Introduction
Neurotransmitters, referred as endogenous chemicals,
play important roles in the regulation of brain functions such as
memory, mode and behaviors (1).
These functions are abnormal when excessive excitatory release of
neurotransmitters occurs, leading to neurodegenerative diseases
such as Alzheimer's disease, Parkinson's disease and Huntington's
disease (2,3). Glutamatergic dysfunction is the key
incentive factor to the occurrence and development of specific
neurodegenerative diseases (4). A
high concentration of glutamate accumulated in the brain resulted
in oxidative toxicity and then, neuron death (5,6). The
mechanism involved in oxidative glutamate toxicity is complex and
largely unknown. Therefore, investigating the molecular mechanism
underpinning the neuronal cell damage upon glutamate-associated
oxidative stress is highly desired, which contributes to
elucidating the pathogenesis of neurodegenerative diseases and
developing therapeutic regimens against these diseases.
Ginkgo biloba extract (GBE), the traditional
Chinese pharmacopoeia, is used world widely as a herbal medicine
against a variety of ailments due to its effect in ameliorating
cerebral blood flow as well as antioxidant, anti-inflammatory and
antiplatelet properties (7,8). It has been marketed in different
formulations, mainly as capsules and Gingko Biloba Dropping
pills. It is well known that GBE has neuroprotective effect, which
is largely due to its antioxidant activity (9). For instance, literature has showed
that GBE exerts its neuroprotective effect via scavenging free
radicals and neutralizing ferryl ion-induced peroxidation (10). However, the detailed mechanism
underpinning the antioxidant activity of GBE against brain nerve
cell injury remains unclear. In this study, we explored the
neuroprotective effect of GBE against oxidative glutamate toxicity
in human neuroblastoma SH-SY5Y cells (11-15),
and also investigated the associated molecular mechanism.
Materials and methods
Chemicals and reagents
GBE was provided by Wanbangde Pharmaceutical Group
Co., Ltd. Chemicals such as L-glutamate, MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide),
DCFH-DA and DMSO were obtained from Sigma-Aldrich; Merck KGaA.
Antibody were obtained from Santa Cruz Biotechnology, Inc. or
Abcam: p-SRC (ab40660, Abcam), SRC (ab109381, Abcam), p-VAV2
(ab86695, Abcam), Vav2 (ab52640, Abcam), p-p66Shc (ab68166, Abcam),
p66Shc (ab33770, Abcam), cytochrome c (ab133504, Abcam),
β-actin (sc-58673, Santa Cruz Biotechnology, Inc.), prohibitin
(sc-377037, Santa Cruz Biotechnology, Inc.), Goat Anti-Rabbit IgG
H&L (HRP) (ab7090, Abcam), Goat Anti-Mouse IgG H&L (HRP)
(ab205719, Abcam). Rac1 activity assay kit was obtained from Cell
Biolabs. Amplex Red hydrogen peroxide/peroxidase assay kit was
obtained from Thermo Fisher Scientific, Inc. Caspase-3 Activity
assay kit was obtained from Abcam. Other chemicals and reagents
used in this study were obtained from Beyotime.
Cell culture and treatment
Human neuroblastoma SH-SY5Y cells were purchased
from the American Type Culture Collection (ATCC). Cells were
maintained in culture medium of DMEM (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (FBS, Gibco;
Thermo Fisher Scientific, Inc.) with addition of antibiotics (100
U/ml penicillin and 100 µg/ml streptomycin, Gibco; Thermo Fisher
Scientific, Inc.) at 37˚C under 5% CO2. Cells were
treated with compounds (Glutamate: 50 mM or GBE: 25, 50 and 100
µg/ml) or transfected with the gene-specific siRNAs (p66Shc: Sense
:5'-AUGAGUCUCUGUCAUCGCUTT-3', antisense:
5'-AGCGAUGACAGAGACUCAUTC-3') using Lipofectamine 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
previous study (16).
Cell viability assay
MTT assay was used to determine cell viability.
Briefly, cells with or without treatment were incubated with 0.5
mg/ml MTT solution (100 µl) for 3 h at 37˚C. The culture medium was
then replaced with DMSO (150 µl). The absorbance was recorded at a
wavelength of 490 nm using the microstrip reader (Bio-Rad
Laboratories).
Oxidative markers analysis
DCFH-DA staining was adopted to assess intracellular
ROS generation. Briefly, cells with or without treatment were
incubated with DCFH-DA (10 µM) for 15 min at 37˚C. ROS
concentration was analyzed using the fluorescence microscope (Leica
Microsystems). Intracellular H2O2 level was
measured by Amplex Red assay as previously reported (17,18).
NOX activity analysis
NOX activity was determined by luminescence assay
using lucigenin as the electron acceptor generated by the NADPH
oxidase complex according to the previous study (19). Cells with or without treatment were
sonicated in PBS buffer containing 1 mM MgCl2, 1 mM EGTA
and protease inhibitors. The lysates (250 µg/ml of protein) were
then incubated with 20 µM lucigenin and 100 µM NADPH. The emitted
luminescence was detected by the luminometer (FluoStar Optima, BMG
Labtech). Typically, the readings of the luminescence increased
linearly within 5 min and the slope of the trend line was defined
as the relative NOX activity.
Cytochrome c release analysis
The cytosol and mitochondrial fractions were
prepared for cytochrome c release analysis according to the
previous study (20). After
treatment, mitochondrial and cytosolic fractions were extracted
from the cells using Cell Mitochondria Isolation Kit (Beyotime)
according to the manufacturer's instructions. The levels of
mitochondrial cytochrome c (Mito Cyto c) and
cytosolic cytochrome c (Cyto Cyto c) were determined
by western blot analysis. β-actin was used as loading control for
cytosolic fraction and prohibitin was used as loading control for
mitochondrial fraction.
Western blot analysis
Total protein extract (20 µg) from cultured cells
was separated by SDS-PAGE and electrophoretically transferred to
polyvinylidene fluoride (PVDF) membrane (Beyotime). Target protein
was probed by primary antibody (1:1,000) followed with horseradish
peroxidase-conjugated secondary antibody (1:500). The protein
signals were detected with chemiluminescence (ECL) detection
reagent (Beyotime) and exposed in a dark room. Image J was used to
quantify band densities with normalization to that of β-actin or
prohibitin.
Caspase activity analysis
Caspase activity was assessed using fluorometric
assay (Beyotime). Cells with or without treatment were lysed and
protein lysate was incubated with caspase-3 substrate DEVD-AFC at
37˚C for 30 min. The caspase activity was assessed with the
spectrofluorometer (Molecular Devices) (excitation wavelength at
400 nm, emission wavelength at 505 nm).
Statistical analysis
Statistical analysis was performed using SPSS 16.0
software package. All data were done in triplicates and the results
were from three independent studies. Results of multiple
experiments were expressed as means ± SD. Statistical comparisons
were made by Student's t-test between two groups and one-way ANOVA
followed by Tukey's post hoc test among multiple groups. P<0.05
was accepted as statistically significant.
