Introduction
Proliferative vitreoretinopathy (PVR) is
characterized by the formation of fibrotic and tractive membranes
that can lead to the reattachment of retina; it is a severe
complication of rhegmatogenous retinal detachment (RRD) surgery
(1). The formation mechanism of
avascular epiretinal membrane and subretinal membrane in PVR
remains unknown. It has been reported that retinal pigment
epithelial (RPE) cells are the main cells in the epiretinal and
subretinal membrane (2). After the
RRD forms, RPE cells are exposed to environments full of cytokines
and growth factors, upon which they undergo type 2
epithelial-mesenchymal transition (EMT), migrate to the epiretinal
and subretinal spaces, proliferate and form avascular membranes
(3). These findings, including our
previous studies (4,5) indicated that EMT was involved in PVR
progression.
MicroRNAs (miRNAs or miRs) are small non-coding RNAs
that are involved in transcriptional and post-transcriptional
regulation of gene expression (6).
miRNAs play important roles in both the maintenance of homeostasis
and the development of diseases (7,8). In
the retina, more than 150 miRNAs have been determined to be
involved in PVR (9).
In our previous study, an EMT model of RPE cell
induction by transforming growth factor (TGF)-β1 was established
and by using miRNA microarray, it was revealed that miR-29b was the
most important miRNA. It was also demonstrated that miR-29b
inhibited EMT in the RPE cell process through its target protein
kinase B (Akt2) (10).
Nevertheless, the mechanism of miR-29b function and its role in PVR
clinical membranes remains unknown.
Akt and the nuclear factor-κB (NF-κB) signaling
pathways play vital roles in regulating cell growth, survival,
proliferation, metabolism, and inflammation (11,12).
In NIH3T3 cells, TNF-α activated the NF-κB pathway and Akt was a
downstream target of NF-κB (13).
In oral squamous cell carcinoma cell lines (KB and KOSCC-25B),
phosphorylated (p)-Akt was upstream of the NF-κB pathway (14). In our previous study, it was
revealed that Snail was an important factor in TGF-β1-induced RPE
cell EMT. EMT was inhibited when Snail was silenced (5). When Snail was overexpressed, RPE cell
EMT was triggered, characterized by increased expression of
α-smooth muscle actin (α-SMA) and decreased expression of
E-cadherin and Zona occludin-1 (ZO-1) (15). The present study explored the
interactions among Akt2, NF-κB, Snail expression, and miR-29b in
RPE cell EMT.
The expression levels of Akt2, α-SMA, E-cadherin and
miR-29b in epiretinal membranes of PVR patients were assessed.
Furthermore, the downstream regulatory pathway of miR-29b with Akt2
in TGF-β1-induced ARPE-19 cell EMT was explored. The results of the
present study may provide insights into the role of miR-29b in RPE
cell EMT and epiretinal membrane formation in PVR.
Materials and methods
Ethical approval
All patients provided written informed consent for
the preservation and analysis of their tissues for research
purposes. The present study was approved by the Ethics Committee of
Shanghai Tenth People's Hospital (Shanghai, China) and complied to
the guidelines of the Declaration of Helsinki (version 2013)
(16). The ethics approval no. was
SHSY-IEC-KY-4.0/17-79/01.
Cell culture
ARPE-19 cells were obtained from the Eye Institute
of Tongji University. ARPE-19 cells at passage 4-6 were cultured in
DMEM/F12 (Invitrogen; Thermo Fisher Scientific, Inc.) with 10%
fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.) at
37˚C in an incubator containing 5% CO2. For TGF-β1
(HumanZyme, Inc.; ProteinTech Group, Inc.) treatment, ARPE-19 cells
were subjected to serum-free medium at 37˚C for 16 h. TGF-β1 was
added to the medium at 10 ng/ml. A total of 3.5 µM of the NF-κB
inhibitor BAY11-7082 (Beyotime Institute of Biotechnology) was
added to the medium at 37˚C for 1 h before TGF-β1 treatment.
RNA transfection
ARPE-19 cells were transfected with 100 nM miR-29b
mimic (3'-UUGUGACUAAAGUUUACCACGAU-5') and miR-negative control
mimic (miR-NC mimic; 3'-AUGCAGUAUACUGUUAAUGACUC-5') (Guangzhou
RiboBio Co., Ltd.) using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) at room temperature
for 6 h following the manufacturer's instructions. ARPE-19 cells
were starved overnight in serum-free medium before the addition of
TGF-β1.
Plasmid DNA transfection
The plasmids of Akt2-targeting short hairpin RNA
(sh)-Akt2 and sh-control (both from Shanghai GeneChem Co., Ltd.)
were transfected into ARPE-19 cells (2.5 µg plasmids or controls in
2.5x105 cells) at 37˚C for 48 h. The HET kit (Biowit
Technologies Ltd.) was used for the transfections according to the
protocols provided by the manufacturer.
