Introduction
Polycystic ovary syndrome (PCOS) is a universal
endocrine disorder, with an incidence of 5-10% in women of
childbearing age globally (1,2). The
major clinical symptoms in patients with PCOS are subfertility,
amenorrhea, seborrhea and acanthosis (3,4). The
etiopathogenesis of PCOS is complicated and its exact mechanism has
not been elucidated (5). Therefore,
it is critical to understand the mechanisms of PCOS and provide new
insight into PCOS therapy.
Propofol is an anesthetic drug frequently-used
during clinical practice (6,7). It
has been reported that propofol exerts anti-inflammatory and
anti-tumor effects on several cancer types, including gastric
(8), colorectal (9) and pancreatic (10). Moreover, previous studies have
revealed that propofol may suppress cancer cell growth. Gao et
al (10) concluded that
propofol could inhibit pancreatic cancer progression under hypoxia
by ADAM8. Yang et al (11)
demonstrated that propofol could suppress lung cancer cell growth
and promote cell apoptosis by altering microRNA (miR)-486
expression. However, whether propofol plays similar roles in PCOS
remains unclear. Therefore, in the present study, the role of
propofol was investigated in ovarian granulosa cell viability and
apoptosis and the associated molecular pathways were
determined.
Previous studies have suggested that the quantity of
growing follicles in PCOS is associated with ovarian granulosa cell
proliferation and genetic factors, such as miRNAs (12). miRNAs, with a length of 21 to 23
nucleotides, can impact biological functions by binding to the
3'-untranslated region (UTR) of target mRNAs (13). miRNAs participate in the progression
of several diseases, including PCOS, and have therefore become
promising targets for PCOS treatment. Specific miRNA examples
include miR-320(14) miR-99a
(15) and miR-21(16). miR-451a is located on chromosome
17q11.2. Previous studies indicated that miR-451a was downregulated
in various tumors based on the expression of the Ago2 protein, such
as papillary thyroid cancer (17),
hepatocellular carcinoma (18),
non-small cell lung cancer (19)
and PCOS (20). Moreover, miR-451a
can regulate cell proliferation, apoptosis and metastasis and
improve the therapeutic effects of drugs. Based on this available
information, the present study explored the role and potential
regulatory mechanism of miR-451a in PCOS.
Granulosa cells (GCs), follicular cells surrounding
oocytes, ignite a continuous cross-talk between somatic and germ
cells (21). GC dysfunction may be
involved in various reproductive endocrine disorders including PCOS
(22-24).
Granulosa cells have been widely used to investigate PCOS in
vitro (24,25). And human ovarian granulosa cell-like
KGN cells are often used as a model of ovarian granulosa cells to
study PCOS in vitro (25,26).
The present study aimed to elucidate the effects of
propofol on ovarian granulosa cell proliferation and apoptosis and
illustrate the mechanisms associated with this process.
Materials and methods
Cell culture
Human ovarian granulosa cell-like KGN cells were
purchased from ATCC. The cells were maintained in DMEM/F12 medium
(Gibco; Thermo Fisher Scientific, Inc.) with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillinG and streptomycin
sulfate (Invitrogen; Thermo Fisher Scientific, Inc.) in a
humidified incubator with 5% CO2 at 37˚C. For
drug-toxicity assays, the cells were cultured in 6-well plates and
exposed to different concentrations of propofol (0, 1, 5 and 10
µg/ml) for 48 h (27).
Cell transfection and reagents
A total of 50 mimic control
(5'-AUCUGAACGGAUCCUUAUUAAC-3'), 50 miR-451a mimic
(5'-AAACCGUUACCAUUACUGAGUU-3'), 100 inhibitor control
(5'-UUGUACUACAAAAGUACUG-3') or 100 nM miR-451a inhibitor
(5'-AACUCAGUAAUGGUAACGGUUU-3') sequences were obtained from
Shanghai GenePharma Co., Ltd. and transfected into KGN cells
(5x104 cells per well) using Lipofectamine®
2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) as
determined by the manufacturer's instructions. Following
transfection, the cells were obtained for subsequent analysis. At
48 h after cell transfection, subsequent experiments were
performed.
Measurement of cell viability
MTT assay was employed to assess the viability of
KGN cells. The cells were transfected with mimic control, miR-451a
mimic, inhibitor control and miR-451a inhibitor sequences or
exposed to propofol. Subsequently, 20 µl MTT (Sigma-Aldrich; Merck
KGaA) was added into each well and the samples were incubated for 4
h. The culture medium was removed and the cells were dissolved in
150 µl DMSO (Sigma-Aldrich; Merck KGaA) in the dark for 10 min.
Absorbance was determined at 570 nm using a microplate reader
(Bio-Rad Laboratories, Inc.).
Flow cytometry analysis
KGN cells were stimulated with propofol or
transfected with mimic control or miR-451a mimic for 48 h and the
induction of apoptosis was measured by flow cytometry.
Subsequently, the cells were washed, centrifuged and harvested and
Annexin V-FITC and PI (Annexin-V/PI Apoptosis Detection kit;
Beyotime Institute of Biotechnology) were added at room temperature
for 15 min in the dark as described by the manufacturer's protocol.
A standard flow cytometer (BD Biosciences) was used to measure the
number of apoptotic cells. The data were analyzed using Cell Quest
software (version 5.1; BD Biosciences).
