1. Introduction and aim
Basilar artery occlusion (BAO) accounts for
approximately 1% of all cases of acute ischemic stroke (AIS) or
transient ischemic attacks (TIA), and for 5% of all intracranial
large vessel occlusions (LVO) (1,2).
Although endovascular treatment is the gold-standard for anterior
circulation LVO, its positive impact on the vertebrobasilar system
is still a matter of debate (3).
Hopefully, ongoing randomized trials will shed more light on this
devastating pathology (4). Due to
the misleading nature of prodromal symptoms related to BAO,
clinical diagnosis is often delayed, leading to prolonged time
intervals to imaging evaluation and treatment (5).
Cross-sectional imaging has proven its diagnostic
and prognostic utility for anterior circulation LVO shifting the
paradigm from time is brain to imaging is brain (6). Computed tomography (CT) is the main
imaging modality for the diagnosis of suspected AIS owing to its
widespread availability in emergency departments. CT has a
sensitivity of up to 80% for the detection of early ischemic
changes (EIC) in middle cerebral artery (MCA) territory strokes
(7). For posterior circulation
ischemia, however, detection of EIC is difficult, mainly due to
posterior fossa radiologic particularities, such as beam hardening
artifacts, which limit the sensitivity to only 55% for cerebellar
EIC, and 33% for brainstem EIC, respectively (8). Magnetic resonance imaging (MRI) is
superior to CT in terms of sensitivity, especially in posterior
fossa ischemia. However, the lack of available MRI machines in
smaller hospitals or in developing countries, and limitations
related to artifacts, uncooperative or severe patients, limit the
feasibility of MRI evaluation in posterior ischemic strokes
(9). Our aim is to review the
imaging signs, or scores described on non-contrast CT (NCCT) and CT
angiography (CTA) in acute BAO in order to provide an integrated
view on their diagnostic and prognostic strengths and
weaknesses.
2. Literature research methods
We performed a non-systematic PubMed search for
papers between 1980 and 2019, using the terms ‘basilar artery
occlusion’ and ‘basilar artery thrombosis’ and ‘imaging’. Inclusion
criteria were retrospective or prospective studies that evaluated
diagnostic and prognostic computed-tomography changes in patients
with BAO. The search generated 445 results. Furthermore, we
identified papers related to imaging markers by searching the
reference list of articles retrieved by our initial search. Only
English reports were included. The final reference list is based on
the relevance and originality of this review's objective and
includes 14 total studies.
3. Hyperdense Basilar Artery (HDBA)
sign
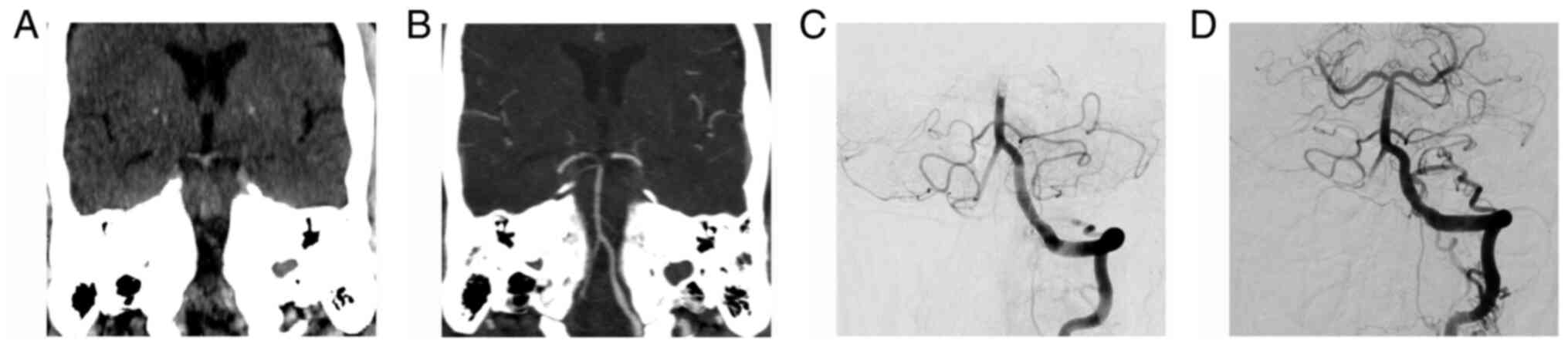
A hyperdense vessel is seen as a region of high
attenuation on NCCT scans (Fig. 1).
