Introduction
Circulatory diseases, the leading causes of death
among the elderly in the EU, are major public health concerns that
contribute to a burden on health care services; the main diseases
being ischemic heart disease and ischemic cerebrovascular disease
(1). Behind these diseases is
usually atherosclerosis, developed following a complex
pathophysiological process leading to endothelial dysfunction,
intimal thickening, inflammation and vascular calcification
(2,3). Some studies have demonstrated that the
prevalence of acute coronary events is higher in patients with
peripheral arterial disease (PAD) than in those without PAD;
therefore, PAD can be considered as a coronary artery disease
equivalent (4-6).
Coronary and carotid artery atherosclerosis have been extensively
investigated in several studies that have focused on the role of
calcification in plaque instability pushing the involvement of the
femoral-popliteal axis (FPA) in the background, despite the
clinical importance of atherosclerosis at its level; calcification
in the lower extremity is considered the iceberg of cardiovascular
disorders, which in diabetes or chronic kidney disease doubles the
risk of cardiovascular mortality and quadruples the risk of
amputation, the most dreaded complication (4,7).
Vascular calcification, the major complication of atherosclerosis,
results from the deposition of calcium phosphate salt in the form
of hydroxyapatite in the plaque and arterial wall. Yet, the
etiology of calcification remains unclear and is an area of active
research. Although previous analyses have associated
‘macrocalcification’ with plaque stability (8,9),
recent studies have suggested that superficial and multiple
calcifications and ulceration are associated with intra-plaque
hemorrhage, and they may be a substitute for higher risk lesions
(10,11). Some studies have investigated the
role of size and location of calcification regarding the restenosis
rate after surgical treatment. Compared with the low percentage of
restenosis after coronary drug-eluted stent replacement (10-15%)
(12), restenosis after lower limb
artery stenting reaches 40-50% (13,14),
while the variable rate of this complication is between 1 and 36%
after intervention at the carotid level (15).
Numerous factors such as demography, comorbidity,
sex, cardiovascular risk factors, high-degree stenosis, metabolic
and hemodynamic factors, biochemical parameters, the lesion length,
the underlying site-specific wall structure and the nature
(including calcification) of the atheromatous plaque alter the
restenosis rate after endarterectomy or stent implantation, of
which the crucial role is attributed to the appearance and extent
of calcification (16,17). Atherosclerotic plaque calcification
varies according to each arterial bed; femoral plaques exhibit a
significantly different type of calcification pattern and osteoid
metaplasia than carotid plaques (18), which suggests the role of a
site-dependent mechanism in plaque calcification. Clinically, two
types of plaque calcification have distinct implications in the
progression and regression of atherosclerosis: Macrocalcification,
which leads to plaque stability, and microcalcification with a
pivotal role in plaque rupture and consequent complications
(19,20). Recent research has demonstrated that
the spatial distribution of mineral mass, calcification size and
location are important determinants of the plaque rupture risk
(21,22).
The effect of calcification is considered biphasic,
from pro-inflammatory properties of ‘microcalcification’ to
anti-inflammatory properties of ‘macrocalcification’. Plaque
rupture has been shown to be positively correlated with
microcalcifications, and conversely with extensive calcifications.
Macroscopic calcification is easily detected and quantified
(calcium scores as predictive value for cardiovascular incidence)
using the CT scan method. In contrast, microcalcification, the
early stage of plaque calcification, is observed only with positron
emission tomography (PET)/CT imaging and optical coherence
tomography (diagnostic methods that are not used in daily practice)
(9).
However, the CT analysis of calcification patterns
is limited by the resolution and blooming artefacts. In this
context, the histopathological examination of the endarterectomy
specimens provides useful information to the clinician to develop a
treatment strategy.
This present study is a detailed cross-sectional
morphological comparative characterization of the intra-plaque
calcification of advanced atherosclerotic lesions based on
morphometric methods involving the most important segments of the
FPA and CA.
Materials and methods
Patients and tissue fragments
In this prospective comparative study, tissue
fragments harvested by conventional transluminal angioplasty from
patients diagnosed with symptomatic PAD and CA atherosclerosis were
included. The material was collected from different patients
between January 2017 and December 2018, at the Vascular Surgery
Clinic, within the Mureș County Emergency Clinical Hospital
(Romania). A total of 101 cases were selected for the clinic
pathological study, based on strict criteria which included
patients with complete clinical documentation and the written
consent of enrolment in the study and an appropriate quantitative
and structural specimen for histological examination. Prior to
surgery, for the clinical assessment of the severity of the CA and
the FPA stenosis, a CT angiography and a Doppler ultrasonography
were performed. The subjects were first divided into two groups:
Group 1 (n=21) included patients with symptomatic CA stenosis
(either transient ischemic attack or stroke on the ipsilateral side
of the carotid stenosis); and group 2 (n=80), patients with
stenosis in different segments of the FPA presenting claudication
or critical limb ischemia. The atherosclerotic plaques from the FPA
were collected from the following levels: The proximal third of the
superficial femoral artery (pSFA), adjacent to the inguinal
ligament (Poupart's ligament); the distal segment of the
superficial femoral artery (dSFA) at the level of the Hunter's
canal and the proximal segment of the popliteal artery (PA),
respectively. All samples from the carotid artery (CA) and FPA were
immediately fixed in 10% neutral buffered formalin and sent for
histological processing. The study was conducted according to the
principles of the Helsinki Declaration and was approved by the
Ethics Committee of the ‘George Emil Palade’ University of
Medicine, Pharmacy, Science and Technology of Târgu-Mureș, Romania
(no. 884 and 11420/30.04.2020). All patients signed an informed
consent for inclusion in the study.
Histological processing
The specimens were processed using a standard method
to produce paraffin sections for staining with hematoxylin and
eosin (H&E), and the calcified specimens were treated with an
ethylenediaminetetraacetic acid (EDTA) solution (pH 7.0).
Establishment of the histological
grade of the atherosclerotic plaques
The histological grade of the atherosclerotic plaque
was evaluated on 4-µm thick and H&E-stained sections according
to the modified American Heart Association (AHA) classification
based on 9, well-defined categories, of which 6 types correspond to
atheromatous plaque (23).
Briefly, type IV is considered to be the first
advanced stage of the disease with confluent extracellular lipid
core. An atheromatous plaque with fibrous cap associated with
prominent fibrosis is included in type V. All atheromatous plaques
with surface damage (ulceration and thrombosis) must be classified
as type VI. A fibrocalcified plaque with extensive calcification is
categorized as type VII. In type VIII, fibrous changes predominate.
