Introduction
Lead intoxication is an environmental health
problem, with extremely severe consequences upon the human body
(1). Acute lead poisoning manifests
clinically with intense acute abdominal pain, being a challenging
diagnosis in emergency situations (2). Development a quick and reliable method
for lead determination in blood and urine, is important in clinical
practice, both in diagnostics, but also in monitoring chelating
therapy.
The main sources of lead that result in lead
contamination include: Paint; leaded petrol; drinking water; car
batteries, cables, glass; printers with lead-based technology;
manufacture and use of war ammunition (3,4). In
melters, the main danger is melting. The risk of exposure to lead
increases with increasing temperature in industrial processes
(5).
The main routes of absorption of lead are gastric
and pulmonary. Gastric absorption in adults is about 10-15% of the
total amount ingested and 40% in children. At the lung level it is
absorbed at ~50-70% of the inhaled dose. In organic form, lead from
tetraethyl lead is also absorbed at the skin level (6). Inhalation absorption depends on the
form of lead (vapors or particles). Approximately 90% of inhaled
particulate lead is absorbed into the respiratory tract (7). Once absorbed, 99% is transported by
the bloodstream and binds to hemoglobin in red blood cells
(2,5).
The distribution is completed in three compartments,
namely in the blood compartment, mineralized tissues (bones, teeth)
and soft tissues (kidneys, bone marrow, liver and brain). Lead in
the blood is distributed at a percentage of 99% in erythrocytes and
1% in plasma and is available for transport to tissues. The blood
concentration does not reflect the actual amount of lead in the
body, but ~90% is stored in tissues, with a maximum half-life of
≤30 years (8). Because lead is
mobilized from these tissues, individuals who have been exposed may
have high concentrations of lead, from a few months to several
years from the time of exposure cessation (7). The level of lead in the blood, a
traditional indication of absorption, reflects only recent exposure
as the half-life in the blood is 36 days (3). In individuals with chronic exposure,
there is a small correlation between a level once determined at a
control and the cumulative absorption index or loading of the lead
organism.
Suspicion of lead poisoning is confirmed by its
concentration in blood and protoporphyrin red blood cells. At low
concentrations, lead influences the synthesis of the heme in the
sense of lowering it. Due to the fact that the level of erythrocyte
protoporphyrin is not pathognomonic in children at levels of
approximately 25 µg/dl, the best method remains to determine the
level of blood lead (9,10). Lead binds to the sulfur groups of
many enzymes by inactivating them. Lead poisoning has a
multisystemic, hematological, cardiovascular, renal, hepatic,
digestive and neurotoxic impact (5). Acute intoxication at blood levels
>50 µg/dl causes saturnine colic, and biochemical dosing of lead
when there is clinical suspicion is extremely important in
emergency situation, in the differential diagnosis of acute
abdomen, to avoid white laparotomies in patients with severe
abdominal colic pain (11).
This work aims to present a method of determination
for lead in blood and urine using graphite furnace atomic
absorption spectrometry (GF-AAS) with a background correction.
Atomic absorption spectrometry with graphite furnace atomization
(GF-AAS) is a leading technique in analytical chemistry as a
routine low-level assay for lead and other heavy metals, for a wide
variety of sample types (12).
Materials and methods
Instrumentation
Lead analysis was performed using a graphite furnace
atomic absorption spectrophotometer (GF-AAS) Varian Spectra AA-880,
with a hollow cathode lamp (Agilent Technologies, Inc.) and a
deuterium lamp (Agilent Technologies, Inc.) for background
correction, coupled to a GTA-100 atomizer and a programmable sample
dispenser (Varian). Addition instrumentation included: A
monochromator (fully automatic computer-controlled Czerny-Tuner
micromotor, focal length 0.33 mm; automatic sample dispenser PSD
Varian with 54 positions for samples, standards, modifiers, quality
control and buffer, maximum injected quantity 100 µl, injection
precision 0.2 µl, automatic dilution and mixing, automatic
re-injection of samples); Neslab CFT 33 water cooler for graphite
oven working at temperature 15-25˚C; a nitrogen
generator (Dominik Hunter) (purity, 99.999%); EBA 200 Hettich
Centrifuge, Eppendorf automatic pipette 1,000 µl. Biochemical
parameters were determined from blood on the Vitros 650 System
(Ortho Clinical Diagnostics) and complete blood count on a
Celltac-F Hematology Analyzer (Nihon Kohden).
Samples and reagents
Biological samples (20 urine samples and 20 blood
samples) were collected during hospitalization (23 days) from a
patient admitted to the Intensive Care II Unit, Toxicology
Department within the Bucharest Emergency Clinical Hospital.
Written informed consent was obtained after the study protocol was
previously discussed and explained to the patient.
All chemicals were of analytical or
certified-reagent grades. Lead standard solution
Certipur® (Merck) (1,000 mg/l Pb) was used. A lead stock
solution (100 µg/l) was prepared daily in 0.01% nitric acid.
