Introduction
Gastric cancer is one of the most common and
incurable types of cancer (1,2).
Gastric cancer is associated with high rates of incidence and
mortality, which imposes a considerable economic burden on society
with 1 million new cases annually in the United States (1,2).
However, the overall survival rate of patients with early stages of
gastric cancer is significantly higher compared with that in
patients with advanced stages of gastric cancer (3). Therefore, diagnosis of gastric cancer
in the early stages is critical for improving the survival rate. In
addition to upper gastrointestinal endoscopy and the detection of
common tumor biomarkers, including carcinoembryonic antigen (CEA),
cancer antigen (CA)72-4 and CA19-9 in the serum (4-6),
long non-coding RNAs (lncRNAs) is garnering interest for reported
their role in the early diagnosis, therapy and prognosis of gastric
cancer (7,8).
The effects of lncRNAs in gastric cancer are
gradually becoming unraveled. Wei and Wang (9) showed that lncRNA maternally expressed
3 was highly expressed in gastric cancer tissues compared with that
in adjacent normal tissues (9). In
addition, other studies revealed that lncRNA small nucleolar host
gene (SNHG)1 and SNHG7 overexpression could promote gastric cancer
cell proliferation (10,11). A study demonstrated that B3GALT5-AS1
expression was markedly increased in patients with gastric cancer
compared with those with normal colonic epithelia (12). Moreover, high serum B3GALT5-AS1
levels were found to be associated with TNM stage and lymph node
metastasis (13), suggesting a role
of B3GALT5-AS1 in gastric cancer occurrence and progression.
Another study suggested that serum levels of B3GALT5-AS1 could also
serve as a diagnostic biomarker of colorectal cancer (14). Indeed, B3GALT5-AS1 was demonstrated
to be localized predominantly in the nucleus, where it could
directly bind to the promoter of microRNA (miR)-203 to upregulate
the expression of the target genes of miR-203, which in turn
suppresses colon cancer metastasis to the liver (12). However, to date, the regulatory
mechanism of B3GALT5-AS1 in gastric cancer remains elusive.
It has been previously demonstrated that casein
kinase 2 a1 (CSNK2A1) is associated with cell invasion and
migration in several types of cancer, including lung (15) and breast cancer (16). Additionally, overexpression of
CSNK2A1 in gastric cancer cells enhanced cell proliferation,
invasion and migration (17),
suggesting a regulatory role of CSNK2A1 in gastric cancer.
Therefore, in the present study, the expression
profile of B3GALT5-AS1 was determined in gastric cancer cell lines.
Additionally, whether B3GALT5-AS1 served a role in gastric cancer
cell proliferation, migration, invasion and apoptosis and its
potential relationship with CSNK2A1 was assessed.
Materials and methods
Cell culture
Gastric cancer cell lines, AGS, HGC-27 and MKN-45
were purchased from the China Infrastructure of Cell Line
Resources, Institute of Basic Medical Sciences, Chinese Academy of
Medical Sciences. The human gastric mucosa cell line GES-1 (cat.
no. CL-0563) was obtained from Procell Life Science &
Technology Co., Ltd.. All cells were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) and maintained in an
incubator with 5% CO2 at 37˚C.
Plasmid transfection
For B3GALT5-AS1 knockdown, two short hairpin RNAs
(shRNA/sh), namely sh-B3GALT5-AS1-1 (5'-GCATAAGAGAGACCAACTTGG-3')
and sh-B3GALT5-AS1-2 (5'-GCAAGACAGCGCATTGATTGG-3'), were
constructed using the pGPU6/Neo plasmid (Shanghai GenePharma Co.,
Ltd.). Scrambled shRNA [shRNA-negative control (NC)] served as the
NC (5'-ACTTGCGCTTGCGAAAATCTATATAGC-3'). The pcDNA-MFHAS1 plasmid
(Shanghai GenePharma Co., Ltd.) was used for CSNK2A1 overexpression
(ov-CSNK2A1). The empty vector, ov-NC, served as the negative
control for the overexpression experiments. Cells were seeded into
six-well plates at a density of 5x105 cells/well. When
they reached 80% confluence, cells were transfected with 3 µg
shRNA-encoding plasmids or 5 µg overexpression plasmids using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Following incubation for 48 h at 37˚C, transfected cells were used
for subsequent experiments.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the four cell lines
(GES-1, AGS, HGC-27 and MKN-45) using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Following reverse
transcription using AMV Reverse Transcription kit (cat. no. A3500;
Promega Corporation), this reaction was performed at 25˚C for 5
min, 50˚C for 20 min, and then 75˚C for 5 min. The FastStart
Universal SYBR Green Master kit (Roche Diagnostics GmbH) was used
for qPCR in an ABI PRISM 7900 HT system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The primer sequences used were as
follows: B3GALT5-AS1 forward, 5'-GATCCACGTCCAGGCTCACT-3' and
reverse, 5'-GTGCTGGCTGTCAGGATGAG-3'; CSNK2A1 forward,
5'-GAACGCTTTGTCCACAGTGA-3' and reverse, 5'-TATCGCAGCAGTTTGTCCAG-3'
and GAPDH forward, 5'-AACAGCGACACCCACTCCTC-3' and reverse,
5'-GGAGGGGAGATTCAGTGTGGT-3'. The thermocycling conditions for PCR
reaction was as follows: Initial denaturation for 5 min at 95˚C,
followed by 40 cycles of 30 sec at 95˚C and 45 sec at 65˚C. The
relative mRNA expression was calculated using the 2-∆∆Cq
method (18) and GAPDH served as
the internal control.
Bioinformatics analysis
The starBase 2.0 database (http://starbase.sysu.edu.cn/) was used to predict the
binding sites between B3GALT5-AS1 and CSNK2A1.