Reverse transcription-quantitative
(RT-q)PCR analysis
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract total RNA from KGN
cells following the manufacturer's protocol. Subsequently, cDNA was
synthesized from the total RNA using a cDNA Synthesis kit (Takara
Bio, Inc.). RT conditions were as follows: 25˚C for 5 min, 42˚C for
60 min and 80˚C for 2 min. RT-qPCR was conducted using an ABI 7500
Real-Time PCR System (Agilent Technologies, Inc.) with SYBR Green
Master mix (Takara Bio, Inc.). The samples were initially incubated
at 95˚C for 10 min to denature the cDNA strand and 37 cycles were
performed including denaturation at 95˚C for 30 sec, annealing at
60˚C for 60 sec and extension at 72˚C for 15 sec. A final extension
was conducted at 72˚C for 10 min. GAPDH was used as the internal
reference for mRNA and U6 for miRNA. Primer sequences for PCR were
as follows: GAPDH forward, 5'-TTTGGTATCGTGGAAGGACTC-3' and reverse,
5'-GTAGAGGCAGGGATGATGTTCT-3'; U6 forward,
5'-GCTTCGGCAGCACATATACTAAAAT-3' and reverse,
5'-CGCTTCACGAATTTGCGTGTCAT-3'; Wnt3a forward,
5'-GTTCGGGAGGTTTGGG-3' and reverse, 5'-CCAGGAAAGCGGACCAT-3';
β-catenin forward, 5'-GTTGTACTGCTGGGACCCTT-3' and reverse,
5'-CCCAAGCATTTTCACCAGCG-3'; and miR-451a forward,
5'-ACACTCCAGCTGGGAAACCGTTACCATTACT-3' and reverse,
5'-CTGGTGTCGTGGAGTCGGCAA-3'. The gene expression levels were
calculated using the 2-ΔΔCq method (28).
Western blot analysis
Following treatment with propofol, total protein was
isolated from KGN cells using RIPA lysis solution (Beyotime
Institute of Biotechnology). The BCA protein quantitative kit
(Beijing Solarbio Science & Technology Co., Ltd.) was employed
to measure the protein concentration in accordance with the
manufacturer's instructions. Subsequently, the extracted protein
samples (40 µg per lane) were loaded on 10% SDS-PAGE and
transferred on PVDF membranes. The membranes were blocked with 5%
skimmed milk at room temperature for 1 h and incubated with the
following primary antibodies: Wnt3a (cat no. ab219412; Abcam),
β-catenin (cat no. ab16051; Abcam), cleaved caspase3 (cat no.
ab32042; Abcam), pro-caspase3 (cat no. ab32499; Abcam) and GAPDH
(cat no. ab9485; Abcam) at a dilution of 1:1,000 overnight at 4˚C.
The following morning, the membranes were incubated with the
corresponding secondary antibody (cat no. ab7090; 1:2,000; Abcam)
for 1 h at room temperature. The protein bands were exposed using
EasyBlot ECL Kit (Shanghai BestBio) and analyzed using Image J
