Introduction
Cardiac arrest (CA) refers to loss of heart function
that results in an abrupt halt of effective blood flow to the body;
the morbidity and mortality due to CA have increased worldwide
(1). The annual incidence of sudden
CA (SCA) is ~3 million; however, the survival rate of SCA is <1%
(2). Roberts et al (3) have reported that dysfunctions in
various organs are common after CA following return of the
spontaneous circulation (ROSC). In particular, kidney injury that
results from CA following ROSC is a complex process; for heart
disease patients admitted to hospitals, impairment of renal injury
is common and is associated with a high mortality rate (4). Acute kidney injury (AKI) occurs
frequently in patients with CA, occurring in ~50% of cases of CA
(5). Geri et al (6) reported that the incidence of acute
renal injury ranges from 12 to 40% in patients with CA. Most
studies have focused on myocardial dysfunction and brain injury
following ROSC after CA; however, renal injury has not been widely
studied (7,8).
Oxidative stress contributes to the pathogenicity of
renal ischemia/reperfusion injury (RI/RI) (9). Furthermore, RI/RI-induced oxidative
stress generates high levels of reactive oxygen species (ROS).
Subsequently, overproduction of ROS results in mutation of DNA,
apoptosis, necrosis and lipid peroxidation, causing cellular death
in numerous ways (10,11). A signaling pathway determined to
have anti-oxidative stress properties and scavenge ROS production
under oxidative stress conditions is nuclear erythroid-related
factor-2 (Nrf2)/heme oxygenase (HO-1). Nrf2, an inducible
transcription factor, binds with the antioxidant response element
(ARE) located on the promoter regions of numerous antioxidant and
detoxifying genes such as HO-1(12). Previous studies (13,14)
mainly focused on histopathological and pathophysiological ailments
and the expression levels of Nrf2 and HO-1 in rats induced by
RI/RI; however, this type of study in a rat model of asphyxial CA
is rare.
The first successful TH application in humans
following ROSC after CA was reported near the end of the 1950s
(15). Since then, TH has been the
most successful treatment for CA (16). Mild TH (MTH) increases the survival
rate and attenuates neurological outcomes in patients with CA who
achieve ROSC (17). Furthermore,
MTH protects the heart, liver and kidney from damage (18). Previous studies demonstrated that
MTH provides damage protection against oxidative stress (18-20).
However, other studies reported that the neurological outcome and
survival rate following ROSC after CA were not significantly
different from those of patients who did not achieve ROSC (21-23).
As TH has a controversial effect in patients with CA, an experiment
was performed in the present study to confirm such an effect
following ROSC.
Tujjar et al (24) demonstrated AKI in patients with CA
following ROSC after CA, although the mechanism of kidney damage
had remained elusive. Several studies reported increased expression
of Nrf2 and HO-1 in the RI/RI experimental animal model (13,14).
Thus, these parameters were selected for the present study to
assess the antioxidative effects of TH in asphyxial CA-induced
RI/RI following ROSC. The present study aimed to investigate the
renoprotective effect of TH on asphyxial CA-induced RI/RI in
rats.
Materials and methods
Experimental animals
Male Sprague-Dawley (SD) rats (total n=48;
bodyweight, 270-300 g; 10-weeks-old) were supplied by the
Experimental Animal Center Jeonbuk National University (Jeonju,
South Korea). They were housed in a conventional manner with
adequate temperature (23±2˚C) and humidity (60±10%) control under a
12-h light/dark cycle. They were provided with food and water ad
libitum. All of the experimental procedures were approved by
the Institutional Animal Care and Use Committee of Jeonbuk National
University (approval no. JBNU 2019-005). Experimental animals were
divided as into two major groups: Sham group (not subjected to CA
surgery) (n=6) and rats subjected to CA surgery (total n=42). Rats
that underwent CA surgery were further divided into the following
groups: i) Normothermia + CA (Normo.) group (n=14); ii) Normo. with
2 h TH immediately after ROSC and gradual increase of the
temperature to the normal temperature until sacrifice (Hypo 2 h)
(n=12); iii) Normo. group with 4 h TH following cardiopulmonary
resuscitation (CPR) and gradual rewarming to the normal
temperature, which was maintained until the sacrifice of the rats
(Hypo 4 h) (n=9); iv) Normo. group with 6 h TH immediately after
ROSC and rewarming was performed to attain normal temperature,
which was maintained until the rats were sacrificed (Hypo 6 h)
(n=7). The number of rats in each group was selected according to
the expected survival rate of each group with a target of having 6
animals per group at the end of the protocol; in contrast to the
estimated high survival rate at 2 and 4 h of TH (25).