Pathologically it represents, in the appropriate clinical setting,
an acute thrombus within the artery. The hyperdense appearance of
fresh intravascular clots is determined by the extravasation of
serum out of the clot, followed by a relative increase in
concentration of red blood cells. Higher densities are mainly
caused by the protein fraction of hemoglobin, much less by its iron
content, which contributes only to 7-8% to its attenuation
(10). Thrombi retrieved from
patients with a dense MCA have, on average, a higher content of red
blood cells (11-14)
and are more frequently associated with a cardioembolic stroke
subtype (15). These observations
are not confirmed for BAO.
The HDBA sign can be a useful tool for the detection
of BAO, with a sensitivity ranging between 68 and 94% and a
specificity between 80 and 98% (16-18).
Sensitivity is higher if the clinical diagnosis of BAO is highly
probable (14) or if the
attenuation of the hyperdense vessel is measured. Optimal measured
cut-off values that best discriminate a HDBA, range between 40 and
46 HU (17,18). Another method by which to increase
detection of an HDBA, that was confirmed for dense MCAs, is to
obtain a CT slice thickness of less than 2 mm or more appropriate 1
mm, and to view them on 5-mm-thick maximum intensity projection
(MIP) reconstructions (19). One
possible limitation of early studies evaluating the diagnostic
value of the HDBA sign, is the use of a larger slice thickness, of
2 mm (16) or 4-5 mm (16,17),
respectively. It is well known that a larger slice thickness
increases partial volume averaging with cerebrospinal fluid and
adjacent brain parenchyma, and can lead to false-negative NCCT
results, potentially missing more hyperdense clots (18).
A dense basilar artery basilar artery (BA) sign is
also valuable as a prognostic marker. It correlates well with
discharge NIHSS and can independently predict short- (OR, 5.5; 95%
CI, 2.2-13.6; P<0.001) (20) and
long-term outcome (OR, 5.3; 95% CI, 1.1-33.3; P=0.05) (16). Nevertheless, in the absence of the
HDBA sign, a brainstem ischemia or LVO should not be excluded;
further contrast-enhanced vessel imaging is mandatory for the
correct radiologic evaluation of patients with suspected posterior
circulation stroke.
4. Posterior Circulation Acute Stroke
Prognosis Early CT Score (pc-ASPECTS)
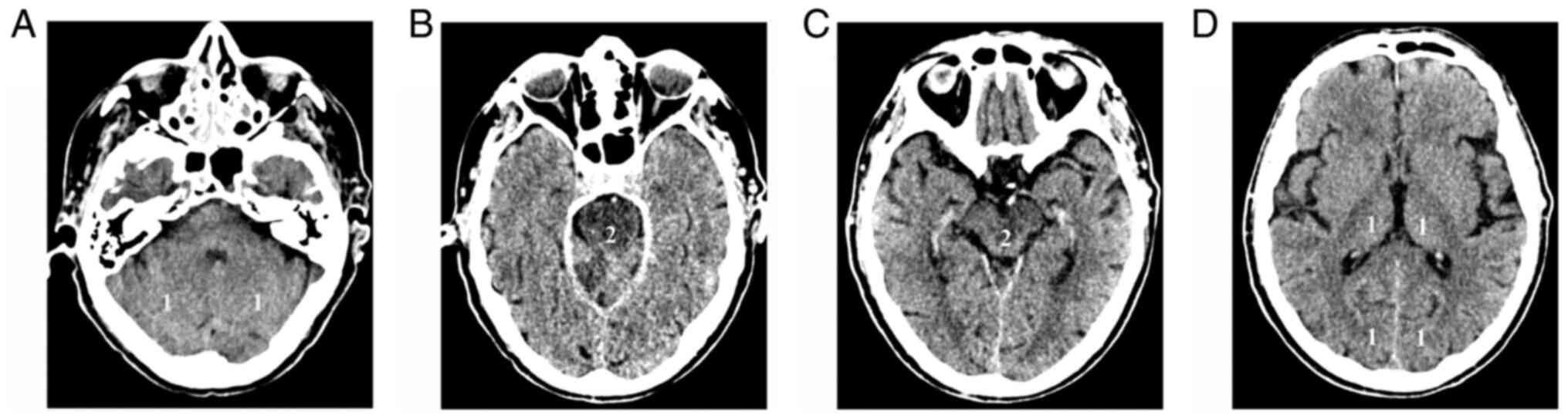
pc-ASPECTS is a 10-point score that evaluates EIC
changes in the thalamus, occipital cortex, cerebellar hemispheres,
midbrain, and pons. For the first three, 1 point is subtracted for
each part if EIC are present, while 2 points each are subtracted if
the midbrain or the pons are affected (21) (Fig.