Complete occlusion (type IX) in our study was not taken into
account.
Determination of the degree (type) of
calcification of the plaques
Focusing on the calcification pattern, the plaques
were included in four categories depending on the calcified patch
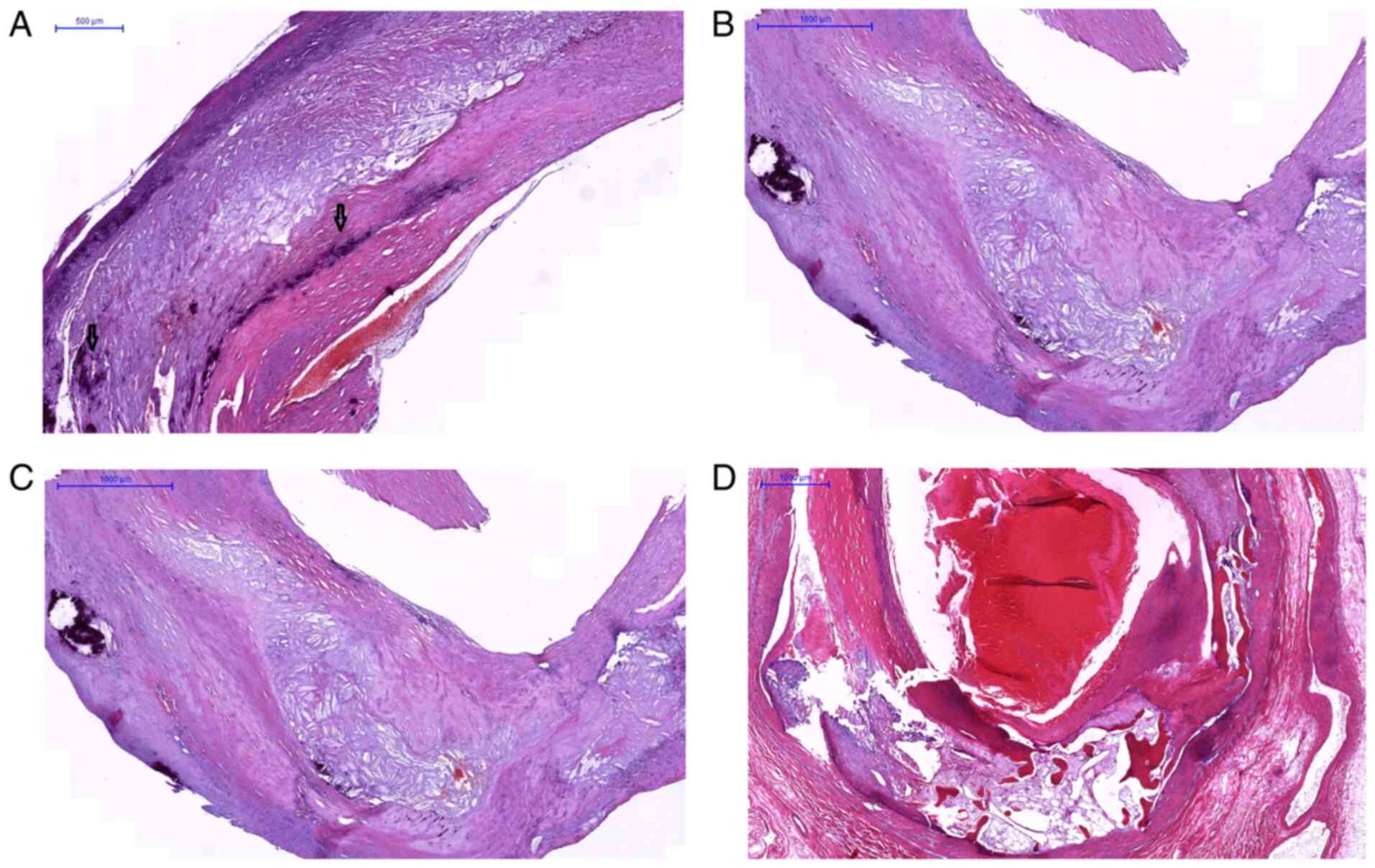
distribution, size and shape: a) Sheet-like calcification (defined
as numerous micronodules/scattered small mineral foci forming a
calcification front within fibrosis (Fig. 1A); b) nodular calcification
(single/multiple stratified mineral deposits with a nodular aspect
(Fig. 1B); c) extensive (confluent)
calcification (conglomerate of mineral material with irregular
edges (Fig. 1C); and d) osteoid
metaplasia (mature bone with lamellar structure and bone marrow
(Fig. 1D). Although there is no
conventional standard of size, there is a general consensus that
categorizes microcalcifications and macrocalcifications based on
nodules of <50 and ≥50 µm, respectively (19).
The site-specific prevalence for each type of
calcification was compared. The comparative study of the histology
of atherosclerotic plaque and degree of calcification depending on
the location (carotid and femoral-popliteal axis) was observed by
digital morphometry. The selected calcified plaques (81 cases) were
grouped according to the involved arterial segments (CA, pSFA, dSFA
and PA). Their associated H&E stained slides were digitally
scanned at x20 magnification with a Mirax Scanner and were examined
with the associated Panoramic Viewer 1.15.4 software (3DHISTECH
Ltd., Budapest, Hungary). A comparative microscopic examination was
performed by the parallel evaluation of the plaque thickness (using
x4 magnification) at the CA and the pSFA.
