1. Introduction
Giant-cell arteritis (GCA) or temporal arteritis and
Takayasu's arteritis are the main known examples of large-vessel
vasculitis (1). GCA is the most
common form of systemic vasculitis and affects both medium and
large vessels, including mainly the aorta and its major
subdivisions, especially the branches of the carotid arteries. GCA
appears almost exclusively after the age of 50 years and its
incidence increases constantly with advancing age, reaching a peak
between 70 and 80 years. The lifetime risk of developing GCA was
estimated at 1% in women and 0.5% in men (1,2).
Although permanent vision loss is considered to be
the most feared complication of GCA, the main factors responsible
for mortality in this pathology are cerebral ischemia and aortic
aneurysms and dissections (3-5).
GCA is a rare cause of stroke, but it is difficult
to differentiate from other, more common etiologies, such as
atherosclerosis because their characteristics and risk factors are
similar (6).
There are several publications in the literature,
mainly small case series reporting stroke cases secondary to GCA,
but there are few population-based studies, using various
methodologies, making their results fairly heterogeneous (7).
Temporal artery biopsy is considered the
gold-standard for the diagnosis of GCA, revealing necrotizing
arteritis with a predominance of mononuclear cell infiltrations or
granulomatosis with multinucleated giant cells. The classification
of GCA developed by the American College of Rheumatology in 1990
includes five criteria: Age of 50 years or older, new-onset
headache, temporal artery tenderness or decreased pulsation,
erythrocyte sedimentation rate of at least 50 and abnormal temporal
artery biopsy results. If at least three of these five criteria are
present in a single case, the diagnosis can be made with a
sensitivity of 93.5% and a specificity of 91.2% (8).
In the last few years duplex ultrasound has become
an important tool for the diagnosis of temporal arteritis. The
presence of a non-compressible, hypoechoic, mostly concentric
arterial wall thickening referred to as the ‘halo’ sign is easily
detectible with ultrasonography (9). The ‘halo sign’ is evidenced, not only
at the level of the temporal arteries, but also at the level of the
vertebral arteries and, in rare cases, at the level of the carotid
arteries too. Sometimes in stroke patients, the accidentally
detected halo sign at the level of the vertebral arteries during a
routine cervical ultrasound examination may be the first clue for
GCA (10).
The aim of the present review was to summarise the
relevant literature of temporal arteritis-related stroke. A
database search was performed with the following terms: ‘Stroke’,
‘temporal arteritis’ and ‘giant cell arteritis’. The majority of
the relevant articles were found in PubMed. In addition, we also
performed screening of Embase, CINAHL (EBSCO) and Cochrane Library
databases. Articles with unavailable full text were excluded from
the analysis.
Searches were limited to articles written in
English.
2. Epidemiology
The majority of relevant studies proved that,
GCA-related stroke mostly occurs between the onset of arteritis
symptoms and one month after the initiation of steroid therapy
(11).
Gonzalez-Gay et al retrospectively analysed
the data of a large series of biopsy-proven GCA patients over a
27-year period of time (12). In
total, 287 patients fulfilled the inclusion criteria and 2.8% of
these patients experienced stroke between onset of the symptoms and
four weeks after the initiation of steroid therapy. The frequency
of stroke was significantly higher in male patients, and the best
predictors of stroke were permanent vision loss and a history of
hypertension prior to the diagnosis of GCA. Furthermore, anaemia at
the time of diagnosis was associated with protection against stroke
(12).
Samson et al conducted a population-based
study to evaluate the epidemiology of stroke patients with GCA
using two prospective databases (13). They found that 7% of 57
biopsy-proven patients developed stroke. The authors explained this
higher incidence relative to that of a previously reported study
(12) was the consequence of their
population-based search design, which allowed the inclusion both of
hospitalised and non-hospitalised patients (13). In addition, they found that the main
risk factors for stroke were male sex, history of vision loss,
smoking, hypertension and high haemoglobin level.
Authors de Boysson et al conducted a
retrospective multicentre case-control study using data from four
GCA referral centres (14). The
incidence of GCA-related stroke ranged from 3 to 7%, which is
consistent with the findings of other authors in different
geographical regions, such as Salvarani et al, with a rate
of 2.7% in 180 GCA patients (11);
Zenone and Puget, who reported a rate of 6.1% in 98 patients
(15); and Nesher et al, who
reported a rate of 7.4% in 175 patients (16).
The first systematic review and meta-analysis of
cohort studies focused on the risk of stroke in GCA was published
by Ungprasert et al (7).
Those authors demonstrated a significant (1.4-fold) increase of
risk of stroke in GCA cases compared with non-GCA cases (7).
3. Pathogenesis
Numerous studies investigated the genetic
susceptibility in GCA, and they found a correlation with certain
human leukocyte antigen (HLA) alleles, mainly the HLA-DRB1*0401 and
DRB1*0404 haplotypes (17). Several
non-HLA genetic loci have also been associated with genetic
susceptibility to GCA, underscoring the polygenic nature of the
genetic background (17).
