Fu WQ, Zhao S and Zhao X:
Regulation of insulin-like growth factor 1 receptor signaling by
microRNA-4458 in the development of lumbar disc degeneration. Am J
Transl Res. 8:2309–2316. 2016.PubMed/NCBI
|
|
61
|
Zhang DY, Wang ZJ, Yu YB, Zhang Y and
Zhang XX: Role of microRNA-210 in human intervertebral disc
degeneration. Exp Ther Med. 11:2349–2354. 2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Cai P, Yang T, Jiang X, Zheng M, Xu G and
Xia J: Role of miR-15a in intervertebral disc degeneration through
targeting MAP3K9. Biomed Pharmacother. 87:568–574. 2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Liu J, Yu J, Jiang W, He M and Zhao J:
Targeting of CDKN1B by miR-222-3p may contribute to the development
of intervertebral disc degeneration. FEBS Open Bio. 9:728–735.
2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Wang W, Guo Z, Yang S, Wang H and Ding W:
Upregulation of miR-199 attenuates TNF-α-induced Human nucleus
pulposus cell apoptosis by downregulating MAP3K5. Biochem Biophys
Res Commun. 505:917–924. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Kang JD, Georgescu HI, McIntyre-Larkin L,
Stefanovic-Racic M, Donaldson WF III and Evans CH: Herniated lumbar
intervertebral discs spontaneously produce matrix
metalloproteinases, nitric oxide, interleukin-6, and prostaglandin
E2. Spine (Phila Pa 1976). 21:271–277. 1996.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Séguin CA, Pilliar RM, Roughley PJ and
Kandel RA: Tumor necrosis factor-alpha modulates matrix production
and catabolism in nucleus pulposus tissue. Spine (Phila Pa 1976).
30:1940–1948. 2005.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Ohba T, Haro H, Ando T, Wako M, Suenaga F,
Aso Y, Koyama K, Hamada Y and Nakao A: TNF-alpha-induced NF-kappaB
signaling reverses age-related declines in VEGF induction and
angiogenic activity in intervertebral disc tissues. J Orthop Res.
27:229–235. 2009.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Clouet J, Vinatier C, Merceron C,
Pot-Vaucel M, Hamel O, Weiss P, Grimandi G and Guicheux J: The
intervertebral disc: From pathophysiology to tissue engineering.
Joint Bone Spine. 76:614–618. 2009.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Gu SX, Li X, Hamilton JL, Chee A, Kc R,
Chen D, An HS, Kim JS, Oh CD, Ma YZ, et al: MicroRNA-146a reduces
IL-1 dependent inflammatory responses in the intervertebral disc.
Gene. 555:80–87. 2015.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Lv F, Huang Y, Lv W, Yang L, Li F, Fan J
and Sun J: MicroRNA-146a Ameliorates inflammation via TRAF6/NF-κB
pathway in intervertebral disc cells. Med Sci Monit. 23:659–664.
2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Chen Z, Han Y, Deng C, Chen W, Jin L, Chen
H, Wang K, Shen H and Qian L: Inflammation-dependent downregulation
of miR-194-5p contributes to human intervertebral disc degeneration
by targeting CUL4A and CUL4B. J Cell Physiol. 234:19977–19989.
2019.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Qin C, Lv Y, Zhao H, Yang B and Zhang P:
MicroRNA-149 suppresses inflammation in nucleus pulposus cells of
intervertebral discs by regulating MyD88. Med Sci Monit.
25:4892–4900. 2019.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Zhang Y, Yang J, Zhou X, Wang N, Li Z,
Zhou Y, Feng J, Shen D and Zhao W: Knockdown of miR-222 inhibits
inflammation and the apoptosis of LPS-stimulated human
intervertebral disc nucleus pulposus cells. Int J Mol Med.