Induction of CA and CPR
The induction of CA and CPR was performed according
to the preferred protocol (26). In
short, a rodent ventilator (Harvard Apparatus) was used to
anesthetize the rats with 2-3% isoflurane and for mechanical
ventilation. Peripheral oxygen saturation (SpO2) was
checked by connecting the pulse oximetry with the right leg. For
electrocardiographic (ECG) assessment, ECG probes were inserted in
the limbs and data were monitored regularly. The cannulation of the
left femoral artery was for monitoring the mean arterial pressure
(MAP) and the right femoral vein was for intravenous
administration. Vecuronium bromide (2 mg/kg; Gensia Sicor
Pharmaceuticals) was injected intravenously after a stabilization
period of 5 min (27,28). Furthermore, mechanical ventilation
was also stopped for the induction of asphyxial CA. The MAP
reaching below 20 mmHg resulting in pulseless electrical activity
is defined as CA and it took 3-4 min for CA induction. After 5 min
of CA with the administration of a bolus injection of epinephrine
(0.005 mg/kg) and sodium bicarbonate (1 mg/kg), CPR was performed
with an rodent CPR machine (Jeung Do Bio & Plant Co., Ltd.) on
the chest of the rat at the depth of one-third of the
anteroposterior region with mechanical chest compression at the
rate of 300/min to provide 100% oxygen supply until the MAP became
60 mmHg (29). ECG was monitored
continuously and the temperature was maintained according to the
experimental protocol (Fig.
1A).
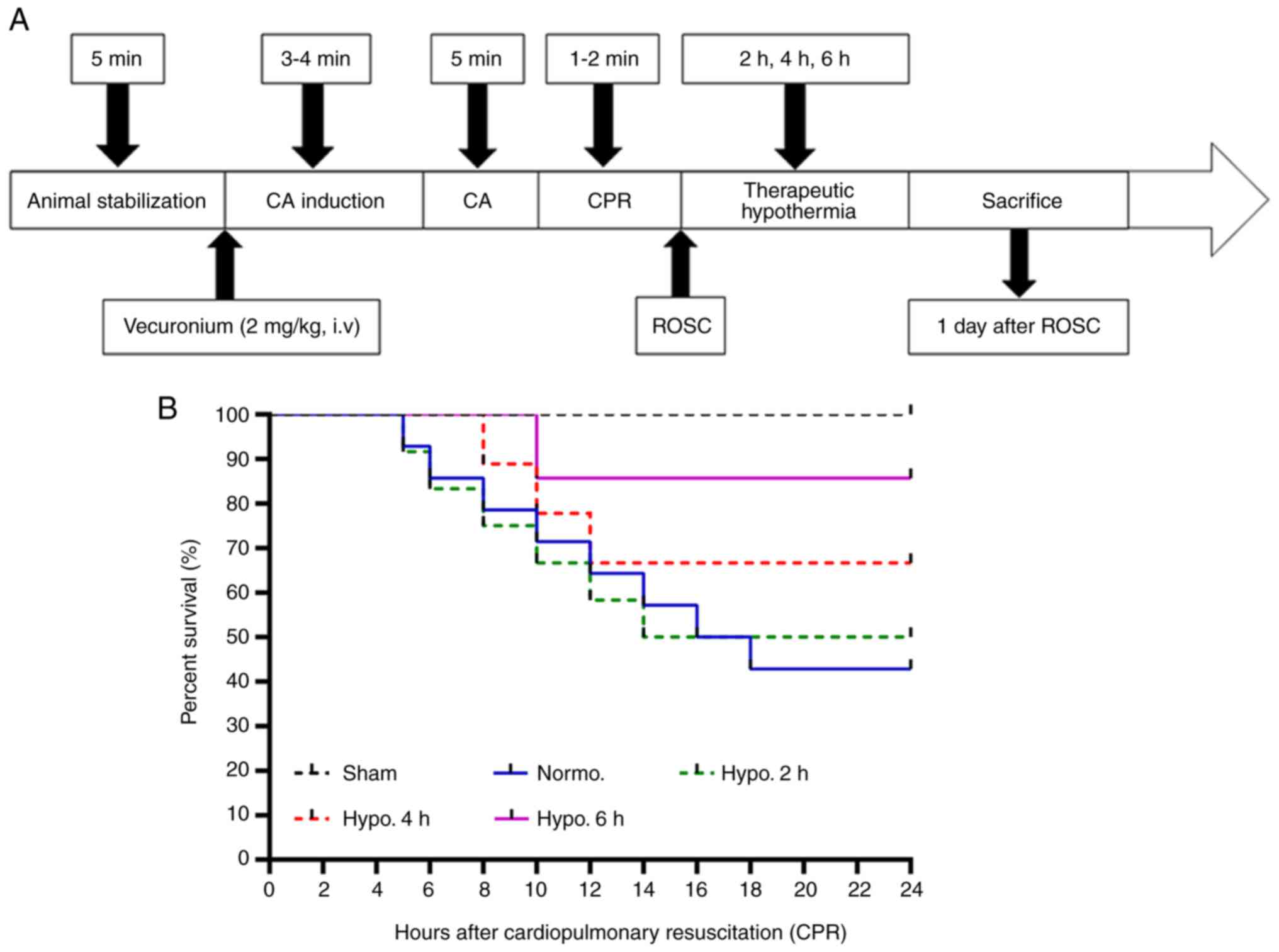 | Figure 1Schematic diagram and survival rate.