The first step of autoimmune vascular damage is the
activation of indigenous dendritic cells (DCs) in the vessel wall
by an unknown trigger (e.g., environmental infectious agent,
autoantigen) (18). Then, the
activated DCs are enabled to present antigens, leading to the
activation of cluster of differentiation (CD)4+
T-lymphocytes through major histocompatibility complex (MHC) class
II expression and costimulatory molecules (CD80 and CD86). DCs can
control which vessels are affected as different arteries express
variable combinations of Toll-like receptors (TLRs). TLRs are
transmembrane proteins that play important roles in the innate
immune system. They recognize molecules shared by microorganisms,
danger or pathogen-associated molecular patterns released from
damaged tissue (19).
After T-cell activation, CD4+ T cells and
macrophages infiltrate the media and secrete proinflammatory
cytokines, leading to further T-cell differentiation: T-helper (Th)
1 and Th-17 cells. Interleukin (IL)-12 and IL-18 promote Th1
differentiation and the production of interferon (IFN)-γ, which has
an important role in macrophage activation and granuloma formation.
Additionally, IL-6, IL-1β, IL-21 and IL-23 stimulate Th-17
differentiation, resulting in the expression of IL-17A (19), a proinflammatory cytokine, which has
a pleiotropic effect on macrophages, vascular smooth muscle cells
(VSMCs), endothelial cells (ECs) and fibroblasts as well (20,21).
Macrophages have an important role in the vasculitic
process; thus, multinucleated giant cells and granulomatous
infiltration are pathognomonic features in GCA-related vascular
lesions. The T cells and macrophages infiltrate the arterial wall
through the vasa vasorum. M1-phenotype macrophages secrete
proinflammatory cytokines (IL-1, IL-6) and matrix
metalloproteinases (MMPs), leading to degradation of the
extracellular matrix. The increased proteolytic activity secondary
to the expression of MMP-2 and MMP-9 leads to disruption of the
internal elastic lamina (IEL), which is the pathological hallmark
of GCA. Macrophages also produce reactive oxygen species, leading
to VSMCs and EC damage (17).
Additionally, M2-phenotype macrophages secrete different growth
factors (e.g., vascular endothelial growth factor, platelet-derived
growth factor and fibroblast growth factor), leading to
myofibroblast proliferation and migration towards the intima and
the production of extracellular matrix proteins, provoking intimal
thickening and vessel occlusion (17).
The growth factors secreted by macrophages promote
angiogenesis, induce neovascularisation and amplify vascular
inflammation. According to the literature, neoangiogenesis may also
have a compensatory effect and it may prevent ischemia and reduce
neuro-ophthalmological complications (22).
VSMCs are not only direct targets of the
inflammatory attack, but they may also participate in the
generation and maintenance of the pathological process.
Specifically, VSMCs have the ability to secrete MMPs, leading to
IEL disruption (18).
ECs, especially the micro-ECs in the vasa vasorum,
also play an important role in vascular remodelling and they are
major targets of inflammatory cytokines. IL-6 has important effects
on EC proliferation, tubular formation and the induction of
angiogenesis. ECs are themselves able to produce proinflammatory
cytokines and adhesion molecules in response to paracrine signals,
thus mediating leukocyte trafficking and migration (17,18).
All this complex amplification cascades and the
pathological processes result in the remodelling of the vessel
wall, which leads to stenosis, occlusion or aneurysm formation in
the affected ateries.
4. Stroke characteristics
The majority of stroke cases secondary to GCA are
ischemic stroke in nature. There are only a few isolated cases,
when development of subarachnoid haemorrhage secondary to GCA was
reported (23-25).
In the general population, the carotid-territory
strokes are more frequent than the vertebrobasilar (VB) territory
strokes; in population-based epidemiologic stroke studies their
ratio was 5:1, respectively (26).
However, in the active phase of the disease (between the onset of
GCA symptoms and one month after the initiation of steroid therapy)
the stroke cases secondary to GCA primarily developed in the VB
territory. For example, Gonzalez-Gay et al reported in a
population-based study including a total of 287 GCA patients that
seven out of the eight stroke cases occurred in VB territory.
Moreover, in six of the seven cases the patients were male
(12). In a population-based cohort
study published by Salvarani et al, all of the five stroke
cases among a series of 180 patients with biopsy-proven GCA showed
VB localisation (11). Similar
results were reported by Samson et al (75% of GCA-related
strokes) (13), by Pariente et
al (11/18 stroke cases) (27)
and by de Boysson et al (73% of cases) (14). Notably, studies, which includes all
cases of stroke in GCA patients, regardless of whether it occurs in
the active stage of the disease or not, tend to report higher
frequency of stroke in the carotid localisation, similar to the
data of the general population (28).
Elhfnawy et al prospectively screened
consecutive patients with VB ischemic stroke for GCA, looking for
the halo sign in the temporal and vertebral arteries (29). Among 65 consecutive VB stroke
patients, two cases were identified as positive for the halo sign
in both temporal arteries and at least one vertebral artery. Those
authors concluded that older age, anaemia, elevated inflammatory
markers and the presence of multiple VB steno-occlusive lesions
could be considered red flags for GCA in patients with VB stroke
(29).
García-García et al in a prospective study
used ultrasound in 1,237 consecutive stroke cases to detect the
halo sign (30). Five out of the
1,237 patients had fulfilled the diagnostic ultrasound criteria of
GCA and the diagnosis was also confirmed with biopsy. All 5 cases
presented as a VB-territory stroke. The outcome was favourable in 4
cases, while one patient died due to aspiration pneumonia. Authors
of that study concluded that the recognition of the halo sign in
the vertebral arteries is essential to establish a proper and early
diagnosis.