44:1357–1365. 2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Sun J, Hong J, Sun S, Wang X, Peng Y, Zhou
J, Huang Y, Li S, Chen W, Li C, et al: Transcription factor 7-like
2 controls matrix degradation through nuclear factor κB signaling
and is repressed by microRNA-155 in nucleus pulposus cells. Biomed
Pharmacother. 108:646–655. 2018.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Shen L, Xiao Y, Wu Q, Liu L, Zhang C and
Pan X: TLR4/NF-κB axis signaling pathway-dependent up-regulation of
miR-625-5p contributes to human intervertebral disc degeneration by
targeting COL1A1. Am J Transl Res. 11:1374–1388. 2019.PubMed/NCBI
|
|
76
|
Lu A, Wang Z and Wang S: Role of
miR-589-3p in human lumbar disc degeneration and its potential
mechanism. Exp Ther Med. 15:1616–1621. 2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Zhang Q, Weng Y, Jiang Y, Zhao S, Zhou D
and Xu N: Overexpression of miR-140-5p inhibits
lipopolysaccharide-induced human intervertebral disc inflammation
and degeneration by downregulating toll-like receptor 4. Oncol Rep.
40:793–802. 2018.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Dong W, Liu J, Lv Y, Wang F, Liu T, Sun S,
Liao B, Shu Z and Qian J: miR-640 aggravates intervertebral disc
degeneration via NF-κB and WNT signalling pathway. Cell Prolif.
52(e12664)2019.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Li G, Tang X, Chen H, Sun W and Yuan F:
miR-148a inhibits pro-inflammatory cytokines released by
intervertebral disc cells by regulating the p38/MAPK pathway. Exp
Ther Med. 16:2665–2669. 2018.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Wang H, Hao P, Zhang H, Xu C and Zhao J:
MicroRNA-223 inhibits lipopolysaccharide-induced inflammatory
response by directly targeting Irak1 in the nucleus pulposus cells
of intervertebral disc. IUBMB Life. 70:479–490. 2018.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Kong L, Sun M, Jiang Z, Li L and Lu B:
MicroRNA-194 inhibits lipopolysaccharide-induced inflammatory
response in nucleus pulposus cells of the intervertebral disc by
targeting TNF receptor-associated factor 6 (TRAF6). Med Sci Monit.
24:3056–3067. 2018.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Zhou J, Liang A, Hong J, Sun J, Lin X,
Peng Y, Wang X, Sun S, Xiao D, Xu K and Ye W: MicroRNA-155
suppresses the catabolic effect induced by TNF-α and IL-1β by
targeting C/EBPβ in rat nucleus pulposus cells. Connect Tissue Res.
60:165–177. 2019.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Cao Z and Chen L: Inhibition of miR-27a
suppresses the inflammatory response via the p38/MAPK pathway in
intervertebral disc cells. Exp Ther Med. 14:4572–4578.
2017.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Yang X and Li X: Nucleus pulposus tissue
engineering: A brief review. Eur Spine J. 18:1564–1572.
2009.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Le Maitre CL, Pockert A, Buttle DJ,
Freemont AJ and Hoyland JA: Matrix synthesis and degradation in
human intervertebral disc degeneration. Biochem Soc Trans.
35:652–655. 2007.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Ye D, Dai L, Yao Y, Qin S, Xie H, Wang W
and Liang W: miR-155 inhibits nucleus pulposus cells' degeneration
through targeting ERK 1/2. Dis Markers.
2016(6984270)2016.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Zhang WL, Chen YF, Meng HZ, Du JJ, Luan
GN, Wang HQ, Yang MW and Luo ZJ: Role of miR-155 in the regulation
of MMP-16 expression in intervertebral disc degeneration. J Orthop
Res. 35:1323–1334. 2017.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Jing W and Jiang W: MicroRNA-93 regulates
collagen loss by targeting MMP3 in human nucleus pulposus cells.
Cell Prolif. 48:284–292. 2015.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Hua WB, Wu XH, Zhang YK, Song Y, Tu J,
Kang L, Zhao KC, Li S, Wang K, Liu W, et al: Dysregulated
miR-127-5p contributes to type II collagen degradation by targeting
matrix metalloproteinase-13 in human intervertebral disc
degeneration. Biochimie. 139:74–80. 2017.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Yang S, Li L, Zhu L, Zhang C, Li Z, Guo Y,
Nie Y and Luo Z: Aucubin inhibits IL-1β- or TNF-α-induced
extracellular matrix degradation in nucleus pulposus cell through
blocking the miR-140-5p/CREB1 axis. J Cell Physiol.