2). Evaluation of the score is difficult on NCCT, due to
posterior fossa beam hardening artefacts, particularly in the
brainstem. CTA source images (CTASI) are a better tool for the
visualization of ischemia. Parenchymal hypodensity on CTASI most
likely represents a region of cerebral blood volume reduction
(CBV). The extent of this region correlates well with admission and
24-h NIHSS, and with the 90-day functional outcome. Furthermore,
ischemic lesion volume on CTASI does not differ significantly from
DWI volume on MRI. If applied on CTASI, pc-ASPECTS is more
sensitive and specific (65 and 82%) (22). In their retrospective cohort of 46
patients with BAO, Puetz et al dichotomized the patients
into two groups: CTASI pc-ASPECTS ≥8 and <8. Patients with a
score ≥8 were 11 times more likely to have a good outcome (mRS ≤3):
52% achieved a good functional outcome at three months, whereas
only 4% if the score was <8 (unadjusted RR, 12.1; 95% CI,
1.7-84.9). Mortality was reduced by 60% if the score was ≥8. If
successful recanalization was obtained, 70% of the patients
achieved a good prognosis if pc-ASPECTS was ≥8, and only 9% if the
score was <8 (unadjusted RR, 7.7; 95% CI, 1.1-52.1) (21).
In patients from the Basilar Artery International
Cooperation Study (BASICS) group, a significant association was
found between pc-ASPECTS ≥8, favorable outcome, functional
independence (mRS ≤2) and reduced mortality (23). However, after adjustment for age,
NIHSS and tissue-type plasminogen activator (tPA), only functional
independence (RR, 2.0; 95% CI, 1.1-3.8) and mortality (RR, 0.7; 95%
CI, 0.5-0.98) were significantly associated, but not a favorable
outcome. In a post hoc analysis, the same group was further
dichotomized by pc-ASPECTS of <6 and ≥6. It was found that a
score of ≥6 was an independent predictor of a favorable outcome,
even after adjustment for age, NIHSS, and treatment modality (RR,
3.1; 95% CI, 1.2-7.5) (23). In
patients with BAO presenting with coma, the discriminative
prognostic power of pc-ASPECTS of <8 OR, ≥8 was not found to be
significantly associated with a favorable outcome or mortality, if
adjusted for age, NIHSS and treatment modality (24). Even in a larger cohort comprising
231 patients with acute BAO, the prognostic value of pc-ASPECTS in
predicting functional independence at 3 months was not significant
(25).
Pc-ASPECTS dichotomized by <8 or ≥8 was also
evaluated on CT perfusion (CTP) maps in 27 patients from the BASICS
registry (26). The most frequent
changes were seen in 93% of cases on mean transit time (MTT)
parameter maps (95% CI, 76-99). Cerebral blood volume (CBV)
pc-ASPECTS <8 was evident in 3 cases, all of whom died. However,
none of the perfusion changes were associated with functional
outcome, likely due to the small number of available cases
(26).
5. Pons-Midbrain Index (PMI)
Schaefer et al more precisely attributed
functional outcome to lesions located in the pons and midbrain, for
which they developed the Pons-Midbrain Index, a scoring system
based on CTASI: 0, if no ischemic changes are visible, 1 if <50%
of the territory is hypodense, 2 if >50% of the parenchyma is
hypodense. Brain structures scored were the medulla, pons,
midbrain, thalami, occipital lobes, inferior parietal lobes and
middle temporal lobes. Each structure was scored separately. The
only regions that were correlated with death and disability were
the pons and midbrain, which taken together as a sum of the scores,
they called the PMI. A PMI ≥3, or <3, was independently
associated with mortality, or survival, respectively (27).
