Introduction
Aldosterone-producing adenoma (APA) is a benign
adenoma that causes autonomous secretion of aldosterone (1). Its symptoms include fatigue; the
prevalence of APA in patients with hypertension is 8% (1). Few patients die of APA and most cases
of APA-associated deaths are caused by the complications, including
hypertensive cerebral haemorrhage, arrhythmia caused by
hypokalaemia, renal failure and electrolyte disturbance (1). The primary method for treating APA is
surgical resection; however, poor regulation of blood pressure
following surgery is a long-term clinical problem as hypertension
can lead to vascular remodeling (2). If the surgery occurred after the
vascular remodeling or medication did not fully treat hypertension,
the rate of post-operative complications may increase (2). Aldosterone may induce vascular
remodelling; it can directly lead to vascular remodelling and
induce the apoptosis of vascular smooth muscle cells. The apoptosis
of vascular endothelial cells and an imbalance in their
proliferation may lead to vascular remodelling (3).
Fibulin-5 belongs to the fibulin family of proteins,
which are extracellular matrix proteins and may serve an important
role in aldosterone-induced vascular remodelling. Fibulin-5 is a
multifunctional protein that serves an important role in
maintaining the formation and stabilization of elastic fibres, as
well as the proliferation, migration and apoptosis of human aortic
vascular smooth muscle cells (4,5). This
experiment stimulates the vascular remodeling which occurs in PA.
It also shows that the mice lack of fibulin-5 get more severe
vascular remodeling and fibulin-5 can improve it (6). In the presence of aldosterone,
fibulin-5 deposition in the rat aorta significantly increases,
suggesting that fibulin-5 may serve an important role in
aldosterone-induced vascular remodelling (7). Furthermore, the regulation of
fibulin-5 during apoptosis differs in different types of cells or
in different environments in the same cell (8-12).
Therefore, it is important to clarify the effect of fibulin-5 on
the apoptosis of human ascending aortic smooth muscle cells treated
with aldosterone.
Materials and methods
Cells and reagents
Human ascending aortic smooth muscle cells
(HA-VSMCs) were purchased from the Advanced Research Center,
Central South University. Cells were cultured in complete medium
[RPMI 1640 medium (Hyclone; GE Healthcare Life Sciences) containing
12% fetal bovine serum (both Gibco; Thermo Fisher Scientific,
Inc.)] at 37˚C. All cells were routinely resuscitated and passaged
and cells used in the following experiment were all in the
logarithmic growth phase.
Apoptosis-inducing agents, including cycloheximide,
which acted as a positive control, and aldosterone were purchased
from Sigma-Aldrich; Merck KGaA, dissolved in dimethyl sulfoxide,
and stored at 4˚C. Puromycin was purchased from InvivoGen,
dissolved in complete medium, sterilized using a 0.22 µm filter and
stored at -20˚C. Lipofectamine® 2000 was purchased from
Invitrogen; Thermo Fisher Scientific, Inc. Fibulin-5 monoclonal
antibody was purchased from Abcam. The Lenti-Pac™ HIV Expression
Packaging kit, All-in-One™ First-Strand cDNA Synthesis kit and
SYBR-Green Master with Rox kit were all purchased from GeneCopoeia,
Inc.
Construction of interference and
overexpression vectors
The interference and overexpression vectors were
designed and constructed by GeneCopoeia, Inc. The fibulin-5
overexpressing vector was constructed using the OmicsLink™
expression cloning vector EX-Z5658-Lv105. The control plasmid used
was EX-NEG-Lv105. The fibulin-5 gene (1,347 bps; NM_006329) was
cloned into the vector, competent cells were transformed and the
culture was expanded by selecting a colony and culture in LB liquid
culture medium supplemented with 100 µg/ml ampicillin for 12-16 h
at 37˚C at 200 rpm. Extracted plasmids were sequenced for
lentivirus packaging. The interference vector was constructed using
the OmicsLink™ short hairpin (sh)RNA Expression Clone vector
psi-LVRU6P. The fibulin-5 shRNA sequence was
5'-GGATACTCACTGTTACCATTC-3' and the scramble shRNA sequence of the
control vector was 5'-GCTTCGCGCCGTAGTCTTA-3'.
Lentiviral packaging of the fibulin-5
interference and overexpression vector
Using the Lenti-Pac™ HIV Expression Packaging kit
(GeneCopoeia), the interference plasmid and overexpression plasmid
were transfected into 293T cells with DNA-lipofectamine 2000
Reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Complete
medium was replaced 8 h after transfection. The culture
supernatants rich in lentiviral particles were collected following
48 h culture and stored at -80˚C.