Morphometric analysis of mineral
deposits
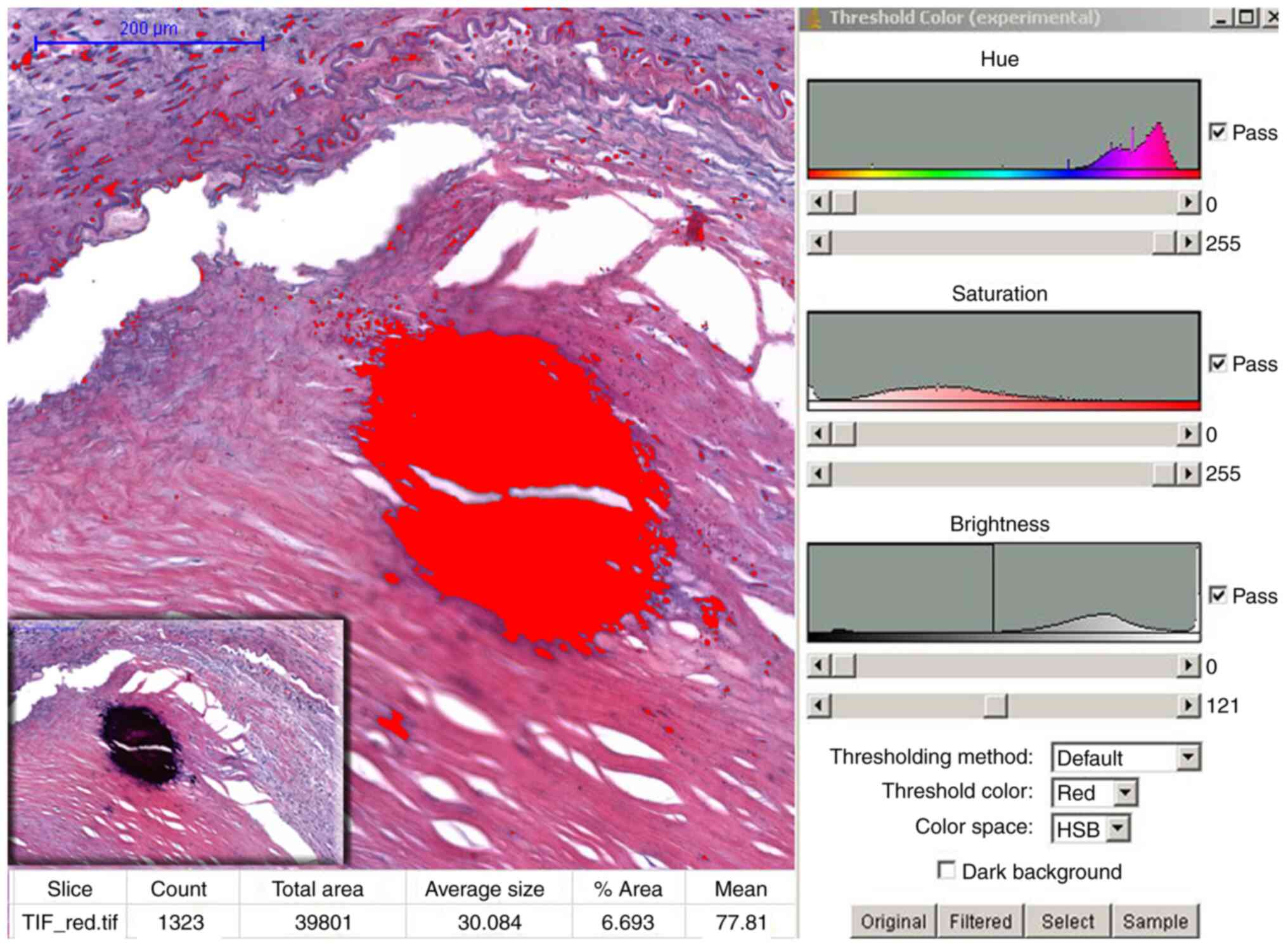
In order to quantifying the total calcified
(positive) surface area (%) of the atheromatous plaques in the two
locations mentioned above, the scanned histological sections were
captured with objective 4, saved in Tiff format, transferred to the
NIH's Image J 1.46 program (National Institutes of Health, USA) and
followed by processing of the obtained image using HSB (hue,
saturation, brightness) color filtering (24). Depending on the size and extent of
calcification, 3 to 5 different representative locations were
selected along each tissue. Calcified patches were tagged as
positive surface; the percentage of the total examined area noted
as the pathological calcium score (pCS) (Fig. 2).
Statistical analysis
The descriptive and comparative statistics were
performed with GraphPad Prism 8 (GraphPad Software, Inc.). Multiple
groups were compared through the Kruskal-Wallis ANOVA test, and
group comparisons (involved arterial segment and pCS) were made
with the non-parametric Mann-Whitney U test. A correlation analysis
was performed according to Spearman. The level of statistical
significance was set at P<0.05.
Results
Patient characteristics
During the aforementioned period, 138 patients were
diagnosed with PAD, of which 101 met the criteria for enrollment in
the study. The mean age of the patients was 66.35±8.31 (range
48-85), 45.54% of the cases being included in the age category
60-69 years. The distribution of patients by sex showed a male
predominance (77.2% vs. 22.8%) with a significant difference
(P<0.001) between the two sexes.
Among all patients, 67.33% had claudication and
32.67% had acute limb-ischemia. When reviewing the cardiovascular
risk factors, 75.24% had hypertension, 29.7% had diabetes, 78.21%
had hyperlipidemia, 62.37% had a history of tobacco use, and 32.67%
were obese (body mass index >30.0). A total of 66% of the
hypertensive patients showed an atherosclerosis of the FPA. There
was no significant difference (P>0.05) between the sexes in
terms of cardiovascular risk factors.
Anatomical location of the
stenosis
Based on the imaging examinations (CT angiography
and Doppler ultrasonography) results, we classified the cases
dependent on the narrowed arterial segment in four categories: I,
21 CA plaques (20.8%); II, 23 plaques from the pSFA (22.77%); III,
46 plaques from the dSFA (45.54 %); and IV, 11 PA plaques
(10.89%).
Establishment of the histological
grade of the atherosclerotic plaques in the different arterial
segments
According to the modified AHA classification
criteria, a large part of the atherosclerotic plaques were
classified based on the morphological aspect in type VII and VIII
(70 cases).
Regarding the localization, we found that the
plaques included in AHA VIII type (45 cases) developed
predominantly at the level of the superficial femoral artery
[proximal third and the distal segment (62.5%) compared to the CA
(23.8%) (P<0.001)]. This significant difference was maintained
even when they were divided into low grade (AHA IV, V) and high
grade categories (AHA VI, VII and VIII) (P<0.05).