Patients and samples
All patients with PVR (n=17; aged 45-66 years; 7
females and 10 males) were from the Shanghai Tenth People's
Hospital (Shanghai, China) between January 2018 and August 2019.
Patients with diabetes, acute inflammation or systemic immune
diseases were excluded. After vitrectomy surgery, the epiretinal
membranes were removed with internal limiting membrane (ILM)
forceps. The epiretinal membranes were immediately stored at
-80˚C.
Reverse transcription-quantitative PCR
(RT-q)PCR
The total RNA of epiretinal membranes was extracted
using the RNAprep pure Micro kit (cat. no. DP 420; Tiangen Biotech
Co., Ltd.) according to the manufacturer's protocol. A reverse
transcription kit (cat. no. RR036A; Takara Bio, Inc.) was used to
synthesize cDNA according to the manufacturer's instructions. SYBR
Green-based RT-qPCR was performed with a 7500 Fast RT-PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Specific
primers (GENTEC Corporation) were used to determine the expression
of EMT-related genes. GAPDH was used as an internal control. The
thermocycling conditions were as follows: 94˚C for 30 sec, 40
cycles of 94˚C for 5 sec and 60˚C for 30 sec. The relative amount
of each gene was measured using the 2-ΔΔCq method as
reported in a previous study (15).
All experiments were repeated 3 times. Primer sequences, which were
also used in a previous study (10), are presented in Table I.
 | Table IPrimer sequences for reverse
transcription-quantitative PCR amplification. |
Table I
Primer sequences for reverse
transcription-quantitative PCR amplification.
| Gene | Forward (5'-3') | Reverse (5'-3') |
|---|
| E-cadherin |
CAGTCTAGGCCAGTGCATCA |
TTTGCCCTCTGCTTTGTTCT |
| α-SMA |
AGCAGGCCAAGGGGCTATATAA |
CGTAGCTGTCTTTTTGTCCCATT |
| Akt2 |
CGCCTGCCCTTCTACAACCA |
ATGACCTCCTTGGCATCGCT |
| GAPDH |
AGAAGGCTGGGGCTCATTTG |
AGGGGCCATCCACAGTCTTC |
Western blot analysis
Western blot analysis was performed as previously
described (17). The antibodies and
the dilutions were as follows: Rabbit anti-RPE-65 (1:1,000; cat.
no. ab231782; Abcam), anti-Akt2 (1:500; cat. no. ab131168; Abcam),
anti-p-Akt2 (ser474; 1:500; cat. no. ab38513; Abcam), anti-Snail
(1:1,000; cat. no. ab216347; Abcam), anti-p65 (1:1,000; cat. no.
AF1234; Beyotime Institute of Biotechnology), anti-p-p65 (ser536;
1:1,000; cat. no. AF5881; Beyotime Institute of Biotechnology), and
anti-GAPDH (1:500; cat. no. AB-P-R001; Goodhere Biological
Technology Group). Anti-rabbit IgG secondary antibodies (1:10,000;
cat. no. W4011; Promega Corporation) were then applied and
incubated at room temperature for 1 h. Image Quant LAS 4000 (GE
Healthcare Life Sciences; Cytiva) and Image J analysis software
(version. no. 1.52t; National Institutes of Health) were used to
quantify band intensities.
Statistical analysis
At least three independent experiments were
performed for each analysis. Data are presented as the mean ±
standard deviation. One-way analysis of variance (ANOVA), Dunnett's
t-test and Tukey's post hoc test were used to analyze the data of
multiple groups. The association between miR-29b and EMT biomarkers
was evaluated using Pearson's correlation analysis. P<0.05 was
considered to indicate a statistically significant difference. SPSS
16.0 (SPSS, Inc.) was used for significance analysis.