RNA immunoprecipitation (RIP)
assay
RIP assay was performed using the Millipore Magna
RIP™ RNA kit (EMD Millipore). Briefly, MKN-45 cells were
resuspended in RIP lysis buffer (EMD Millipore) and incubated on
ice for 5 min. Subsequently, the cell lysate (100 µl per antibody
per RIP) was incubated with an anti-CSNK2A1 antibody (1:1,000; cat.
no. ab70774; Abcam) or IgG antibody (0.2 µg/ml; cat. no. ab190475;
Abcam) and 40 µl protein A/G magnetic beads (EMD Millipore) at 4˚C
overnight. The protein-RNA complexes were digested with 2 µl
proteinase K buffer at 55˚C for 30 min. The beads buffer was spun
down at 2,000 x g for 30 sec, and the supernatant was transferred
to a fresh tube. Finally, the extracted RNA was subjected to
RT-qPCR.
RNA pull-down assay
RNA pull-down assay was performed using
Pierce™ Magnetic RNA-Protein Pull-Down Kit (Thermo
Fisher Scientific, Inc.). Briefly, MKN-45 cells were lysed by RIPA
lysis buffer (Sigma-Aldrich; Merck KGaA). B3GALT5-AS1 primers were
5'-CCTTGAGAGACGAAGCAC-3' (sense) and 5'-ATTTCACGGATGAGACGAC-3'
(antisense). B3GALT5-AS1 was labeled with biotin using Pierce RNA
3' End Desthiobiotinylation Kit (Thermo Fisher Scientific, Inc.)
and then bound to streptavidin magnetic beads (~10 µg), before this
complex was incubated with 1 ml cell lysates at 4˚C for 1 h.
RNA-protein complexes were eluted by adding 30 µl SDS sample buffer
to the beads and heating at 95˚C for 5 min. The samples were cooled
on ice for 1 min, 2 µl Benzonase added, and then incubated for 15
min at room temperature. The sample buffer was heated again at 95˚C
for 5 min. The magnetic beads were spun down at room temperature at
12,000 x g for 1 min. The presence of CSNK2A1 protein in the
RNA-protein complexes was analyzed by western blotting.
Western blotting
Total proteins were isolated from cell lysates using
RIPA lysis buffer (Sigma-Aldrich; Merck KGaA) and then quantified
using a bicinchoninic acid protein assay kit (Thermo Fisher
Scientific, Inc.). Subsequently, 20 µg protein extracts per lane
were separated by 10% SDS-PAGE and transferred onto nitrocellulose
membranes (EMD Millipore). Following blocking with 5% skim milk at
room temperature for 2 h, membranes were incubated with antibodies
against CSNK2A1 (1:1,000; cat. no. ab70774; Abcam), matrix
metalloproteinase (MMP)2 (1:2,000; cat. no. AF0577; Affinity
Biosciences), MMP9 (1:2,000; cat. no. AF5228; Affinity
Biosciences), phosphorylated (p)-c-Met (1:1,000; cat. no. AF3128;
Affinity Biosciences), c-Met (1:1,000; cat. no. AF6128; Affinity
Biosciences), Bcl-2 (1:5,000; cat. no. AF6139; Affinity
Biosciences), Bax (1:5,000; cat. no. AF0120; Affinity Biosciences),
cleaved caspase-3 (1:2,000; cat. no. AF7022; Affinity Biosciences),
caspase-3 (1:2,000; cat. no. AF6311; Affinity Biosciences) or GAPDH
(1:5,000; cat. no. AF7021; Affinity Biosciences) at 4˚C overnight.
The next day, the membranes were incubated with corresponding
HRP-conjugated secondary antibodies (1:5,000; cat. no. S0001;
Affinity Biosciences) at room temperature for 2 h. The protein
bands were visualized using the Immobilon Western Chemilum HRP
substrate (EMD Millipore) and analyzed using Image Lab Software 3.0
(Bio-Rad Laboratories, Inc.).
Cell Counting Kit 8 (CCK-8) assay
For CCK-8 assays, MKN-45 cells were seeded into a
96-well plates at a density of 5,000 cells per well and incubated
at 37˚C overnight. The next day, 10 µl CCK-8 reagent (Dojindo
Molecular Technologies, Inc.) was added into each well and cells
were incubated for 2 h at 37˚C. Finally, the optical density (OD)
in each well was measured using a microplate reader (Bio-Rad
Laboratories, Inc.) at a wavelength of 450 nm.
Cell invasion assay
The upper chamber of the Transwell chamber (Corning,
Inc.) was coated with 100 µl Matrigel (1 mg/ml; BD Biosciences) and
incubated at 37˚C for 4 h. The cell density was then adjusted to
2x105 cells/ml and 100 µl cell suspension in serum-free
RPMI-1640 medium was added to the upper chamber and cultured at
37˚C in the presence of 5% CO2 for 24 h. The lower
chamber was supplemented with 600 µl RPMI-1640 medium containing
20% FBS. At 24 h post-incubation, invasive cells were fixed with 4%
paraformaldehyde at room temperature for 0.5 h, stained with 0.1%
crystal violet at 37˚C for 10 min and photographed in randomly
selected nine fields of view under a light microscope
(magnification, x100).
Cell migration assay
MKN-45 cells were seeded into a six-well plate
(5x105 cells/well) and when they reached 100%
confluence, scratches were created using a 20-µl pipette tip. The
medium was then replaced with fresh medium containing 1% FBS and
cells were cultured at 37˚C for 24 h (19). Finally, images of the migrated cells
were captured at 0 and 24 h in randomly selected fields of view
under a light microscope (magnification, x100). The migration
distance was calculated as the width of the scratch at 24 h minus
the width of the scratch at 0 h. The relative migration rate was
calculated by normalizing to the control group.