The Basilar Artery International Cooperation Study
(BASICS) group looked at the death rate among patients with BAO who
presented with coma. Among 78 patients with BAO and coma, 49% died,
and 17% had a favorable outcome (mRS 0-3) if PMI was <3, as
opposed to 76% deaths, and only 14% favorable outcome if PMI was
≥3(24). However, after adjusting
for age, NIHSS and treatment type, only mortality was associated
with a PMI <3 (RR, 0.67; 95% CI, 0.46-1.00), but not a favorable
outcome. Notably, a subgroup of patients, despite presenting with
coma, with extensive pontine-mesencephalic ischemia (PMI ≥3) had a
favorable outcome at 1 month, comprising 14%. Based on this
observation, the authors argue against the use of the PMI to
exclude patients with BAO and coma from intravenous or
intraarterial treatment (24).
6. Posterior Circulation CT Angiography
(pc-CTA) score
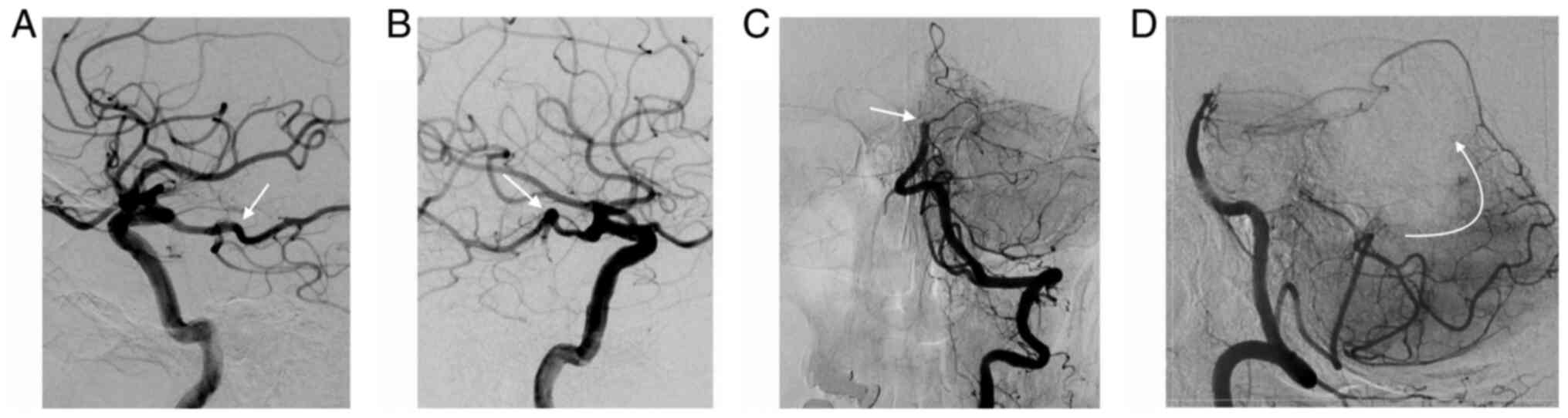
The Posterior Circulation CT Angiography (pc-CTA)
score is a 6-point scoring system that evaluates the extent of the
occlusion and, indirectly, the collateral circulation (28) (Fig.
3). One point is assigned for each of the following occluded
segments: One of the vertebral arteries, proximal segment of the BA
[from the vertebrobasilar junction to the origin of the
anterior-interior cerebellar arteries (AICA)], middle segment of
the BA [from the origin of the AICA to the superior cerebellar
arteries (SCA)], distal BA (from the SCA to the tip) and the
posterior cerebral arteries (PCA). A pc-CTA score of 0 means that
all vascular segments are open; a score of 6, that all are occluded
(28).
In their retrospective series of 15 patients, the
primary endpoint was the correlation between the score and patient
outcome at 3 months, as assessed by the modified Rankin Scale
(mRS). A pc-CTA score <3 was associated with a good outcome (mRS
≤3) in all patients. In contrast, the contrary, if the score was
≥3, patient outcome was worse (28). In a retrospective study performed by
Alemseged et al (29) a
pc-CTA ≥3 predicted a poor outcome if adjusted for age (OR, 1.6;
95% CI, 1.1-2.1; P=0.008), but not for NIHSS.