Results
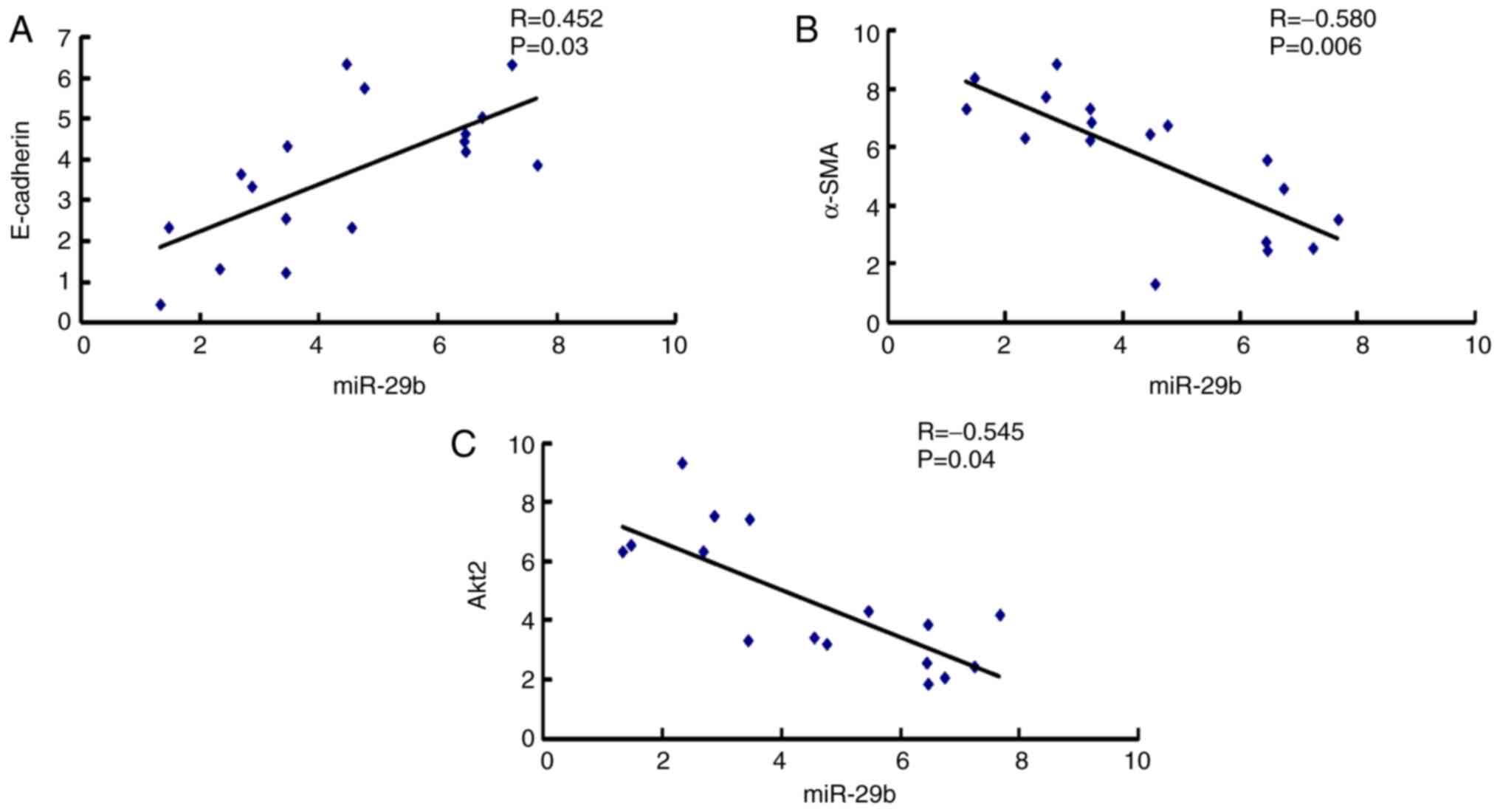
Correlation between miR-29b and EMT
markers in epiretinal membranes of patients with PVR
Epiretinal membranes are the characteristic of the
PVR process that pulls the retina and leads to retinal detachment
(1). To investigate the
relationship between miR-29b and EMT during the formation process
of epiretinal membranes, the correlation between miR-29b and the
mRNA expression of E-cadherin, α-SMA and Akt2 in epiretinal
membranes of patients with PVR was assessed. As demonstrated in
Fig. 1A, miR-29b was positively
correlated with E-cadherin mRNA expression, while there was a
negative correlation between miR-29b and α-SMA mRNA expression
(Fig. 1B), and between miR-29b and
Akt2 mRNA expression (Fig. 1C).
These results indicated that EMT played a vital role in the
development of PVR, and that miR-29b and its target Akt2
participated in this process.
miR-29b inhibits the expression of
p-p65 and Snail in ARPE-19 cells
The downstream pathways of miR-29b and Akt2 were
further investigated. The NF-κB pathway has been widely studied in
the EMT process of other cells (18,19).
Snail was revealed to play a critical part in the EMT process of
ARPE-19 cells in our previous study (5). It was concluded that p65, p-p65 and
Snail were downstream of miR-29b and Akt2 in ARPE-19 cells. As
demonstrated in Fig. 2A and
B, the expression of RPE-65 did not
decrease at passage 6 of ARPE-19 cells. Therefore, ARPE-19 cells at
passages 4-6 were used in this study. miR-29b expression was
significantly upregulated after miR-29b mimic was transfected into
ARPE-19 cells (Fig. 2C). The
results also revealed that TGF-β1 induced the expression of Akt2,
p-Akt2, p-p65 and Snail while the addition of miR-29b significantly
inhibited these expression levels (Fig.