Results
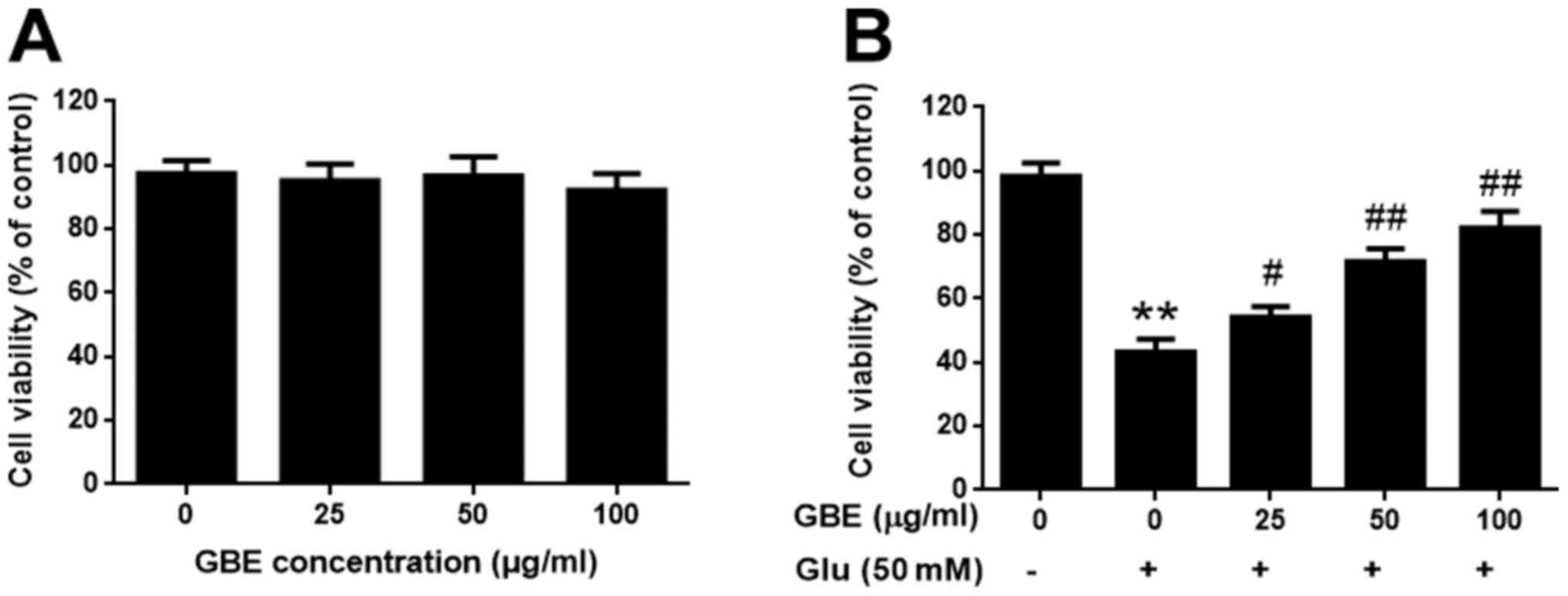
GBE attenuates glutamate-induced
cytotoxicity
The SH-SY5Y cells were pre-treatment with or without
GBE at a range of concentrations (0, 25, 50, 100 µg/ml) for 24 h,
and followed with glutamate incubation (50 mM, 6 h). Cell viability
was evaluated using MTT assay. Upon GBE pre-treatment (up to 100
µg/ml), cell viability was not significantly affected, which
suggested that GBE is non-toxic to the cells (Fig. 1A). Comparing the groups treated with
glutamate, GBE dose-dependently attenuated the glutamate-induced
cytotoxicity (Fig. 1B). Such
findings demonstrated the protective effect of GBE against
glutamate-induced toxicity in SH-SY5Y cells.
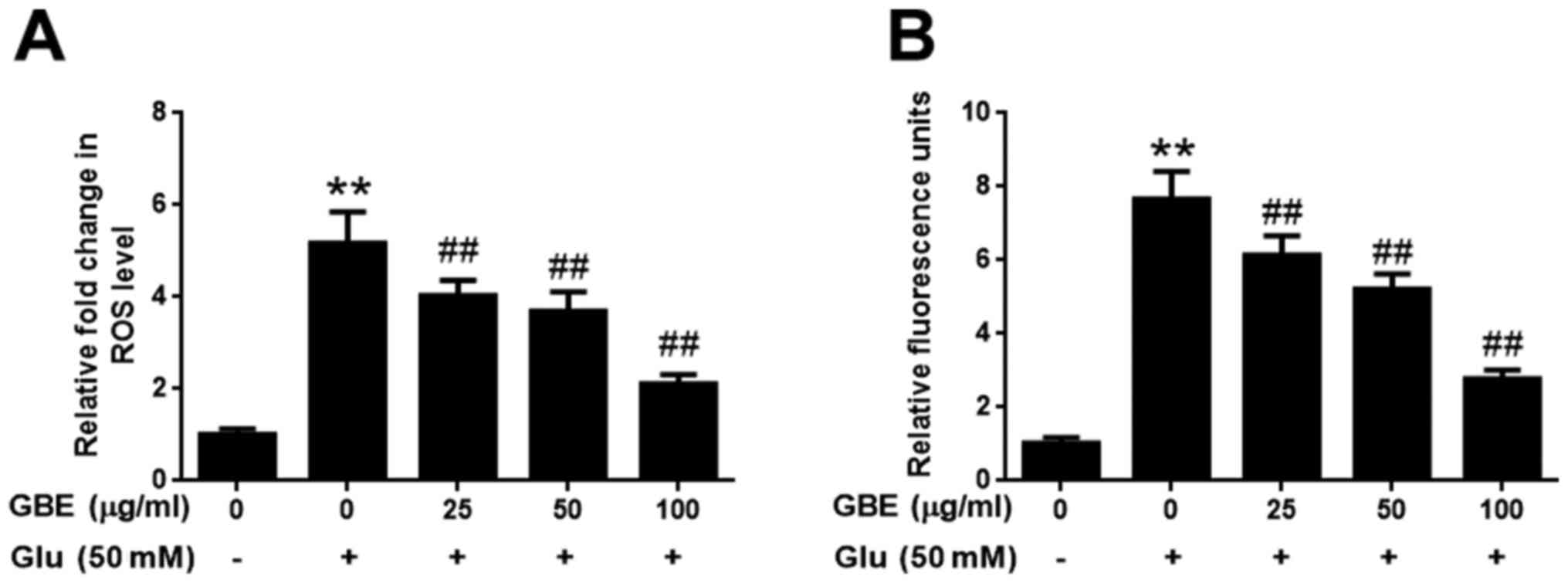
GBE attenuates glutamate-induced
oxidative injury
The effect of GBE against glutamate-induced
oxidative injury in SH-SY5Y cells was further evaluated with the
measurement of ROS and H2O2. As shown in
Fig. 2, exposure of cells to
glutamate (50 mM) for 2 h resulted in the significantly upregulated
levels of intracellular ROS and H2O2,
potentiating the oxidative toxicity of glutamate. However, cells
pre-treated with GBE (0, 25, 50, 100 µg/ml, 24 h) prior to
glutamate incubation resulted in a dose-dependent attenuation of
ROS and H2O2 generation (Fig. 2). These results indicated that GBE
exerted its protective effect against glutamate-induced toxicity by
attenuating oxidative stress in SH-SY5Y cells.