GCA could lead to bilateral damage of the vertebral
arteries, such as bilateral occlusion, which may result in severe
stroke with a high mortality rate. Rüegg et al analysed a
series of cases with bilateral vertebral artery occlusion-three out
of the total number of eight were his own cases (31). The mortality rate was 75%, the mean
age of onset was 69 years, with male predominance (n=5 men and n=3
women). All patients presented with new-onset headache, four
patients presented with fever and the erythrocyte sedimentation
rate was substantially elevated in all the cases. The extradural
part of the vertebral arteries between the V2 and proximal V4
segments were affected in all the cases. In isolated cases,
bilateral vertebral artery occlusion could be the initial clinical
manifestation of GCA (31,32). The early diagnosis and prompt
initiation of corticosteroid and antithrombotic treatment could
lead to a favourable prognosis even in older patients (33).
It appeared that the intracranial arteries were
spared in GCA because they contain no or little IEL, according to
previous literature. By contrast, the supra-aortic arteries contain
IEL from the aortic arch to the place of entry into the dura mater
and it may extend 5 mm distally too (34,35).
The newer literature, based on data from contrast-enhanced 3-Tesla
magnetic resonance imaging (MRI) demonstrated the frequent
involvement of intradural arteries, mainly the internal carotid
arteries (ICA). Siemonsen et al (36) in a prospective study evaluated 28
patients with suspected GCA, using an MRI protocol focused on the
assessment of the intradural arteries. 3-Tesla MRI with
fat-saturated pre- and postcontrast T1-weighted sequences were
adapted to detect mural thickening and contrast enhancement of the
vessel wall, which are considered to be the signs of mural
inflammation. Ten out of 25 patients presented with vessel wall
enhancement (VWE) of the intradural ICA, and all these cases were
positive for GCA. Five patients presented bilateral, and four
patients presented unilateral VWE of the vertebral arteries,
respectively. The basilar artery did not show VWE in any of the
cases. Moreover, involvement of the intradural vessels did not
correlate with the intracranial steno-occlusive lesions, nor the
cerebral ischemic lesions (36).
Several publications proved that the ischemic
cerebrovascular events are mainly related to the stenosis or
occlusion of the extradural vessels rather than to the intradural
vasculitis (35,37).
There is no clear explanation for the more frequent
involvement of the VB territory predominance in GCA-related
strokes. The smaller diameter of the vertebral arteries relative to
the carotid arteries and the higher vulnerability of these vessels
to high-grade stenosis or occlusion could be a plausible
explanation (34).
Small case series reported that multiple cerebral
ischemic lesions secondary to GCA during the active phase of the
disease could lead to multi-infarct dementia (34,38).
Carotid and vertebral artery dissections with severe
cerebral ischemic complications secondary to GCA had been published
in isolated case reports. The radiological distinction between the
vasculitic vessel wall changes and the mural hematoma secondary to
the dissection is challenging (39-43).
5. Diagnosis
The diagnosis of GCA in stroke patients may be
challenging, especially if stroke is the first clinical
manifestation. Anorexia, malaise, weight loss, fever, headache or
arthralgia in the prior medical history of the patient may turn
attention to the diagnosis of GCA, but these nonspecific symptoms
could easily be overlooked in case of acute stroke. Both C-reactive
protein level and the erythrocyte sedimentation rate are
significantly higher in the majority of the GCA cases, but these
findings are also nonspecific (13). However, duplex ultrasonography,
which is a standard diagnostic tool in acute stroke, could help to
establish the diagnosis of CGA.
The presence of the halo sign at the level of the
cervical vessels, mainly in the vertebral arteries and less often
in the carotid arteries may be the first proof of GCA. In these
cases, the Doppler ultrasound examination of the superficial
temporal arteries is mandatory, because the classic sonography
signs, e.g., hypoechoic halo sign, compression sign, of GCA are
usually present at this level in the majority of cases.