Construction of the fibulin-5 stably
transmitted HA-VSMC cell line
Following 72 h fibulin-5 interference, HA-VSMC cells
were infected with overexpression and control lentivirus particles
(MOI, 5) using polybrene™ (EMD Millipore; Merck KGaA) and 1.5 µg/ml
puromycin to kill any cells that were not successfully transfected.
The medium was changed every 2 days and continuous screening
continued for 2 weeks with complete medium to expand the cell
culture. Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) was used to verify the expression of fibulin-5
mRNA and western blotting was performed to evaluate the expression
of fibulin-5 protein.
Detection of a stable transfection
effect
RNA was extracted using TRIzol® (Thermo
Fisher Scientific, Inc.) from each group of cells in the
logarithmic growth phase. The concentration and purity of RNA were
determined using a spectrophotometer at a wavelength of 260 and 280
nm. RT was performed using the All-in-One™ First-Strand cDNA
Synthesis kit (GeneCopoeia) under the following reaction
conditions: 42˚C for 60 min and 70˚C for 5 min. Primers were
designed and synthesized by GeneCopoeia. qPCR was performed using
the SYBR-Green Master with Rox kit (GeneCopoeia). The reaction
conditions for qPCR were 95˚C for 10 min, followed by 40 cycles of
95˚C for 15 sec, 60˚C for 30 sec and 72˚C for 30 sec. mRNA
expression in each group was quantified using the 2-ΔΔCq
method (13).
Western blotting
The expression level of fibulin-5 was detected in
the human ascending aortic smooth muscle cells from the following
groups by western blotting: HA-VSMC, mFibulin-5, mFibulin-5 Ctrl,
shFibulin-5 Ctrl, and shFibulin-5. Briefly, protein samples were
obtained using RIPA lysis buffer (Beyotime Institute of
Biotechnology) containing protease inhibitor cocktail (EMD
Millipore; Merck KGaA), following which protein concentrations were
measured using Bicinchoninic Acid Protein Assay Kit (Beyotime
Institute of Biotechnology). Samples at 30 mg/lane were separated
by 10% SDS-PAGE and then transferred onto a PVDF membrane (EMD
Millipore; Merck KGaA). β-Actin was used as a loading control. The
membrane was then blocked with 5% fat-free milk at room temperature
for 40 min and separately with either mouse anti-Fibulin-5
(1:1,000; cat. no. ab66339; Abcam) or mouse anti-β-actin (1:5,000;
cat. no. ab6276; Abcam) primary antibodies overnight at 4˚C. The
membranes were then incubated with peroxidase-conjugated goat
anti-mouse IgG secondary antibody at room temperature for 1 h
(1:10,000; cat. no. A4416; Sigma-Aldrich; Merck KGaA). Protein
bands were visualized using SuperSignal™ West Femto Maximum
Sensitivity Substrate (Thermo Fisher Scientific, Inc). ImageJ
software (version 1.46; National Institutes of Health) was used for
grey scale analysis and to calculate relative protein
expression.
Assessment of apoptosis in HA-VSMCs
following treatment with aldosterone
HA-VSMC cells in the logarithmic growth phase were
plated in a 6-well plate at a density of 5x105.
Following incubation, cells were treated with 0, 1, 10, 100 and
1000 µM aldosterone for 1, 2, 3, 4 and 5 days. Cells were then
collected. Cells treated with 4 mg/l actinomycin for 3 h were used
as apoptotic positive controls as actinomycin (Beijing Solarbio
Science & Technology Co., Ltd.) represses transcription and
induce necrosis; untreated cells were used as negative controls.
Levels of apoptosis were measured using the Annexin V-fluorescein
isothiocyanate (FITC) apoptosis detection kit (NeoBioscience
Technology Co., Ltd.) and a flow cytometer. Cells
(5x105/ml) were resuspended in 1X binding buffer and
stained with Annexin V-FITC and propidium iodide for 5 min at room
temperature, followed by flow cytometry detection. The rate of
apoptosis was analysed using CellQuest software (version 3.3; BD
Biosciences).
Assessment of fibulin-5 interference
and overexpression on aldosterone-induced apoptosis
Cells overexpressing, knockdown and control cells in
the logarithmic growth phase were seeded into a 6-well plate
(5x105 cells/well) following overnight incubation were
treated with different concentrations of aldosterone for 48 h.