Determination of the degree (type) of
calcification of the plaques
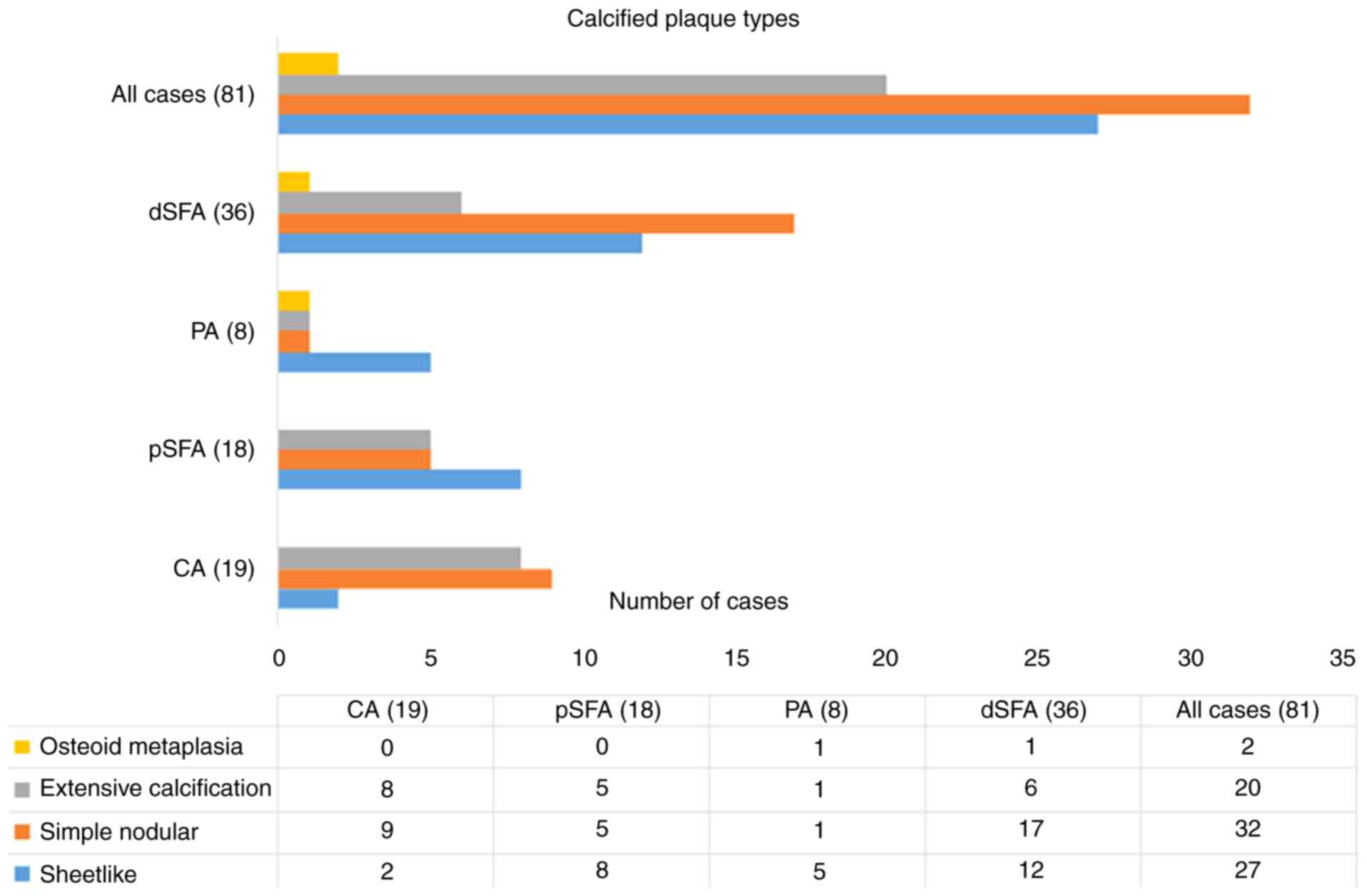
Of the 101 cases, only 20 did not show
calcifications in the examined plaques. Regarding the degree of
intra-plaque calcification, 27 cases were classified as subtype I
(sheet-like calcification); 32 cases were classified as subtype II,
simple nodular calcification; 20 cases were classified as subtype
III with extensive calcification; and 2 cases were categorized as
subtype IV with osteoid metaplasia. Most cases of plaque
calcifications (n=36) were from the FPA (44.45%), of which 17
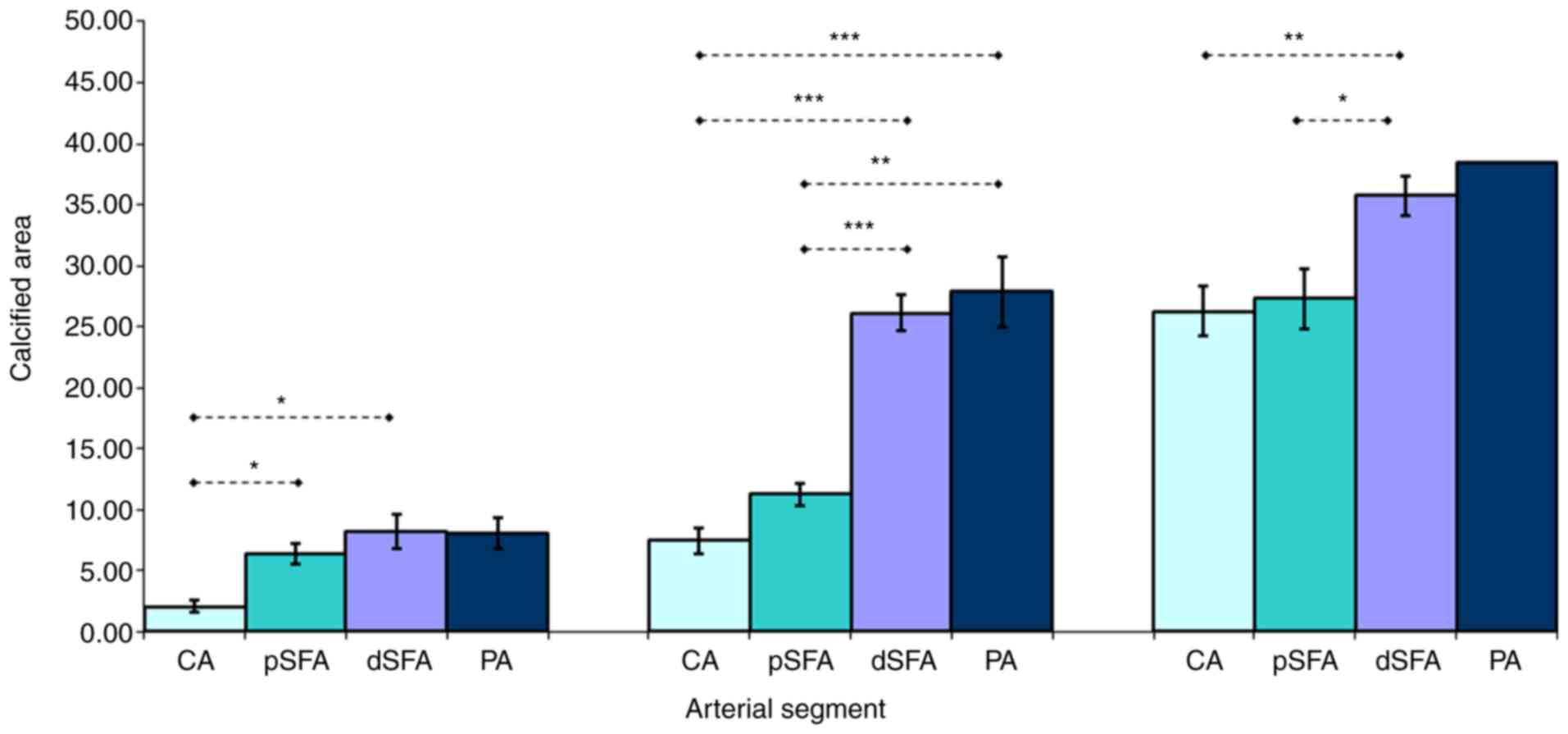
(47.23%) fell into subtype II (nodular calcification) (Fig. 3). Statistical analysis indicated a
significant difference between CA and FPA plaques in terms of
nodular calcification (type II) in favor of FPA (P<0.05).