Cell apoptosis assay
Apoptotic cells were assessed using an One Step
TUNEL Apoptosis Assay kit (Beyotime Institute of Biotechnology).
Briefly, MKN-45 cells (3x105 cells/ml) were fixed with
4% paraformaldehyde at room temperature for 30 min and then
incubated with 0.3% Triton X-100 at room temperature for 5 min.
Subsequently, each well of the 24-well plate was supplemented with
50 µl TUNEL detection reagent for 60 min at 37˚C in the dark. DAPI
staining solution (10 µg/ml) was used to visualize all nuclei for 5
min at 37˚C. The coverslips were then washed with PBS and mounted
on slides with anti-fading solution. The apoptotic cells (green
fluorescence) in three random fields were observed under a
fluorescence microscope (magnification, x100).
Statistical analysis
All data in the study are expressed as the mean ± SD
and analyzed using an independent unpaired t-test or one-way ANOVA
followed by a Tukey's post hoc test. Each experiment was repeated ≥
three times. P<0.05 was considered to indicate a statistically
significant difference.
Results
Interaction between B3GALT5-AS1 and
CSNK2A1
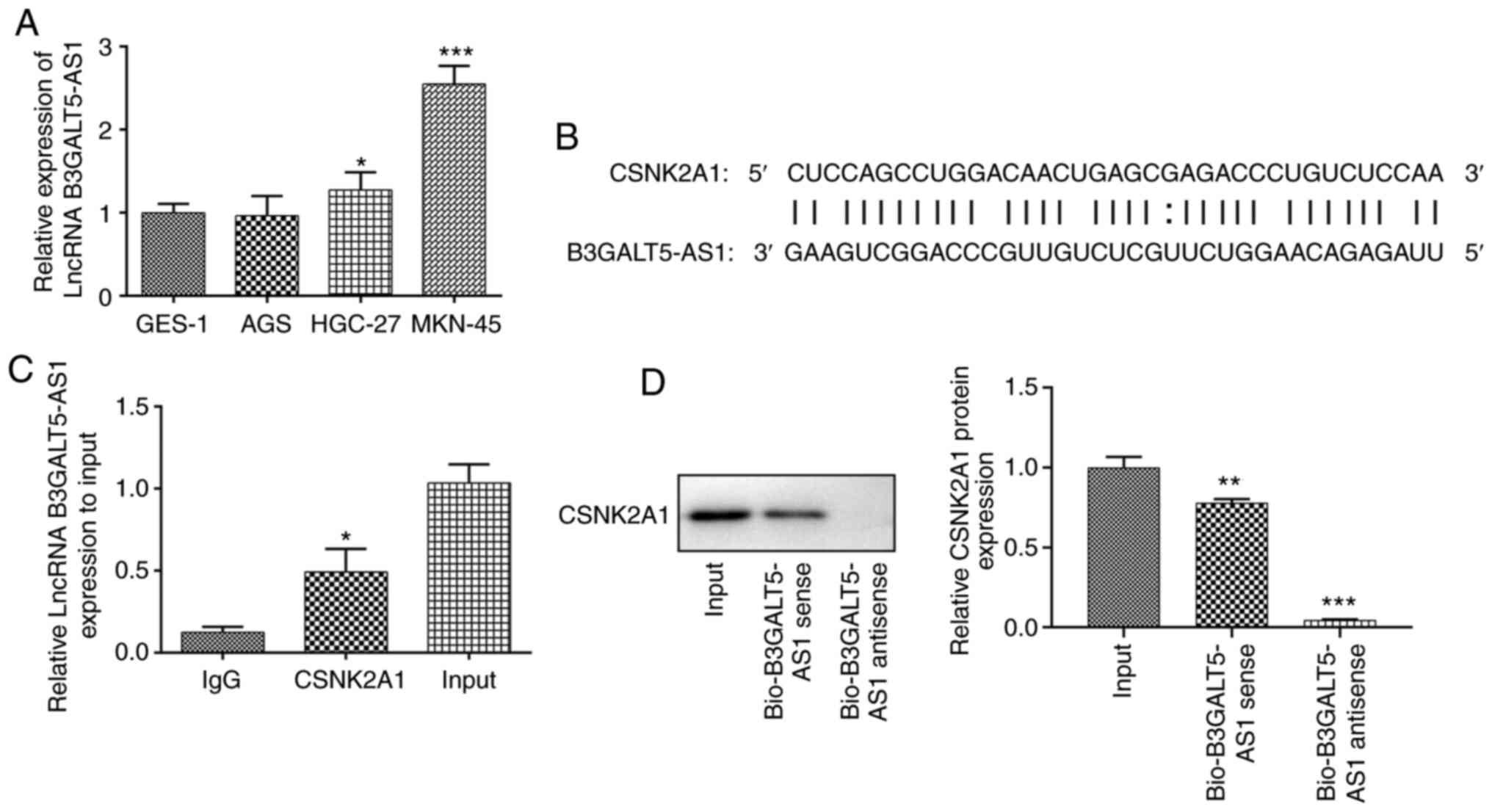
The expression levels of B3GALT5-AS1 were first
determined in a range of gastric cell lines. The expression levels
of B3GALT5-AS1 in HGC-27 cells were higher compared with that in
the human gastric mucosa cell line GES-1 (Fig. 1A). Compared with that in GES-1
cells, B3GALT5-AS1 expression was significantly higher in MKN-45
cells (Fig. 1A). It has been
previously suggested that B3GALT5-AS1 may originate from autocrine
gastric tumor cells and is closely associated with gastric tumor
metastasis (13). However, the
specific regulatory mechanism underlying the effect of B3GALT5-AS1
on tumor metastasis remains unclear. Therefore, based on the
expression of B3GALT5-AS1, the MKN-45 cell line was used for the
subsequent experiments. mRNA of CSNK2A1 was located in
Chr20:453618-453657. Bioinformatics analysis predicted that
B3GALT5-AS1 could bind to the sequence of CSNK2A1 (Fig. 1B). Notably, bioinformatics analysis
predicted binding sites between the sequences of B3GALT5-AS1 and
CSNK2A1, suggesting that B3GALT5-AS1 may modulate CSNK2A1
expression by direct binding (12,20).