234:13639–13648. 2019.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Yang S, Li L, Zhu L, Zhang C, Li Z, Guo Y,
Nie Y and Luo Z: Bu-Shen-Huo-Xue-Fang modulates nucleus pulposus
cell proliferation and extracellular matrix remodeling in
intervertebral disk degeneration through miR-483 regulation of Wnt
pathway. J Cell Biochem. 120:19318–19329. 2019.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Zhang B, Guo W, Sun C, Duan HQ, Yu BB, Mu
K, Guan YY, Li Y, Liu S, Liu Y, et al: Dysregulated MiR-3150a-3p
promotes lumbar intervertebral disc degeneration by targeting
aggrecan. Cell Physiol Biochem. 45:2506–2515. 2018.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Yan N, Yu S, Zhang H and Hou T: Lumbar
disc degeneration is facilitated by MiR-100-mediated FGFR3
suppression. Cell Physiol Biochem. 36:2229–2236. 2015.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Zhou T, Lin H, Cheng Z, Ji C, Zhang C and
Tian J: Mechanism of microRNA-146a-mediated IL-6/STAT3 signaling in
lumbar intervertebral disc degeneration. Exp Ther Med.
14:1131–1135. 2017.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Liu W, Zhang Y, Xia P, Li S, Feng X, Gao
Y, Wang K, Song Y, Duan Z, Yang S, et al: MicroRNA-7 regulates
IL-1β-induced extracellular matrix degeneration by targeting GDF5
in human nucleus pulposus cells. Biomed Pharmacother. 83:1414–1421.
2016.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Kang L, Yang C, Yin H, Zhao K, Liu W, Hua
W, Wang K, Song Y, Tu J, Li S, et al: MicroRNA-15b silencing
inhibits IL-1β-induced extracellular matrix degradation by
targeting SMAD3 in human nucleus pulposus cells. Biotechnol Lett.
39:623–632. 2017.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Liu W, Xia P, Feng J, Kang L, Huang M,
Wang K, Song Y, Li S, Wu X, Yang S and Yang C: MicroRNA-132
upregulation promotes matrix degradation in intervertebral disc
degeneration. Exp Cell Res. 359:39–49. 2017.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Wang WJ, Yang W, Ouyang ZH, Xue JB, Li XL,
Zhang J, He WS, Chen WK, Yan YG and Wang C: MiR-21 promotes ECM
degradation through inhibiting autophagy via the PTEN/akt/mTOR
signaling pathway in human degenerated NP cells. Biomed
Pharmacother. 99:725–734. 2018.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Yang RS, Wang YH, Ding C, Su XH and Gong
XB: MiR-146 regulates the repair and regeneration of intervertebral
nucleus pulposus cells via Notch1 pathway. Eur Rev Med Pharmacol
Sci. 23:4591–4598. 2019.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Shi C, Wu L, Lin W, Cai Y, Zhang Y, Hu B,
Gao R, Im HJ, Yuan W, Ye X, et al: MiR-202-3p regulates
interleukin-1β-induced expression of matrix metalloproteinase 1 in
human nucleus pulposus. Gene. 687:156–165. 2019.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Wang C, Zhang ZZ, Yang W, Ouyang ZH, Xue
JB, Li XL, Zhang J, Chen WK, Yan YG and Wang WJ: MiR-210
facilitates ECM degradation by suppressing autophagy via silencing
of ATG7 in human degenerated NP cells. Biomed Pharmacother.
93:470–479. 2017.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Chai X, Si H, Song J, Chong Y, Wang J and
Zhao G: miR-486-5p inhibits inflammatory response, matrix
degradation and apoptosis of nucleus pulposus cells through
directly targeting FOXO1 in intervertebral disc degeneration. Cell
Physiol Biochem. 52:109–118. 2019.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Wang J, Liu X, Sun B, Du W, Zheng Y and
Sun Y: Upregulated miR-154 promotes ECM degradation in
intervertebral disc degeneration. J Cell Biochem: Mar 1, 2019 (Epub
ahead of print). doi: 10.1002/jcb.28471.
|
|
104
|
Grunhagen T, Wilde G, Soukane DM,
Shirazi-Adl SA and Urban JP: Nutrient supply and intervertebral
disc metabolism. J Bone Joint Surg Am. 88 (Suppl 2):S30–S35.