(A) Schematic representation of the asphyxial CA model in rats and
measurements obtained during the animal stabilization period
(baseline), induction of CA, CA, CPR time, ROSC, TH duration and
time-point of sacrifice. (B) The survival rate of the experimental
rats was compared using Kaplan-Meier analysis (P<0.05). The
Normo. group had a survival rate of 42.8% and it increased
significantly at 2 h (50%), 4 h (66.6%) and 6 h (85.7%) of TH after
CPR. Groups: Normo., Normothermia + CA; Hypo., TH after ROSC
following CA. CPR, cardiopulmonary resuscitation; CA, cardiac
arrest; TH, therapeutic hypothermia; ROSC, return of spontaneous
circulation. |
Temperature management among the
groups
The body temperature of the Normo. group was
maintained during and after surgery at 37±0.5˚C and this
temperature was further maintained until the rats were sacrificed.
In the 2 h of TH group, CA was performed at a normal temperature
(37±0.5˚C) and then the body temperature was maintained at 33±0.5˚C
immediately after CPR and the same temperature was maintained for 2
h, following which rapid rewarming was performed with the heating
pad until the normal temperature (37±0.5˚C) was achieved and the
rats were returned to their cages until they were sacrificed
(Fig. S1). In the 4 and 6 h of TH
groups, the duration of maintaining the body temperature at
33±0.5˚C CA after CPR was extended to 4 and 6 h, respectively. The
body temperature was monitored by a rectal temperature sensor
(30). After 24 h of CPR, all rats
were sacrificed (13,31).
Detection of blood urea nitrogen (BUN)
and serum creatinine (Cr)
All of the rats were anesthetized with 30% urethane
(1,400 mg/kg, intraperitoneal) at 24 h after ROSC and
transcardially perfused with 4% paraformaldehyde and 5 ml of blood
was collected from the inferior vena cava. The blood was
centrifuged at 1,413 x g for 15 min at 4˚C and serum was obtained
for the determination of BUN and Cr with an Olympus AU 2700
Analyzer (Olympus Optical Co., Ltd.).
Measurement of malondialdehyde (MDA)
content of renal tissues
The MDA concentration in renal tissues was measured
according to a previously published protocol by our group (32). In short, homogenization and
centrifugation of the renal tissues were performed at 8,832 x g for
10 min at 4˚C and the supernatant was collected and stored at -80˚C
for MDA analysis. Subsequently, the MDA content of the renal
tissues was measured according to the instructions of a commercial
kit (TBARS assay kit; Cayman Chemical).
Tissue processing
For transcardial perfusion, 0.1 M PBS (pH 7.4) was
used following 4% paraformaldehyde in 0.1 M phosphate buffer (pH
7.4). Kidneys were harvested from each rat and fixed with 10%
neutral buffered formalin. Subsequently, the kidney was sagittally
cut, embedded in paraffin and sectioned at 5 µm.
Histopathological changes
H&E staining was performed to observe the
histopathological changes in the kidney according to the published
protocol (33). The kidney sections
were mounted on glass slides, dehydrated with ethanol after
staining with H&E and mounted with Canada balsam (Kanto
Chemical). Periodic acid Schiff (PAS) staining was performed
according to the kit (cat. no. PAS-1-IFU; ScyTek Laboratories,
Inc.) manufacturer's protocol. A Leica DM 2500 microscope (Leica
Microsystems) was used to image the sections at fixed
magnifications of x400 (H&E) and x1,000 (PAS), and in each
group, 10 specific areas were captured. Analysis of glomerular
lesions was performed according to a previously published procedure
(25). The scoring was as follows:
Normal, 0; <25% damage, 1; 26-50% damage, 2; 51-75% damage, 3;
and 76-100% damage, 4(25).
Histopathological analysis of lesions was performed according to a
published procedure (34). In
short, histopathological changes were evaluated by quantitative
measurement of interstitial tubular injury and through counting the
number of apoptotic and necrotic cells, tubular brush border loss,
dilatation of tubules, cast formation and infiltration of
neutrophils. The injury scoring was as follows: 0, None; 1, 0-10;
2, 11-25; 3, 26-45; 4, 46-75; and 5, 76-100% (34).