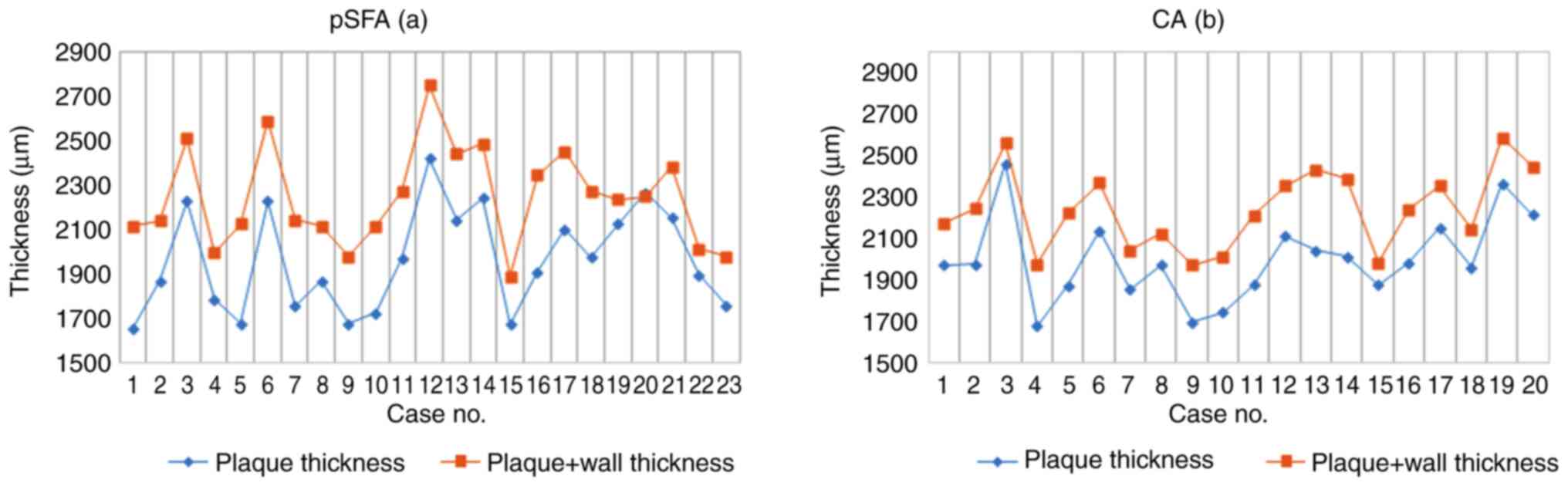
The morphometric analysis of plaque thickness in
carotid and femoral endarterectomy specimens did not show
significant differences in the average thickness of the plaques at
the level of the pSFA (2,000.67±202.98 µm; range 1,680.15 to
2,462.3 µm) compared to CA (1,977.17±231.88 µm; range 1,654.34 to
2,424.15 µm) (P<0.05). This mild variation was also underlined
by the values of the ratio plaque thickness/total thickness in the
two mentioned locations (0.89 vs. 0.87) (Fig. 4).
Comparative morphometric analysis of
mineral deposits in the femoral and carotid artery plaques
In the present study, we included only calcified
plaques (from 81 cases), that showed a higher frequency among men
(P<0.001). Image analysis of the total mineralized area of
atherosclerotic plaques from the four locations showed different
results concerning the amount of deposited mineral salts. Ignoring
the calcification subtype, there was no significant difference
between the pCS of pSFA (13.55±9.58) and the CA plaques
(14.84.55±10.96) (P=0.704). In contrast, with the decrease in the
vascular caliber, a statistically significant difference appeared
between the pCS of the CA plaques (14.84.55±10.96) and dSFA
(21.63±11.39) plaques (P=0.038), respectively the pSFA (13.55±9.58)
and dSFA (21.63±11.39) plaques (P=0.013) and the pSFA (13.55±9.58)
and PA (22.47±12.27) (P=0.048). Another noteworthy result was the
increase in the mineralized surface at the level of the different
FPA plaque segments, in parallel with the narrowing of the vascular
lumen diameter.
We compared the calcified area of the plaques
developed in the above segments also according to the subtype of
calcification. Regarding the sheet-like plaque calcification
pattern in the paired comparison of the FPA segments (pSFA vs.
dSFA, pSFA vs. PA), we found no significant difference (P=0.134 and
P=0.49). Because in the CA plaques this pattern developed in only
two cases, this segment was not included in the paired comparison.