GBE attenuates glutamate-induced
redoxosome activation
To examine the molecular mechanism responsible for
the neuroprotective effect of GBE, redoxosome activity was
measured. As shown in Fig. 3,
glutamate treatment (50 mM, 2 h) induced the upregulated
phosphorylation of Src tyrosine and Vav2 tyrosine, which in turn
induced the increased expression of active Rac1-GTP, leading to
NADPH oxidase (NOX) activation in the Rac1-GTP-dependent manner.
However, the incubation of Rac inhibitor (NSC23766, 80 µM, 6 h) or
NOX inhibitor (VAS2870, 10 µM, 6 h) potently protected the cells
from the cytotoxic effect of glutamate (Fig. 4). Furthermore, the pre-treatment of
GBE (0, 25, 50, 100 µg/ml, 24 h) prior to glutamate incubation (50
mM, 6 h) resulted in a dose-dependent attenuation of redoxosome
activation.
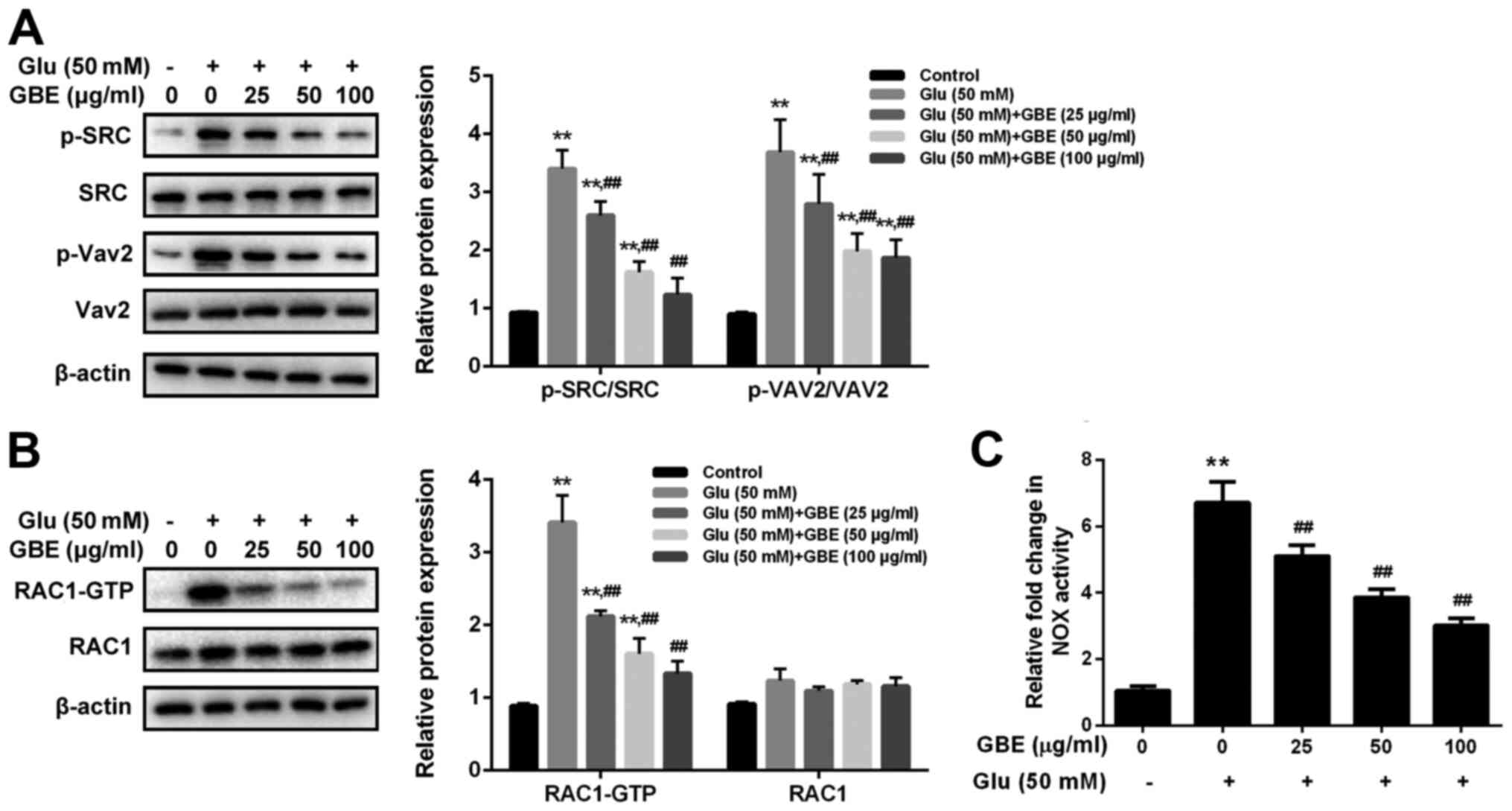 | Figure 3Protective effect of GBE against
Glu-induced redoxosome activation in SH-SY5Y cells. Cells were
pretreated with various concentrations of GBE (0, 25, 50, 100
µg/ml) for 24 h and then exposed to glutamate (50 mM) for 2 h. (A)
Protein levels of p-SRC, SRC, p-Vav2 and Vav2 were evaluated by
western blot analysis. (B) Representative Rac1 western blot is
shown. (C) NADPH oxidase activity was measured.