Concentrated nitric acid (Lach-Ner), with a lead content
(<0.00005%) below the GF-AAS detection limit, was used.
Solutions were prepared with grade doubly distilled, de-ionized
water in polypropylene calibrated flasks. The required volumes were
measured with air displacement pipettes (Eppendorf Research plus
Models) with premium grade polypropylene tips. All glassware was
cleaned with acid and rinsed thoroughly with distilled or unionized
water before use.
Blood samples
Sodium heparin (Lilly) with a lead content below the
GF-AAS detection limit was used. For analysis, 200 µl of blood was
mixed together with 800 µl 5% anti-foam B (Sigma-Aldrich; Merck
KGaA) solution and 1,000 µl 1.6 M solution of 65% HNO3.
They were allowed to stabilize for 10 min and then centrifuged for
5 min at 2,884 x g relative centrifugal force (RCF). The
supernatant was collected and analyzed on the graphite furnace
atomic absorption (GF-AAS) system.
Urine samples
A volume of 9 ml of urine was treated with 1 ml of
65% HNO3. The sample was allowed to stabilize for 20 min
and then centrifuged for 10 min at 2,884 x g relative centrifugal
force (RCF). The supernatant was then analyzed on the GF-AAS
system. There were several trials using different working
parameters, to reach optimum conditions (Table I).
 | Table IWorking parameters for determining
lead by GF-AAS. |
Table I
Working parameters for determining
lead by GF-AAS.
| No. | Parameter | Method |
|---|
| 1. | Injection mode | Automated
dilution |
| 2. | Calibration
mode | Concentration |
| 3. | Type of
measurement | Peak height |
| 4. | Replicate
standard | 3 |
| 5. | Replicate
sample | 3 |
| 6. | Smoothing | 9 |
| 7. | Wavelength | 283.3 nm |
| 8. | Slit | 0.5 |
| 9. | Lamp current | 10 mA |
| 10. | Background
correction | Yes |
| 11. | Standard 1 | 10 µg/l |
| 12. | Standard 2 | 20 µg/l |
| 13. | Standard 3 | 50 µg/l |
| 14. | Standard 4 | 100 µg/l |
| 15. | Recalibration
rate | 30 |
| 16. | Calibration
algorithm | New rational |
| 17. | Total volume | 15 µl |
| 18. | Sample volume | 10 µl |
| 19. | Dilution
coefficient | 2 |
Results
Optimization of the working
parameters
The atomization temperature was established by
varying the atomization temperature between 1,600 and 2,100˚C. As
expected, the lead signal increased with the increase in the
atomization temperature up to 2,000˚C. For temperatures
>2,100˚C, the signals remained almost constant, indicating that
maximum atomization efficiency can be achieved in this range
(Table II).
 | Table IIFurnace GTA-100 Varian operating
conditions for lead measurements. |
Table II
Furnace GTA-100 Varian operating
conditions for lead measurements.
| Step | Temperature
(˚C) | Time (sec) | Flow
(liters/min) | Gas type | Read | Signal storage |
|---|
| | 40 | 1.0 | 3.0 | Normal | No | No |
| | 85 | 5.0 | 3.0 | Normal | No | No |
| | 95 | 40.0 | 3.0 | Normal | No | No |
| | 120 | 10.0 | 3.0 | Normal | No | No |
| | 120 | 5.0 | 3.0 | Normal | No | No |
| | 400 | 5.0 | 3.0 | Alternate | No | No |
| | 400 | 1.0. | 3.0 | Alternate | No | No |
| | 400 | 2.0 | 0.0 | Alternate | No | Yes |
| | 2,100 | 1.0 | 0.0 | Alternate | Yes | Yes |
| | 2,100 | 2.0 | 0.0 | Alternate | Yes | Yes |
| | 2,100 | 3.0 | 3.0 | Normal | No | Yes |
| | 40 | 2.0 | 3.0 | Normal | No | No |
| | 40 | 5.0 | 0.0 | Normal | No | No |
The application of the optimized temperature program
made possible elimination of the whole matrix of the sample before
the atomization step, as confirmed by the low background signals
observed in the measurement of lead (13-15).
To determine the performance parameters for the method (linearity,
accuracy, precision and robustness) standard calibration solutions
were used in the concentration range 10-100 µg/l (14). The detection and quantification
limits were established according to ICH (International Conference
on Harmonization) recommendations (16,17).