(National Institutes of Health).
Statistical analysis
The measurement data are presented as the mean ± SD
from at least three independent experiments. Statistical analyses
were performed using SPSS 19.0 statistical software. One-way ANOVA
with Tukey's post-hoc test was used to compare differences among
the groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Propofol inhibits KGN cell
proliferation and induced cell apoptosis
In order to investigate the function of propofol in
KGN cells, different concentrations of this compound were used to
stimulate the cells for 48 h. Subsequently, MTT and flow cytometry
analyses were conducted to evaluate cellular growth and apoptosis,
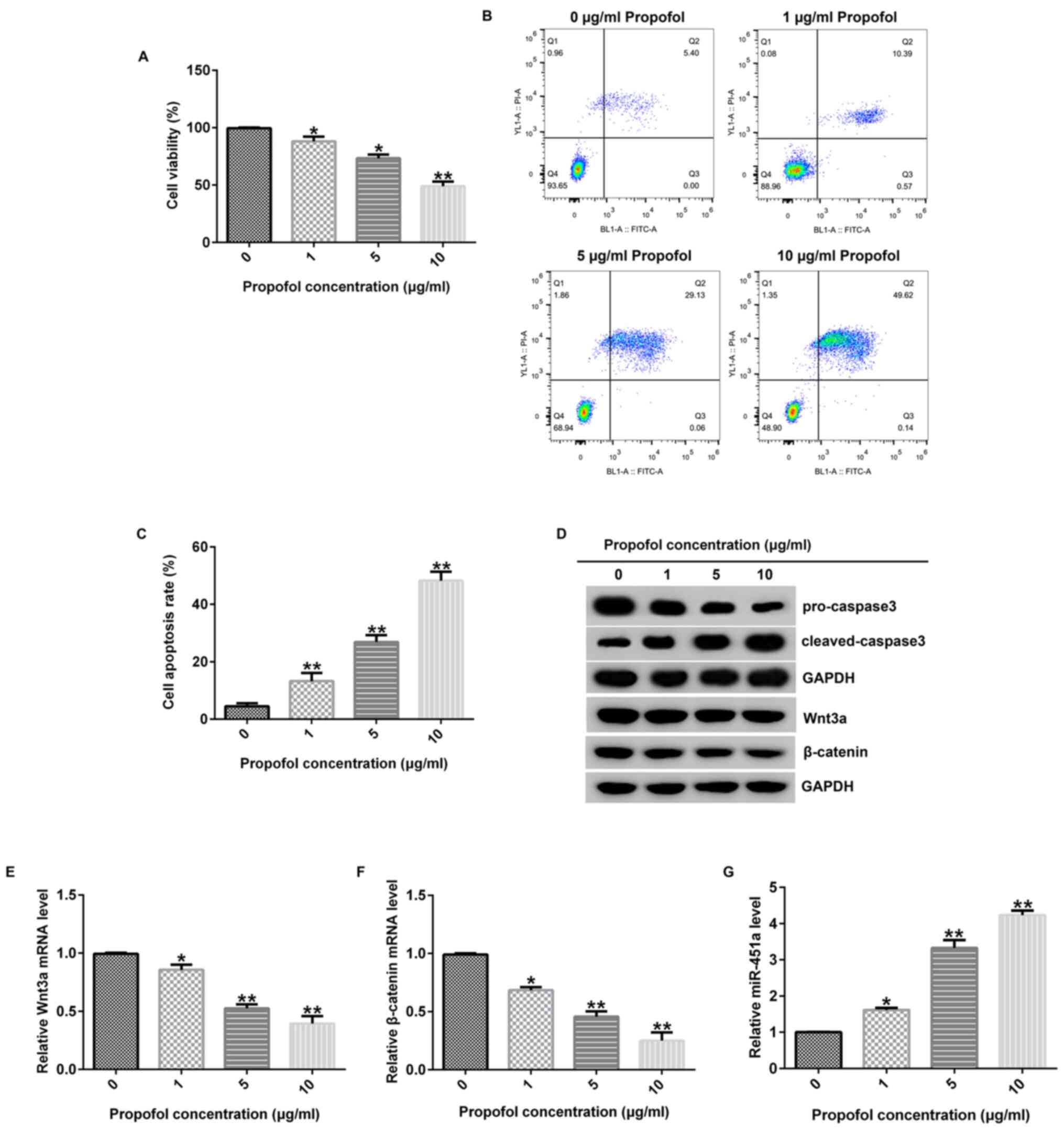
respectively. MTT assay revealed that the reduction of KGN cell
viability with propofol treatment in comparison to the control was
significant (Fig. 1A). In addition,
flow cytometry revealed that propofol treatment led to an increase
of apoptosis in KGN cells (Fig. 1B
and C). Moreover, western blot
analysis revealed that propofol markedly increased
cleaved-caspase-3 expression and reduced pro-caspase 3 levels in
KGN cells compared with levels in untreated cells (Fig. 1D). Furthermore, the influence of
propofol on the Wnt/β-catenin signaling pathway were investigated
and the data suggested that propofol inhibited Wnt3a and β-catenin
protein (Fig. 1D) and mRNA
expression levels (Fig. 1E and
F) in KGN cells in comparison with
a control. These findings suggested that propofol inhibited cell
viability and induced apoptosis of KGN cells and that this was
potentially via the Wnt/β-catenin signaling pathway. In addition,
the data suggested that propofol enhanced miR-451a expression
levels in KGN cells in comparison with control (Fig. 1G), suggesting that it may have the
potential to suppress PCOS progression.
miR-451a mimic inhibits KGN cell
viability and induced cell apoptosis
To examine the effects of miR-451a on KGN cells,
mimic control or miR-451a mimic sequences were transfected into KGN
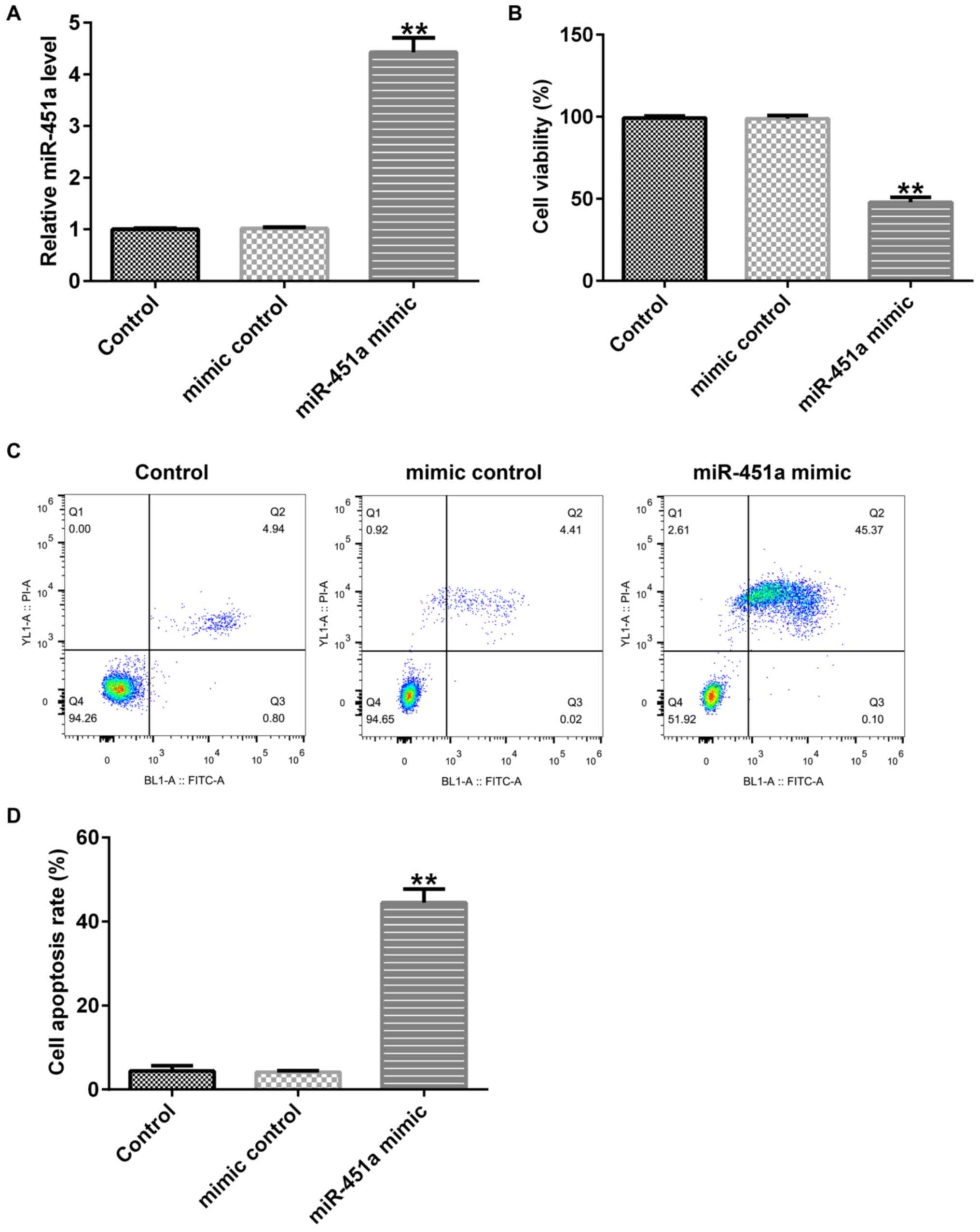
cells and the cells were incubated for 48 h. Upregulation of
miR-451a promoted miR-451a expression in KGN cells compared with
that noted in untreated control and mimic control cells (Fig. 2A). Furthermore, cell proliferation
was inhibited (Fig. 2B) and
apoptosis was promoted in KGN cells following miR-451a mimic
transfection (Fig. 2C and D). These data suggested that miR-451a may
participate in the development of PCOS.