The simple nodular calcification pattern did not show significant
differences between the pCS of the CA and the pSFA plaques
(P=0.06). In contrast, pCSs of the CA were much lower than those in
dSFA and PA, respectively, segments at which calcification occupied
significantly larger areas (P=0.0007 and P=0.0009). At the level of
pSFA segments, the nodular calcification showed significant
differences in favor of the PA (P=0.007). Extensive calcification
surface area did not differ between the CA and pSFA plaques
(P=0.83), but the pCS of the CA plaques was lower than those
measured in dSFA (P=0.004). A less pronounced, but significant
difference was observed between the pCS of the pSFA and dSFA
plaques (P=0.017) (Table I and
Fig. 5).
 | Table IComparison of total calcified area in
different arterial segments. |
Table I
Comparison of total calcified area in
different arterial segments.
| | Arterial
segment |
|---|
| Total calcified
area (pCS) | CA plaque
(n=19) | pSFA plaque
(n=18) | dSFA plaque
(n=35) | PA plaque
(n=9) |
|---|
| I. Sheet-like
calcification | 2.09±0.47 | 6.34±0.85 | 8.27±0.75 | 8.07±1.25 |
| II. Nodular
calcification |
7.5±1.06e,f |
11.27±0.88d,g |
26.09±1.47e,g |
27.89±2.90d,f |
| III. Extensive
calcification |
26.28±2.00c |
27.33±2.46b |
35.73±1.59b,c | 38.52±0.00 |
| Average
calcification |
14.88±2.51a |
13.54±2.26b |
21.63±1.93a,b | 22.47±4.09 |
Discussion
The majority of studies focusing on calcification
are based on calcium score (CS) determination by imaging; few of
them use morphometric analysis of mineral content on the
histopathological sections of the endarterectomy samples involving
one type of artery (21) or two
types (18).
According to our knowledge, this comparative study
between the carotid artery (CA) and the femoral-popliteal axis
(FPA) is the first to follow the extent of calcification depending
on the variation of the vascular caliber.
Our patients undergoing carotid and femoral
endarterectomy represented a select risk group with high grade
artery stenosis; a large part of the atherosclerotic plaques being
classified based on morphological aspects of type VII and VIII,
over three quarters of them with intra-plaque calcification.
Preliminary histological studies demonstrated considerable
differences between plaques with identical degrees of stenosis,
respectively. Certain plaque features are associated with an
increased risk of ischemic event (25). At the same time, it has been shown
that carotid plaque thickness or plaque volume is more strongly
associated with ischemic events than the degree of stenosis
(26). Although vascular
calcification in the lower extremities is commonly a hallmark of
peripheral arterial disease (PAD) and critical limb ischemia (CLI),
the true prevalence of vascular calcification in symptomatic PAD
patients remains undefined. Regarding plaque histology, in our
comparative study, we observed that femoral and carotid plaques
showed different morphology. The establishment of the histological
grade of the atherosclerotic plaques in the examined arterial
segments showed that high grade American Heart Association (AHA)
categories develop predominantly at the level of the superficial
femoral artery (SFA) compared to the CA. This site-dependent
pattern of atherosclerotic plaques is probably the result of the
structure of the vascular wall and of differences in hemodynamics.
The pathophysiological studies underlined that artery
type-dependent plaque features result from the complex effect of
blood flow, represented by peak/mean wall shear rates (higher in CA
than FPA) (27) and variations in
the speed of blood circulation, that influence the residence time
with endothelial cells of lipoproteins, inflammatory cells and
molecules which have an important role in the mechanism of
atherosclerosis (28). Another
finding of our study refers to calcification. Different femoral
artery segments were more susceptible to calcification in
comparison with the CA. Vascular calcification occurs in all
atherosclerotic lesions independently of their arterial location,
but in peripheral arteries differs quantitatively. Compared to the
CA atherosclerosis, the FPA exhibited increased calcification
(29). At the same time the
location of calcification in plaque structures also plays an
important role in plaque progression. In advanced plaques, even
small calcifications of the thin fibrous cap may lead to plaque
destabilization and rupture. By contrast, large calcification areas
in the intimal-medial boundary do not contribute to lesion
instability, but may be involved in the development of stable
atherosclerotic disease (30).
Represented by a relatively equal number, we
initially compared the atherosclerotic plaque structures in two
arterial segment, the bifurcation of CA and proximal (p)SFA
arteries with similar morphology regarding the wall structure (both
being transitional type between the elastic and the muscular
artery) (31). Despite the fact
that the thickness of the carotid and femoral atherosclerotic
plaques did not differ in the two localizations, we found
differences in terms of nodular and extensive calcification types
in favor of the pSFA plaques. Regarding nodular calcification,
similar results have been previously noted by Herisson et al
(18), but they did not
sufficiently characterize the ratio between the mineral mass and
the surrounding environments around the calcification within
plaques. Based on the findings in the literature according to which
superficial calcifications are independently associated with plaque
vulnerability, and the type of calcification extension (dispersed
or compacted), dimensions, shapes and positions may play different
roles in plaque evolution (11), we
continued our study by determining the total calcified surface area
(as pCS) of remote plaques by morphometry and we focused on the
extent of calcification in terms of spectrum between the sheet-like
nodular-extensive calcification. In the present study we showed
that the calcified area varied depending on the arterial segment
affected by atherosclerosis; the total calcified area was higher in
femoral plaques than in carotid plaques, but without a significant
difference between the plaques of the two arteries with the same
structure (pSFA and CA). At the same time, we noted a significant
increase in mineralized surface at the level of the different FPA
segments in parallel with a decrease in the vascular lumen
diameter. Differences in the total calcification of plaques
depending on the involved arterial segments were consistent with
the histological grade, according to which most of the femoral
plaques were classified as fibrocalcific (VII and VIII AHA types),
whereas carotid plaques were classified as fibrous cap atheroma (IV
and V types) (18).
At the same time, we found that, even when the three
femoral segment plaques and carotid plaques explain different
calcification pattern, the total mineralized area did not depend on
the shape and pattern of the plaques, but rather on vascular
caliber; in parallel to the narrowing of the lumen, the calcified
area of the plaque increased. This finding was also supported by
the results obtained in the case of an extensive calcification
pattern, in the sense that the mineralized area did not differ
between the CA and pSFA plaques, but in contrast, the pCS of the CA
plaques was lower than those measured in distal (d)SFA.
Although several molecular aspects of this mechanism
have been elucidated regarding the two types of arterial
calcification in the different vessel types (large elastic vs.
smaller muscular arteries) and parts (proximal vs. distal)
(32,33), few morphologic studies have focused
on the plaque calcification in the different segments of the FPA in
comparison with the CA. The most significant results are in the
study of Herisson et al (18), who focused on the calcification
pattern, complemented with quantitative measurements of calcium and
lipids within the plaque. This complex approach of calcification
underscored the high prevalence of sheet-like and nodular
calcification parallel with significantly higher amounts of calcium
at the level of FA plaques in comparison with CA plaques, and did
not lead to the staging of calcification according to different
segments of AFP.
In studies that included the measurement of calcium
scores and occlusion grade, it was reported that patients with PAD
presenting increased occlusion and calcification scores were
strongly associated with more severe stages of ischemia (34), and independently predicted
amputation and mortality (35-37).