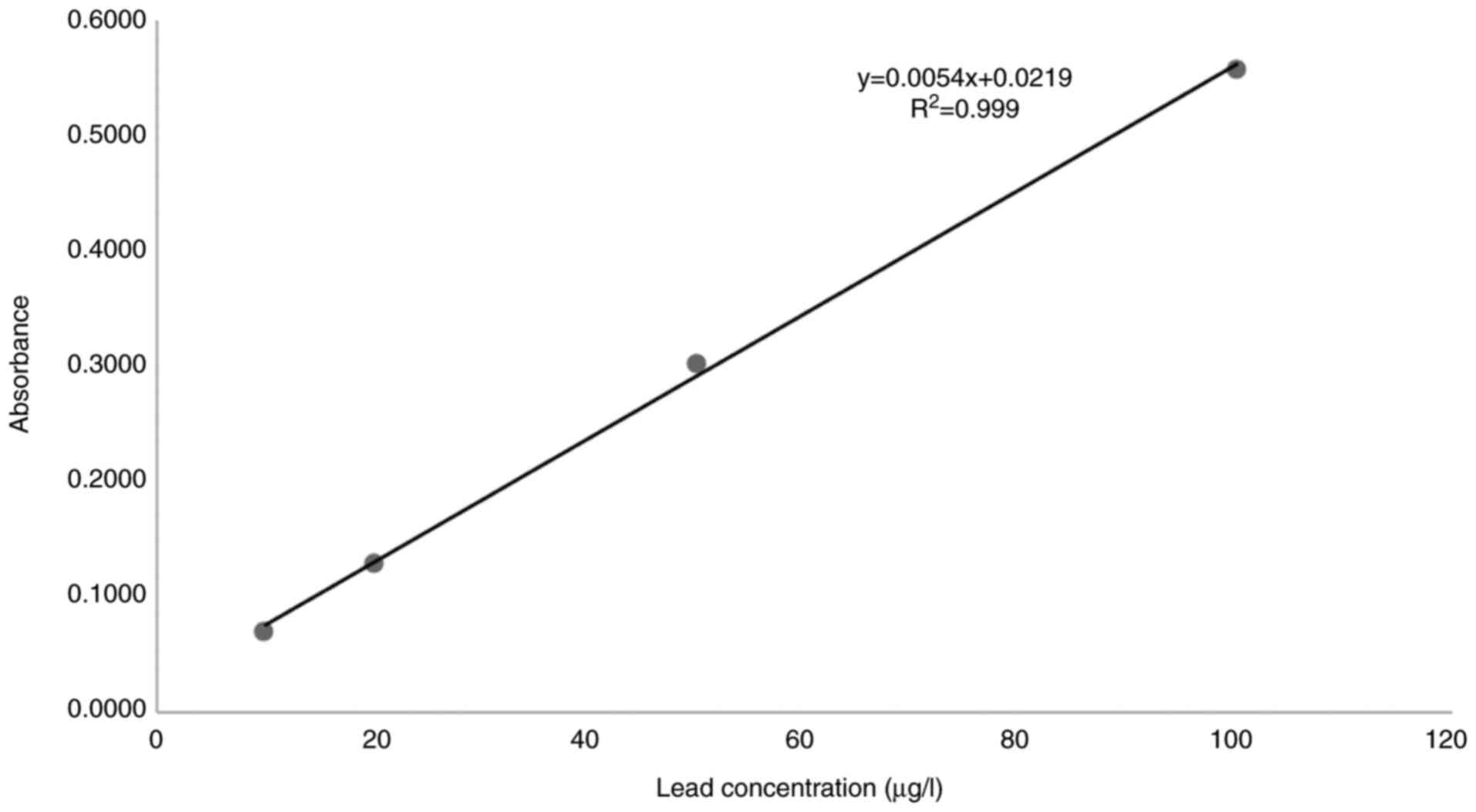
A linear relationship was found between the
absorbance at 283.3 nm and the concentration of lead in the range
of 10.0 to 100 µg/l. The representative linear equation was y =
0.0054x + 0.0219 where: y is the absorbance, x is the lead
concentration (µg/l), calculated by the least squares method. The
regression coefficient (R2) standard curve was 0.9990
(Fig. 1) indicating good
linearity.
The performance parameters of the linear regression
equation are presented in Table
III. The parameters of the GF-AAS analysis method of lead are
presented in Table IV, and the
calibration curve can be observed in Fig. 1. The limit of detection (LOD) and
the limit of quantification (LOQ) were detected for the method
based on the standard deviation of 6 readings of the standard
solution blank and on the slope of the analytical curve (Table III).
 | Table IIIPerformance parameters of the linear
regression equation. |
Table III
Performance parameters of the linear
regression equation.