7. Posterior Circulation Collateral Score
(pc-CS)
The investigators enrolled in the Basilar Artery
International Cooperation Study (BASICS) developed this CT
angiography score [Posterior Circulation Collateral Score (pc-CS)]
as a prognostic tool for identifying patients who present with a
poor outcome (30). The score
allots 1 point for each patent posterior inferior cerebellar artery
(PICA), anterior-inferior cerebellar artery (AICA), superior
cerebellar artery (SCA) and posterior communicating artery (Pcom)
if its diameter is smaller than the ipsilateral PCA, or 2 points if
it has a diameter equal to, or larger than the ipsilateral PCA. A
score of 10 implies that all the aforementioned branches are
patent, while a score of 0, means that all of them are not
visible/occluded. Three intervals of severity were defined for the
pc-CS: Poor 0-3, intermediate 4-5, and good 6-10. Patients with a
poor score had higher median NIHSS at admission and had more
frequently severe symptoms (tetraplegia, locked-in state or coma),
than patients with an intermediate or a good score. Moreover, there
was a 25% lower risk of poor outcome in patients with a good pc-CS
score, compared to those with a poor score, after adjustment for
age, time to treatment and treatment modality (RR, 0.74; 95% CI,
0.58-0.96). There was no significant difference between poor
outcome compared to intermediate outcome. An important finding of
their analysis was the crucial role played by the presence or
absence of PComs, or their diameter. A poor outcome is more
frequently encountered if one or both PComs are absent, and if
their diameter is small (30).
Ravindren et al further emphasized the
crucial importance of the PComs in BAO, by analyzing the presence
or absence of collaterals, represented by the PCom and PICA-AICA
anastomosis. Patients with collaterals had an almost 3-times higher
likelihood of a good outcome at 3 months (OR, 2.73; 95% CI,
1.01-7.39), while absence of both PComs was associated with a 60%
decreased chance of good functional outcome at 90 days (OR, 0.39;
95% CI, 0.17-0.93). Furthermore, even unilateral absence of a PCom
led to a 2-fold increase in mortality (OR, 2.17; 95% CI, 1.14-4.13)
(25).
When time-to-treatment ≤6 or >6 h and
revascularization status were taken into account, all patients with
a favorable pc-CS had a good outcome (OR, 9.4; 95% CI, 1.4-64;
P=0.02). Conversely, an unfavorable pc-CS was associated with good
outcome only in patients treated within 6 h from symptom onset, and
not beyond (OR, 5.5; 95% CI, 1.4-2; P=0.01) (31).
8. Basilar Artery on Computed Tomography
Angiography (BATMAN) Prognostic Score
BATMAN is a 10-point score that incorporates
thrombus burden and extent of primary collaterals. It allocates 1
point for the patency of either the vertebral artery, proximal BA
segment, middle BA, distal BA, for each PCA and 2 points for each
PCom larger than 1 mm in diameter. If smaller, i.e. hypoplastic, 1
point is given to each. If a fetal PCom is present, it receives 3
points (29). The optimal cut-off
value to discriminate between good and bad functional outcome is a
score of 7. A BATMAN score <7 is an independent predictor of
poor outcome (OR, 6.9; 95% CI, 1.4-33; P=0.01) and an increased
risk of mortality (OR, 7.4; 95% CI, 1.2-44; P=0.03) after
adjustment for age and NIHSS. The score did not influence
recanalization success between the two groups. If recanalization
was not achieved, the rates of poor outcome were similar. However,
if recanalization was successful, only 24% of the patients had a
poor outcome if BATMAN was ≥7, compared to 76% if <7. Compared
to the prognostic scores reviewed earlier, BATMAN performed better
than pc-CS in terms of accuracy and interrater agreement, but not
better than pc-CTA (29).
In a recent paper, Alemseged et al (31) assessed the prognostic value of
BATMAN correlated with time-to-treatment (TTT) and recanalization
status (mTICI). In patients with a favorable score treated within
or even beyond 6 h, complete revascularization (mTICI 2b-3) was
associated with a good outcome (adjusted OR, 15.8; 95% CI, 1.4-175;
P=0.02). However, in cases with an unfavorable score,
revascularization was associated with good outcome only if obtained
within 6 h (OR, 15; 95% CI, 1.9-124; P=0.01); findings that support
the relevance of collaterals and thrombus load in late time-windows
for BAO.