The halo sign was first described by Schmidt et
al in 1995(44). The swollen
vessel wall of the temporal arteries is characterised by ultrasound
as a hypoechoic circumferential mural thickening localised around
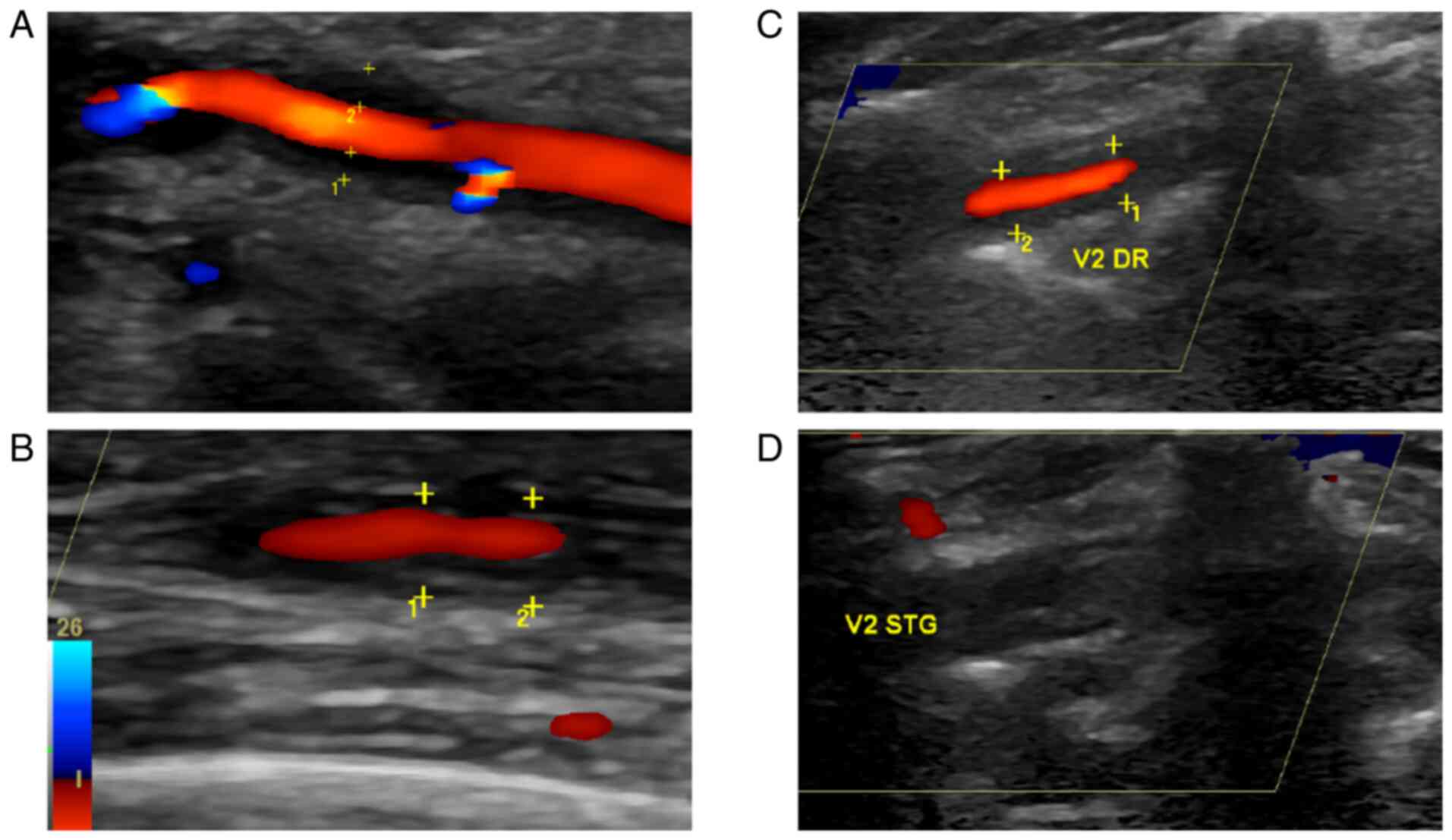
the lumen, with a diameter ranging from 0.3 to 0.5 mm (Fig. 1). The halo sign can be present, not
only at the level of the superficial temporal arteries, but at the
level of the vertebral, occipital, subclavian or axillary arteries
too, and in rare cases, at the level of the carotid arteries
(45). Vessel stenosis or occlusion
can also be evidenced at the level of temporal arteries, but the
diagnostic value of such is lower. The ultrasound examination can
also help to navigate or to plan the precise localization of biopsy
(44).
The other temporal arteritis related ultrasound sign
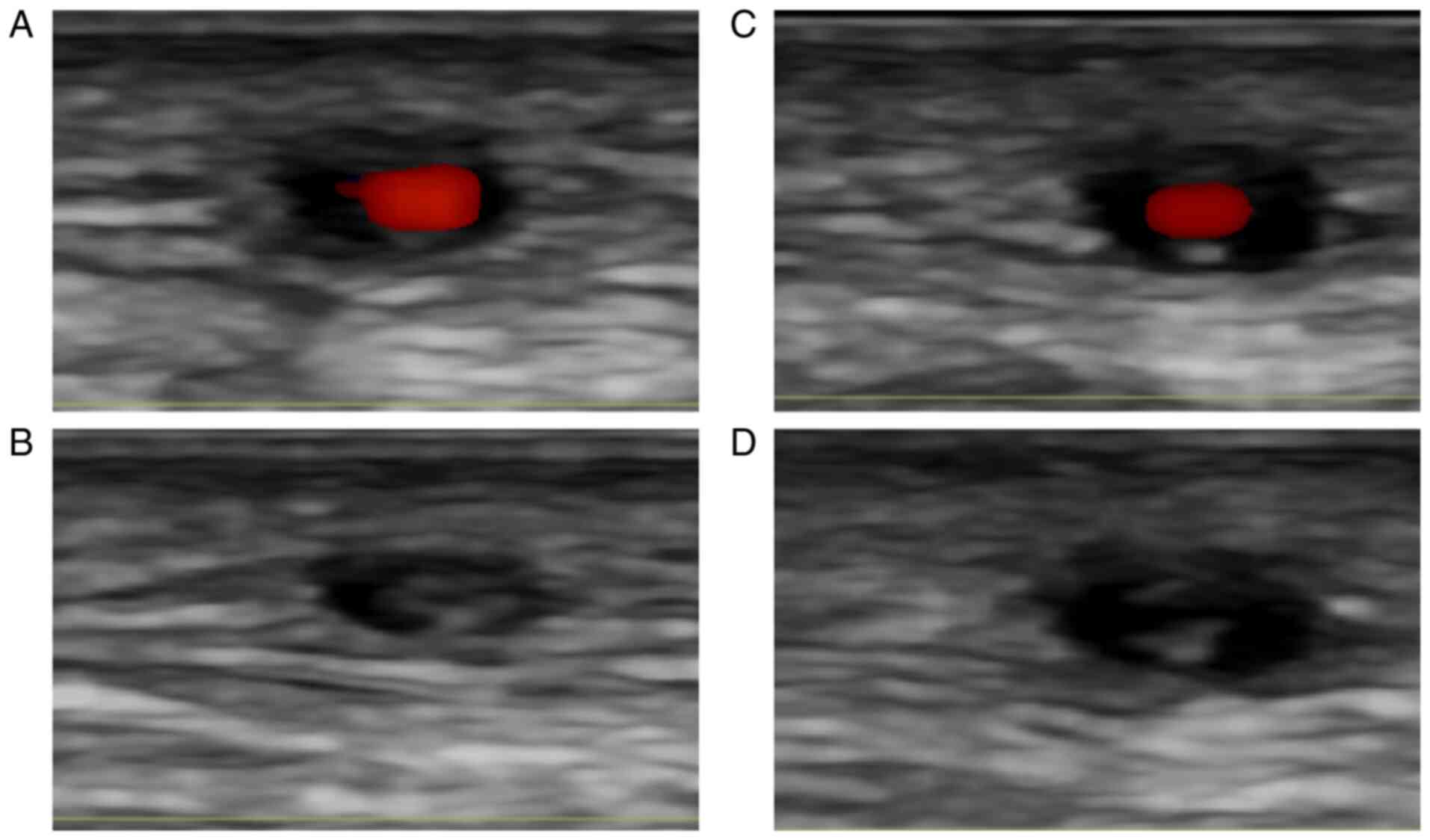
is the so-called ‘compression sign’. This term refers to the
continuous feature of a hypoechoic halo during the compression of
the vessel lumen by the ultrasound transducer (Fig. 2). This sign has high specificity for
the diagnosis (46).
The main advantages of the ultrasound examination
are its non-invasive nature, fast availability, lack of radiation
exposure and the possibility of simultaneous image acquisition and
interpretation. Conversely, the disadvantage of this method is its
operator-dependent nature, the need for high-quality equipment, the
adequate probe setting and the standardisation requirement.
The so-called ‘fast-track clinics’ in Europe and in
the United States with well-trained ultrasound specialists may help
to initiate treatment before the appearance of severe complications
(47,48).
Temporal artery biopsy remains the gold standard of
the diagnosis; however, it is more time-consuming compared to
ultrasonography. Moreover, false-negative results are not uncommon,
due to the segmental involvement of the vessel walls. The mural
edema and vessel wall enhancement were demonstrated with
high-resolution contrast-enhanced MRI and MR angiography (49).
Finally, positron-emission tomography can also be
useful in diagnosis in selected cases (50).
6. Treatment options
The GCA-related stroke is a rare condition;
therefore, there are no evidence-based guidelines or standard