| Denomination | Conc. (µg/l) | Abs | Media Abs | Abs without
blank | R2
coefficient of determination | Slope | Standard deviation
of slope (STD) | Ordinate at origin
b | Standard deviation
(STD) of the ordinate at origin |
|---|
| Blank | 0 | 0.0086 | 0.0085 | | 0.9986 | 0.0054 | 0.0001 | 0.0219 | 0.0068 |
| | | 0.0091 | | | | | | | |
| | | 0.0079 | | | | | | | |
| Standard 1 | 10 | 0.0726 | 0.0788 | 0.0703 | | | | | |
| | | 0.0839 | | | | | | | |
| | | 0.0799 | | | | | | | |
| Standard 2 | 20 | 0.1393 | 0.1387 | 0.1302 | | | | | |
| | | 0.1363 | | | | | | | |
| | | 0.1406 | | | | | | | |
| Standard 3 | 50 | 0.2943 | 0.3095 | 0.3009 | | | | | |
| | | 0.3151 | | | | | | | |
| | | 0.3190 | | | | | | | |
| Standard 4 | 100 | 0.5744 | 0.5654 | 0.5569 | | | | | |
| | | 0.5630 | | | | | | | |
| | | 0.5589 | | | | | | | |
 | Table IVValidation parameters. |
Table IV
Validation parameters.
| Cation |
Pb2+ |
|---|
| Linearity
range | 10-100 µg/l |
| Regression
equation | y=0.0054x +
0.0219 |
| Correlation
coefficient (R2) | 0.9990 |
| Intercept | 0.0219 |
| Slope | 0.0054 µg/l |
| SE of
intercept | 0.0068 |
| SD of
intercept | 0.02633572 |
| LOD | 4.15 µg/l |
| LOQ | 12.59 µg/l |
LOD is the lowest concentration of an analyte that
can be detected while LOQ is defined as the lowest concentration of
an analyte that can be determined at the acceptable level of
precision and accuracy and were calculated according to the formula
below: LOD = 3.3x (SD of intercept/slope) and LOQ = 10x (SD of
intercept/slope) (Table IV).
The accuracy of the assays (BIAS), expressed as the
consistency between the real value and the analytical result, was
calculated using three sources of blank matrix samples fortified at
each level of concentration analyzed in duplicate. The calculated
coefficient of variance (CV%) which describes the precision of the
analytical method is shown in Table
V. The CV% did not exceed 15% at each concentration and the
BIAS was <15% at each level of concentration, which ensured a
superior trust grade for each determination using this method. In
other words, the difference between the real value and the
determined value of concentration was minimal.
 | Table VThe performance parameters of the
analytical method for lead determination. |
Table V
The performance parameters of the
analytical method for lead determination.
| Level | Standard solution
concentration µg/ml | Measured
concentration µg/ml | Average measured
concentration µg/ml | Standard
deviation | BIAS | CV% |
|---|
| 1 | 10 | 9.41 | 10.56 | 1.06 | -5.90 | 10.04 |
| | 10 | 11.50 | | | 15.00 | |
| | 10 | 10.76 | | | 7.60 | |
| 2 | 20 | 21.78 | 21.67 | 0.41 | 8.90 | 1.89 |
| | 20 | 21.22 | | | 6.10 | |
| | 20 | 22.02 | | | 10.10 | |
| 3 | 50 | 50.53 | 53.34 | 2.46 | 1.06 | 4.61 |
| | 50 | 54.38 | | | 8.76 | |
| | 50 | 55.11 | | | 10.22 | |
| 4 | 100 | 102.47 | 100.81 | 1.49 | 2.47 | 1.48 |
| | 100 | 100.36 | | | 0.36 | |
| | 100 | 99.60 | | | -0.40 | |
Clinical application
Lead level monitoring in humans is of great
importance due to its high toxicity. In order to detect lead in
whole blood, the authors developed a method using GF-AAS, for
quantifying the lead level in blood which can be used for
monitoring lead levels during chelation treatment time. The
permissible concentration of lead in the blood is up to 20 µg/dl,
and in urine ≤40 µg/l.
Acute lead poisoning manifests with acute abdominal
pain, nausea and vomiting, being a neglected cause in the
differential diagnosis of acute surgical abdomen. A careful
anamnesis, revealing previous toxicological history, occupational
risk or hobbies that might be associated with lead exposure, may
reveal important information, but it may not be always accurate,
due to patient multiple comorbidities, ignorance of the possible
toxic risk or neuropsychiatric disorders. A frequent associated
condition is anemia, due to the increased fragilization of the
erythrocyte membranes and hemolysis. In suspect cases or patients
with a non-conclusive clinical and imagistic exam, a prompt and
reliable determination of lead concentration in blood is extremely
important to avoid ‘white’ laparotomies and associated
perioperative morbidity.
The therapeutic approach is based on sustaining
vital functions and increased lead elimination through urine.
Chelating therapy is usually initiated when lead concentration in
blood is more than 50 µg/dl, due to the possible dangerous side
effects.
The most commonly used chelating agents in lead
intoxication include: Dimercaptosuccinic acid, dimercaprol and
CaNa2 EDTA. Among the current drawbacks of the chelation
therapy, the clinician must take into account the hepatotoxicity
and nephrotoxicity, essential metal loss, headaches, nausea,
arrythmias, hypotension or hypertension, bone marrow depression,
convulsions or even cardio-respiratory arrest (17,18).