Cells were grown under a humidified atmosphere containing 5%
CO2 at 37˚C. Cells were collected and levels of
apoptosis were measured using flow cytometry. Cells treated with
actinomycin for 3 h were used as apoptotic positive controls and
untreated cells were used as negative controls.
Statistical analysis
Results were plotted using Graph Pad Prism 6.01
(GraphPad Software, Inc.) and analyzed using one-way analysis of
variance followed by a Student-Newman-Keuls test. Data were
analysed using the SPSS 21.0 statistical software package (IBM
Corp.) and all data are presented as the mean ± standard deviation
of three independent experiment.
Results
Determination of fibulin-5
interference and overexpression in stable cell lines
The fibulin-5 overexpression and interference
lentiviruses were used to infect HA-VSMC cells. Cells were then
screened with puromycin for 2 weeks, subsequently mRNA and protein
were extracted from cells for analysis. The expression of fibulin-5
mRNA and protein in each treated group was detected by RT-qPCR and
western blotting, respectively. The results demonstrated that the
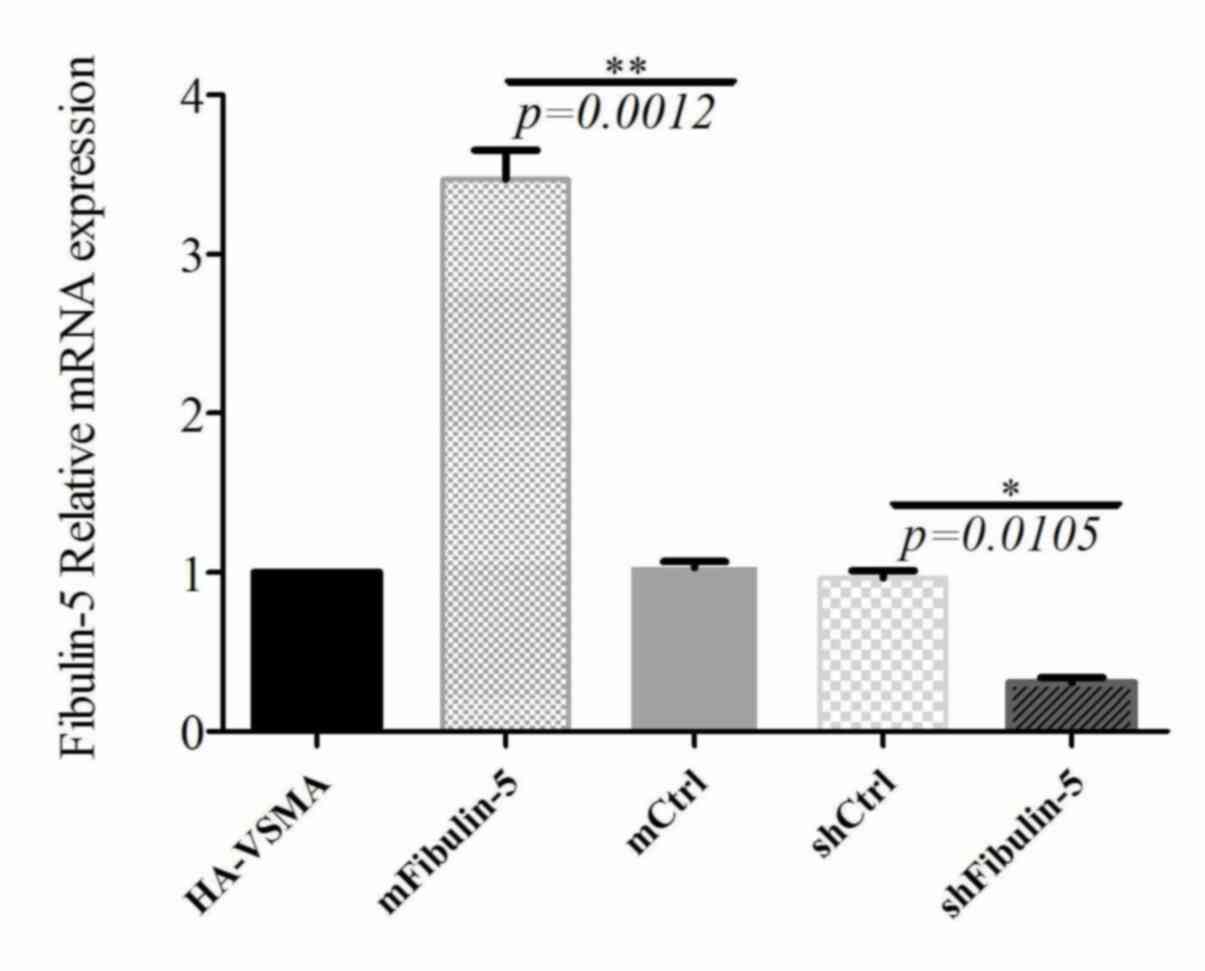
expression fibulin-5 in cell lines overexpressing fibulin-5 was
significantly higher (~3 times) compared with the control groups
and the expression of mRNA in shFibulin-5 interference cells was
significantly downregulated by 69% (Fig. 1). Furthermore, the results of
western blotting confirmed that the expression of fibulin-5 protein
in cells transfected with overexpressing lentivirus was
significantly increased (2 times) compared with control cells
(Fig. 2). However, the expression
of fibulin-5 in cells transfected with shFibulin-5 was
significantly downregulated by ~80% compared with the control
group. These results confirm that stable cell lines exhibiting
fibulin-5 overexpression and inhibition were successfully
constructed and that they could be used in subsequent
experiments.