miR-451a inhibitor eliminates the
effects of propofol on KGN cell growth and on the induction
apoptosis
To evaluate the association between miR-451a
expression and propofol, inhibition experiments were carried out.
KGN cells were transfected with miR-451a inhibitor and inhibitor
control sequences for 6 h using Lipofectamine® 2000
reagent. Following transfection, KGN cells were exposed to 10 µg/ml
propofol for 48 h. The cells were divided into the following
groups: Control, propofol, propofol + inhibitor control and
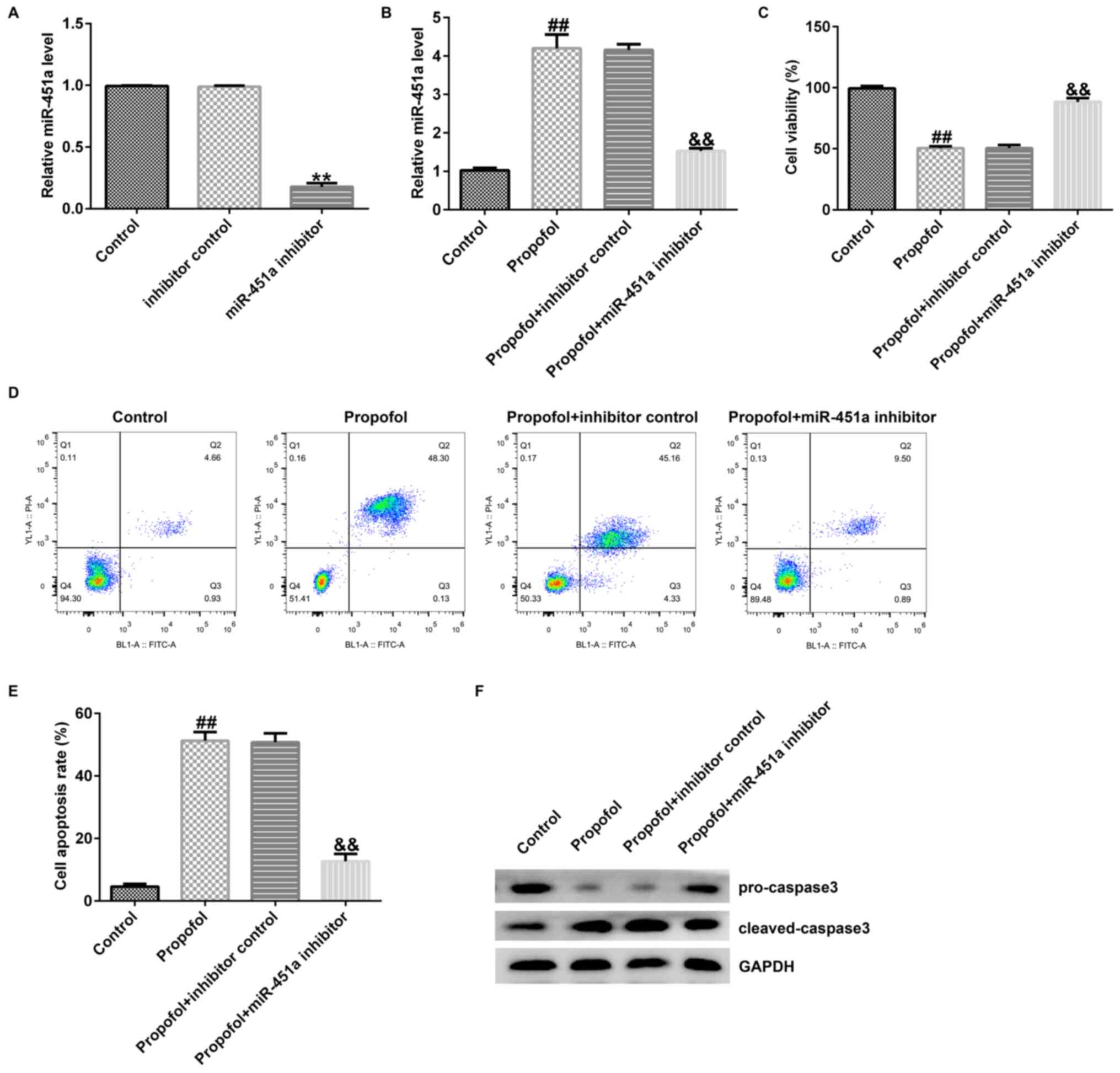
propofol + miR-451a inhibitor. RT-qPCR revealed that miR-451a
inhibitor treatment markedly inhibited miR-451a levels in KGN cells
compared with those of the control cells (Fig. 3A). However, miR-451a expression
levels were significantly increased in propofol-treated KGN cells
compared with those of the control cells (Fig. 3B). The opposite effects were noted
in the propofol + miR-451a inhibitor group (Fig. 3B). In addition, cell growth,
apoptosis and the expression levels of specific apoptotic proteins
were assessed using MTT, flow cytometry and western blot assays,
respectively. The data revealed that propofol prominently
suppressed KGN cell growth (Fig.