recommendations for the treatment. The role of antiplatelet therapy
in the prevention of severe ischemic complications of GCA is
debated. Nesher et al retrospectively analysed 175
consecutive cases of GCA, and 21% of the patients got a low-dose
aspirin treatment at the time of the diagnosis of GCA (51). The cranial ischemic complications
were less frequent in the aspirin-treated group compared to the
non-treated group. As such, authors of that study concluded that
low-dose aspirin decreases the rate of visual complications and
stroke in patients with GCA. A retrospective study published by Lee
et al included 143 patients with GCA and the mean follow-up
time was up to four years (52).
They reported that the antiplatelet or anticoagulant therapy
significantly reduced the frequency of the cranial ischemic events
compared to the non-treated group, i.e., 16.2 vs. 48%,
respectively, while there was no significant difference between the
two groups regarding to the haemorrhagic complications. On the
other hand, two other studies failed to prove a beneficial effect
of the antiplatelet therapy on the occurrence of severe visual
complication or stroke in newly diagnosed GCA (11,53).
Martínez-Taboada et al published a cumulative
meta-analysis based on the data of 914 GCA patients. According to
their results the antithrombotic therapy applied prior to the
diagnosis of GCA did not provide a protective effect against severe
ischemic complications (54).
However, the combined antithrombotic and corticosteroid treatment
applied after the diagnosis of GCA resulted in a slightly
favourable outcome.
High-dose glucocorticoids are the core therapy in
patients with GCA-related severe complications and it should be
instantly initiated once the diagnosis of GCA is strongly
suspected. The superior efficacy of methylprednisolone pulse
therapy in comparison with high-dose oral prednisone is not clearly
proven, but it is widely used (14,55).
The possible beneficial effects of anticoagulant
treatment are not well-documented (52), while the influence of statins on the
risk reduction of cardiovascular complications of GCA was not
proven. Moreover, the possible use of statins in terms of the
glucocorticoid-sparing effect was not evident (55).