9. Current challenges and future
directions
Major limitations of the aforementioned studies are
related to their retrospective design and relatively small number
of patients (see Tables SI and
SII). Furthermore, the
heterogenous treatment modalities (intravenous tPA, intraarterial
thrombolysis, mechanical thrombectomy) and different clinical and
imaging outcome parameters reported (mRS ≤2 or ≤3, mTICI), limit
the generalizability of the scores, especially in the new era of
mechanical and aspiration thrombectomy. There are also restrictions
imposed by spiral computed tomography physics; posterior fossa beam
hardening artefacts decrease sensitivity of diagnostic tests,
particularly for the visualization of parenchymal structures.
Therefore, prospective trials, using standardized
computed tomographic parameters, clinical outcome and
recanalization scales are mandatory in order to consolidate the
diagnostic and prognostic value of these imaging markers; emphasis
should be placed on collateral capacity, thrombus burden and
thrombus location. Moreover, occlusion etiology should be
dichotomized into atherosclerotic vs. cardioembolic, in light of
their different treatment strategies and prognosis (32). There is a growing body of evidence
that supports, in addition to the classic non-contrast CT and CT
angiography, the inclusion of perfusion imaging in the
armamentarium aimed at the patient with posterior fossa ischemia
(26,33-35).
It improves detection of infratentorial ischemia and provides
further prognostic information. Ultimately, machine learning
software could be used to integrate the different imaging
parameters and to guide treatment decision-making.
10. Conclusions
Acute BAO remains a devastating disease in spite of
recent technological improvements. Computed tomography markers can
aid and accelerate the diagnosis of this complex clinical entity,
leading to shorter time-to-treatment intervals. They can provide
valuable prognostic knowledge to better inform patients and their
relatives about the consequences of the disease. However, none of
the computed tomography markers should be used to exclude patients
from intravenous and intraarterial therapy.
Supplementary Material
Summary of the diagnostic properties
attributed to the reviewed markers.
Prognostic properties of the scores
reviewed for HDBA, ps-ASPECTS, PMI, pc-CTA, PC-CS and BATMAN.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All information included in this review is
documented by relevant references.
Authors' contributions
RCF conceived the concept of the review; LM, AS, and
ZB performed the literature search, analyzed the relevant
literature and wrote the manuscript. RCF, ZB, and AS contributed to
the interpretation of the data and the revision of the manuscript,
and provided critical review for the manuscript. All authors read
and approved the final manuscript for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Rares Cristian Filep: ORCID ID:
0000-0002-7422-1178.
References
|
1
|
Mattle HP, Arnold M, Lindsberg PJ,
Schonewille WJ and Schroth G: Basilar artery occlusion. Lancet
Neurol. 10:1002–1014. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kayan Y, Meyers PM, Prestigiacomo CJ, Kan
P and Fraser JF: Society of NeuroInterventional Surgery: Current
endovascular strategies for posterior circulation large vessel
occlusion stroke: Report of the Society of NeuroInterventional
Surgery Standards and Guidelines Committee. J Neurointerv Surg.
11:1055–1062. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Turc G, Bhogal P, Fischer U, Khatri P,
Lobotesis K, Mazighi M, Schellinger PD, Toni D, de Vries J, White P
and Fiehler J: European stroke organisation (ESO) - European
society for minimally invasive neurological therapy (ESMINT)
guidelines on mechanical thrombectomy in acute ischemic stroke. J
Neurointerv Surg. 11:535–538. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
van der Hoeven EJRJ, Schonewille WJ, Vos
JA, Algra A, Audebert HJ, Berge E, Ciccone A, Mazighi M, Michel P,
Muir KW, et al: The basilar artery international cooperation study
(BASICS): Study protocol for a randomised controlled trial. Trials.
14(200)2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sarraj A, Medrek S, Albright K,
Martin-Schild S, Bibars W, Vahidy F, Grotta JC and Savitz SI:
Posterior circulation stroke is associated with prolonged
door-to-needle time. Int J Stroke. 10:672–678. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Puig J, Shankar J, Liebeskind D, Terceño
M, Nael K, Demchuk AM, Menon B, Dowlatshahi D, Leiva-Salinas C,
Wintermark M, et al: From ʻtime is brain’ to ʻimaging is brain’: A
paradigm shift in the management of acute ischemic stroke. J
Neuroimaging. 30:562–571. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Grotta JC, Chiu D, Lu M, Patel S, Levine
SR, Tilley BC, Brott TG, Haley EC Jr, Lyden PD, Kothari R, et al:
Agreement and variability in the interpretation of early CT changes
in stroke patients qualifying for intravenous rtPA therapy. Stroke.