Aldosterone induces the apoptosis of
HA-VSMCs
The effect of different concentrations of
aldosterone (1, 10, 100 and 1,000 µM) on the apoptosis of HA-VSMCs
at different time points (48 h and 5 days) was determined using the
Annexin V-FITC/propidium iodide Apoptosis Detection kit. The
results indicated that treatment with <10 µM aldosterone for 5
days did not induce apoptosis in HA-VSMC cells (Fig. 3). The effect on the apoptosis of
HA-VSMCs following treatment with 1,000 µM aldosterone for 24 h was
not significant (data not shown); however, 48 h treatment with
1,000 µM aldosterone was effective at inducing apoptosis. After 72
h aldosterone treatment, the toxicity was more notable, where the
number of C-+ cells increased markedly. Increased apoptotic rate in
C+-\C++ cells was also observed (Fig.
3), in accordance with the results of previous studies
(14,15).
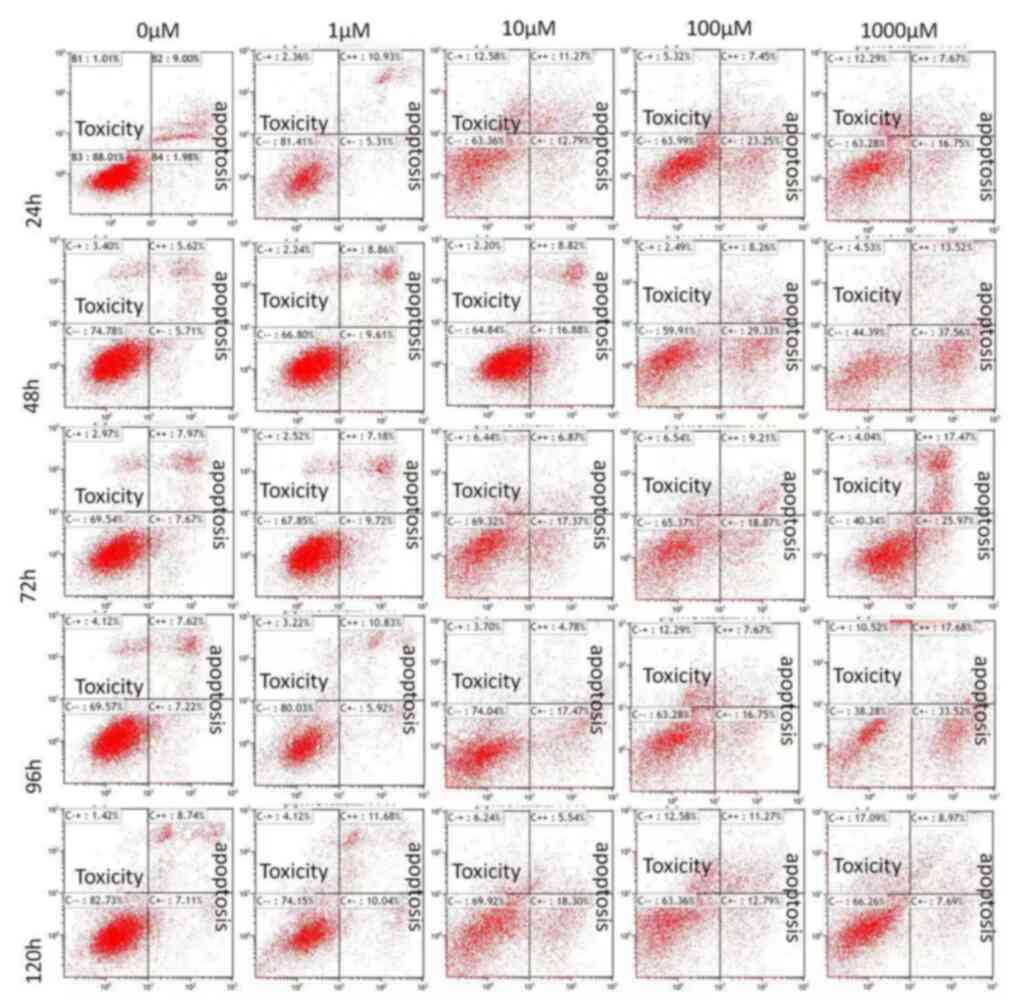 | Figure 3HA-VSMCs were treated with different
concentrations of Aldo (0, 1, 10, 100 and 1,000 µM) for different
durations (1, 2, 3, 4 and 5 days). Apoptosis was measured using the
Annexin V-fluorescein isothiocyanate/propidium iodide Apoptosis
Detection kit. HA-VSMC, human ascending aortic smooth muscle cells;
Aldo, aldosterone; B1, B2, B3 and B4, normal cell control groups;
C-+, dead cells; C++: Late apoptotic cells; C--, living cells; C+-,
early apoptotic cells. |
Effect of fibulin-5 on the
aldosterone-induced apoptosis of HA-VSMC
Under the same conditions (logarithmic growth phase
and fibulin-5 interference and overexpression), stable strains and
the corresponding control cell group were treated with 100 and
1,000 µM aldosterone for 48 h. The apoptosis rate of each group was