The spectrum of glucocorticoid-sparing agents used
in GCA treatment is large, encompassing classical immunosuppressant
drugs such as azathioprine, methotrexate, cyclophosphamide,
cyclosporine and mycophenolate mofetil together with newer agents
including tocilizumab, ustekinumab, abatacept and adalimumab
(19). Some authors have
recommended using immunosuppressive drugs as first-line therapy
because of steroid-induced side effects and the risk of relapse
(56).
7. Conclusions
Stroke is a rare but outcome-defining complication
of GCA, because it is one of the leading factors, which influence
the mortality and disability rates. GCA-related stroke typically
develops in the VB territory and is more frequent in patients who
have ophthalmic ischemic symptoms. In the context of constitutional
symptoms and elevated inflammatory markers, clinicians must search
for GCA in elderly stroke patients because the prompt institution
of corticosteroid treatment and antithrombotic therapy may improve
the prognosis. The widely available imaging modality of duplex
ultrasound may easily guide the diagnosis if the suggestive halo
sign is identified in the extracranial vessels. The diagnosis of
this entity requires a high level of suspicion.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
ZB, RB, AS and RCF conceived and designed the study;
ZB, SM, RCF, AS performed the literature search and assessed the
authenticity of raw data; ZB, SM, AM, LB, AS, and SA analyzed the
relevant literature and wrote the manuscript; ZB, RB, RCF, AS and
SA contributed to the interpretation of data and the revision of
the manuscript, provided critical review for the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
None to declare.
References
|
1
|
Crowson CS, Matteson EL, Myasoedova E,
Michet CJ, Ernste FC, Warrington KJ, Davis JM III, Hunder GG,
Therneau TM and Gabriel SE: The lifetime risk of adult-onset
rheumatoid arthritis and other inflammatory autoimmune rheumatic
diseases. Arthritis Rheum. 63:633–639. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gonzalez-Gay MA, Vazquez-Rodriguez TR,
Lopez-Diaz MJ, Miranda-Filloy JA, Gonzalez-Juanatey C, Martin J and
Llorca J: Epidemiology of giant cell arteritis and polymyalgia
rheumatica. Arthritis Rheum. 61:1454–1461. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Aiello PD, Trautmann JC, McPhee TJ,
Kunselman AR and Hunder GG: Visual prognosis in giant cell
arteritis. Ophthalmology. 100:550–555. 1993.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Font C, Cid MC, Coll-Vinent B, López-Soto
A and Grau JM: Clinical features in patients with permanent visual
loss due to biopsy-proven giant cell arteritis. Br J Rheumatol.
36:251–254. 1997.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kermani TA, Warrington KJ, Crowson CS,
Ytterberg SR, Hunder GG, Gabriel SE and Matteson EL: Large-vessel
involvement in giant cell arteritis: A population-based cohort
study of the incidence-trends and prognosis. Ann Rheum Dis.
72:1989–1994. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Arboix A, Bechich S, Oliveres M,
García-Eroles L, Massons J and Targa C: Ischemic stroke of unusual
cause: Clinical features, etiology and outcome. Eur J Neurol.
8:133–139. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ungprasert P, Wijarnpreecha K, Koster MJ,
Thongprayoon C and Warrington KJ: Cerebrovascular accident in
patients with giant cell arteritis: A systematic review and
meta-analysis of cohort studies. Semin Arthritis Rheum. 46:361–366.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hunder GG, Bloch DA, Michel BA, Stevens
MB, Arend WP, Calabrese LH, Edworthy SM, Fauci AS, Leavitt RY and
Lie JT: , et al: The American College of Rheumatology 1990
criteria for the classification of giant cell arteritis. Arthritis
Rheum. 33:1122–1128. 1990.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Schmidt WA: Ultrasound in the diagnosis
and management of giant cell arteritis. Rheumatology (Oxford). 57
(Suppl 2):ii22–ii31. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bajkó Z, Bălaşa R, Szatmári S, Rusu S,
Moţăţăianu A and Maier S: The role of ultrasound in the diagnosis
of temporal arteritis. Neurol Neurochir Pol. 49:139–143.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Salvarani C, Della Bella C, Cimino L,
Macchioni P, Formisano D, Bajocchi G, Pipitone N, Catanoso MG,
Restuccia G, Ghinoi A and Boiardi L: Risk factors for severe
cranial ischemic events in an Italian population-based cohort of
patients with giant cell arteritis. Rheumatology (Oxford).
48:250–253. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gonzalez-Gay MA, Vazquez-Rodriguez TR,
Gomez-Acebo I, Pego-Reigosa R, Lopez-Diaz MJ, Vazquez-Triñanes MC,
Miranda-Filloy JA, Blanco R, Dierssen T, Gonzalez-Juanatey C and
Llorca J: Strokes at time of disease diagnosis in a series of 287
patients with biopsy-proven giant cell arteritis. Medicine
(Baltimore). 88:227–235. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Samson M, Jacquin A, Audia S, Daubail B,
Devilliers H, Petrella T, Martin L, Durier J, Besancenot JF,
Lorcerie B, et al: Stroke associated with giant cell arteritis: A
population-based study. J Neurol Neurosurg Psychiatry. 86:216–221.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
de Boysson H, Liozon E, Larivière D,
Samson M, Parienti JJ, Boutemy J, Maigné G, Martin Silva N, Ly K,
Touzé E, et al: Giant cell arteritis-related stroke: A
retrospective multicenter case-control study. J Rheumatol.