3C), promoted apoptosis (Fig.
3D and E), enhanced cleaved
caspase 3 levels and reduced pro-caspase 3 expression (Fig. 3F) compared with the corresponding
effects noted in control cells. However, these data were reversed
following downregulation of miR-451a. The results indicated that
miR-451a expression may regulate the effects of propofol in KGN
cells.
miR-451a inhibitor eliminates the
inhibition effect of propofol on the Wnt/β-catenin signaling
pathway in KGN cells
To further illustrate the underlying mechanisms of
cell apoptosis following treatment of KGN cells with propofol, the
expression levels of apoptosis-associated genes and proteins were
determined in these cells using RT-qPCR and western blot assays,
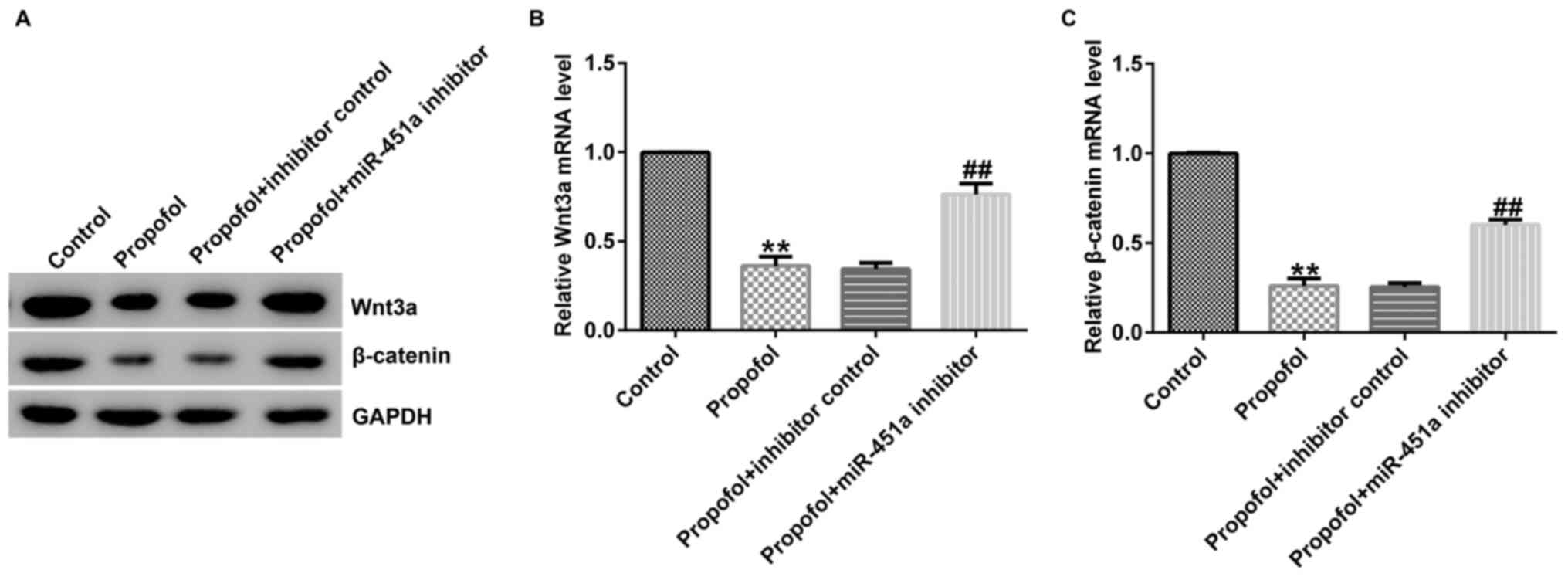
respectively. Lower Wnt3a and β-catenin protein (Fig. 4A) and mRNA levels (Fig. 4B and C) were observed in propofol-stimulated KGN
cells than those noted in the control group, suggesting that
miR-451a may regulate the protective function of propofol in
ovarian granulosa cell proliferation and apoptosis through the
Wnt/β-catenin pathway.
Discussion
Propofol is one of the most frequently used
anesthetics (6,7). This compound exhibits anti-tumorigenic
or anti-phlogotic effects on several cancer cell lines. Yu et
al (29) demonstrated that
propofol exhibited neuroprotective effects on glutamic acid treated
PC12 cells by downregulating miR-19a expression. Moreover, Li et
al (9) indicated that propofol
inhibited colorectal cancer cell viability and metastasis by
regulating the miR-124-3p.1/AKT3 axis. PCOS is a common endocrine
disorder with approximate incidence of 10% in women worldwide
(1). The common clinical
manifestations of PCOS include hyperandrogenemia, chronic
anovulation and sclerocystic ovaries (2,3).