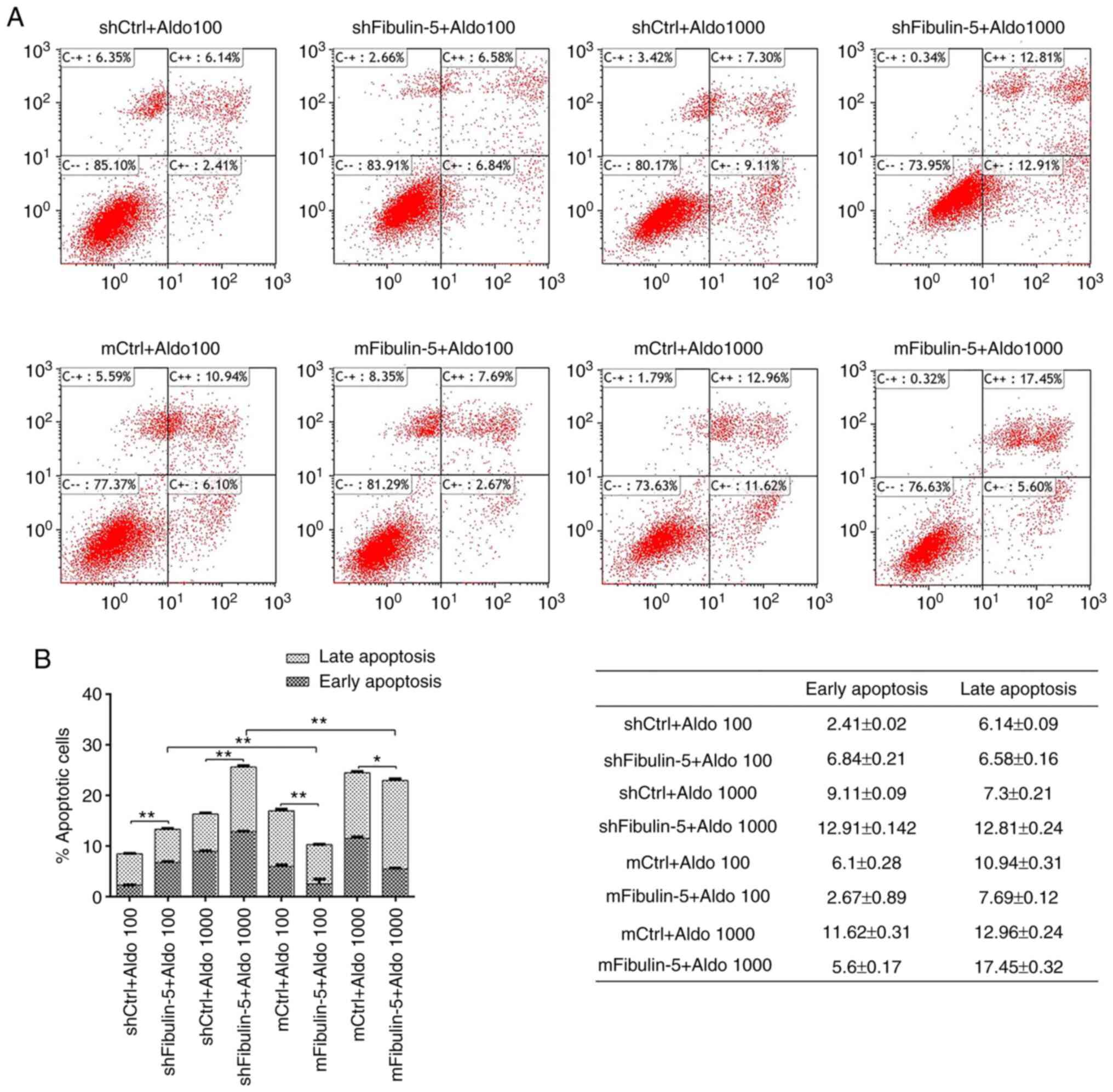
detected by flow cytometry. As presented in Fig. 4, the rates of apoptosis were
significantly increased following treatment with high
concentrations of aldosterone (1,000 µM) in fibulin-5 knockdown
cells compared with their respective control. However, fibulin-5
knockdown cells were significantly increased compared with their
respective cells following treatment with 100 µM Aldo. The rate of
apoptosis in cells overexpressing fibulin-5 significantly decreased
following stimulation with low concentrations of aldosterone
compared with their respective controls.
These results suggest that fibulin-5 inhibits
aldosterone-induced apoptosis. When the aldosterone concentration
is high, the inhibitory activity of fibulin-5 is saturated, thereby
reducing its inhibitory activity.
Discussion
PA is the most common cause of secondary
hypertension. It was previously thought that patients with PA only
accounted for 1-2% of the total population with hypertension but it
has been demonstrated that patients with PA account for >10% of
the total hypertensive population (16). APA is the most common type of PA and
accounts for 70-80% of all cases of PA. The primary method of
treating APA is via surgical resection; however, a long-term
follow-up study revealed that the postoperative hypertension
control rate was only 33-87% and has become a long-term clinical
problem. Vascular remodelling may be responsible for stimulating
hypertension; however, the specific mechanism remains to be
elucidated.
Aldosterone directly stimulates vascular remodelling
and a number of previous studies have indicated that aldosterone
damages the cardiovascular system (17-19).