44:297–303. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zenone T and Puget M: Characteristics of
cerebrovascular accidents at time of diagnosis in a series of 98
patients with giant cell arteritis. Rheumatol Int. 33:3017–3023.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nesher G, Berkun Y, Mates M, Baras M,
Nesher R, Rubinow A and Sonnenblick M: Risk factors for cranial
ischemic complications in giant cell arteritis. Medicine
(Baltimore). 83:114–122. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Koster MJ and Warrington KJ: Giant cell
arteritis: Pathogenic mechanisms and new potential therapeutic
targets. BMC Rheumatol. 1(2)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Piggott K, Biousse V, Newman NJ, Goronzy
JJ and Weyand CM: Vascular damage in giant cell arteritis.
Autoimmunity. 42:596–604. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Harky A, Fok M, Balmforth D and Bashir M:
Pathogenesis of large vessel vasculitis: Implications for disease
classification and future therapies. Vasc Med. 24:79–88.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Espígol-Frigolé G, Corbera-Bellalta M,
Planas-Rigol E, Lozano E, Segarra M, García-Martínez A,
Prieto-González S, Hernández-Rodríguez J, Grau JM, Rahman MU and
Cid MC: Increased IL-17A expression in temporal artery lesions is a
predictor of sustained response to glucocorticoid treatment in
patients with giant-cell arteritis. Ann Rheum Dis. 72:1481–1487.
2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Terrier B, Geri G, Chaara W, Allenbach Y,
Rosenzwajg M, Costedoat-Chalumeau N, Fouret P, Musset L, Benveniste
O, Six A, et al: Interleukin-21 modulates Th1 and Th17 responses in
giant cell arteritis. Arthritis Rheum. 64:2001–2011.
2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cid MC, Hernández-Rodríguez J, Esteban MJ,
Cebrián M, Gho YS, Font C, Urbano-Márquez A, Grau JM and Kleinman
HK: Tissue and serum angiogenic activity is associated with low
prevalence of ischemic complications in patients with giant-cell
arteritis. Circulation. 106:1664–1671. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Takahashi I, Takamura H, Gotoh S, Sasaki H
and Ishikawa T: Giant cell arteritis with subarachnoid haemorrhage
due to the rupture of inflammatory aneurysm of the posterior
inferior cerebellar artery. Acta Neurochir (Wien). 138:893–894.
1996.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dawson ET, Brown DA and Rabinstein AA:
Headache, TIA and subarachnoid haemorrhage: Dissecting an unusual
cause for stroke-like symptoms. BMJ Case Rep.
2017(bcr2017219927)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gál R, Bălaşa R, Bajkó Z, Maier S, Simu I
and Bălaşa A: Lethal subarachnoid and intracerebral haemorrhage
associated with temporal arteritis. A case report. J Crit Care Med
(Targu Mures). 3:153–157. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Turney TM, Garraway WM and Whisnant JP:
The natural history of hemispheric and brainstem infarction in
Rochester, Minnesota. Stroke. 15:790–794. 1984.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pariente A, Guédon A, Alamowitch S,
Thietart S, Carrat F, Delorme S, Capron J, Cacciatore C, Soussan M,
Dellal A, et al: Ischemic stroke in giant-cell arteritis: French
retrospective study. J Autoimmun. 99:48–51. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Caselli RJ, Hunder GG and Whisnant JP:
Neurologic disease in biopsy-proven giant cell (temporal)
arteritis. Neurology. 38:352–359. 1988.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Elhfnawy AM, Elsalamawy D, Abdelraouf M,
Schliesser M, Volkmann J and Fluri F: Red flags for a concomitant
giant cell arteritis in patients with vertebrobasilar stroke: A
cross-sectional study and systematic review. Acta Neurol Belg.
120:1389–1398. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
García-García J, Ayo-Martín Ó,
Argandoña-Palacios L and Segura T: Vertebral artery halo sign in
patients with stroke: A key clue for the prompt diagnosis of giant
cell arteritis. Stroke. 42:3287–3290. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rüegg S, Engelter S, Jeanneret C, Hetzel
A, Probst A, Steck AJ and Lyrer P: Bilateral vertebral artery
occlusion resulting from giant cell arteritis: Report of 3 cases
and review of the literature. Medicine (Baltimore). 82:1–12.
2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Säve-Söderbergh J, Malmvall BE, Andersson
R and Bengtsson BA: Giant cell arteritis as a cause of death.
Report of nine cases. JAMA. 255:493–496. 1986.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bajko Z, Filep RC, Maier S, Motataianu A,
Andone S and Balasa R: Bilateral vertebral artery occlusion without
stroke secondary to giant cell arteritis. Acta Reumatol Port.
44:270–272. 2019.PubMed/NCBI
|
|
34
|
Solans-Laqué R, Bosch-Gil JA,
Molina-Catenario CA, Ortega-Aznar A, Alvarez-Sabin J and
Vilardell-Tarres M: Stroke and multi-infarct dementia as presenting
symptoms of giant cell arteritis: Report of 7 cases and review of
the literature. Medicine (Baltimore). 87:335–344. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wilkinson IM and Russell RW: Arteries of
the head and neck in giant cell arteritis. A pathological study to
show the pattern of arterial involvement. Arch Neurol. 27:378–391.