2D and E). No significant
difference in the expression of p65 was observed. Collectively, the
expression levels of p-Akt2, p-p65 and Snail expression were
affected by miR-29b. Additionally, p-p65 and Snail were probably
involved in the EMT of ARPE-19 cells.
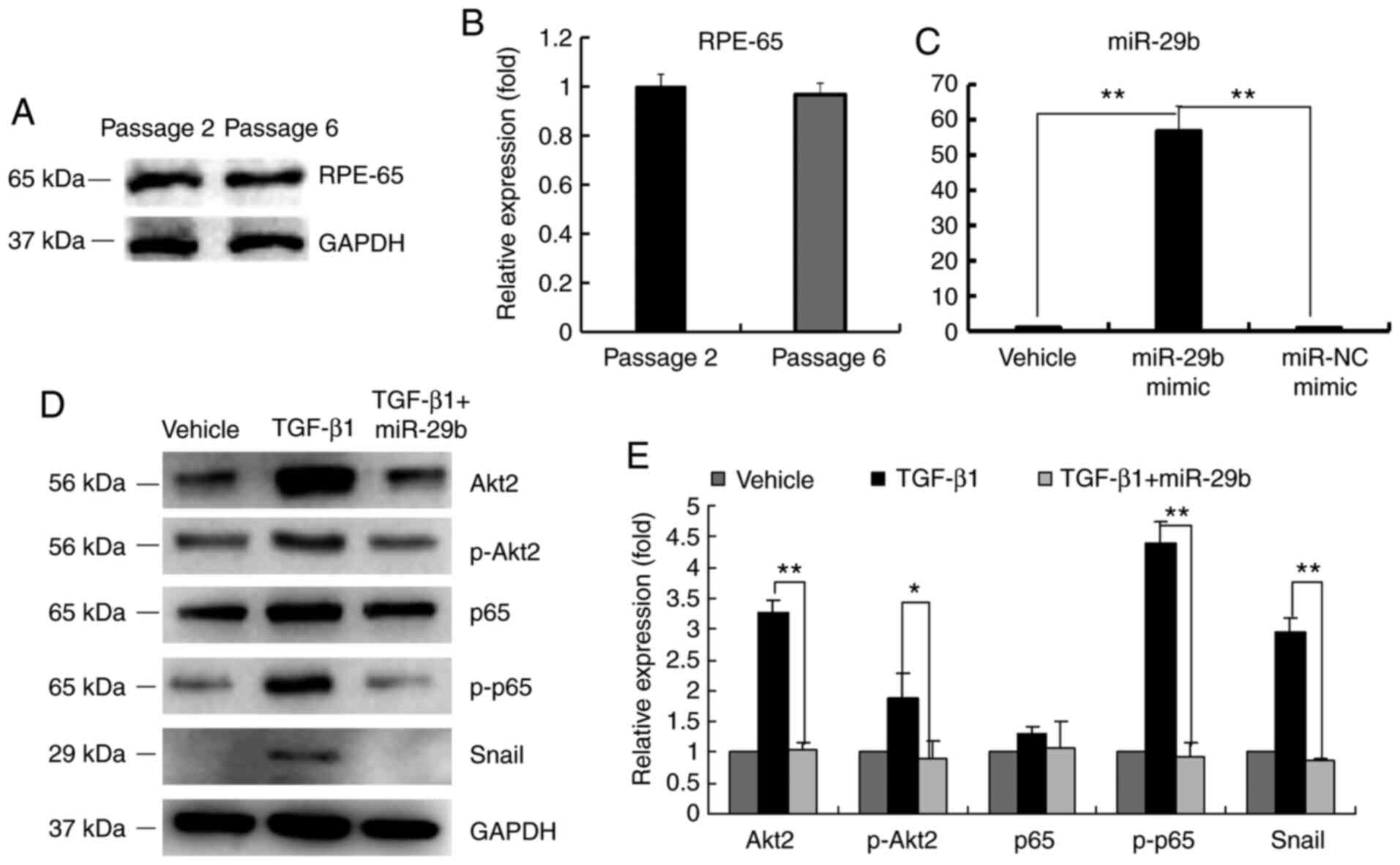 | Figure 2Effect of miR-29b on Akt2, p-p65 and
Snail expression. (A) Western blot analysis of RPE-65. (B)
Graphical representation of the relative abundance of RPE-65. (C)
miR-29b expression after ARPE-19 cells were transfected with
miR-29b mimic and miR-NC mimic. (D) Western blot analysis of Akt2,
p-Akt2, p65, p-p65 and Snail expression with transfection of
miR-29b before TGF-β1 treatment. (E) Graphical representation of
the relative abundance of Akt2, p-Akt2, p65, p-p65 and Snail
expression. All experiments were performed in triplicate.