Another aspect is the redistribution of the lead among the body
compartments, which may generate important fluctuation of blood and
urine concentration during the first days of therapy. Prolonged
treatment with CaNa2 EDTA results in depletion of
essential metals, especially Zn, Cu and Mn, requiring oral Zn
supplementation (17).
Chelating therapy requires close monitorization and
advanced medical skill (5). In our
department, patients admitted for acute lead poisoning are closely
followed up by daily laboratory tests including: Hemoleucogram,
transaminase, urea, creatinine, electrolytes (Na+,
K+, Ca++), as well as lead concentration in
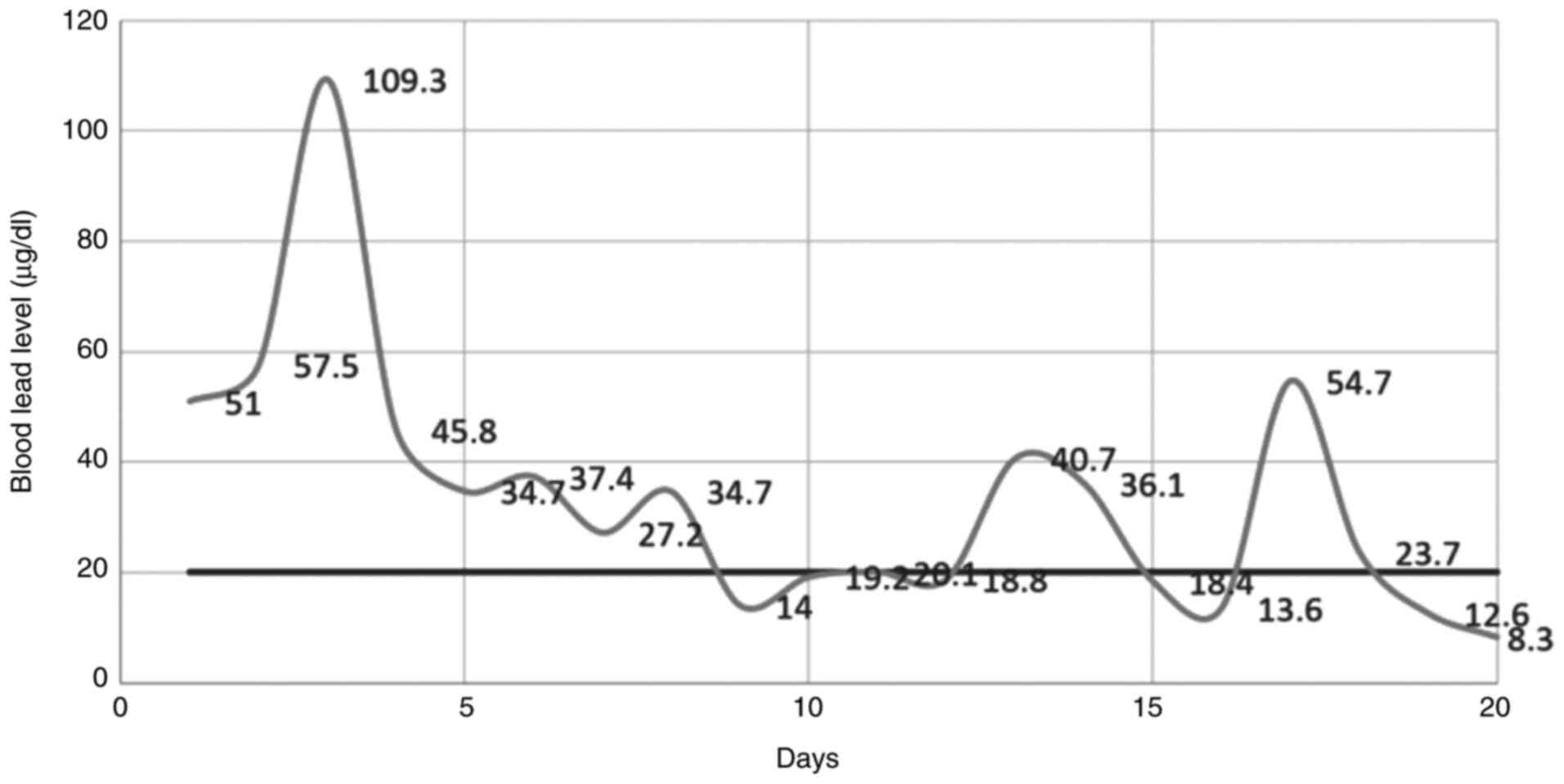
the blood and urine. The optimized method based on GF-AAS technique
proves to be useful for monitoring chelation therapy in the cases
of acute lead poisoning. It allows a close follow-up of the dynamic
concentrations of lead in the blood and urine, as presented in
Fig. 2.
Discussion
Saturnine colic is an infrequent cause of
differential diagnosis of acute abdomen, which may appear in cases
of acute lead poisoning, with blood lead levels exceeding 50-80
µg/dl (2,19-22).