Immunohistochemistry (IHC)
IHC analysis of Nrf2/HO-1 was performed according to
a previously published protocol by our group (32). In short, deparaffinization and
dehydration of the paraffin sections were performed in xylene and
ethanol. Antigen retrieval was performed with citrate buffer and 3%
hydrogen peroxide was used for inactivation of endogenous
peroxidase activity. Goat serum (cat. no. S-1000-20; Vector
Laboratories, Inc.) was used for blocking of the tissue, followed
by incubation with anti-rabbit polyvalent Nrf2 (cat. no. BP1-32822;
Novusbio) and HO-1 (cat. no. ab13243; Abcam) antibody (dilution,
1:500). Subsequently, sections were incubated with the biotinylated
secondary antibody (dilution, 1:250; cat. no. BA-4000-1.5) and
Vectastain ABC reagent (cat. no. PK-4000) (both Vector
Laboratories, Inc.) at room temperature for 1 h. Diaminobenzidine
was applied to the sections in the dark until the development of a
brown color. After counterstaining with hematoxylin staining for
2-3 sec at room temperature, the sections were dehydrated and
cleared in ethanol and xylene and then mounted on a glass slide. A
Leica DM 2500 microscope (Leica Microsystems) was used to image the
sections at a fixed magnification of x400. From each group, 10
specific areas were captured. ImageJ threshold analysis software
version 1.52a (National Institutes of Health) was used to measure
the relative optical density (ROD%).
Statistical analysis
GraphPad Prism 5.0 (GraphPad Software, Inc.) was
used to analyze the data. Values are expressed as the mean ±
standard error or the mean. The survival rate of the rats was
analyzed using Kaplan-Meier curves and log-rank tests. Furthermore,
values were expressed as the median with interquartile range and
statistical comparisons were performed using the Kruskal Wallis
test followed by Dunn's post-hoc test. P<0.05 was considered to
indicate statistical significance.
Results
Physiological variables of the
rats
The physiological parameters were insignificant
between the Sham and Normo. groups (P>0.05). There was also no
significant difference between the Normo. group and the groups with
2, 4 and 6 h of TH (P>0.05; Table
I). CA was confirmed with isoelectric ECG and SpO2
(Table I).
 | Table IPhysiological variables of rats after
asphyxial CA. |
Table I
Physiological variables of rats after
asphyxial CA.
| Physiological
variable | Baseline | Normo. | Hypo. 2 h | Hypo. 4 h | Hypo. 6 h |
|---|
| Body weight, g | 281±14.21 | 286±14.51 | 283±9.91 | 275±20.39 | 279±15.01 |
| Asphyxial time to
CA, sec | N. A. | 139±34.68 | 145±23.71 | 156±31.41 | 149±24.84 |
| CPR time, sec | N. A. | 68±10.89 | 63±15.67 | 75±7.85 | 73±11.12 |
| Heart rate,
beats/min | 331±10.91 | 339±19.54 | 335±18.74 | 338±10.31 | 333±8.79 |
| Room temperature,
˚C | N. A. | 24±0.70 | 25±0.49 | 25±0.57 | 24±0.81 |
Survival rate
The survival rate of the rats was determined at 1
day post-CA. Kaplan-Meier analysis demonstrated a significant
difference in the survival rate (P<0.05). In the sham group, the
survival rate was 100%; however, the survival rate in the Normo.
group was 42.8% at 1 day after ROSC. The survival rate of rats
increased significantly in the groups with 2 h (50%), 4 h (66.6%)
and 6 h (85.7%) of TH after ROSC (P=0.0267 treatment groups vs.
Normo. group; Fig. 1B).
Serum BUN and Cr
Serum BUN and Cr levels, two major indexes of renal
function, were significantly increased in the Normo. group as
compared with those in the sham group (P=0.0018 and 0.0036,
respectively). After 2 h (P>0.05) and 4 h (P>0.05), the
levels of BUN and Cr decreased when compared with those in the
Normo. group but the change was not significant. However, BUN and
Cr significantly decreased at 6 h of TH as compared to the Normo.
group (P=0.0068 and 0.0189, respectively; Fig. 2A and B).