As shown in Fig. 1C, the
interaction between B3GALT5-AS1 and CSNK2A1 was evaluated using RIP
assays. Further evidence of a CSNK2A1-complex interaction was found
via a RNA pull down assay (Fig.
1D). Since B3GALT5-AS1 was found to be enriched in the
CSNK2A1-complex, direct binding of CSNK2A1 to B3GALT5-AS1 was
reproduced in vitro by a RNA pull-down assay.
Efficiency of B3GALT5-AS1 knockdown
and CSNK2A1 overexpression
Since B3GALT5-AS1 expression was the highest in
MKN-45 cells, these cells were transfected with plasmids encoding
shRNAs to knockdown the expression B3GALT5-AS1. As shown in
Fig. 2A, between the two shRNA
clones, shRNA-B3GALT5-AS1-1 exhibited the more potent silencing
effect compared with that mediated by shRNA-B3GALT5-AS1-2. The
protein expression level of CSNK2A1 in MKN-45 cells transfected
with shRNA-B3GALT5-AS1-1 was significantly lower compared with that
in the shRNA-NC group. However, no significant differences were
observed in the protein expression of CSNK2A1 between the control
and shRNA-NC groups (Fig. 2B).
Subsequently, MKN-45 cells were transfected with a CSNK2A1
overexpression plasmid. mRNA and protein expression of CSNK2A1 were
both significantly upregulated following cell transfection with
ov-CSNK2A1 compared with those transfected with ov-NC (Fig. 2C and D).
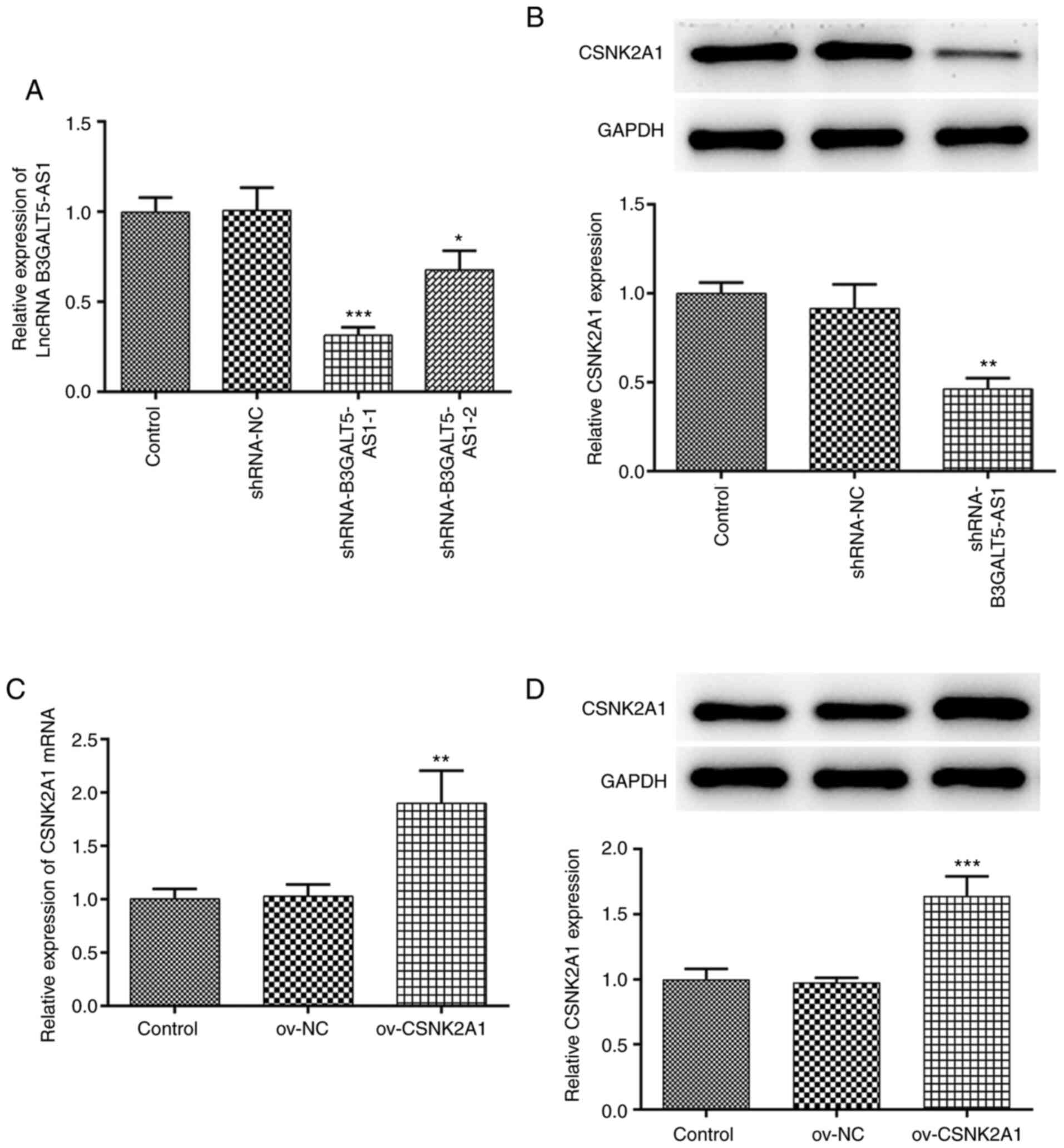 | Figure 2B3GALT5-AS1 silencing and CSNK2A1
overexpression on MKN-cells. (A) Expression levels of B3GALT5-AS1
in MKN-45 cells following transfection with shRNA-NC,
shRNA-B3GALT5-AS1-1 or shRNA-B3GALT5-AS1-2 were determined by
RT-qPCR. (B) Western blot analysis showing the protein expression
levels of CSNK2A1. *P<0.05, **P<0.01
and ***P<0.001 vs. shRNA-NC. (C) mRNA expression
levels of CSNK2A1 following transfection with ov-NC or ov-CSNK2A1
were assessed by RT-qPCR. (D) Western blot analysis showing the
protein expression levels of CSNK2A1 following transfection of
MKN-45 cells with ov-NC or ov-CSNK2A1. **P<0.01 and
***P<0.001 vs. ov-NC. B3GALT5-AS1,
β-1,3-galactosyltransferase 5-AS1; CSNK2A1, casein kinase 2 a1; NC,