If undiagnosed, it can lead to avoidable surgeries, by mimicking
acute appendicitis, perforated ulcer, acute pancreatitis or bowel
obstruction (21-23).
The abdominal pain is intense, colliquative, in the periumbilical
area, resistant to usual antispastics (2,20-22).
The pain diminishes at profound palpation of the abdomen, with no
tenderness or contracture, being a key element of differential
diagnosis with surgical acute abdomen. Other signs and symptoms are
anorexia, nausea, vomiting, constipation or very rarely, diarrhea
(23,24). Radiological abdominal exam shows
hydroaeric images, with alternative sectors of spastic and moderate
dilated intestinal loops. The patient may experience transient
increased blood pressure, which comes back to normal after
ceasement of abdominal pain with chelating therapy. Oliguria,
increased serum urea and leukocytosis may be associated with acute
poisoning (19).
It is well known that exposure to lead causes
dose-dependent decreases in heme synthesis by inhibiting the enzyme
δ-aminolaevulinic acid dehydrase (δ-ALAD). Hematologic tests such
as hemoglobin concentration may suggest toxicity, but this is not
specific for lead (25). The higher
the level of blood lead levels, the lower the hemoglobin in the
blood and the level of erythrocytes due to the increase in
lead-induced membrane fragility resulting in the development of
anemia (26). The toxicity of lead
on the human hematological system has been established in numerous
studies. Other effects of lead on the hematological system are
decreased activity of erythrocyte enzymes (pyrimidine
5'-nucleotidase) and altered levels of plasma erythropoietin
(27).
The effects of acute and chronic lead intoxication
upon the nervous system have been studied for 100 years. Lead is a
highly neurotoxic element, both for central nervous system and
peripheric nerves. Even concentrations below 10 µg/dl, in children,
are inversely correlated with the intelligence quotient (IQ). There
are well-defined clinical features encountered in both adults and
children: Decreased learning ability, memory loss, cognitive
deterioration, reduced neural signaling and demyelination. At blood
levels >70 µg/dl in children and >100 µg/dl in adults lead
toxicity is increased and may cause paresis or paralysis and
saturnine encephalopathy, with sudden seizures, changes in
consciousness, coma and death (28,29).
Furthermore, several studies have confirmed the pathogenic role of
lead intoxication in Alzheimer disease and glaucoma, by increasing
the tissular oxidative stress, through depletion of glutathione and
thiol pools, as well as by disrupting the antioxidant defense
system (30-35).
Several studies revealed that probiotics may be useful for
alleviation and treatment of lead toxicity, reducing the specific
side-effects in heavily polluted areas (36,37).
Accidental or occupational lead intoxication is an important public
health problem, causing a significant burden especially in low- and
middle-income countries (38). The
Institute for Health Metrics and Evaluation (IHME) estimated that
in 2017, lead exposure accounted for 1.06 million deaths and 24.4
million years of healthy life lost worldwide due to long-term
effects on health (39). The most
severe include the neurologic and cardiovascular effects of acute
or chronic lead poisoning: 63.2% of the global burden of idiopathic
developmental intellectual disability, 10.3% of the global burden
of hypertensive heart disease, 5.6% of the global burden of the
ischemic heart disease and 6.2% of the global burden of stroke
(40).
Graphite furnace atomic absorption spectrometry
(GF-AAS) is increasingly becoming the method of choice for the
determination of lead and other heavy metals in blood and urine, as
well as in other biological products (40-43),
with several improvements developed in time to increase its power
of detection and determination for lower concentrations.
Whole blood lead levels are the most widely used and
most generally accepted measure of absorbed dose. A repeatable,
reliable, cost-efficient method is an important tool for lead
intoxication screening and chelation therapy monitoring in clinical
practice.
GF-AAS is simpler, less expensive, quicker and more
accurate than neutron activation or emission spectrometric
technique. The absorbance signals obtained for lead in the
optimizing conditions presented ensures a well-defined profile and
a low background (42-44).
The reported method shows high precision and
accuracy, as well as a wide applicability in routine lead
determination and research assays. The methods developed are
valuable for clinical diagnostics and biological monitoring of
work-related exposure.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MST, GC, OA, ADS and DS were responsible for the
conception and design of this study. DMP, CGS, CT, AMD, CDB, DOC
and BS were responsible for the data collection and analysis. MST,
GC, OA, CT and DS were in charge of drafting the manuscript. AMD,
MC, ADS, DOC and RS revised critical perspectives for important
intellectual content. The final version for publication was read
and approved by all the authors.