30:1528–1533. 1999.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hwang DY, Silva GS, Furie KL and Greer DM:
Comparative sensitivity of computed tomography vs. magnetic
resonance imaging for detecting acute posterior fossa infarct. J
Emerg Med. 42:559–565. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Schramm P, Schellinger PD, Klotz E,
Kallenberg K, Fiebach JB, Külkens S, Heiland S, Knauth M and Sartor
K: Comparison of perfusion computed tomography and computed
tomography angiography source images with perfusion-weighted
imaging and diffusion-weighted imaging in patients with acute
stroke of less than 6 h’ duration. Stroke. 35:1652–1658.
2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
New PF and Aronow S: Attenuation
measurements of whole blood and blood fractions in computed
tomography. Radiology. 121 (3 Pt. 1):635–640. 1976.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liebeskind DS, Sanossian N, Yong WH,
Starkman S, Tsang MP, Moya AL, Zheng DD, Abolian AM, Kim D, Ali LK,
et al: CT and MRI early vessel signs reflect clot composition in
acute stroke. Stroke. 42:1237–1243. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bajkó Z, Maier S, Moţăţăianu A, Balasa R,
Vasiu S, Stoian A and Andone S: Stroke secondary to traumatic
carotid artery injury - A case report. J Crit Care Med (Targu
Mures). 4:23–28. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bajkó Z, Bălaşa R, Moţăţăianu A, Bărcuţean
L, Stoian A, Stirbu N and Maier S: Malignant middle cerebral artery
infarction secondary to traumatic bilateral internal carotid artery
dissection. A case report. J Crit Care Med (Targu Mures).
2:135–141. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Filep RC, Bajko Z, Simu IP and Stoian A:
Pseudo-dissection of the internal carotid artery in acute ischemic
stroke. Acta Neurol Belg. 120:469–472. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim SK, Baek BH, Lee YY and Yoon W:
Clinical implications of CT hyperdense artery sign in patients with
acute middle cerebral artery occlusion in the era of modern
mechanical thrombectomy. J Neurol. 264:2450–2456. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Goldmakher GV, Camargo EC, Furie KL,
Singhal AB, Roccatagliata L, Halpern EF, Chou MJ, Biagini T, Smith
WS, Harris GJ, et al: Hyperdense basilar artery sign on unenhanced
CT predicts thrombus and outcome in acute posterior circulation
stroke. Stroke. 40:134–139. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Connell L, Koerte IK, Laubender RP,
Morhard D, Linn J, Becker HC, Reiser M, Brueckmann H and
Ertl-Wagner B: Hyperdense basilar artery sign-a reliable sign of
basilar artery occlusion. Neuroradiology. 54:321–327.
2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ernst M, Romero JM, Buhk JH, Cheng B,
Herrmann J, Fiehler J and Groth M: Sensitivity of hyperdense
basilar artery sign on non-enhanced computed tomography. PLoS One.
10(e0141096)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Riedel CH, Zoubie J, Ulmer S,
Gierthmuehlen J and Jansen O: Thin-slice reconstructions of
nonenhanced CT images allow for detection of thrombus in acute
stroke. Stroke. 43:2319–2323. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tan X and Guo Y: Hyperdense basilar artery
sign diagnoses acute posterior circulation stroke and predicts
short-term outcome. Neuroradiology. 52:1071–1078. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Puetz V, Sylaja PN, Coutts SB, Hill MD,
Dzialowski I, Mueller P, Becker U, Urban G, O'Reilly C, Barber PA,
et al: Extent of hypoattenuation on CT angiography source images
predicts functional outcome in patients with basilar artery
occlusion. Stroke. 39:2485–2490. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bhatia R, Bal SS, Shobha N, Menon BK,
Tymchuk S, Puetz V, Dzialowski I, Coutts SB, Goyal M, Barber PA, et
al: CT angiographic source images predict outcome and final infarct
volume better than noncontrast CT in proximal vascular occlusions.