**P<0.01 vs. control; ##P<0.01 vs. Glu
alone. Glu, glutamate; GBE, Ginkgo biloba extract; p,
phosphorylated; SRC, proto-oncogene tyrosine-protein kinase Src;
Vav2, guanine nucleotide exchange factor VAV2. |
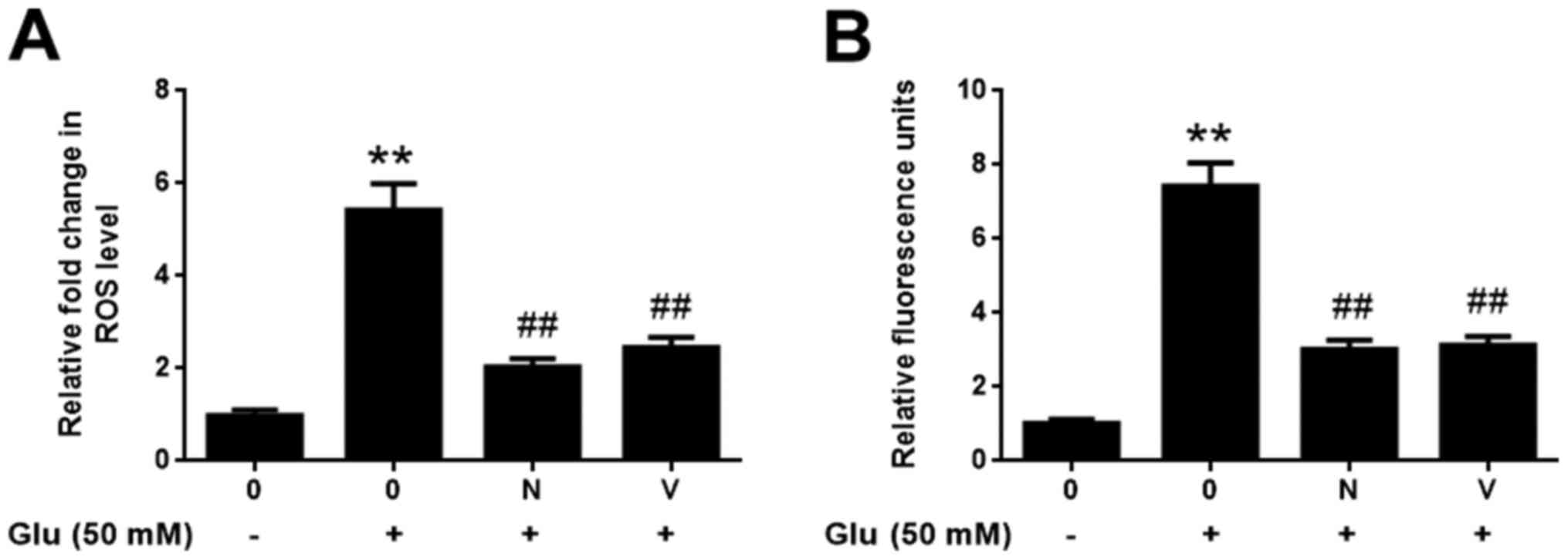
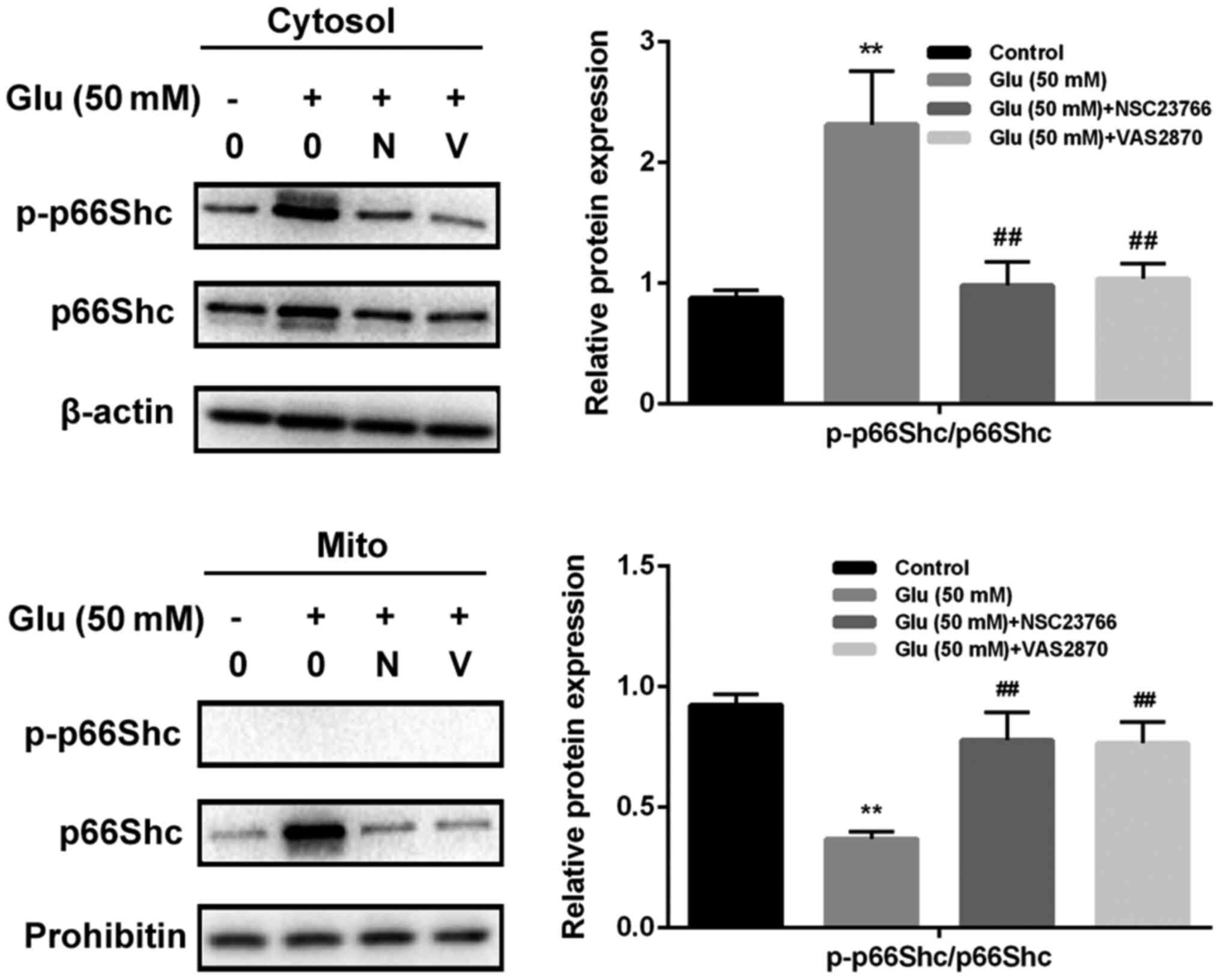
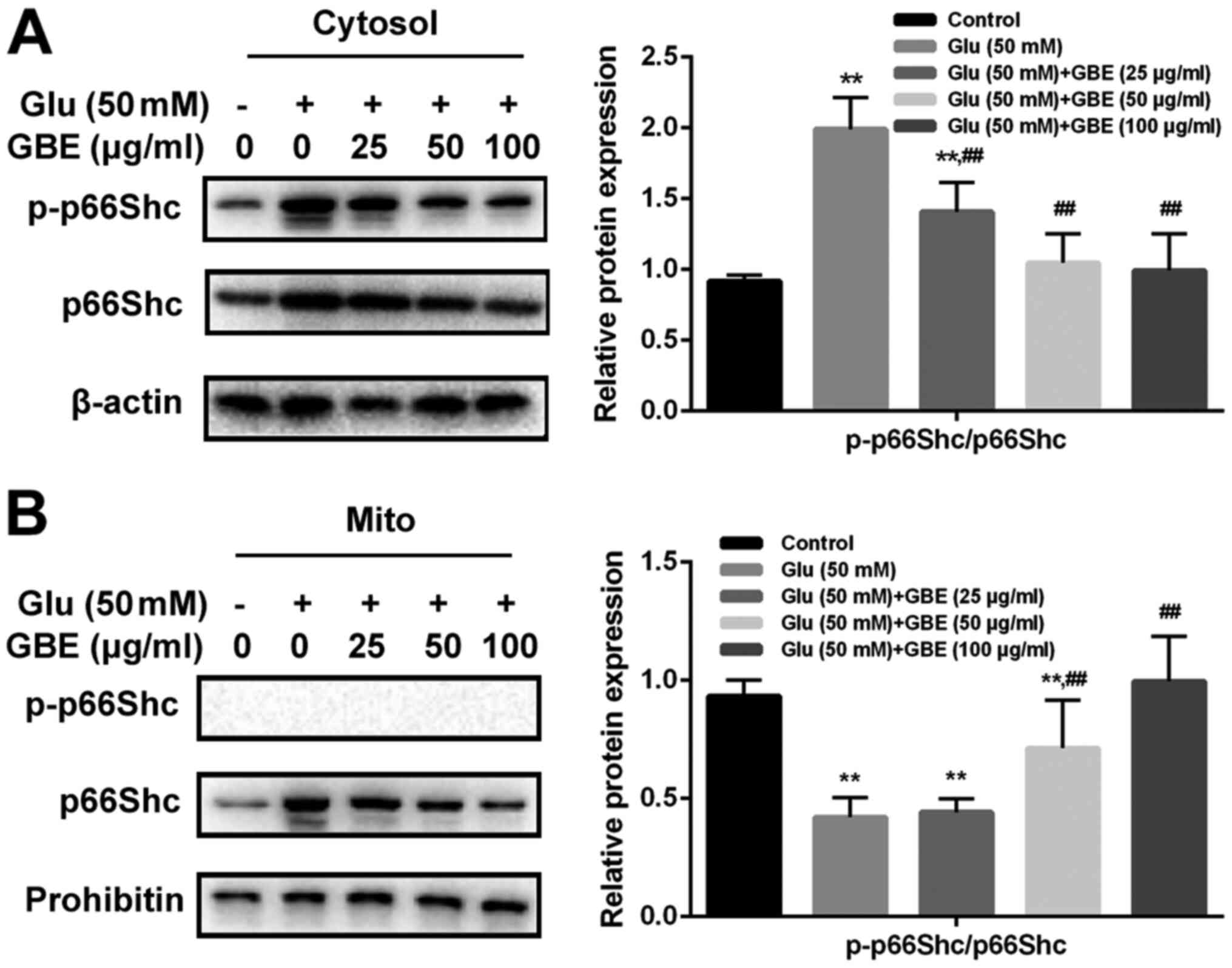
GBE inhibits redoxosome-dependent
p66Shc activation
To investigate whether glutamate-induced redoxosome
activation impacts on p66Shc activity, the expressions of p-p66Shc
and distribution of p66Shc were measured with or without GBE
pre-treatment. As shown in the Fig.
5, glutamate treatment (50 mM, 2 h) induced p66Shc serine36
phosphorylation and mitochondrial translocation of p66Shc. However,
Rac inhibitor (NSC23766, 80 µM, 6 h) or NOX inhibitor (VAS2870, 10
µM, 6 h) incubation blocked the effect of glutamate on p66Shc