2006.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Huang YC, Urban JP and Luk KD:
Intervertebral disc regeneration: Do nutrients lead the way? Nat
Rev Rheumatol. 10:561–566. 2014.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Moon SM, Yoder JH, Wright AC, Smith LJ,
Vresilovic EJ and Elliott DM: Evaluation of intervertebral disc
cartilaginous endplate structure using magnetic resonance imaging.
Eur Spine J. 22:1820–1828. 2013.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Urban JP, Smith S and Fairbank JC:
Nutrition of the intervertebral disc. Spine (Phila Pa 1976).
29:2700–2709. 2004.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Ariga K, Miyamoto S, Nakase T, Okuda S,
Meng W, Yonenobu K and Yoshikawa H: The relationship between
apoptosis of endplate chondrocytes and aging and degeneration of
the intervertebral disc. Spine (Phila Pa 1976). 26:2414–2420.
2001.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Holm S, Holm AK, Ekström L, Karladani A
and Hansson T: Experimental disc degeneration due to endplate
injury. J Spinal Disord Tech. 17:64–71. 2004.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Chen H, Wang J, Hu B, Wu X, Chen Y, Li R
and Yuan W: MiR-34a promotes Fas-mediated cartilage endplate
chondrocyte apoptosis by targeting Bcl-2. Mol Cell Biochem.
406:21–30. 2015.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Zhan B, Zhan Y, Wang W, Zhan Y and Liu B:
Expression of miR-625 and Fas in cervical vertebral cartilage
endplate. Exp Ther Med. 15:513–519. 2018.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Sheng B, Yuan Y, Liu X, Zhang Y, Liu H,
Shen X, Liu B and Chang L: Protective effect of estrogen against
intervertebral disc degeneration is attenuated by miR-221 through
targeting estrogen receptor α. Acta Biochim Biophys Sin (Shanghai).
50:345–354. 2018.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Stokes IA and Iatridis JC: Mechanical
conditions that accelerate intervertebral disc degeneration:
Overload versus immobilization. Spine (Phila Pa 1976).
29:2724–2732. 2004.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Zheng Q, Li XX, Xiao L, Shao S, Jiang H,
Zhang XL, Sun LY and Xu HG: MicroRNA-365 functions as a
mechanosensitive microRNA to inhibit end plate chondrocyte
degeneration by targeting histone deacetylase 4. Bone.
128(115052)2019.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Xiao L, Xu S, Xu Y, Liu C, Yang B, Wang J
and Xu H: TGF-β/SMAD signaling inhibits intermittent cyclic
mechanical tension-induced degeneration of endplate chondrocytes by
regulating the miR-455-5p/RUNX2 axis. J Cell Biochem.
119:10415–10425. 2018.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Liu MH, Sun C, Yao Y, Fan X, Liu H, Cui
YH, Bian XW, Huang B and Zhou Y: Matrix stiffness promotes
cartilage endplate chondrocyte calcification in disc degeneration
via miR-20a targeting ANKH expression. Sci Rep.
6(25401)2016.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Feng G, Yang X, Shang H, Marks IW, Shen
FH, Katz A, Arlet V, Laurencin CT and Li X: Multipotential
differentiation of human anulus fibrosus cells: An in vitro study.
J Bone Joint Surg Am. 92:675–685. 2010.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Yeh CH, Jin L, Shen F, Balian G and Li XJ:
miR-221 attenuates the osteogenic differentiation of human annulus
fibrosus cells. Spine J. 16:896–904. 2016.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Fan Y, Zhao L, Xie W, Yi D, He S, Chen D
and Huang J: Serum miRNAs are potential biomarkers for the
detection of disc degeneration, among which miR-26a-5p suppresses
Smad1 to regulate disc homeostasis. J Cell Mol Med. 23:6679–6689.
2019.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Sherafatian M, Abdollahpour HR,
Ghaffarpasand F, Yaghmaei S, Azadegan M and Heidari M: MicroRNA
expression profiles, target genes, and pathways in intervertebral
disk degeneration: A meta-analysis of 3 microarray studies. World
Neurosurg. 126:389–397. 2019.PubMed/NCBI View Article : Google Scholar
|