*P<0.05 and **P<0.01. miR-29b,
microRNA-29b; p-, phosphorylated; RPE, retinal pigment epithelial;
NC, negative control; TGF, transforming growth factor. |
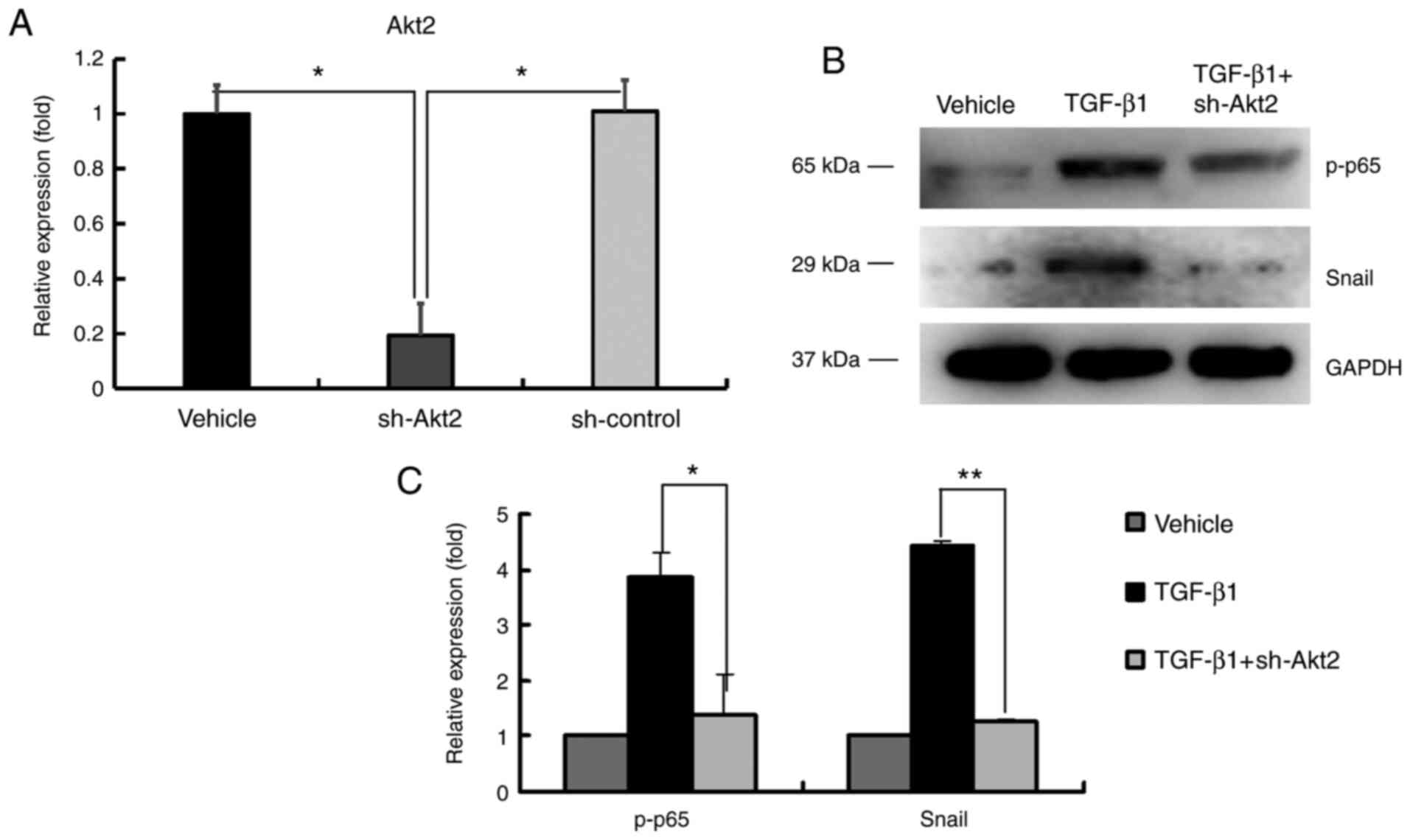
Downregulated Akt2 inhibits the
expression of p-p65 and Snail in ARPE-19 cells
To further study the relationship of Akt2, p-p65 and
Snail in ARPE-19 cells, the expression of Akt2 was silenced, while
p-p65 and Snail expression was determined. As demonstrated in
Fig. 3A, Akt2 was significantly
downregulated after sh-Akt2 transfection in ARPE-19 cells. TGF-β1
induced the expression of p-p65 and Snail; however, the expression
levels of p-p65 and Snail were downregulated after Akt2 expression
was inhibited by sh-Akt2 (Fig. 3B
and C). The aforementioned results
indicated that p-p65 and Snail participated in the downstream
pathway of miR-29b and Akt2.