However, the therapeutic methods for PCOS patients are limited and
its pathogenesis is not fully explored (5).
In the present study, the function of propofol was
assessed in ovarian granulosa cells. KGN cells were cultured
following treatment with various concentrations of propofol (0, 1,
5, and 10 µg/ml) for 48 h. Propofol suppressed cellular growth and
promoted cell apoptosis in KGN cells. Cleaved-caspase 3 and
pro-caspase 3 are important regulators in cell apoptotic signaling
and lead to apoptotic cell death (30). The data presented in the current
report are in line with our previous investigations demonstrating
that propofol enhanced cleaved-caspase 3 expression and inhibited
pro-caspase 3 levels in ovarian granulosa cells (31,32).
Accumulating evidence has indicated that the change in the
expression levels of Wnt/β-catenin signaling proteins may affect
the expression levels of proteins involved in various diseases
(33,34). The Wnt/β-catenin signaling pathway
participates in specific cell functions, including cell growth
(35), differentiation (36) and apoptosis (37). Therefore, the expression levels of
the proteins associated with the Wnt/β-catenin pathway were
investigated. The results of western blot analysis and RT-qPCR
indicated that propofol reduced Wnt3a and β-catenin protein and
mRNA expression levels. These results revealed that propofol may
inhibit cell proliferation and promote cell apoptosis via the
Wnt/β-catenin signaling pathway, suggesting that it could serve as
an appropriate target for PCOS treatment.
miRNAs are small RNAs, which exert key roles by
abnormal expression in various human diseases such as cancer
(38) and neurodegenerative and
cardiovascular disease (39).
Previous studies have indicated that miRNAs are associated with
several processes involved in tumorigenesis, including cell
differentiation, proliferation and apoptosis (40,41).
miR-451a has been reported as a noninvasive biomarker in several
types of cancer. For example, Liu et al (42) demonstrated that miR-451a inhibited
breast cancer cell proliferation and improved tamoxifen sensitivity
by regulating macrophage migration inhibitory factor. The results
of the present study are in agreement with these findings
indicating that miR-451a expression was upregulated in
propofol-treated KGN cells compared with that noted in the control
cells. This in turn suggested that miR-451a levels may influence
the function of propofol in PCOS. However, the expression level of
miR-451a in ovarian tissue or ovarian granulosa cells from PCOS
patients and normal women was not assessed in the present study,
which was a limitation.
In addition, the influence of miR-451a on ovarian
granulosa cells was explored. KGN cells were transfected with mimic
control or miR-451a mimic for 48 h. The transfection efficiency was
assessed by RT-qPCR analysis. The expression of miR-415a was
increased in KGN cells compared with that of the control cells.
Furthermore, the results from the MTT and flow cytometry assays
suggested that miR-451a mimic inhibited KGN cell viability and
facilitated apoptosis. Moreover, miR-451a inhibitor and inhibitor
control sequences were transfected in KGN cells for 6 h and
miR-451a expression was determined using RT-qPCR. The data
demonstrated that miR-451a expression was downregulated in KGN
cells following transfection. These results indicated that miR-451a
may be involved in the progression of PCOS.
Several reports have shown that the mechanism of
action of propofol is regulated by specific genes during disease
progression (43,44). For example, Ren and Zhang (43) suggested that propofol accelerated
colorectal cancer cell apoptosis by alleviating the inhibition of
lncRNA HOXA11-AS on miRNA let-7i. Sun et al (44) reported that propofol inhibits JEG-3
choriocarcinoma cell proliferation and metastasis by upregulating
miR-495. Therefore, inhibition experiments were carried out to
evaluate whether miR-451a regulates the effects of propofol. KGN
cells were transfected with inhibitor control or miR-451a inhibitor
sequences for 6 h. Following transfection, KGN cells were treated
with 10 µg/ml propofol for 48 h. The cells were divided into the
four following groups: Control, propofol, propofol + inhibitor
control and propofol + miR-451a inhibitor. The results demonstrated
that propofol increased miR-451a expression, reduced KGN cell
growth, promoted apoptosis, enhanced cleaved caspase 3 levels and
reduced pro-caspase 3 expression compared to the corresponding
effects noted in the control cells. However, these effects were
reversed following inhibition of miR-451a. The results revealed
that miR-451a expression regulated the functions of propofol in KGN
cells.
The interaction of miR-451a and propofol was also
examined with regard to the regulation of the Wnt/β-catenin
pathway. The data suggested that propofol suppressed the mRNA and
protein expression levels of β-catenin and Wnt3a in KGN cells
compared with those noted in the control cells. Moreover, the
opposite effects were noted in the propofol + miR-451a inhibitor
group compared with those of the propofol + inhibitor control
group.
In conclusion, the present study provided evidence
that propofol exerts anti-proliferative and apoptosis-inducing
effects on ovarian granulosa cells by regulating miR-451a
expression. This effect contributed partially to the deactivation
of the Wnt/β-catenin pathway. The findings indicate that propofol
may be a promising target for PCOS treatment. However, this study
was only a preliminary in vitro study of propofol and
miR-451 association. In order to clarify the role of
propofol/miR-451a in polycystic ovary syndrome in-depth research is
needed. For example, the target genes of mir-451a must be analyzed
and rescue experiments are needed to prove the mechanism of
miR-451a's effect on KNG cells. Whether propofol/miR-451a directly
regulates the Wnt/β-catenin pathway in KNG cells requires further
in-depth research. Additionally, the role of propofol/miR-451a in
polycystic ovary syndrome should be investigated in vivo.