negative control; RT-qPCR, reverse transcription-quantitative PCR;
LncRNA, long non-coding RNA; Ov, expression; shRNA, short hairpin
RNA. |
B3GALT5-AS1/CSNK2A1 axis controls cell
migration and invasion
Subsequently, effects of gene knockdown or
overexpression on the migratory and invasive behavior of MKN-45
cells were investigated. Cell viability was significantly decreased
following cell transfection with shRNA-B3GALT5-AS1-1 compared with
those transfected with shRNA-NC at 72 h. However, co-transfection
with shRNA-B3GALT5-AS1-1 and ov-CSNK2A1 partially but significantly
elevated cell viability compared with that in the
shRNA-B3GALT5-AS1-1 + ov-NC group (Fig.
3A). MMPs are involved in the remodeling of the extracellular
matrix and basement membrane, which is a key process in the
invasion and metastasis of cancer cells (21,22).
The protein expression levels of MMP2 and MMP9 were significantly
reduced after cell transfection with shRNA-B3GALT5-AS1-1 compared
with that in the shRNA-NC group, which was significantly reversed
by CSNK2A1 plasmid co-transfection (Fig. 3B). Transwell assays showed that the
invasive ability of MKN-45 cells was significantly decreased
following B3GALT5-AS1 knockdown compared with that in the shRNA-NC
group (Fig. 3C). B3GALT5-AS1
silencing also significantly attenuated the migratory ability of
MKN-45 cells compared with that in cells transfected with shRNA-NC
(Fig. 3C). For both migration and
invasion, CSNK2A1 plasmid co-transfection significantly reversed
the inhibitory effects of B3GALT5-AS1 knockdown (Fig. 3C).
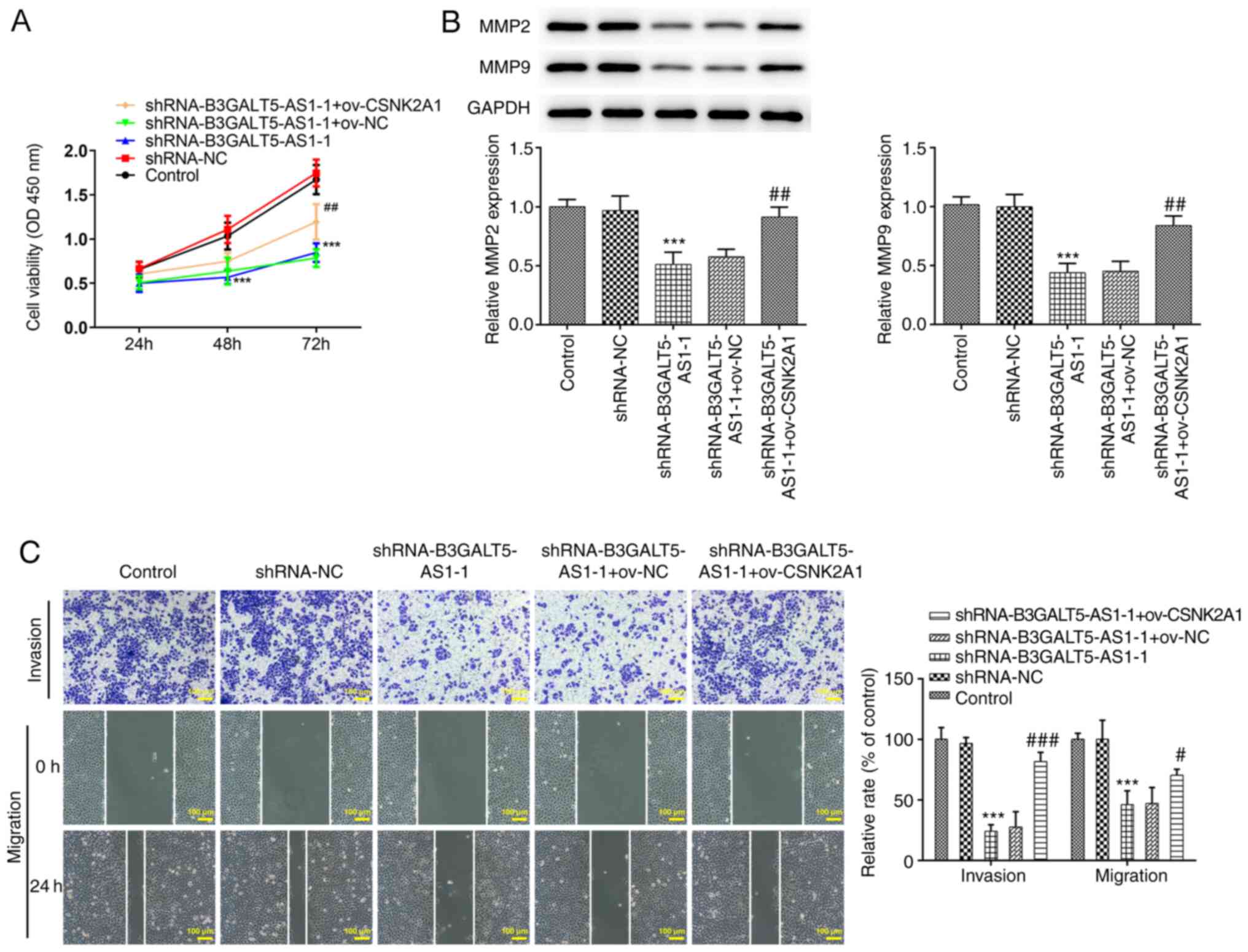 | Figure 3B3GALT5-AS1/CSNK2A1 axis regulates
MKN-45 cell migration and invasion. (A) Cell viability in the
different transfection groups was evaluated by Cell Counting Kit-8
assay. (B) Western blot analysis showing the protein expression
levels of MMP2 and MMP9 in different groups. (C) Cell invasion and
migration in different groups of cells after transfection. Scale
bar, 100μm, ***P<0.001 vs. shRNA-NC;
#P<0.05, ##P<0.01 and
###P<0.001 vs. shRNA-B3GALT5-AS1-1 + ov-NC.