activation. Moreover, cells pre-treated with GBE (0, 25, 50, 100
µg/ml, 24 h) prior to glutamate incubation (50 mM, 2 h) led to a
dose-dependent attenuation of p66Shc activation (Fig. 6).
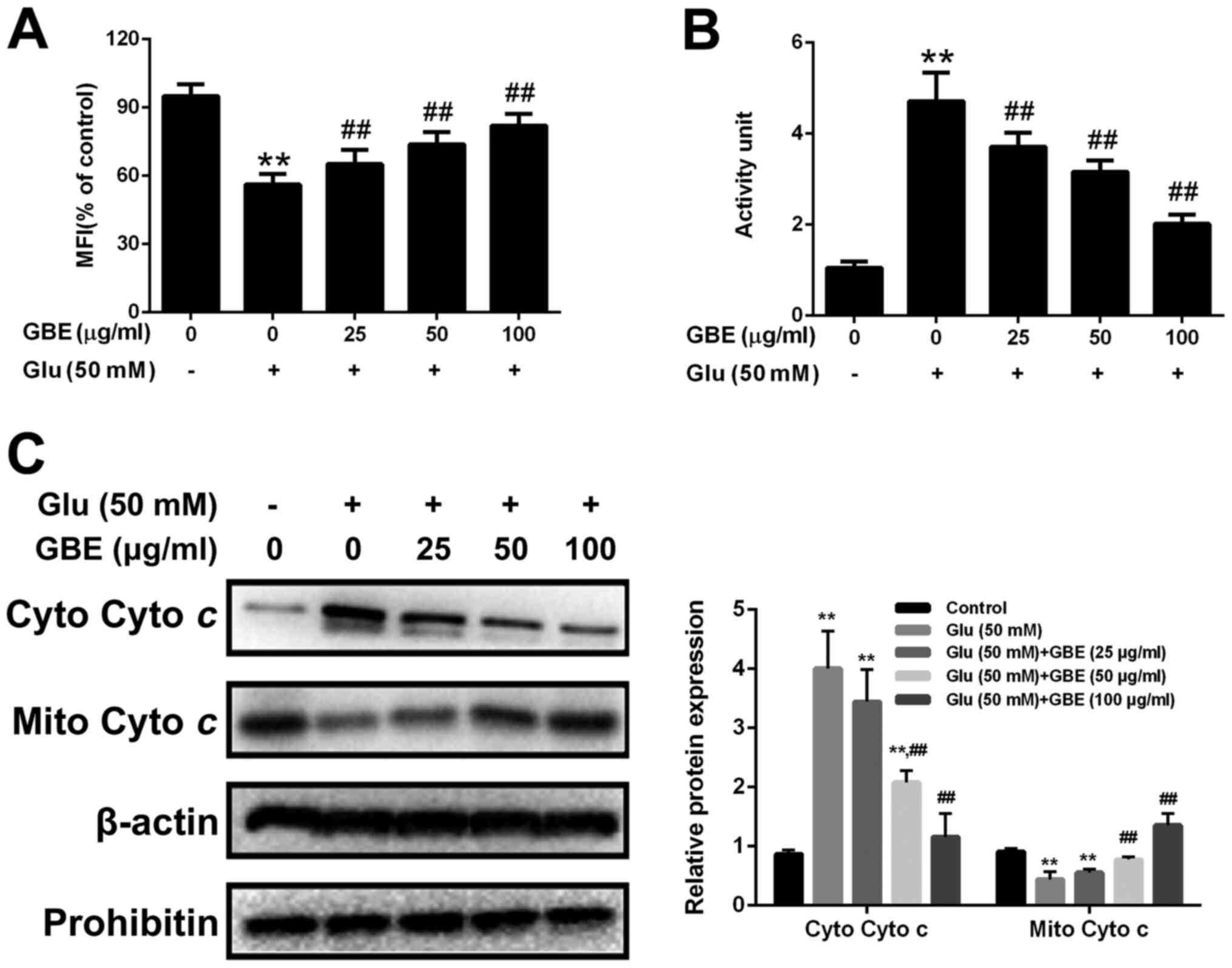
GBE attenuates glutamate-induced
mitochondrial dysfunction
Mitochondria translocation of p66Shc induces
mitochondrial dysfunction. Thus, we also assessed the mitochondrial
function in cells with or without GBE pre-treatment. As shown in
the Fig. 7, glutamate treatment (50
mM, 6 h) resulted in the reduced mitochondrial membrane potential
(MMP), increased cytochrome c release and activation of
caspase-3. However, p66Shc inhibition blocked glutamate-induced
mitochondrial dysfunction. Furthermore, cells pre-treated with GBE
(0, 25, 50, 100 µg/ml, 24 h) prior to glutamate incubation (50 mM,
6 h) showed the decreased mitochondrial dysfunction
dose-dependently (Fig. 8).