Blood vessels can express the mineralocorticoid receptor (MR) and
11β-hydroxysteroid dehydrogenases (20). The latter is an enzyme that ensures
that aldosterone is specifically bound to MR. Aldosterone can lead
to vasoconstriction and elevate blood pressure (21). Hypertension can damage and remodel
blood vessels (21). In a previous
study, to identify the pathogenesis of PA, a micro-osmotic pump
(ALZET 2004) was injected to pump aldosterone into rats and thus
establish an animal model of PA. The systolic blood pressure of
rats increased, plasma aldosterone concentration increased and
renin activity was inhibited following 3 weeks perfusion with
aldosterone. After 8 weeks, the mesenteric arteries of the rats
exhibited marked changed, including wall thickening and a smaller
lumen (3).
Aldosterone induced the apoptosis of VSMCs.
Apoptosis serves an important role in the pathogenesis of vascular
lesions and excessive apoptosis leads to the loss of vascular cells
(18). Previous studies have
demonstrated that aldosterone is able induce apoptosis in
cardiomyocytes and renal tubular epithelial cells (7,17). A
previous study by the current authors identified found that
treatment with aldosterone increased the number of TUNEL-positive
VSMCs in the aorta increased, indicating that aldosterone may
induce the apoptosis of rat aorta VSMCs in vivo.
Furthermore, the expression of the pro-apoptotic proteins Bax,
cytochrome c and caspase-3 increased and the expression of
the apoptosis inhibition protein Bcl-2 decreased (3). These results indicate that aldosterone
induces the apoptosis of VSMCs via the mitochondrial pathway;
however, the specific mechanism of action requires further
clarification.