B3GALT5-AS1, β-1,3-galactosyltransferase 5-AS1; CSNK2A1, casein
kinase 2 a1; MMP, matrix metalloproteinase; LncRNA, long non-coding
RNA; Ov, expression; shRNA, short hairpin RNA; OD, optical
density. |
B3GALT5-AS1/CSNK2A1 axis modulates
cell apoptosis
Furthermore, a TUNEL assay was performed to measure
cell apoptosis. The number of apoptotic cells in the
shRNA-B3GALT5-AS1-1 group was markedly higher compared with that in
the shRNA-NC or control group, which was reversed by CSNK2A1
plasmid co-transfection (Fig. 4A).
The levels of the pro-apoptotic proteins Bax and cleaved caspase-3
were both significantly upregulated following B3GALT5-AS1
knockdown, but their protein levels were significantly restored
after CSNK2A1 overexpression in addition to B3GALT5-AS1 knockdown
(Fig. 4B). By contrast, the
expression levels of the anti-apoptotic proteins Bcl-2 and p-c-Met
were decreased in the shRNA-B3GALT5-AS1-1 group compared with those
in the shRNA-NC group. However, co-transfection of MKN-45 cells
with shRNA-B3GALT5-AS1-1 and ov-CSNK2A1 upregulated Bcl-2 and
p-c-Met compared with cells transfected with shRNA-B3GALT5-AS1-1
and ov-NC (Fig. 4B). Dysregulation
in c-Met has been implicated in the pathogenesis and development of
gastric cancer, where its downregulation can induce apoptosis
(23,24). These results aforementioned suggest
that B3GALT5-AS1 knockdown promotes gastric cancer cell apoptosis
by binding to CSNK2A1.
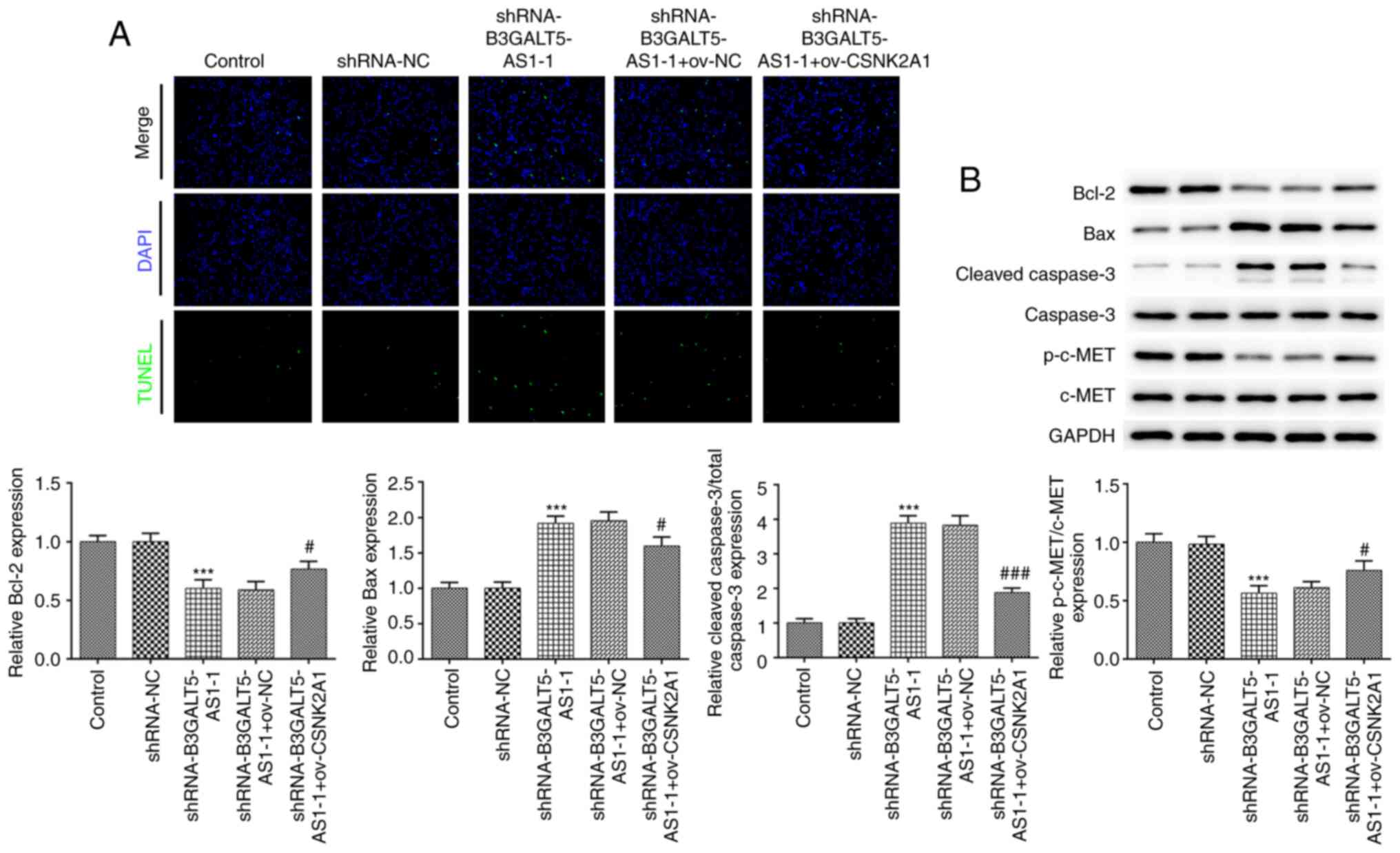 | Figure 4B3GALT5-AS1/CSNK2A1 axis regulates
cell apoptosis. (A) Cell apoptosis in different transfection groups
was assessed using a TUNEL assay. Magnification, x100. (B) Western
blot analysis showing the protein levels of Bcl-2, Bax, cleaved
caspase-3, caspase-3, p-c-Met and c-Met in different groups.