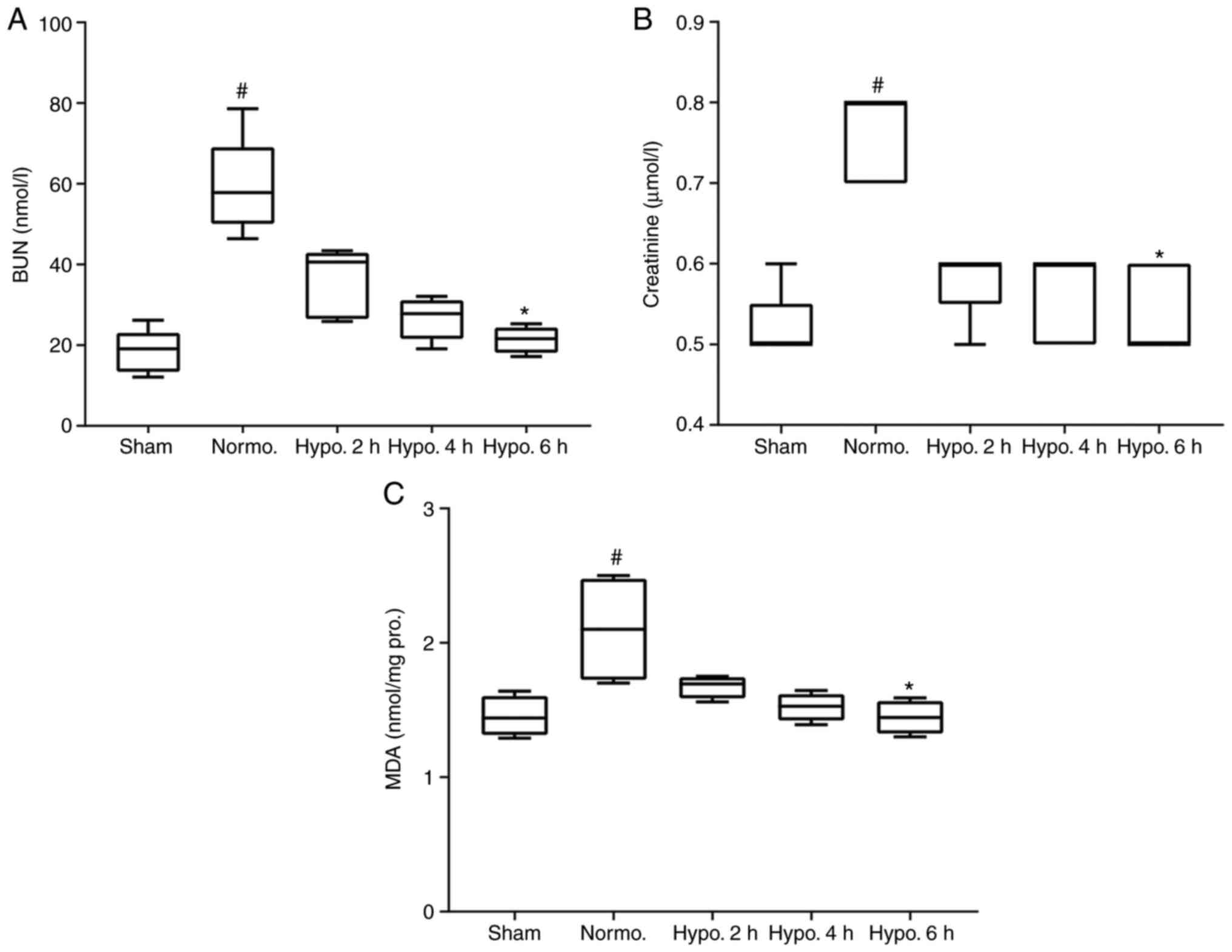 | Figure 2Serum BUN, Cr and MDA. Serum BUN,
serum Cr and MDA content of the renal tissues increased
significantly in the Normo. group compared to the sham group. The
level of (A) BUN, (B) serum Cr and (C) MDA decreased
insignificantly after 2 and 4 h of TH and increased significantly
after 6 h of TH. The level of BUN, Cr and MDA data are presented as
median and interquartile range. #P<0.05 compared with
the Sham group; *P<0.05 compared with the Normo.
group. Groups: Normo., Normothermia + CA; Hypo., TH after return of
spontaneous circulation following CA. BUN, blood urea nitrogen; Cr,
creatinine; MDA, malondialdehyde; TH, therapeutic hypothermia; CA,
cardiac arrest; pro., protein. |
MDA levels in renal tissues
MDA, a final product of lipid peroxidation, which is
induced by ROS production, was measured in renal tissues with an
MDA kit (13). The MDA
concentration significantly increased (P=0.0338) in the Normo.
group compared with that in the sham group. However, its level was
significantly decreased in the 6 h (P=0.0450) of TH groups. Its
level also decreased in the 2 h (P>0.05) and 4 h (P>0.05) of
TH group but not significantly compared with that in the Normo.
group (Fig. 2C).