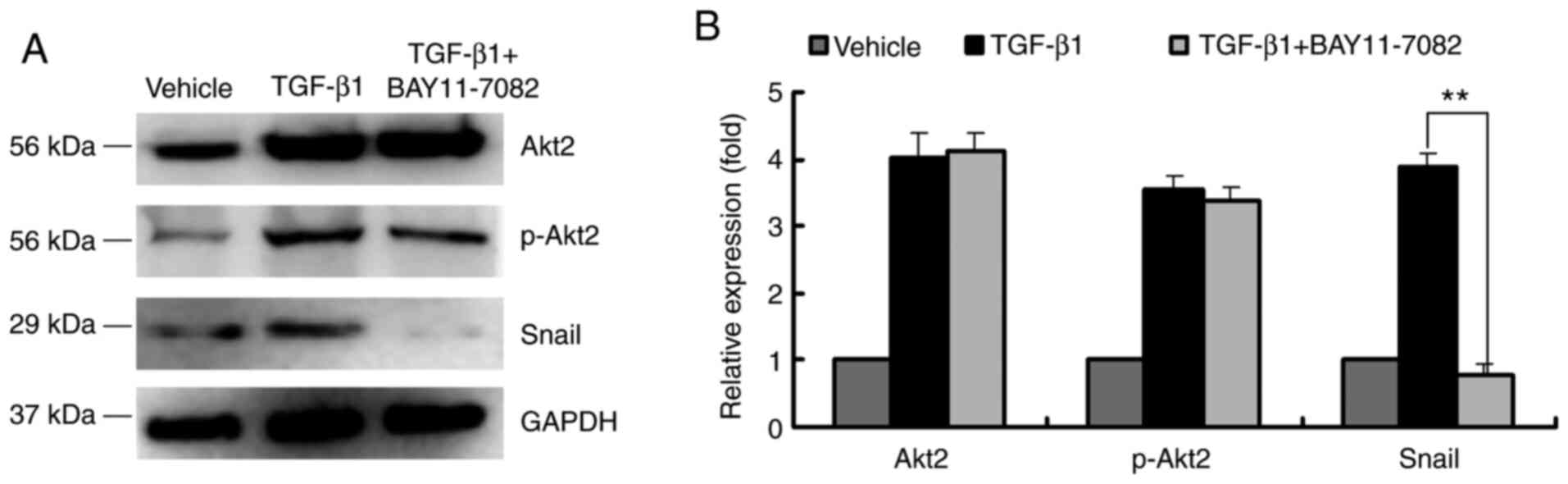
NF-κB inhibitor reduces the expression
of Snail in ARPE-19 cells
It was revealed that Snail is an important
zinc-finger transcription factor in ARPE-19 cell EMT (5). To investigate the regulatory effect of
p-p65 on Snail, the NF-κB inhibitor BAY11-7082 was used to reduce
p-p65 expression. TGF-β1 induced Akt2 and p-Akt2 expression and
this was not affected by the addition of BAY11-7082 (Fig. 4A and B). Moreover, Snail expression was
upregulated after ARPE-19 cells were treated with TGF-β1; however,
this effect was inhibited by BAY11-7082. These results indicated
that the function of Snail in EMT was regulated by NF-κB effector
p-p65 (Fig. 4A and B).
Discussion
EMT of RPE cells in epiretinal membranes is a
potential mechanism of PVR. TGF-β1 has been revealed to play a
vital role in triggering EMT in fibrogenesis and tumor progression
(20,21). In pulmonary fibrosis, a miR-29
family member was significantly reduced and this affected a subset
of fibrosis-related genes including laminins and integrins
(22). Arboleda et al
reported that overexpression of Akt2 in breast cancer cells
resulted in the formation of metastasis and suggested that Akt2
regulated the metastatic genes (23). In the present study, TGF-β1-induced
ARPE-19 cells were used to establish the EMT model and the
relationship between miR-29b and EMT was further investigated. It
was demonstrated that miR-29b and its target Akt2 were closely
related to PVR progression. In epiretinal membranes of patients
with PVR, miR-29b expression was positively correlated with
epithelial marker E-cadherin, while an inverse correlation was
found between the expression of miR-29b, mesenchymal marker α-SMA,
miR-29b and Akt2 mRNA expression. These results indicated that
miR-29b and its target Akt2 participated in epiretinal membrane
formation in PVR.
TGF-β1 is a well-recognized influencing factor of
the NF-κB pathway (24). In the
present study, it was demonstrated that TGF-β1 induced the
expression of p-Akt2 and p-p65, but had no effect on p65.