***P<0.001 vs. shRNA-NC; #P<0.05 and
###P<0.001 vs. shRNA-B3GALT5-AS1-1 + ov-NC.
B3GALT5-AS1, β-1,3-galactosyltransferase 5-AS1; CSNK2A1, casein
kinase 2 a1; p-, phosphorylated; Ov, expression; shRNA, short
hairpin RNA; NC, negative control. |
Discussion
The incidence rate of gastric cancer is particularly
high in developing countries, such as China and India (25). Data suggests that gastric cancer
ranks second in terms of incidence among all malignancies in China
(26,27). Gastric cancer has long been
considered to be a difficult challenge to treat clinically
(28). Therapeutic interventions in
patients with gastric cancer typically involve alterations in diet,
psychological support and changes in daily life habits to optimize
the therapeutic effects and improve the quality of life (29). The moderate diagnostic value of CEA
and CA199 for the early detection of gastric cancer highlights the
importance and urgency of developing novel biomarkers with high
specificity and sensitivity (30).
It has been reported that B3GALT5-AS1 can acts as a diagnostic and
prognostic biomarker of gastric cancer (13).
The present study demonstrated that B3GALT5-AS1 was
highly expressed in MKN-45 cells compared with that in normal
gastric mucosa GES-1 cells. In addition, binding sites between the
sequences of B3GALT5-AS1 and CSNK2A1 were identified. B3GALT5-AS1
silencing repressed the protein expression of CSNK2A1. The
catalytic subunit of CK2α is encoded by CSNK2A1(31) and is associated with cell
proliferation and invasion in colorectal cancer (32). A previous study showed that the
knockdown of CSNK2A1 expression attenuated the proliferative and
invasive capabilities of breast carcinoma cells (16). In the present study, B3GALT5-AS1
knockdown in MKN-45 cells reduced cell viability, which was
reversed by CSNK2A1 overexpression. The reduced MKN-45 cell
migration and invasion, mediated by shRNA-B3GALT5-AS1, were also
restored following CSNK2A1 overexpression. Furthermore, silencing
of B3GALT5-AS1 expression induced cell apoptosis in a manner that
was partially counteracted by CSNK2A1 overexpression. However,
there are some limitations in the present study. These findings
were based on in vitro cell model, which require further
validation using in vivo animal models or human tissues in
future studies. In addition, the underlying mechanism of the
B3GALT5-AS1/CSNK2A1 axis regulating gastric cancer physiology has
not been studied in depth in the present study.
To conclude, the present study demonstrated that
knocking down B3GALT5-AS1 expression, which was upregulated in
gastric cancer cells, could reduce cell viability and inhibit
migration whilst inducing cell apoptosis. These effects could be
restored by increasing the expression of CSNK2A1.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PW and GBS acquired the data. GXD and BQW
contributed to the study design and analysis of the data. PW
drafted the manuscript and BQW revised it critically for important
intellectual content. All authors read and approved the final
manuscript. BQW and PW are responsible for confirming the
authenticity if the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Thrift AP and El-Serag HB: Burden of
gastric cancer. Clin Gastroenterol Hepatol. 18:534–542.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Suzuki T, Kitagawa Y, Nankinzan R and
Yamaguchi T: Early gastric cancer diagnostic ability of ultrathin
endoscope loaded with laser light source. World J Gastroenterol.