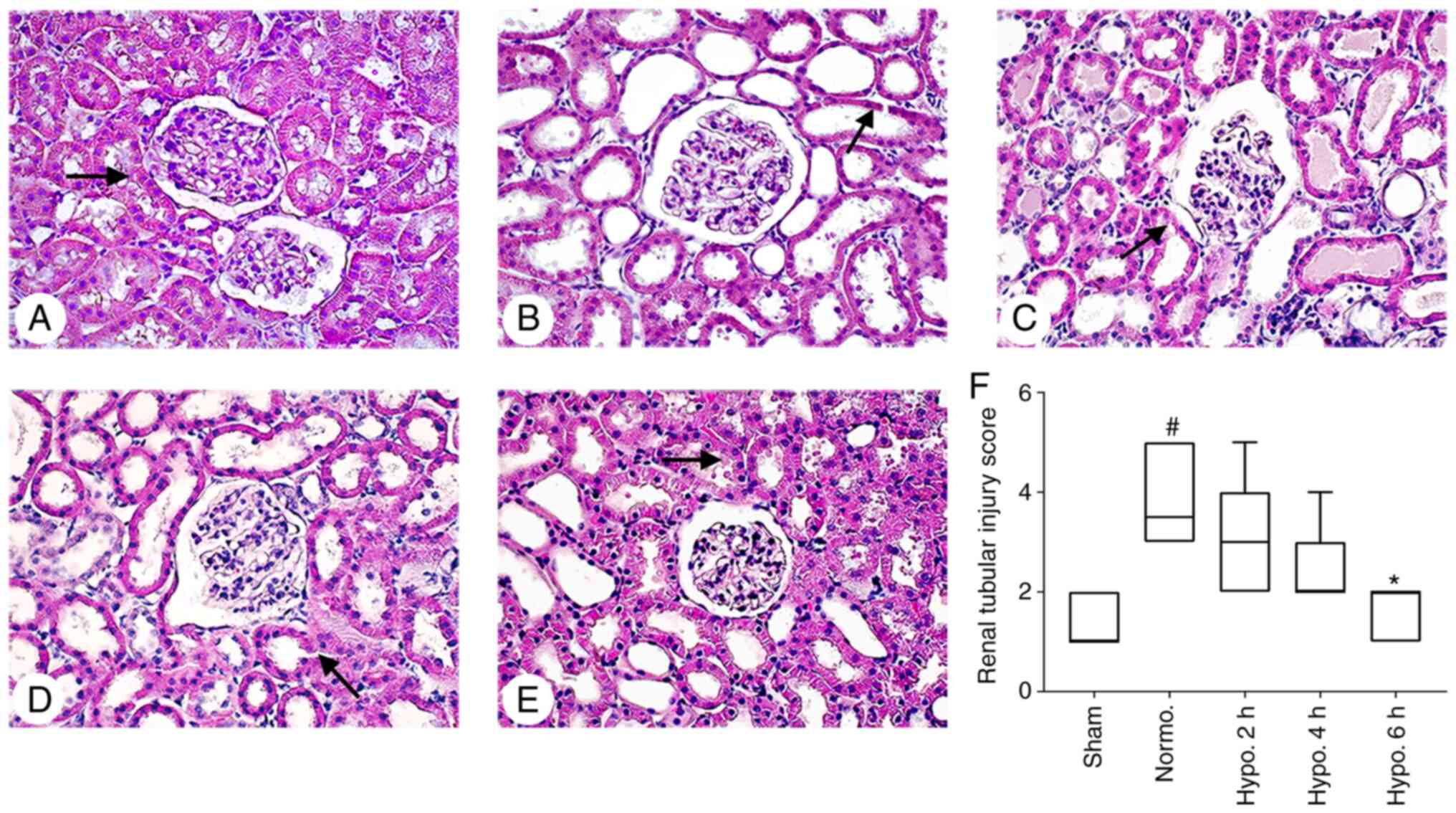
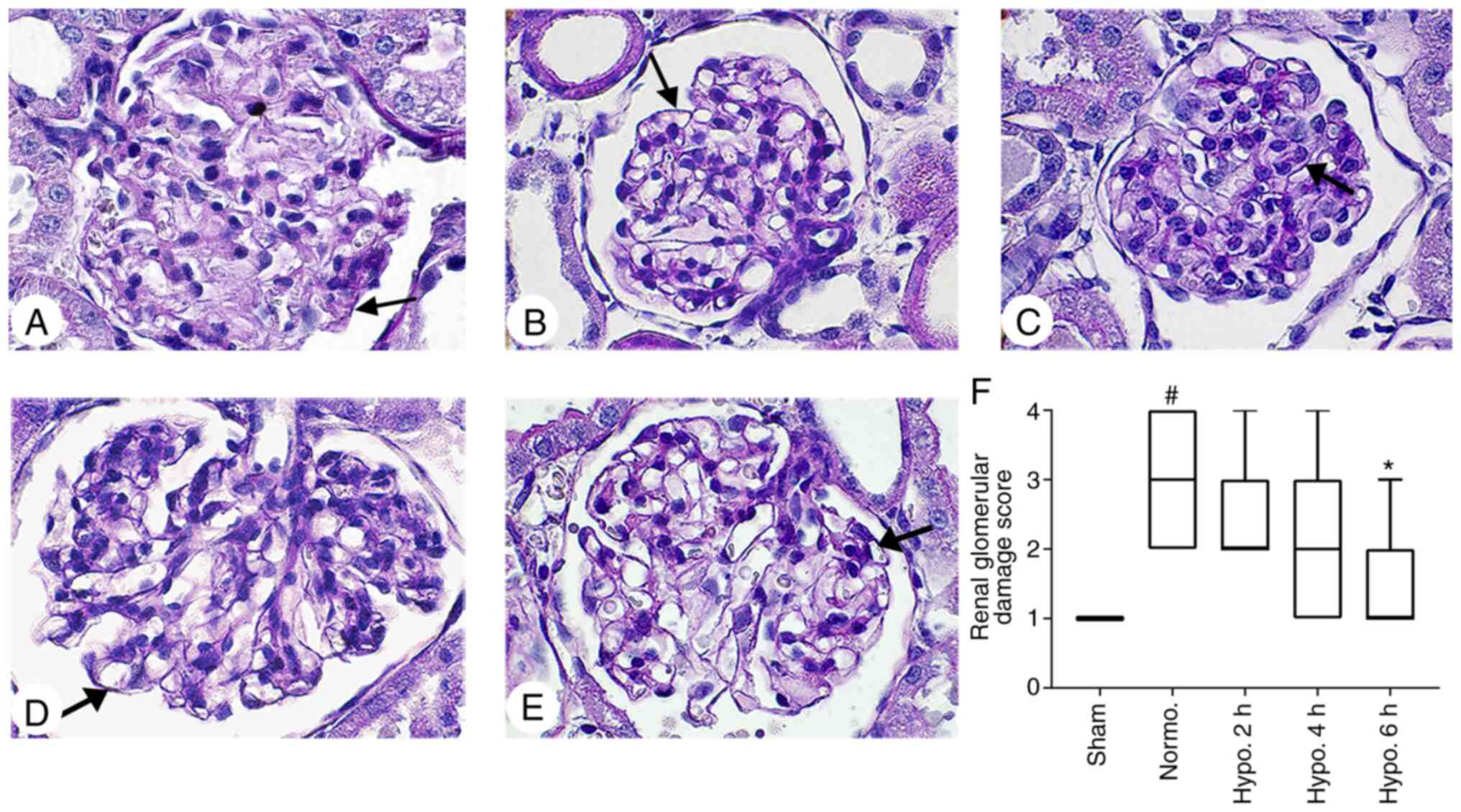
Histopathological damage
Renal histopathological changes were evaluated by
H&E and PAS staining (Figs. 3
and 4). No histopathological
changes were present in the kidneys of the Sham group (34). Kidney lesions were markedly
increased in the Normo. group compared with those in the Sham group
(P<0.0001). Histopathological damage was significantly decreased
(P=0.0046) in the group with 6 h of TH; however, it was not
significantly decreased in the groups with 2 h (P>0.05) and 4 h
(P>0.05) of TH when compared with the Normo. group. The brush
border of renal tubular epithelial cells was severely eroded.
Tubular dilatation and acute renal tubular necrosis were more
evident in the Normo. group when compared to the groups with 2, 4
and 6 h of TH. In addition, dilatation of glomerular capillaries
was severe in the Normo. group (P=0.0064) as compared to the sham
group; however, it was attenuated after 2 h (P>0.05), 4 h
(P>0.05) and 6 h (P=0.0460) of TH (Figs. 3 and 4).
Expression of Nrf2 and HO-1
IHC was performed to investigate the mechanisms of
the renoprotective effects of TH in each group. The expression of
Nrf2 and HO-1 increased in the Normo. group compared to the sham
group but without any statistical significance (P>0.05). The
ROD% of Nrf2 expression increased insignificantly at 2 h
(P>0.05) and 4 h (P>0.05), and was significantly increased at
6 h of TH (P=0.0002; Fig. 5).
Furthermore, HO-1 expression was significantly increased after 6 h
(P=0.007) of TH but not significantly after 2 h (P=0.079) and 4 h
(P=0.1089) of TH when compared with the Normo. group (Fig. 6).