Discussion
Gingko biloba extract (maidenhair tree) is a
traditional Chinese medicine, which has been clinically adopted in
the treatment of various neurodegenerative diseases for hundreds of
years (21). For example, EGb761,
one of the standard extracts isolated from Ginkgo biloba
leaves, has been used in treating neurological diseases including
Alzheimer's disease, Parkinson's disease and Huntington's disease
(22,23). GBE of Gingko Biloba Dropping
is another standard exact, which is prepared through alcohol
extraction and then purification with a macroporous resin column.
Considering national pharmacopoeia standards, the quality indexes
of GBE are quite standardized: Flavonoid glycosides ≥24%, terpene
lactones ≥6%, ginkgolic acids ≤5 or 10 ppm. The most important
flavonoids of GBE are glycosides of kaempferol, quercetin, and
isorhamnetin with glucose or rhamnose. Ginkgolides, only present in
Ginkgo biloba extract but any other living species, can be
divided into types A, B, C, and J (a very small quantity). Its
other clinically important ingredients are not well studied, such
as procyanidins, catechins and organic acids (24).
It is well known that GBE has potent neuroprotective
effect, which is largely due to its anti-oxidant activity. It can
alleviate ischemia, oxidative stress and β-amyloid-induced toxicity
(25). Huang et al have
reported that EGb761 can protect SH-SY5Y neuroblastoma cells
against glutamate toxicity via inhibiting excitotoxicity and
calcium influx as well as reducing the expression of apoptotic
markers (26). However, little is
known about GBE. In this study, we investigated the anti-oxidative
effect of GBE, the main active extract from the Ginkgo
biloba dropping pills, against glutamate-induced toxicity in
SH-SY-5Y cells. We found that GBE has comparable effect to that of
EGb761 (data not shown). More importantly, we investigated the
molecular mechanism of GBE against glutamate-induced oxidative
toxicity. According to the previous studies, 50 mM of glutamate is
effective to induce oxidative toxicity in SH-SY5Y cells; while
0-100 µg/ml of GBE was selected as the desired concentration range
to test for its anti-oxidative effect (27-29).
Redox signaling is a key player in the regulation of
physiological processes (30).
Accumulating evidence reveals that the abnormality of redox
signaling in response to stress leads to the occurrence and
development of neurological disorders and other diseases (31,32).
Redoxosome is a fledgling area of cellular signaling through
superoxide-producing endosomes, which acts via specific redox
modifications on numerous proteins and enzymes (33). Redoxosome activation includes Src
kinase-dependent Vav2 tyrosine phosphorylation, Rac1-GTP activation
and activation of NADPH oxidase (34). In this study, glutamate treatment
remarkably induced oxidative stress by activating redoxosome
signaling, which is associated with Src-VAV2-Rac1-NOX activation;
while Rac inhibitor (NSC23766) or NOX inhibitor (VAS2870) could
potently alleviate glutamate-induced oxidative toxicity.
Consistently, GBE dose-dependently attenuated the oxidative
toxicity associated with glutamate through inactivating redoxosome
signaling in SH-SY5Y cells. p66Shc, a 66 kDa Src collagen homologue
(Shc) adaptor protein, is reported to be downstream effector of
redoxosome signaling in several types of cells (33). Genetic deletion of p66Shc adaptor
protein prevents hyperglycemia-induced oxidative stress in
endothelial cells (35). p66Shc
Ser36 phosphorylation induces its translocation to the
mitochondrial intermembrane space, enabling its interaction with
cytochrome c and promoting the transfer of electrons to
oxygen for the generation of hydrogen peroxide and consequently,
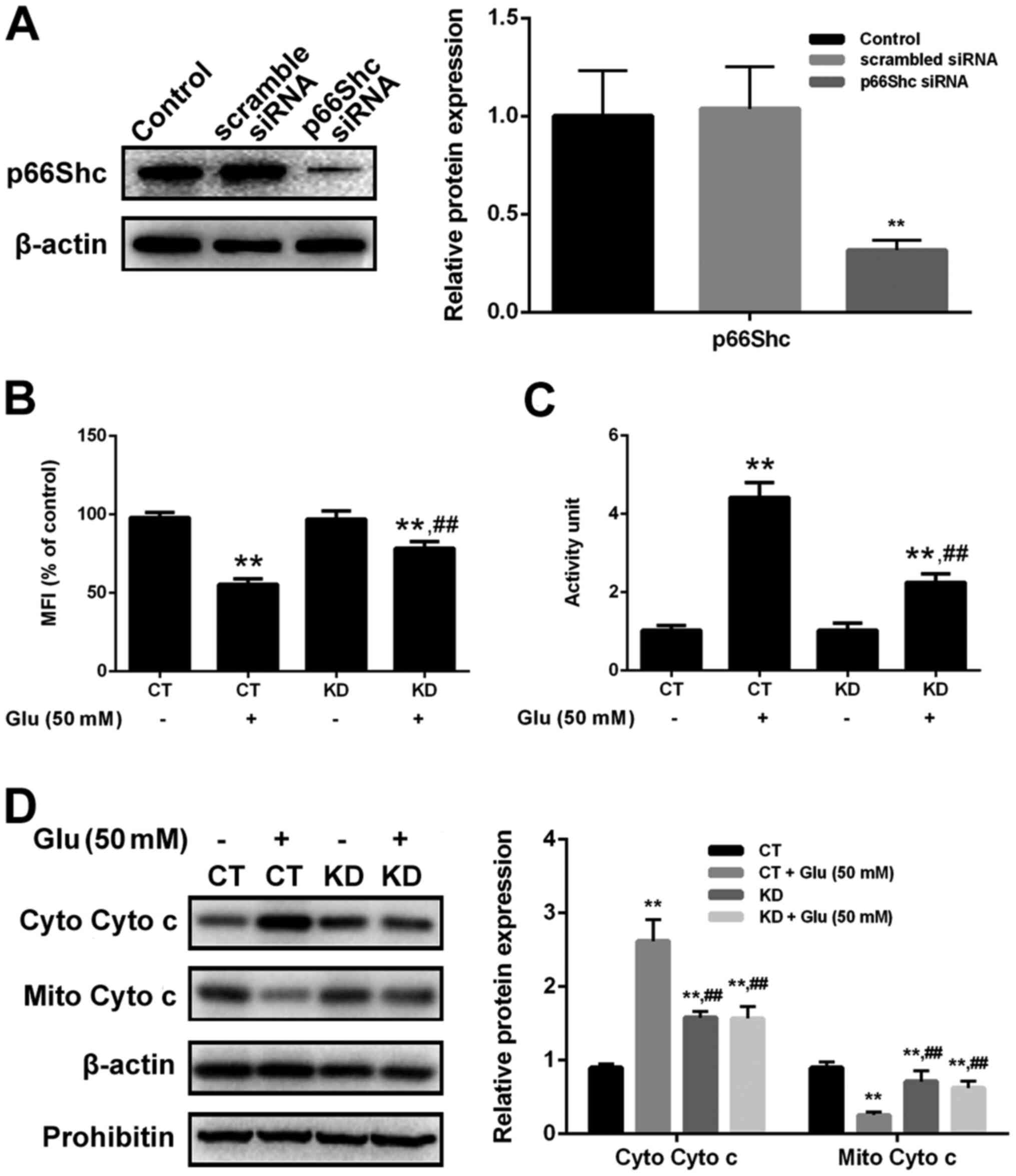
activating programmed cell death (36). In this study, p66Shc inhibition
through siRNA silencing potently prevented cells from the
glutamate-induced oxidative toxicity, indicating that p66Shc
activation directly contributes to glutamate toxicity in a
redoxosome dependent manner. In addition, p66Shc activation reduced
MMP, increased the release of cytochrome c and upregulated
caspase-3. Interestingly, p66Shc activation and mitochondrial
dysfunction can be dose-dependently attenuated with the
pre-treatment of GBE in SH-SY5Y cells. Our results revealed the
novel regulatory mechanism of GBE against glutamate-induced
oxidative toxicity.