Specifically, TGF-β1 promoted p65 phosphorylation, but had little
effect on total p65 expression. When Akt2 was silenced, the
expression levels of the subunit of NF-κB (p-p65) and Snail protein
were reduced. NF-κB inhibitor BAY11-7082 prevented TGF-β1-induced
Snail protein expression; however, it had no effect on Akt2 and
p-Akt2. These data indicated that Akt2 was upstream of p-p65 and
Snail was downstream of p-p65. As reported previously, the
activation of NF-κB subunit p65 was sufficient for induction of EMT
(25), and the transcription factor
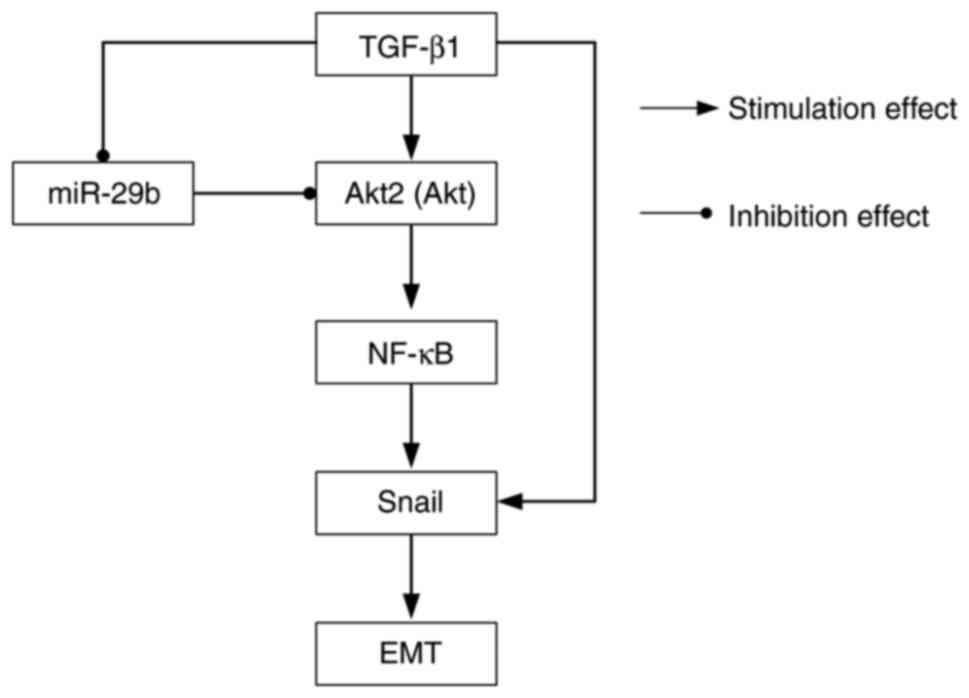
Snail regulated EMT by affecting E-cadherin expression (26). Collectively, these data indicated
that NF-κB and Snail are downstream signals of miR-29b in ARPE-19
cells (Fig. 5). The majority of
experiments were performed using ARPE-19 cells due to their PVR
membrane deficiency, which serves as a limitation to the current
study.
In conclusion, the results of the present study
indicated that miR-29b contributed to the progression of epiretinal
membrane formation in patients with PVR. The effect of miR-29b on
TGF-β1-mediated EMT in ARPE-19 cells was mediated by the
Akt2/p-p65/Snail pathway. The aforementioned findings indicated a
targeted therapeutic strategy for the treatment of PVR. The
Akt2/p-p65/Snail pathway may be the underlying mechanism of miR-29b
in EMT during PVR.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported from the National
Natural Science Foundation of China (grant. no. 81500727) and the
Fundamental Research Funds for the Central Universities (grant. no.
22120180053).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML and FW designed the current study. ML, HL and SY
performed the experiments. XL and CZ analyzed the data. ML wrote
the original manuscript. HL and FW revised the final manuscript. CZ
and FW confirmed the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent for
the preservation and analysis of their tissues for research
purposes. The present study was approved by the Ethics Committee of
Shanghai Tenth People's Hospital (Shanghai, China) and complied to
the guidelines of the Declaration of Helsinki (2013). The ethics
approval no. was SHSY-IEC-KY-4.0/17-79/01.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kwon OW, Song JH and Roh MI: Retinal
detachment and proliferative vitreoretinopathy. Dev Ophthalmol.
55:154–162. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pastor JC, de la Rua ER and Martin F:
Proliferative vitreoretinopathy: Risk factors and pathobiology.