1972.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Siemonsen S, Brekenfeld C, Holst B,
Kaufmann-Buehler AK, Fiehler J and Bley TA: 3T MRI reveals extra-
and intracranial involvement in giant cell arteritis. AJNR Am J
Neuroradiol. 36:91–97. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bogousslavsky J, Deruaz JP and Regli F:
Bilateral obstruction of internal carotid artery from giant-cell
arteritis and massive infarction limited to the vertebrobasilar
area. Eur Neurol. 24:57–61. 1985.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Caselli RJ: Giant cell (temporal)
arteritis: A treatable cause of multi-infarct dementia. Neurology.
40:753–755. 1990.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Parra J, Domingues J, Sargento-Freitas J
and Santana I: Extensive intracranial involvement with multiple
dissections in a case of giant cell arteritis. BMJ Case Rep.
2014(bcr2014204130)2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Proft F, Czihal M, Rémi J, Fesl G,
Woischke C and Koops HS: Fatal spontaneous bilateral vertebral
artery dissection in giant cell arteritis (GCA). J Vasc.
3(2)2017.
|
|
41
|
Bajkó Z, Bălaşa R, Moţăţăianu A, Bărcuţean
L, Stoian A, Stirbu N and Maier S: Malignant middle cerebral artery
infarction secondary to traumatic bilateral internal carotid artery
dissection. A case report. J Crit Care Med (Targu Mures).
2:135–141. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Filep RC, Bajko Z, Simu IP and Stoian A:
Pseudo-dissection of the internal carotid artery in acute ischemic
stroke. Acta Neurol Belg. 120:469–472. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Bajkó Z, Maier S, Moţăţăianu A, Bălaşa R,
Vasiu S, Stoian A and Andone S: Stroke secondary to traumatic
carotid artery injury-A case report. J Crit Care Med (Targu Mures).
4:23–28. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Schmidt WA, Kraft HE, Völker L, Vorpahl K
and Gromnica-Ihle EJ: Colour Doppler sonography to diagnose
temporal arteritis. Lancet. 345(866)1995.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Schmidt WA, Natusch A, Möller DE, Vorpahl
K and Gromnica-Ihle E: Involvement of peripheral arteries in giant
cell arteritis: A color Doppler sonography study. Clin Exp
Rheumatol. 20:309–318. 2002.PubMed/NCBI
|
|
46
|
Aschwanden M, Daikeler T, Kesten F, Baldi
T, Benz D, Tyndall A, Imfeld S, Staub D, Hess C and Jaeger KA:
Temporal artery compression sign-a novel ultrasound finding for the
diagnosis of giant cell arteritis. Ultraschall Med. 34:47–50.
2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Schmidt WA: Role of ultrasound in the
understanding and management of vasculitis. Ther Adv Musculoskelet
Dis. 6:39–47. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Baig IF, Pascoe AR, Kini A and Lee AG:
Giant cell arteritis: Early diagnosis is key. Eye Brain. 11:1–12.
2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Bley TA, Wieben O, Uhl M, Thiel J, Schmidt
D and Langer M: High-resolution MRI in giant cell arteritis:
Imaging of the wall of the superficial temporal artery. AJR Am J
Roentgenol. 184:283–287. 2005.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Pelletier-Galarneau M and Ruddy TD: PET/CT
for diagnosis and management of large-vessel vasculitis. Curr
Cardiol Rep. 21(34)2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Nesher G, Berkun Y, Mates M, Baras M,
Rubinow A and Sonnenblick M: Low-dose aspirin and prevention of
cranial ischemic complications in giant cell arteritis. Arthritis
Rheum. 50:1332–1337. 2004.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Lee MS, Smith SD, Galor A and Hoffman GS:
Antiplatelet and anticoagulant therapy in patients with giant cell
arteritis. Arthritis Rheum. 54:3306–3309. 2006.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Narváez J, Bernad B, Gómez-Vaquero C,
García-Gómez C, Roig-Vilaseca D, Juanola X, Rodriguez-Moreno J,
Nolla JM and Valverde J: Impact of antiplatelet therapy in the
development of severe ischemic complications and in the outcome of
patients with giant cell arteritis. Clin Exp Rheumatol. 26:S57–S62.
2008.PubMed/NCBI
|
|
54
|
Martínez-Taboada VM, López-Hoyos M,
Narvaez J and Muñoz-Cacho P: Effect of antiplatelet/anticoagulant
therapy on severe ischemic complications in patients with giant
cell arteritis: A cumulative meta-analysis. Autoimmun Rev.
13:788–794. 2014.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Bienvenu B, Ly KH, Lambert M, Agard C,
André M, Benhamou Y, Bonnotte B, de Boysson H, Espitia O, Fau G, et
al: Management of giant cell arteritis: Recommendations of the
French study group for large vessel vasculitis (GEFA). Rev Med
Interne. 37:154–165. 2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Larivière D, Sacre K, Klein I, Hyafil F,
Choudat L, Chauveheid MP and Papo T: Extra- and intracranial
cerebral vasculitis in giant cell arteritis: An observational
study. Medicine (Baltimore). 93(e265)2014.PubMed/NCBI View Article : Google Scholar
|