However, the exact mechanism of atherosclerotic calcification,
including the territorial distribution and the size of calcified
regions within atherosclerotic lesions and its role in plaque
vulnerability, remains incompletely understood (21).
Microcalcifications (0.5-50 mm) represent an early
stage in the spectrum of the vascular calcification cascade. They
are considered predictors of cardiovascular events, leading to
rupture of the plaque through the transfer of the stress from the
interface between the fibrous cap and lipid core to the interface
between the fibrous cap and vessel lumen (38). Some authors claim that although
extensive calcifications are associated with plaque stability, the
role of the calcification size in plaque vulnerability may be
biphasic. They explain this phenomenon by the interaction of
mechanical forces with the mass of the plaque. Normally, mechanical
stress is expected to be concentrated at the interfaces between
materials with different stiffness, within a plaque at the
interfaces between calcium deposits and the rest of the plaque
elements. Calcium stiffness is at least four times higher than that
of other plaque components (39),
as a result, if the degree of calcification increases, the calcium
deposits merge and the soft surface area progressively decreases
(11).
We are aware of the limitations of our study. The
relatively small sample number of a single center material
characterizes the CA and FPA plaques at a well-determined moment.
The morphological aspects of the plaques of the two arteries from
different patients compromise the comparison due to their
development in different pathological conditions. At the same time,
most of the lesions came from advanced atherosclerotic plaques,
with extensive calcification associated with a relatively small
number of sheet-like calcifications. Regarding the morphometric
analysis, we determined the total surface and not the total amount
of mineral salts of the plaques, because different patterns were
combined in a single plaque, but we agree that the nodular type of
macrocalcifications has a large volume but a small surface area
(40,41). A technical disadvantage of the study
was the loss of calcification (during cutting) in some sections and
morphology distortion. In some cases, we tried to overcome this by
following the perimeter of the calcified cavity, a method that is
possible only in nodular calcifications.
In conclusion, despite being exposed to similar risk
factors, peripheral arteries develop heterogeneous lesions. Femoral
and carotid plaques show different morphologies and tendencies for
calcification. In the present study, we demonstrated that a
population with similar demographic and biological data develops at
the level of smaller caliber arteries (femoral-popliteal/popliteal
artery) larger areas of calcification than at the level of larger
caliber arteries (femoral/carotid). These results suggest that the
mechanism is site-specific, and wall structure-dependent. In
advanced carotid and FPA atherosclerosis, calcification has a
heterogeneous pattern with a simultaneous presence of large
calcified area and numerous small calcific patches. Due to the
heterogeneous composition of the calcified plaques, extensive
calcification remains controversial in terms of plaque
stability.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data and materials that support the results of
this study are available from the corresponding author (EH).
Authors' contributions
MCC conceived the study, coordinated the surgical
team, performed the surgery, analyzed and interpreted the patient
data. MCC, ER and CM selected the patients and performed the
surgery. EH designed the study, performed histopathological and
morphometric analyses of the biopsies, contributed to data
analysis, performed figure design and provided critical review of
the manuscript and was responsible for the final edited version.
GBM performed the histopathological and morphometric analyses of
the biopsies, contributed to data analysis and was responsible for
the acquisition of the data. VAM acquired the data and revised the
manuscript critically for important intellectual content and
coordinated the research. All authors contributed to data
interpretation and editing the manuscript and read and approved the
final version.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
‘George Emil Palade’ University of Medicine, Pharmacy, Science and
Technology of Târgu-Mureș (no. 884 and 11420/30.04). All patients
provided informed consent for inclusion in the study.
Patient consent for publication
This manuscript does not contain particular cases,
personal information or images which would require patient personal
consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eurostat: Your key to European statistics:
https://ec.europa.eu/eurostat/statistics-xplained/index.php/Causes_of_death_statistics_-people_over_65
https://ec.europa.eu/eurostat/statistics-explained/index.php/Cardiovascular_diseases_statistics.
|
|
2
|
Singh RB, Mengi SA, Xu YJ, Arneja AS and
Dhalla NS: Pathogenesis of atherosclerosis: A multifactorial
process. Exp Clin Cardiol. 7:40–53. 2002.PubMed/NCBI
|
|
3
|
Albanese I, Khan K, Barratt B, Al-Kindi H
and Schwertani A: Atherosclerotic calcification: Wnt is the hint. J
Am Heart Assoc. 7(e007356)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Peace A, van Mil A, Jones H and Thijssen
DHJ: Similarities and differences between carotid artery and
coronary artery function. Curr Cardiol Rev. 14:254–263.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Schiano V, Sirico G, Giugliano G,
Laurenzano E, Brevetti L, Perrino C, Brevetti G and Esposito G:
Femoral plaque echogenicity and cardiovascular risk in claudicants.
JACC Cardiovasc Imaging. 5:348–357. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Golomb BA, Dang TT and Criqui MH:
Peripheral arterial disease: Morbidity and mortality implications.
Circulation. 114:688–699. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Maleckis K, Anttila E, Aylward P, Poulson
W, Desyatova A, MacTaggart J and Kamenskiy A: Nitinol Stents in the
femoropopliteal artery: A mechanical perspective on material,
design, and performance. Ann Biomed Eng. 46:684–704.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kwee RM: Systematic review on the
association between calcification in carotid plaques and clinical
ischemic symptoms. J Vasc Surg. 51:1015–1025. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shioi A and Ikari Y: Plaque Calcification
during atherosclerosis progression and regression. J Atheroscler
Thromb. 25:294–303. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yang J, Pan X, Zhang B, Yan Y, Huang Y,
Woolf AK, Gillard JH, Teng Z and Hui P: Superficial and multiple
calcifications and ulceration associate with intraplaque hemorrhage
in the carotid atherosclerotic plaque. Eur Radiol. 28:4968–4977.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shi X, Gao J, Lv Q, Cai H, Wang F, Ye R
and Liu X: Calcification in atherosclerotic plaque vulnerability:
Friend or Foe? Front Physiol. 11(56)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tang L, Cui QW, Liu DP and Fu YY: The
number of stents was an independent risk of stent restenosis in
patients undergoing percutaneous coronary intervention. Medicine
(Baltimore). 98(e18312)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Laird JR and Yeo KK: The treatment of
femoropopliteal in-stent restenosis: Back to the future. J Am Coll
Cardiol. 59:24–25. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gerardi D, Alfani A, Tesorio T, Cioppa A,
Esposito G and Stabile E: Drug-coated balloon in superficial
femoral artery in-stent restenosis. Postepy Kardiol Interwencyjnej.