Aldosterone induces the deposition of fibulin-5 in
the aorta. Extracellular matrix proteins are an important component
of vascular remodelling. Fibulin-5 is a member of the fibulin
family and is also an important component of the extracellular
matrix. It has been demonstrated that fibulin-5 deposition
increases in PA rat aortic extracellular matrix proteins (7). Fibulin-5 is a multifunctional protein
that serves an important role in cell proliferation, migration and
apoptosis (4,5). The ligation of the carotid artery of
fibulin-5 knockout mice is accompanied by severe neointima
formation and thickening of the outer membrane (6). The results of a previous study
indicated that the deposition of fibulin-5 in the aorta of the rats
significantly increased following treatment with aldosterone,
suggesting that fibulin-5 may serve an important role in
aldosterone-induced vascular remodelling (7).
Fibulin-5 also affects apoptosis. Fibulin-5 has
cell- and environment-specific functions; it is able to stimulate
the synthesis of fibroblast DNA but also inhibit the synthesis of
epithelial cells (8). Fibulin-5
knockout mice exhibited increased intravascular vascular density in
normal tissues and reduced endothelial cell apoptosis (9). A polyvinylchloride sponge increased
the invasion of new blood vessels in fibulin-5 knockout mice
demonstrating that fibulin-5 inhibited cell invasion (10). Fibrosarcoma cells overexpressing
fibulin-5 that were subcutaneously injected into mice inhibited the
growth of tumors, suggesting that fibulin-5 promotes apoptosis
(11). Under hypoxic conditions,
knockout of the fibulin-5 gene significantly increased the
apoptosis of endothelial cells (12). The apoptosis of vascular endothelial
cells in fibulin-5 knockout mice with pancreatic cancer increases,
indicating that fibulin-5 inhibits the apoptosis of endothelial
cells (9). Thus, the regulation of
apoptosis by fibulin-5 is different in different types of cells or
in different environments. The results of the current study
demonstrated that the overexpression of fibulin-5 inhibited
aldosterone-induced apoptosis, particularly at lower concentrations
of aldosterone and promoted aldosterone-induced apoptosis following
fibulin-5 knockdown, particularly at higher concentrations of
aldosterone. These results suggest that fibulin-5 inhibits
aldosterone-induced H-VSMC apoptosis, and this effect may be
associated with concentrations of aldosterone in the body.
In conclusion, the results of the present study
indicate that fibulin-5 serves a role in the inhibition of
aldosterone-induced apoptosis of human aortic smooth muscle cells.
The effect of fibulin-5 in vivo may be associated with
levels of aldosterone in the body. However, the specific mechanism
by which fibulin-5 inhibits apoptosis remains unclear and further
studies are required.
Acknowledgements
Not applicable.
Funding
Funding: The current study was supported by the National Natural
Science Foundation of China (grant no. 81360126) and Joint Special
Fund of Kunming Medical University (grant no. 2013FB163).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JP and XQN were responsible for the application and
design of the whole project, YJY, XJL and XCL jointly completed the
experiments, where YJY was responsible for the research direction
of the project. XJL and XCL performed vector design, cell
transfection and apoptosis measurements. XZ, CZY, and JTT performed
cell culture, reagent preparation, statistical analysis and
literature research. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen HZ, Zhong NS, Lu ZY, et al: The
eighth rdition Textbook of Surgery[M]// published by People's
Education Press: Saunders, 2013:708-709. Primary aldosteronism is
characterized by the overproduction of the mineralocorticoid
hormone aldosterone by the adrenal glands, adrenal cortex
hyperplasia or adrenal carcinoma main cause the disease; Symptoms:
Occasional muscular weakness, muscle spasms, tingling sensations,
or excessive urination the prevalence of APA in hypertension is 10
percent, and the symptom and incidence is like PA.
|
|
2
|
Rossi GP, Bolognesi M, Rizzoni D, Seccia
TM, Piva A, Porteri E, Tiberio GA, Giulini SM, Agabiti-Rosei E and
Pessina AC: Vascular remodeling and duration of hypertension
predict outcome of adrenalectomy in primary aldosteronism patients.
Hypertension. 51:1366–1371. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yan Y, Ouyang J, Wang C, Wu Z, Ma X, Li H,
Xu H, Hu Z, Li J, Wang B, et al: Aortic cell apoptosis in rat
primary aldosteronism model. J Huazhong Univ Sci Technolog Med Sci.