25:1378–1386. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yu J and Zheng W: An alternative method
for screening gastric cancer based on serum levels of CEA, CA19-9,
and CA72-4. J Gastrointest Cancer. 49:57–62. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mori A, Ohashi N, Maruyama T, Tatebe H,
Sakai K, Shibuya T, Inoue H and Okuno M: Cardiovascular tolerance
in upper gastrointestinal endoscopy using an ultrathin scope:
Prospective randomized comparison between transnasal and transoral
procedures. Dig Endosc. 20:79–83. 2008.
|
|
7
|
Necula L, Matei L, Dragu D, Neagu AI,
Mambet C, Nedeianu S, Bleotu C, Diaconu CC and Chivu-Economescu M:
Recent advances in gastric cancer early diagnosis. World J
Gastroenterol. 25:2029–2044. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Song P, Jiang B, Liu Z, Ding J, Liu S and
Guan W: A three-lncRNA expression signature associated with the
prognosis of gastric cancer patients. Cancer Med. 6:1154–1164.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wei GH and Wang X: lncRNA MEG3 inhibit
proliferation and metastasis of gastric cancer via p53 signaling
pathway. Eur Rev Med Pharmacol Sci. 21:3850–3856. 2017.PubMed/NCBI
|
|
10
|
Hu Y, Ma Z, He Y, Liu W, Su Y and Tang Z:
LncRNA-SNHG1 contributes to gastric cancer cell proliferation by
regulating DNMT1. Biochem Biophys Res Commun. 491:926–931.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang S, Liu Y, Li MY, Ng CSH, Yang SL,
Wang S, Zou C, Dong Y, Du J, Long X, et al: FOXP3 promotes tumor
growth and metastasis by activating Wnt/β-catenin signaling pathway
and EMT in non-small cell lung cancer. Mol Cancer.
16(124)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang L, Wei Z, Wu K, Dai W, Zhang C, Peng
J and He Y: Long noncoding RNA B3GALT5-AS1 suppresses colon cancer
liver metastasis via repressing microRNA-203. Aging (Albany NY).
10:3662–3682. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Feng W, Zong W, Li Y, Shen X, Cui X and Ju
S: Abnormally expressed long noncoding RNA B3GALT5-AS1 may serve as
a biomarker for the diagnostic and prognostic of gastric cancer. J
Cell Biochem. 121:557–565. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ding Y, Feng W, Ge JK, Dai L, Liu TT, Hua
XY, Lu X, Ju SQ and Yu J: Serum level of long noncoding RNA
B3GALT5-AS1 as a diagnostic biomarker of colorectal cancer. Future
Oncol. 16:827–835. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim S, Ham S, Yang K and Kim K: Protein
kinase CK2 activation is required for transforming growth factor
β-induced epithelial-mesenchymal transition. Mol Oncol.
12:1811–1826. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bae JS, Park SH, Jamiyandorj U, Kim KM,
Noh SJ, Kim JR, Park HJ, Kwon KS, Jung SH, Park HS, et al:
CK2α/CSNK2A1 phosphorylates SIRT6 and is involved in the
progression of breast carcinoma and predicts shorter survival of
diagnosed patients. Am J Pathol. 186:3297–3315. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jiang C, Ma Z, Zhang G, Yang X, Du Q and
Wang W: CSNK2A1 promotes gastric cancer invasion through the
PI3K-Akt-mTOR signaling pathway. Cancer Manag Res. 11:10135–10143.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kramer N, Walzl A, Unger C, Rosner M,
Krupitza G, Hengstschläger M and Dolznig H: In vitro cell migration
and invasion assays. Mutat Res. 752:10–24. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jiang HM, Dong JK, Song K, Wang TD, Huang
WK, Zhang JM, Yang XY, Shen Y and Zhang J: A novel allosteric site
in casein kinase 2α discovered using combining bioinformatics and
biochemistry methods. Acta Pharmacol Sin. 38:1691–1698.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Huang H, Jin H, Zhao H, Wang J, Li X, Yan
H, Wang S, Guo X, Xue L, Li J, et al: RhoGDIβ promotes Sp1/MMP-2
expression and bladder cancer invasion through perturbing
miR-200c-targeted JNK2 protein translation. Mol Oncol.
11:1579–1594. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Huang H: Matrix metalloproteinase-9
(MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent
advances. Sensors (Basel). 18(3249)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gu ML, Zhou XX, Ren MT, Shi KD, Yu MS,
Jiao WR, Wang YM, Zhong WX and Ji F: Blockage of ETS homologous
factor inhibits the proliferation and invasion of gastric cancer
cells through the c-Met pathway. World J Gastroenterol.
26:7497–7512. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang Q, Zhang H, Ning T, Liu D, Deng T,
Liu R, Bai M, Zhu K, Li J, Fan Q, et al: Exosome-delivered c-Met
siRNA could reverse chemoresistance to cisplatin in gastric cancer.
Int J Nanomedicine. 15:2323–2335. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen W, Sun K, Zheng R, Zeng H, Zhang S,
Xia C, Yang Z, Li H, Zou X and He J: Cancer incidence and mortality
in China, 2014. Chin J Cancer Res. 30:1–12. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zheng R, Zeng H, Zhang S and Chen W:
Estimates of cancer incidence and mortality in China, 2013. Chin J
Cancer. 36(66)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shishida M, Toyota K, Ikeda M, Karakuchi
N, Inoue M, Saito Y and Takahashi T: Clinical complete response
after chemotherapy and palliative surgery for unresectable gastric
cancer. Case Rep Oncol. 13:689–695. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Song Z, Wu Y, Yang J, Yang D and Fang X:
Progress in the treatment of advanced gastric cancer. Tumour Biol.
39(1010428317714626)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lamerz R: Role of tumour markers,
cytogenetics. Ann Oncol. 10 (Suppl 4):S145–S149. 1999.PubMed/NCBI
|
|
31
|
Bodenbach L, Fauss J, Robitzki A, Krehan
A, Lorenz P, Lozeman FJ and Pyerin W: Recombinant human casein
kinase II. A study with the complete set of subunits (alpha, alpha'
and beta), site-directed autophosphorylation mutants and a
bicistronically expressed holoenzyme. Eur J Biochem. 220:263–273.
1994.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zou J, Luo H, Zeng Q, Dong Z, Wu D and Liu
L: Protein kinase CK2α is overexpressed in colorectal cancer and
modulates cell proliferation and invasion via regulating
EMT-related genes. J Transl Med. 9(97)2011.PubMed/NCBI View Article : Google Scholar
|