14:9–14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gaudry M, Bartoli JM, Bal L, Giorgi R, De
Masi M, Magnan PE and Piquet P: Anatomical and technical factors
influence the rate of in-stent restenosis following carotid artery
stenting for the treatment of post-carotid endarterectomy stenosis.
PLoS One. 11(e0161716)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Katano H, Nishikawa Y, Yamada H and Mase
M: Calcification in original plaque and restenosis following
carotid artery stenting. Surg Neurol Int. 8(279)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Katano H, Mase M, Nishikawa Y, Yamada H
and Yamada K: Analysis of recurrent stenosis after carotid
endarterectomy featuring primary plaque calcification.
Neurosurgery. 80:863–70. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Herisson F, Heymann MF, Chetiveaux M,
Charrier C, Battaglia S, Pilet P, Rouillon T, Krempf M, Lemarchand
P, Heymann D and Gouëffic Y: Carotid and femoral atherosclerotic
plaques show different morphology. Atherosclerosis. 216:348–354.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kelly-Arnold A, Maldonado N, Laudier D,
Aikawa E, Cardoso L and Weinbaum S: Revised microcalcification
hypothesis for fibrous cap rupture in human coronary arteries.
PNAS. 110:10741–1046. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jinnouchi H, Sato Y, Sakamoto A,
Cornelissen A, Mori M, Kawakami R, Gadhoke NV, Kolodgie FD, Virmani
R and Finn AV: Calcium deposition within coronary atherosclerotic
lesion: Implications for plaque stability. Atherosclerosis.
306:85–95. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Han RI, Wheeler TM, Lumsden AB, Reardon
MJ, Lawrie GM, Grande-Allen KJ, Morrisett JD and Brunner G:
Morphometric analysis of calcification and fibrous layer thickness
in carotid endarterectomy tissues. Comput Biol Med. 70:210–219.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tavakoli S and Sadeghi MM:
18F-NaF PET and plaque calcification: How complicated
can it be? Circ Cardiovasc Imaging. 12(e008712)2019.
|
|
23
|
Stary HC: Natural history and histological
classification of atherosclerotic lesion: An update. Arterioscler
Thromb Vasc Biol. 20:1177–1178. 2000.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ferreira T and Rasband W: ImageJ User
Guide. Image Processing and Analysis in Java. National Institutes
of Health, 2012. http://rsb.info.nih.gov/ij.
|
|
25
|
Redgrave JNE, Lovett JK, Gallagher PJ and
Rothwell P: Histological assessment of 526 symptomatic carotid
plaques in relation to the nature and timing of ischemic symptoms:
The Oxford plaque study. Circulation. 113:2320–2328.
2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhu G, Hom J, Li Y, Jiang B, Rodriguez F,
Fleischmann D, Saloner D, Porcu M, Zhang Y, Saba L and Wintermark
M: Carotid plaque imaging and the risk of atherosclerotic
cardiovascular disease. Cardiovasc Diagn Ther. 10:1048–1067.
2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Reneman RS, Arts T and Hoeks AP: Wall
shear stress-an important determinant of endothelial cell function
and structure-in the arterial system in vivo discrepancies with
theory. J Vasc Res. 43:251–269. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
VanderLaan PA, Reardon CA and Getz GS:
Site specificity of atherosclerosis: Site selective responses to
atherosclerotic modulators. Arterioscler Thromb Vasc Biol.
24:12–22. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Otsuka F, Sakakura K, Yahagi K, Joner M
and Virmani R: Has our understanding of calcification in human
coronary atherosclerosis progressed? Arterioscler Thromb Vasc Biol.
34:724–736. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chistiakov DA, Myasoedova VA, Melnichenko
AA, Grechko AV and Orekhov AN: Calcifying matrix vesicles and
atherosclerosis. Biomed Res Int. 2017(7463590)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Janzen J: The microscopic transitional
zone between elastic and muscular arteries. Arch Mal Coeur Vaiss.
97:909–914. 2004.PubMed/NCBI
|
|
32
|
Amann K: Media calcification and intima
calcification are distinct entities in chronic kidney disease. Clin
J Am Soc Nephrol. 3:1599–1605. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Allison MA, His S, Wassel CL, Morgan C, Ix
JH, Wright CM and Criqui MH: Calcified atherosclerosis in different
vascular beds and the risk of mortality. Arterioscler Thromb Vasc
Biol. 32:140–146. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zettervall SL, Marshall AP, Fleser P and
Guzman RJ: Association of arterial calcification with chronic limb
ischemia in patients with peripheral artery disease. J Vasc Surg.
67:507–513. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Huang CL, Wu IH, Wu YW, Hwang JJ, Wang SS,
Chen WJ, Lee WJ and Yang WS: Association of lower extremity
arterial calcification with amputation and mortality in patients
with symptomatic peripheral artery disease. PLoS One.
9(e90201)2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Blacher J, Guerin AP, Pannier B, Marchais
SJ and London GM: Arterial calcifications, arterial stiffness, and
cardiovascular risk in end-stage renal disease. Hypertension.
38:938–942. 2001.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Guzman RJ, Brinkley DM, Schumacher PM,
Donahue RMJ, Beavers H and Qin X: Tibial artery calcification as a
marker of amputation risk in patients with peripheral arterial
disease. J Am Coll Cardiol. 51:1967–1974. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Rambhia SH, Liang X, Xenos M, Alemu Y,
Maldonado N, Kelly A, Chakraborti S, Weinbaum S, Cardoso L, Einav S
and Bluestein D: Microcalcifications increase coronary vulnerable
plaque rupturepotential: A patient-based micro-CT fluid-structure
interaction study. Ann Biomed Eng. 40:1443–1454. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lee RT, Grodzinsky AJ, Frank EH, Kamm RD
and Schoen FJ: Structure-dependent dynamic mechanical behavior of
fibrous caps from human atherosclerotic plaques. Circulation.
83:1764–1770. 1993.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang Y, Osborne MT, Tung B, Li M and Li Y:
Imaging cardiovascular calcification. J Am Heart Assoc.
7(e008564)2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Dweck MR, Aikawa E, Newby DE, Tarkin JM,
Rudd JH, Narula J and Fayad ZA: Noninvasive molecular imaging of
disease activity in atherosclerosis. Circ Res. 119:330–340.
2016.PubMed/NCBI View Article : Google Scholar
|