Moreover, the expression of miR-451a in ovarian tissue or ovarian
granulosa cells from PCOS patients should be determined, and the
relationship between miR-451a expression and the
clinicopathological parameters of PCOS patients needs to be
explored. These studies will be performed in the future.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RD and WK contributed to study design, data
collection, statistical analysis, data interpretation and
manuscript preparation. DW contributed to statistical analysis and
data interpretation. LW contributed to statistical analysis and
manuscript preparation. RD, WK, DW and LW confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li X, Zhang T, Li S, Deng Y, Wang L, Tao
T, Wang S, Gu Y, Gu W, Hong J, et al: Correlation between glucose
metabolism and serum steroid hormones in patients with polycystic
ovary syndrome. Clin Endocrinol (Oxf). 92:350–357. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Xie L, Zhang D, Ma H, He H, Xia Q, Shen W,
Chang H, Deng Y, Wu Q, Cong J, et al: The effect of berberine on
reproduction and metabolism in women with polycystic ovary
syndrome: A systematic review and meta-analysis of randomized
control trials. Evid Based Complement Alternat Med.
2019(7918631)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Berger JJ and Bates GJ: Optimal management
of subfertility in polycystic ovary syndrome. Int J Womens Health.
6:613–621. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dong Z, Huang J, Huang L, Chen X, Yin Q
and Yang D: Associations of acanthosis nigricans with metabolic
abnormalities in polycystic ovary syndrome women with normal body
mass index. J Dermatol. 40:188–192. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bednarska S and Siejka A: The pathogenesis
and treatment of polycystic ovary syndrome: What's new? Adv Clin
Exp Med. 26:359–367. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang YF, Li CS, Zhou Y and Lu XH: Effects
of propofol on colon cancer metastasis through STAT3/HOTAIR axis by
activating WIF-1 and suppressing Wnt pathway. Cancer Med.
9:1842–1854. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li X, Huang L, Zhao Z, Bo L, Kang R, Yang
J and Dong Z: The protective effect of the Rho-kinase inhibitor
hydroxyfasudil on propofol-induced hippocampal neuron apoptosis in
neonatal rats. Int J Clin Exp Pathol. 11:4562–4570. 2018.PubMed/NCBI
|
|
8
|
Ni YJ, Lu J and Zhou HM: Propofol
suppresses proliferation, migration and invasion of gastric cancer
cells via regulating miR-29/MMP-2 axis. Eur Rev Med Pharmacol Sci.
23(10177)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li Y, Dong W, Yang H and Xiao G: Propofol
suppresses proliferation and metastasis of colorectal cancer cells
by regulating miR-124-3p.1/AKT3. Biotechnol Lett. 42:493–504.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gao Y, Yu X, Zhang F and Dai J: Propofol
inhibits pancreatic cancer progress under hypoxia via ADAM8. J
Hepatobiliary Pancreat Sci. 26:219–226. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Yang N, Liang Y, Yang P, Yang T and Jiang
L: Propofol inhibits lung cancer cell viability and induces cell
apoptosis by upregulating microRNA-486 expression. Braz J Med Biol
Res. 50(e5794)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xue Y, Lv J, Xu P, Gu L, Cao J, Xu L, Xue
K and Li Q: Identification of microRNAs and genes associated with
hyperandrogenism in the follicular fluid of women with polycystic
ovary syndrome. J Cell Biochem. 119:3913–3921. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vicchio TM, Aliquò F, Ruggeri RM, Ragonese
M, Giuffrida G, Cotta OR, Spagnolo F, Torre ML, Alibrandi A,
Asmundo A, et al: MicroRNAs expression in pituitary tumors:
Differences related to functional status, pathological features,
and clinical behavior. J Endocrinol Invest. 43:947–958.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rashad NM, Ateya MA, Saraya YS, Elnagar
WM, Helal KF, Lashin ME, Abdelrhman AA, Alil AE and Yousef MS:
Association of miRNA-320 expression level and its target gene
endothelin-1 with the susceptibility and clinical features of
polycystic ovary syndrome. J Ovarian Res. 12(39)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Geng Y, Sui C, Xun Y, Lai Q and Jin L:
MiRNA-99a can regulate proliferation and apoptosis of human
granulosa cells via targeting IGF-1R in polycystic ovary syndrome.
J Assist Reprod Genet. 36:211–221. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jiang L, Li W, Wu M and Cao S: Ciculating
miRNA-21 as a biomarker predicts polycystic ovary syndrome (PCOS)
in patients. Clin Lab. 61:1009–1015. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fan X and Zhao Y: miR-451a inhibits cancer
growth, epithelial-mesenchymal transition and induces apoptosis in
papillary thyroid cancer by targeting PSMB8. J Cell Mol Med.
23:8067–8075. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wei GY, Hu M, Zhao L and Guo WS: MiR-451a
suppresses cell proliferation, metastasis and EMT via targeting
YWHAZ in hepatocellular carcinoma. Eur Rev Med Pharmacol Sci.
23:5158–5167. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shen YY, Cui JY, Yuan J and Wang X:
MiR-451a suppressed cell migration and invasion in non-small cell
lung cancer through targeting ATF2. Eur Rev Med Pharmacol Sci.