30:385–390. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Xiao W, Zhou S, Xu H, Li H, He G, Liu Y
and Qi Y: Nogo-B promotes the epithelial-mesenchymal transition in
HeLa cervical cancer cells via Fibulin-5. Oncol Rep. 29:109–116.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kapustin A, Stepanova V, Aniol N, Cines
DB, Poliakov A, Yarovoi S, Lebedeva T, Wait R, Ryzhakov G,
Parfyonova Y, et al: Fibulin-5 binds urokinase-type plasminogen
activator and mediates urokinase-stimulated β1-integrin-dependent
cell migration. Biochem J. 443:491–503. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Spencer JA, Hacker SL, Davis EC, Mecham
RP, Knutsen RH, Li DY, Gerard RD, Richardson JA, Olson EN and
Yanagisawa H: Altered vascular remodeling in fibulin-5-deficient
mice reveals a role of fibulin-5 in smooth muscle cell
proliferation and migration. Proc Natl Acad Sci USA. 102:2946–2951.
2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ding W, Yang L, Zhang M and Gu Y: Reactive
oxygen species-mediated endoplasmic reticulum stress contributes to
aldosterone-induced apoptosis in tubular epithelial cells. Biochem
Biophys Res Commun. 418:451–456. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Schiemann WP, Blobe GC, Kalume DE, Pandey
A and Lodish HF: Context-specific effects of fibulin-5 (DANCE/EVEC)
on cell proliferation, motility, and invasion. Fibulin-5 is induced
by transforming growth factor-beta and affects protein kinase
cascades. J Biol Chem. 277:27367–27377. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Schluterman MK, Chapman SL, Korpanty G,
Ozumi K, Fukai T, Yanagisawa H and Brekken RA: Loss of fibulin-5
binding to beta1 integrins inhibits tumor growth by increasing the
level of ROS. Dis Model Mech. 3:333–342. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sullivan KM, Bissonnette R, Yanagisawa H,
Hussain SN and Davis EC: Fibulin-5 functions as an endogenous
angiogenesis inhibitor. Lab Invest. 87:818–827. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Albig AR, Neil JR and Schiemann WP:
Fibulins 3 and 5 antagonize tumor angiogenesis in vivo. Cancer Res.
66:2621–2629. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Guadall A, Orriols M, Rodríguez-Calvo R,
Calvayrac O, Crespo J, Aledo R, Martínez-González J and Rodríguez
C: Fibulin-5 is up-regulated by hypoxia in endothelial cells
through a hypoxia-inducible factor-1 (HIF-1α)-dependent mechanism.
J Biol Chem. 286:7093–7103. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Qiao W, Zhang W, Shao S, Gai Y and Zhang
M: Effect and mechanism of poly (ADP-ribose) polymerase-1 in
aldosterone-induced apoptosis. Mol Med Rep. 12:1631–1638.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ishizawa K, Izawa Y, Ito H, Miki C, Miyata
K, Fujita Y, Kanematsu Y, Tsuchiya K, Tamaki T, Nishiyama A and
Yoshizumi M: Aldosterone stimulates vascular smooth muscle cell
proliferation via big mitogen-activated protein kinase 1
activation. Hypertension. 46:1046–1052. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Young WF Jr: Minireview: Primary
aldosteronism-changing concepts in diagnosis and treatment.
Endocrinology. 144:2208–2213. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hayashi H, Kobara M, Abe M, Tanaka N,
Gouda E, Toba H, Yamada H, Tatsumi T, Nakata T and Matsubara H:
Aldosterone nongenomically produces NADPH oxidase-dependent
reactive oxygen species and induces myocyte apoptosis. Hypertens
Res. 31:363–375. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Korshunov VA and Berk BC: Smooth muscle
apoptosis and vascular remodeling. Curr Opin Hematol. 15:250–254.
2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Calvier L, Miana M, Reboul P, Cachofeiro
V, Martinez-Martinez E, de Boer RA, Poirier F, Lacolley P, Zannad
F, Rossignol P and López-Andrés N: Galectin-3 mediates
aldosterone-induced vascular fibrosis. Arterioscler Thromb Vasc
Biol. 33:67–75. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Takeda Y, Miyamori I, Yoneda T, Iki K,
Hatakeyama H, Blair IA, Hsieh FY and Takeda R: Production of
aldosterone in isolated rat blood vessels. Hypertension.
25:170–173. 1995.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Leopold JA: Aldosterone, mineralocorticoid
receptor activation, and cardiovascular remodeling. Circulation.
124:e466–e468. 2011.PubMed/NCBI View Article : Google Scholar
|