Prog Retin Eye Res. 21:127–144. 2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Saika S, Yamanaka O, Flanders KC, Okada Y,
Miyamoto T, Shirai K, Kitano A, Miyazaki K, Tanaka S and Ikeda K:
Epithelial-mesenchymal transition as a therapeutic target for
prevention of ocular tissue fibrosis. Endocr Metab Immune Disord
Drug Targets. 8:69–76. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hoerster R, Muether PS, Vierkotten S,
Hermann MM, Kirchhof B and Fauser S: Upregulation of TGF-ß1 in
experimental proliferative vitreoretinopathy is accompanied by
epithelial to mesenchymal transition. Graefes Arch Clin Exp
Ophthalmol. 252:11–16. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li H, Wang HW, Wang F, Gu Q and Xu X:
Snail involves in the transforming growth factor β1-mediated
epithelial-mesenchymal transition of retinal pigment epithelial
cells. PLoS One. 6(e23322)2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kevin C and Nikolaus R: The evolution of
gene regulation by transcription factors and microRNAs. Nat Rev
Genet. 8:93–103. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Inui M, Martello G and Piccolo S: MicroRNA
control of signal transduction. Nat Rev Mol Cell Biol. 11:252–263.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Bhatt K, Mi QS and Dong Z: microRNAs in
kidneys: Biogenesis, regulation, and pathophysiological roles. Am J
Physiol Renal Physiol. 300:F602–F610. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kaneko H and Terasaki H: Biological
involvement of microRNAs in proliferative vitreoretinopathy. Transl
Vis Sci Technol. 6(5)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li M, Li H, Liu XQ, Xu D and Wang F:
MicroRNA-29b regulates TGF-β1-mediated epithelial-mesenchymal
transition of retinal pigment epithelial cells by targeting AKT2.
Exp Cell Res. 345:115–124. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Irie HY, Pearline RV, Grueneberg D, Hsia
M, Ravichandran P, Kothari N, Natesan S and Brugge JS: Distinct
roles of Akt1 and Akt2 in regulating cell migration and
epithelial-mesenchymal transition. J Cell Biol. 171:1023–1034.
2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hotamisligil GS: Endoplasmic reticulum
stress and the inflammatory basis of metabolic disease. Cell.
140:900–917. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Meng F, Liu L, Chin PC and D'Mello SR: Akt
is a downstream target of NF-kappa. B. J Biol Chem.
277:29674–29680. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hong K, Kim JH, Hong JS, Yoon HJ, Lee JI,
Hong SP and Hong SD: Inhibition of Akt activity induces the
mesenchymal-to-epithelial reverting transition with restoring
E-cadherin expression in KB and KOSCC-25B oral squamous cell
carcinoma cells. J Exp Clin Cancer Res. 28(28)2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta DeltaC(T)) method. Methods. 25:402–408.
2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
World Medical Association (WMA):
Declaration of Helsinki. Medical Research Involving Human Subjects.
WMA, Ferney-Voltaire 2013. https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/.
|
|
17
|
Dang H, Ding W, Emerson D and Rountree CB:
Snail induces epithelial-to-mesenchymal transition and tumor
initiating stem cell characteristics. BMC Cancer.
11(396)2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cheng ZX, Wang DW, Liu T, Liu WX, Xia WB,
Xu J, Zhang YH, Qu YK, Guo LQ, Ding L, et al: Effects of the HIF-1α
and NF-κB loop on epithelial-mesenchymal transition and
chemoresistance induced by hypoxia in pancreatic cancer cells.
Oncol Rep. 31:1891–1898. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cichon MA and Radisky DC: ROS-induced
epithelial-mesenchymal transition in mammary epithelial cells is
mediated by NF-kB-dependent activation of snail. Oncotarget.
5:2827–2838. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ma J, Zhang L, Hao J, Li N, Tang J and Hao
L: Up-regulation of microRNA-93 inhibits TGF-β1-induced EMT and
renal fibrogenesis by down-regulation of Orai1. J Pharmacol Sci.
136:218–227. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ma J, Tian K, Wang L, Wang K, Du J, Li D,
Wu Z and Zhang J: High expression of TGF-β1 predicting tumor
progression in skull base chordomas. World Neurosurg.
131:e265–e270. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cushing L, Kuang PP, Qian J, Shao F, Wu J,
Little F, Thannickal VJ, Cardoso WV and Lü J: miR-29 is a major
regulator of genes associated with pulmonary fibrosis. Am J Respir
Cell Mol Biol. 145:287–294. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Arboleda MJ, Lyons JF, Kabbinavar FF, Bray
MR, Snow BE, Ayala R, Danino M, Karlan BY and Slamon DJ:
Overexpression of AKT2/protein kinase B beta leads to up-regulation
of β1 integrins, increased invasion, and metastasis of human breast
and ovarian cancer cells. Cancer Res. 63:196–206. 2003.PubMed/NCBI
|
|
24
|
Yang HW, Kim HJ, Park JH, Shin JM and Lee
HM: Apigenin alleviates TGF-β1-induced nasal mucosa remodeling by
inhibiting MAPK / NF-kB signaling pathways in chronic
rhinosinusitis. PLoS One. 13(e0201595)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Julien S, Puig I, Caretti E, Bonaventure
J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A
and Larue L: Activation of NF-kappaB by Akt upregulates Snail
expression and induces epithelium mesenchyme transition. Oncogene.
26:7445–7456. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000.PubMed/NCBI View
Article : Google Scholar
|