22:5554–5561. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Díaz M, Bassols J, López-Bermejo A, de
Zegher F and Ibáñez L: Low circulating levels of miR-451a in girls
with polycystic ovary syndrome: Different effects of randomized
treatments. J Clin Endocrinol Metab. 105(dgz204)2019.
|
|
21
|
Tatone C and Amicarelli F: The aging
ovary-the poor granulosa cells. Fertil Steril. 99:12–17.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Artimani T, Saidijam M, Aflatoonian R,
Ashrafi M, Amiri I, Yavangi M, SoleimaniAsl S, Shabab N, Karimi J
and Mehdizadeh M: Downregulation of adiponectin system in granulosa
cells and low levels of HMW adiponectin in PCOS. J Assist Reprod
Genet. 33:101–110. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xiong Y, Liu T, Wang S, Chi H, Chen C and
Zheng J: Cyclophosphamide promotes the proliferation inhibition of
mouse ovarian granulosa cells and premature ovarian failure by
activating the lncRNA-Meg3-p53-p66Shc pathway. Gene. 596:1–8.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kong L, Wang Q, Jin J, Xiang Z, Chen T,
Shen S, Wang H, Gao Q and Wang Y: Insulin resistance enhances the
mitogen-activated protein kinase signaling pathway in ovarian
granulosa cells. PLoS One. 12(e0188029)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Huang X, Jin J, Shen S, Xia Y, Xu P, Zou
X, Wang H, Yi L, Wang Y and Gao Q: Modulation of expression of
17-Hydroxylase/17,20 lyase (CYP17) and P450 aromatase (CYP19) by
inhibition of MEK1 in a human ovarian granulosa-like tumor cell
line. Gynecol Endocrinol. 32:201–205. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Han XM, Tian PY and Zhang JL:
MicroRNA-486-5p inhibits ovarian granulosa cell proliferation and
participates in the development of PCOS via targeting MST4. Eur Rev
Med Pharmacol Sci. 23:7217–7223. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Feng S and Sun Y: Protective role of
propofol in endometriosis and its mechanism. Exp Ther Med.
16:3646–3650. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yu S, Xin W, Jiang Q and Li A: Propofol
exerts neuroprotective functions by down-regulating microRNA-19a in
glutamic acid-induced PC12 cells. Biofactors. 46:934–942.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Crowley LC and Waterhouse NJ: Detecting
cleaved caspase-3 in apoptotic cells by flow cytometry. Cold Spring
Harb Protoc: doi:10.1101/pdb.prot087312.
|
|
31
|
Qu ZJ, Qu ZJ, Zhou HB, Xu CS, Zhang DZ and
Wang G: Protective effect of remifentanil on myocardial
ischemia-reperfusion injury through Fas apoptosis signaling
pathway. Eur Rev Med Pharmacol Sci. 23:5980–5986. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wu H, Medeiros LJ and Young KH: Apoptosis
signaling and BCL-2 pathways provide opportunities for novel
targeted therapeutic strategies in hematologic malignances. Blood
Rev. 32:8–28. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Perugorria MJ, Olaizola P, Labiano I,
Esparza-Baquer A, Marzioni M, Marin JJG, Bujanda L and Banales JM:
Wnt-β-catenin signalling in liver development, health and disease.
Nat Rev Gastroenterol Hepatol. 16:121–136. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jiang H, Li Y, Li J, Zhang X, Niu G, Chen
S and Yao S: Long noncoding RNA LSINCT5 promotes endometrial
carcinoma cell proliferation, cycle, and invasion by promoting the
Wnt/β-catenin signaling pathway via HMGA2. Ther Adv Med Oncol.
11(1758835919874649)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen H, Wang S, Chen L, Chen Y, Wu M,
Zhang Y, Yu K, Huang Z, Qin L and Mo D: MicroRNA-344 inhibits
3T3-L1 cell differentiation via targeting GSK3β of Wnt/β-catenin
signaling pathway. FEBS Lett. 588:429–435. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sun H, Gao Y, Lu K, Zhao G, Li X, Li Z and
Chang H: Over-expression of Klotho suppresses liver cancer
progression and induces cell apoptosis by negatively regulating
wnt/β-catenin signaling pathway. World J Surg Oncol.
13(307)2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Iacona JR and Lutz CS: miR-146a-5p:
Expression, regulation, and functions in cancer. Wiley Interdiscip
Rev RNA. 10(e1533)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Vishnoi A and Rani S: MiRNA biogenesis and
regulation of diseases: An overview. Methods Mol Biol. 1509:1–10.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhao M, Sun L, Chen S, Li D, Zhang L, He
P, Liu X, Zhang L, Zhang H, Yang D, et al: Borna disease virus
infection impacts microRNAs associated with nervous system
development, cell differentiation, proliferation and apoptosis in
the hippocampi of neonatal rats. Mol Med Rep. 12:3697–3703.
2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Shen S, Luo X, Gao K, Sun Y, Yao D and Zhu
L: Identification and integrative analysis of microRNAs and mRNAs
involved in proliferation and invasion of pressuretreated human
liver cancer cell lines. Mol Med Rep. 20:375–387. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu Z, Miao T, Feng T, Jiang Z, Li M, Zhou
L and Li H: MiR-451a inhibited cell proliferation and enhanced
tamoxifen sensitive in breast cancer via macrophage migration
inhibitory factor. Biomed Res Int. 2015(207684)2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ren YL and Zhang W: Propofol promotes
apoptosis of colorectal cancer cells via alleviating the
suppression of lncRNA HOXA11-AS on miRNA let-7i. Biochem Cell Biol.
98:90–98. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sun H, Wang Y and Zhang W: Propofol
inhibits proliferation and metastasis by up-regulation of miR-495
in JEG-3 choriocarcinoma cells. Artif Cells Nanomed Biotechnol.
47:1738–1745. 2019.PubMed/NCBI View Article : Google Scholar
|