Stroke. 42:1575–1580. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Puetz V, Khomenko A, Hill MD, Dzialowski
I, Michel P, Weimar C, Wijman CA, Mattle HP, Engelter ST, Muir KW,
et al: Extent of hypoattenuation on CT angiography source images in
basilar artery occlusion: Prognostic value in the Basilar Artery
International Cooperation Study. Stroke. 42:3454–3459.
2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pallesen LP, Khomenko A, Dzialowski I,
Barlinn J, Barlinn K, Zerna C, van der Hoeven EJ, Algra A, Kapelle
LJ, Michel P, et al: CT-angiography source images indicate less
fatal outcome despite coma of patients in the Basilar Artery
International Cooperation Study. Int J Stroke. 12:145–151.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ravindren J, Aguilar Pérez M, Hellstern V,
Bhogal P, Bäzner H and Henkes H: Predictors of outcome after
endovascular thrombectomy in acute basilar artery occlusion and the
6 hr time window to recanalization. Front Neurol.
10(923)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pallesen LP, Gerber J, Dzialowski I, van
der Hoeven EJ, Michel P, Pfefferkorn T, Ozdoba C, Kappelle LJ,
Wiedemann B, Khomenko A, et al: Diagnostic and prognostic impact of
pc-ASPECTS applied to perfusion CT in the Basilar Artery
International Cooperation Study. J Neuroimaging. 25:384–389.
2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Schaefer PW, Yoo AJ, Bell D, Barak ER,
Romero JM, Nogueira RG, Lev MH, Schwamm LH, Gonzalez RG and Hirsch
JA: CT Angiography-Source image hypoattenuation predicts clinical
outcome in posterior circulation strokes treated with
Intra-Arterial therapy. Stroke. 39:3107–3109. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Da Ros V, Meschini A, Gandini R, Del
Giudice C, Garaci F, Stanzione P, Rizzato B, Diomedi M, Simonetti
G, Floris R and Sallustio F: Proposal for a vascular computed
Tomography-Based grading system in posterior circulation stroke: A
Single-Center experience. J Stroke Cerebrovasc Dis. 25:368–377.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Alemseged F, Shah DG, Diomedi M, Sallustio
F, Bivard A, Sharma G, Mitchell PJ, Dowling RJ, Bush S, Yan B, et
al: The basilar artery on computed tomography angiography
prognostic score for basilar artery occlusion. Stroke. 48:631–637.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
van der Hoeven EJ, McVerry F, Vos JA,
Algra A, Puetz V, Kappelle LJ and Schonewille WJ: BASICS registry
investigators. Collateral flow predicts outcome after basilar
artery occlusion: The posterior circulation collateral score. Int J
Stroke. 11:768–775. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Alemseged F, Van der Hoeven E, Di Giuliano
F, Shah D, Sallustio F, Arba F, Kleinig TJ, Bush S, Dowling RJ, Yan
B, et al: Response to Late-Window endovascular revascularization is
associated with collateral status in basilar artery occlusion.
Stroke. 50:1415–1422. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Piechowiak EI, Kaesmacher J, Zibold F,
Dobrocky T, Mosimann PJ, Jung S, Fischer U, Arnold M, Bellwald S,
Heldner MR, et al: Endovascular treatment of tandem occlusions in
vertebrobasilar stroke: Technical aspects and outcome compared with
isolated basilar artery occlusion. J Neurointerv Surg. 12:25–29.
2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bollwein C, Plate A, Sommer WH,
Thierfelder KM, Janssen H, Reiser MF, Straube A and von Baumgarten
L: Diagnostic accuracy of whole-brain CT perfusion in the detection
of acute infratentorial infarctions. Neuroradiology. 58:1077–1085.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fabritius MP, Reidler P, Froelich MF,
Rotkopf LT, Liebig T, Kellert L, Feil K, Tiedt S, Kazmierczak PM,
Thierfelder KM, et al: Incremental value of computed tomography
perfusion for final infarct prediction in acute ischemic cerebellar
stroke. J Am Heart Assoc. 8(e013069)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sporns P, Schmidt R, Minnerup J, Dziewas
R, Kemmling A, Dittrich R, Zoubi T, Heermann P, Cnyrim C, Schwindt
W, et al: Computed tomography perfusion improves diagnostic
accuracy in acute posterior circulation stroke. Cerebrovasc Dis.
41:242–247. 2016.PubMed/NCBI View Article : Google Scholar
|