Ethics approval and consent to
participate
Written informed consent was obtained after the
study protocol was previously discussed and explained to the
patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jaishankar M, Tseten T, Anbalagan N,
Mathew BB and Beeregowda KN: Toxicity, mechanism and health effects
of some heavy metals. Interdiscip Toxicol. 7:60–72. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gopinath B, Kappagantu V, Mathew R and
Jamshed N: Acute lead poisoning: A diagnostic challenge in the
emergency department. BMJ Case Rep. 14(e239740)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wani AL, Ara A and Usmani JA: Lead
toxicity: A review. Interdiscip Toxicol. 8:55–64. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Maizlish N and Rudolph L: California
adults with elevated blood lead levels, 1987 through 1990. Am J
Public Health. 83:402–405. 1993.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Flora SJ and Pachauri V: Chelation in
metal intoxication. Int J Environ Res Public Health. 7:2745–2788.
2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rădulescu A and Lundgren S: A
pharmacokinetic model of lead absorption and calcium competitive
dynamics. Sci Rep. 9(14225)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Internaţional Labor Organization (ILO):
Encyclopaedia of Occupational Health and Safety. 4th edition.
Stellman JM (ed). Geneva, 1998. https://www.ilo.org/global/publications/ilo-bookstore/order-online/multimedia/WCMS_PUBL_9221098184_EN/lang--en/index.htm.
|
|
8
|
Rossi E: Low level environmental lead
exposure-a continuing challenge. Clin Biochem Rev. 29:63–70.
2008.PubMed/NCBI
|
|
9
|
Riva MA, Lafranconi A, D'orso MI and
Cesana G: Lead poisoning: Historical aspects of a paradigmatic
‘occupational and environmental disease’. Saf Health Work. 3:11–16.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Halmo L and Nappe TM: Lead Toxicity.
[Updated 2020 Jul 10]. In: StatPearls [Internet]. Treasure Island
(FL), StatPearls Publishing, Jan, 2021. https://www.ncbi.nlm.nih.gov/books/NBK541097/.
|
|
11
|
Rolston DD: Uncommon sources and some
unsual manifestations of lead poisoning in a tropical developing
country. Trop Med Health. 39:127–132. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tudosie MS, Danciulescu Miulescu R,
Negulescu V, Ionica M, Stefan SD, Corlan G and Macovei R:
Evaluation and modeling of pharmacokinetics of copper ion during
hemodialysis. Farmacia. 61:53–65. 2013.
|
|
13
|
Tudosie MS, Păun SC, Macovei R, Ionica M,
Ardelean L and Macovei A: The role of the atomic absorption
spectrometry in the study of blood lead levels and outlet dialysate
lead levels for chronic kidney disease patients. Therapeutics
Pharmacol Clin Toxicol. 15:211–215. 2011.
|
|
14
|
Kelly RS, Lundh T, Porta M, Bergdahl IA,
Palli D, Johansson AS, Botsivali M, Vineis P, Vermeulen R,
Kyrtopoulos SA, et al: Blood erythrocyte concentrations of cadmium
and lead and the risk of B-cell non-Hodgkin's lymphoma and multiple
myeloma: A nested case-control study. PLoS One.
8(e81892)2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
International Conference on Harmonization
(ICH) of Technical Requirements for the Registration of
Pharmaceuticals for Human Use-3AQ13a Validation of analytical
procedures: Methodology, Geneva, Switzerland, 1997.
|
|
16
|
International Conference on Harmonization
(ICH) of Technical Requirements for the Registration of
Pharmaceuticals for Human Use-Q2(R1) Validation of analytical
procedures: Text and methodology. Geneva, Switzerland, 2005.
|
|
17
|
Gracia RC and Snodgrass WR: Lead toxicity
and chelation therapy. Am J Health Syst Pharm. 64:45–53.
2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ferrero ME: Rationale for the successful
management of EDTA chelation therapy in human burden by toxic
metals. Biomed Res Int. 2016(8274504)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kosnett MJ: ‘Lead’. In Brent J (ed):
Critical Care Toxicology: Diagnosis and Management of the
Critically Poisoned Patient. Gulf Professional Publishing,
2005.
|
|
20
|
Shiri R, Ansari M, Ranta M and
Falah-Hassani K: Lead poisoning and recurrent abdominal pain. Ind
Health. 45:494–496. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Vossoughinia H, Pourakbar A, Esfandiari S
and Sharifianrazavi M: Severe abdominal pain caused by lead
toxicity without response to oral chelators: A case report. Middle
East J Dig Dis. 8:67–72. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Spataru RI, Sirbu A and Sirbu D: Forensic
ramifications in diagnosing and treating high forms of the
Hirschsprung's disease. Rom J Leg Med. 21:105–110. 2013.
|
|
23
|
Tsai MT, Huang SY and Cheng SY: Lead
poisoning can be easily misdiagnosed as acute porphyria and
nonspecific abdominal pain. Case Rep Emerg Med.