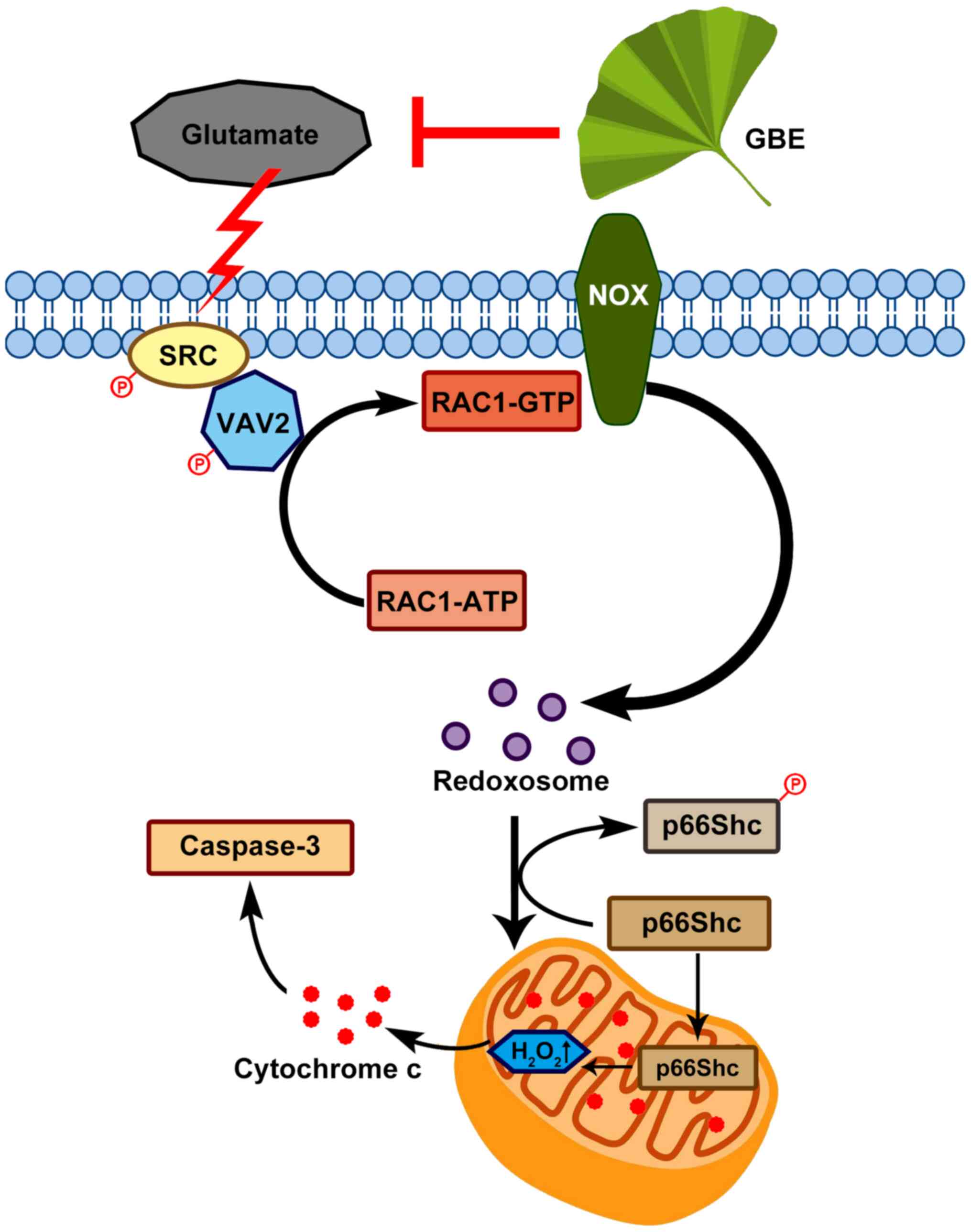
In conclusion, this study demonstrated the
neuroprotective effect of GBE against glutamate-induced oxidative
toxicity in SH-SY5Y cells, which effect is possibly mediated
through redoxosome-p66Shc signaling (Fig. 9). This provides new information in
the pathogenesis of various neurological diseases that are induced
by oxidative damage, and also form the basis of the clinical use of
GBE in treating these diseases. In addition, this study suggests
that redoxosome-p66Shc signaling can be a potential therapeutic
target in the prevention/treatment of neurological pathologies.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by grants from the Project of
National Natural Science Foundation of China (grant no. 81700852),
the Young Talent's Subsidy Project in Science and Education of the
Department of Public Health of Jiangsu Province (grant no.
QNRC2016627), Six Talent Peaks Project in Jiangsu Province (grant
no. WSW-047), Six-one Scientific Research Project (grant no.
LGY2019087), Innovation Capacity Development Plan of Jiangsu
Province (grant no. BM2018023) and the Project of Wuxi Municipal
Health Bureau (grant no. Q201813).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KW and FZ designed the experiments. KW, JN and XZ
carried out the experiments. KW, JN and XZ confirmed the
authenticity of all the raw data. YL and LZ analyzed the
experimental results. KW, FZ and JN wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hyman SE: Neurotransmitters. Curr Biol.
15:R154–R158. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kanazawa I: Neurotransmitters and
neurodegenerative disorders. Clin Ther. 7:48–58. 1984.PubMed/NCBI
|
|
3
|
Shen J: Impaired neurotransmitter release
in Alzheimer's and Parkinson's diseases. Neurodegener Dis. 7:80–83.
2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Meldrum BS: Glutamate as a
neurotransmitter in the brain: Review of physiology and pathology.