2017(9050713)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nicolli A, Mina GG, De Nuzzo D, Bortoletti
I, Gambalunga A, Martinelli A, Pasqualato F, Cacciavillani M,
Carrieri M and Trevisan A: Unusual domestic source of lead
poisoning. Int J Environ Res Public Health. 17(4374)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mărginean CO, Meliţ LE, Moldovan H, Lupu
VV and Mărginean MO: Lead poisoning in a 16-year-old girl: A case
report and a review of the literature (CARE compliant). Medicine
(Baltimore). 95(e4916)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hegazy AA, Zaher MM, Abd El-Hafez MA,
Morsy AA and Saleh RA: Relation between anemia and blood levels of
lead, copper, zinc and iron among children. BMC Res Notes.
3(133)2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
NAS: 2013. Potential Health Risks to DOD
Firing-Range Personnel from Recurrent Lead Exposure. Washington,
DC: National Academy of Sciences, National Research Council.
http://nap.edu/18249. Accessed February 19,
2020.
|
|
28
|
Kim Y, Yoo CI, Lee CR, Lee JH, Lee H, Kim
SR, Chang SH, Lee WJ, Hwang CH and Lee YH: Evaluation of activity
of erythrocyte pyrimidine 5'-Nucleotidase (P5N) in lead exposed
workers: With focus on the effect on hemoglobin. Ind Health.
40:23–27. 2002.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ellenhorn MJ: Ellenhorn's Medical
Toxicology. Diagnosis and Treatment of Human Poisoning. 2nd
edition. Williams & Wilkins a Waverly Comp, Baltimore, MD,
1997.
|
|
30
|
Stanciu AE, Stanciu MM and Vatasescu RG:
NT-proBNP and CA 125 levels are associated with increased
pro-inflammatory cytokines in coronary sinus serum of patients with
chronic heart failure. Cytokine. 111:13–19. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fathabadi B, Dehghanifiroozabadi M, Aaseth
J, Sharifzadeh G, Nakhaee S, Rajabpour-Sanati A, Amirabadizadeh A
and Mehrpour O: Comparison of blood lead levels in patients with
alzheimer's disease and healthy people. Am J Alzheimers Dis Other
Demen. 33:541–547. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang W, Moroi S, Bakulski K, Mukherjee B,
Weisskopf MG, Schaumberg D, Sparrow D, Vokonas PS, Hu H and Park
SK: Bone lead levels and risk of incident primary open-angle
glaucoma: The VA normative aging study. Environ Health Perspect.
126(087002)2018.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Arnold C: Looking backward: Long-term lead
exposure and risk of glaucoma. Environ Health Perspect.
127(54001)2019.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Yuki K, Dogru M, Imamura Y, Kimura I,
Ohtake Y and Tsubota K: Lead accumulation as possible risk factor
for primary open-angle glaucoma. Biol Trace Elem Res. 132:1–8.
2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jomova K and Valko M: Advances in
metal-induced oxidative stress and human disease. Toxicology.
283:65–87. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bhattacharya S: Probiotics against
alleviation of lead toxicity: Recent advances. Interdiscip Toxicol.
12:89–92. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Radu N, Roman V and Tanasescu C:
Biomaterials obtained from probiotic consortia of microorganisms.
Potential applications in regenerative medicine. Mol Crystals
Liquid Crystals. 628:115–123. 2017.
|
|
38
|
Kordas K, Ravenscroft J, Cao Y and McLean
EV: Lead exposure in low and middle-income countries: Perspectives
and lessons on patterns, injustices, economics, and politics. Int J
Environ Res Public Health. 15(2351)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Valko M, Jomova K, Rhodes CJ, Kuča K and
Musílek K: Redox- and non-redox-metal-induced formation of free
radicals and their role in human disease. Arch Toxicol. 90:1–37.
2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
World Health Organization (WHO): Lead
poisoning and health. August 23, 2019. https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health.
Accessed February 15, 2021.
|
|
41
|
Horng CJ, Tsai JL, Horng PH, Lin SC, Lin
SR and Tzeng CC: Determination of urinary lead, cadmium and nickel
in steel production workers. Talanta. 56:1109–1115. 2002.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ivanenko NB, Solovyev ND, Ivanenko AA and
Ganeev AA: Application of Zeeman graphite furnace atomic absorption
spectrometry with high-frequency modulation polarization for the
direct determination of aluminum, beryllium, cadmium, chromium,
mercury, manganese, nickel, lead, and thallium in human blood. Arch
Environ Contam Toxicol. 63:299–308. 2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhong WS, Ren T and Zhao LJ: Determination
of Pb (Lead), Cd (Cadmium), Cr (Chromium), Cu (Copper), and Ni
(Nickel) in Chinese tea with high-resolution continuum source
graphite furnace atomic absorption spectrometry. J Food Drug Anal.
24:46–55. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Pozzatti M, Borges RA, Dessuy MB, Vale MGR
and Welz B: Determination of cadmium, chromium and copper in
vegetables of the Solanaceae family using high-resolution continuum
source graphite furnace atomic absorption spectrometry and direct
solid sample analysis. Anal Methods. 9:329–337. 2017.
|