J Nutr. 130 (4S Suppl):1007S–1015S. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Maher P, van Leyen K, Dey PN, Honrath B,
Dolga A and Methner A: The role of Ca2+ in cell death
caused by oxidative glutamate toxicity and ferroptosis. Cell
Calcium. 70:47–55. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lewerenz J and Maher P: Chronic glutamate
toxicity in neurodegenerative diseases-what is the evidence? Front
Neurosci. 9(469)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nash KM and Shah ZA: Current perspectives
on the beneficial role of Ginkgo biloba in neurological and
cerebrovascular disorders. Integr Med Insights. 10:1–9.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mashayekh A, Pham DL, Yousem DM, Dizon M,
Barker PB and Lin DD: Effects of Ginkgo biloba on cerebral blood
flow assessed by quantitative MR perfusion imaging: A pilot study.
Neuroradiology. 53:185–191. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ahlemeyer B and Krieglstein J:
Neuroprotective effects of Ginkgo biloba extract. Cell Mol Life
Sci. 60:1779–1792. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Droy-Lefaix MT: Effect of the antioxidant
action of Ginkgo biloba extract (EGb 761) on aging and oxidative
stress. Age (Omaha). 20:141–149. 1997.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Park SE, Kim S, Sapkota K and Kim SJ:
Neuroprotective effect of rosmarinus officinalis extract on human
dopaminergic cell line, SH-SY5Y. Cell Mol Neurobiol. 30:759–767.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gismondi A, Trionfera E, Canuti L, Di
Marco G and Canini A: Royal jelly lipophilic fraction induces
antiproliferative effects on SH-SY5Y human neuroblastoma cells.
Oncol Rep. 38:1833–1844. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lantto TA, Colucci M, Závadová V, Hiltunen
R and Raasmaja A: Cytotoxicity of curcumin, resveratrol and plant
extracts from basil, juniper, laurel and parsley in SH-SY5Y and
CV1-P cells. Food Chem. 117:405–411. 2009.
|
|
14
|
Rahman MA, Yang H, Lim SS and Huh SO:
Apoptotic effects of melandryum firmum root extracts in human
SH-SY5Y neuroblastoma cells. Exp Neurobiol. 22:208–213.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Izuta H, Shimazawa M, Tazawa S, Araki Y,
Mishima S and Hara H: Protective effects of Chinese propolis and
its component, chrysin, against neuronal cell death via inhibition
of mitochondrial apoptosis pathway in SH-SY5Y cells. J Agric Food
Chem. 56:8944–8953. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhu X, Xue L, Yao Y, Wang K, Tan C, Zhuang
M, Zhou F and Zhu L: The FoxM1-ABCC4 axis mediates carboplatin
resistance in human retinoblastoma Y-79 cells. Acta Biochim Biophys
Sin (Shanghai). 50:914–920. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhu P, Tong BM, Wang R, Chen JP, Foo S,
Chong HC, Wang XL, Ang GY, Chiba S and Tan NS: Nox4-dependent ROS
modulation by amino endoperoxides to induce apoptosis in cancer
cells. Cell Death Dis. 4(e552)2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Karamitros CS, Lim J and Konrad M: An
amplex red-based fluorometric and spectrophotometric assay for
L-asparaginase using its natural substrate. Anal Biochem.
445:20–23. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang Y and Lou MF: The regulation of NADPH
oxidase and its association with cell proliferation in human lens
epithelial cells. Invest Ophthalmol Vis Sci. 50:2291–2300.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yang J, Liu X, Bhalla K, Kim CN, Ibrado
AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by
Bcl-2: Release of cytochrome c from mitochondria blocked. Science.
275:1129–1132. 1997.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Christen Y: Ginkgo biloba and
neurodegenerative disorders. Front Biosci. 9:3091–3104.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Bastianetto S, Ramassamy C, Doré S,
Christen Y, Poirier J and Quirion R: The Ginkgo biloba extract (EGb
761) protects hippocampal neurons against cell death induced by
beta-amyloid. Eur J Neurosci. 12:1882–1890. 2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Müller WE, Eckert A, Eckert GP, Fink H,
Friedland K, Gauthier S, Hoerr R, Ihl R, Kasper S and Möller HJ:
Therapeutic efficacy of the Ginkgo special extract
EGb761® within the framework of the mitochondrial
cascade hypothesis of Alzheimer's disease. World J Biol Psychiatry.
20:173–189. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ren C, Ji YQ, Liu H, Wang Z, Wang JH,
Zhang CY, Guan LN and Yin PY: Effects of Ginkgo biloba extract
EGb761 on neural differentiation of stem cells offer new hope for
neurological disease treatment. Neural Regen Res. 14:1152–1157.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yao ZX, Han Z, Drieu K and Papadopoulos V:
Ginkgo biloba extract (Egb 761) inhibits beta-amyloid production by
lowering free cholesterol levels. J Nutr Biochem. 15:749–756.
2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Huang DS, Lin HY, Lee-Chen GJ, Hsieh-Li
HM, Wu CH and Lin JY: Treatment with a Ginkgo biloba extract, EGb
761, inhibits excitotoxicity in an animal model of spinocerebellar
ataxia type 17. Drug Des Devel Ther. 10:723–731. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lee HJ, Spandidos DA, Tsatsakis A, Margina
D, Izotov BN and Yang SH: Neuroprotective effects of scrophularia
buergeriana extract against glutamate-induced toxicity in SH-SY5Y
cells. Int J Mol Med. 43:2144–2152. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sun X, Shi X, Lu L, Jiang Y and Liu B:
Stimulus-dependent neuronal cell responses in SH-SY5Y neuroblastoma
cells. Mol Med Rep. 13:2215–2220. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ba XH and Min LQ: Effects of Ginkgo biloba
extract on the apoptosis of oxygen and glucose-deprived SH-SY5Y
cells and its mechanism. Indian J Pharmacol. 47:101–104.
2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bekeschus S, Bräutigam L, Wende K and
Hanschmann EM: Oxidants and redox signaling: Perspectives in cancer
therapy, inflammation, and plasma medicine. Oxid Med Cell Longev.
2017(4020253)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hsieh HL and Yang CM: Role of redox
signaling in neuroinflammation and neurodegenerative diseases.
Biomed Res Int. 2013(484613)2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Franco R and Vargas MR: Redox biology in
neurological function, dysfunction, and aging. Antioxid Redox
Signal. 28:1583–1586. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Karunakaran U, Elumalai S, Moon JS and Won
KC: CD36 dependent redoxosomes promotes ceramide-mediated
pancreatic β-cell failure via p66Shc activation. Free Radic Biol
Med. 134:505–515. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Spencer NY and Engelhardt JF: The basic
biology of redoxosomes in cytokine-mediated signal transduction and
implications for disease-specific therapies. Biochemistry.
53:1551–1564. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kumar S: P66Shc and vascular endothelial
function. Biosci Rep. 39(BSR20182134)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang M, Tang J, Shan H, Zhang Q, Yang X,
Zhang J and Li Y: P66Shc mediates mitochondrial dysfunction
dependent on PKC activation in airway epithelial cells induced by
cigarette smoke. Oxid Med Cell Longev. 2018(5837123)2018.PubMed/NCBI View Article : Google Scholar
|