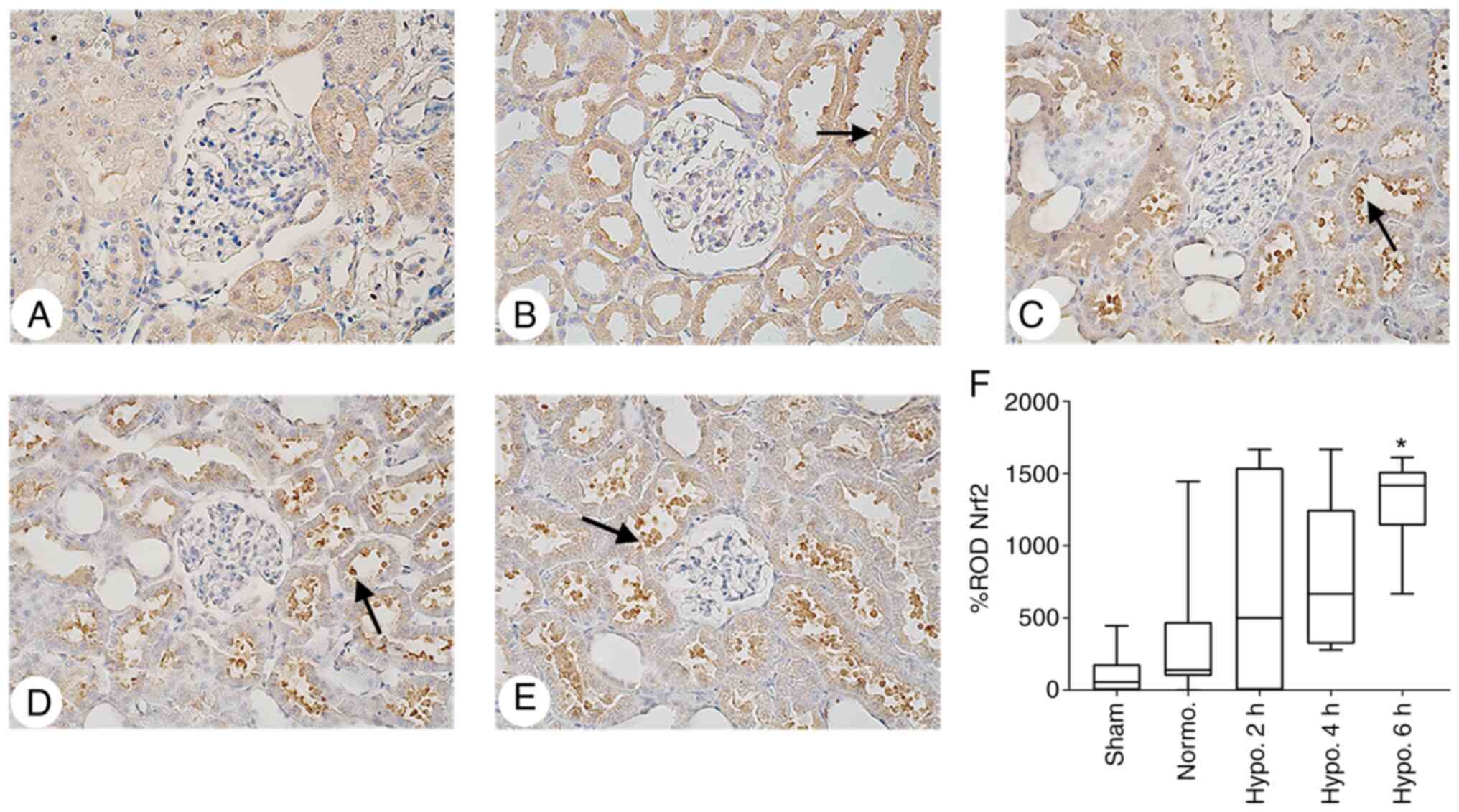 | Figure 5IHC analysis of Nrf2 expression in
renal tissues. Representative IHC staining images for Nrf2 in the
kidney tissues of (A) the Sham group, (B) Normo. group, (C) 2 h TH
group, (D) 4 h TH group and (E) 6 h TH group (positive staining
indicated by arrows; magnification, x400). It was revealed that TH
increased the expression of Nrf2 in the renal cortex. (F) The ROD%
of Nrf2 expression was significantly increased at 6 h of TH. ROD%
of Nrf2 data are expressed as the median with interquartile range.
*P<0.05 compared with the Normo. group. Groups:
Normo., Normothermia + CA; Hypo., TH after return of spontaneous
circulation following CA. TH, therapeutic hypothermia; CA, cardiac
arrest; Nrf2, nuclear erythroid-related factor-2; IHC,
immunohistochemical; ROD, relative optical density. |
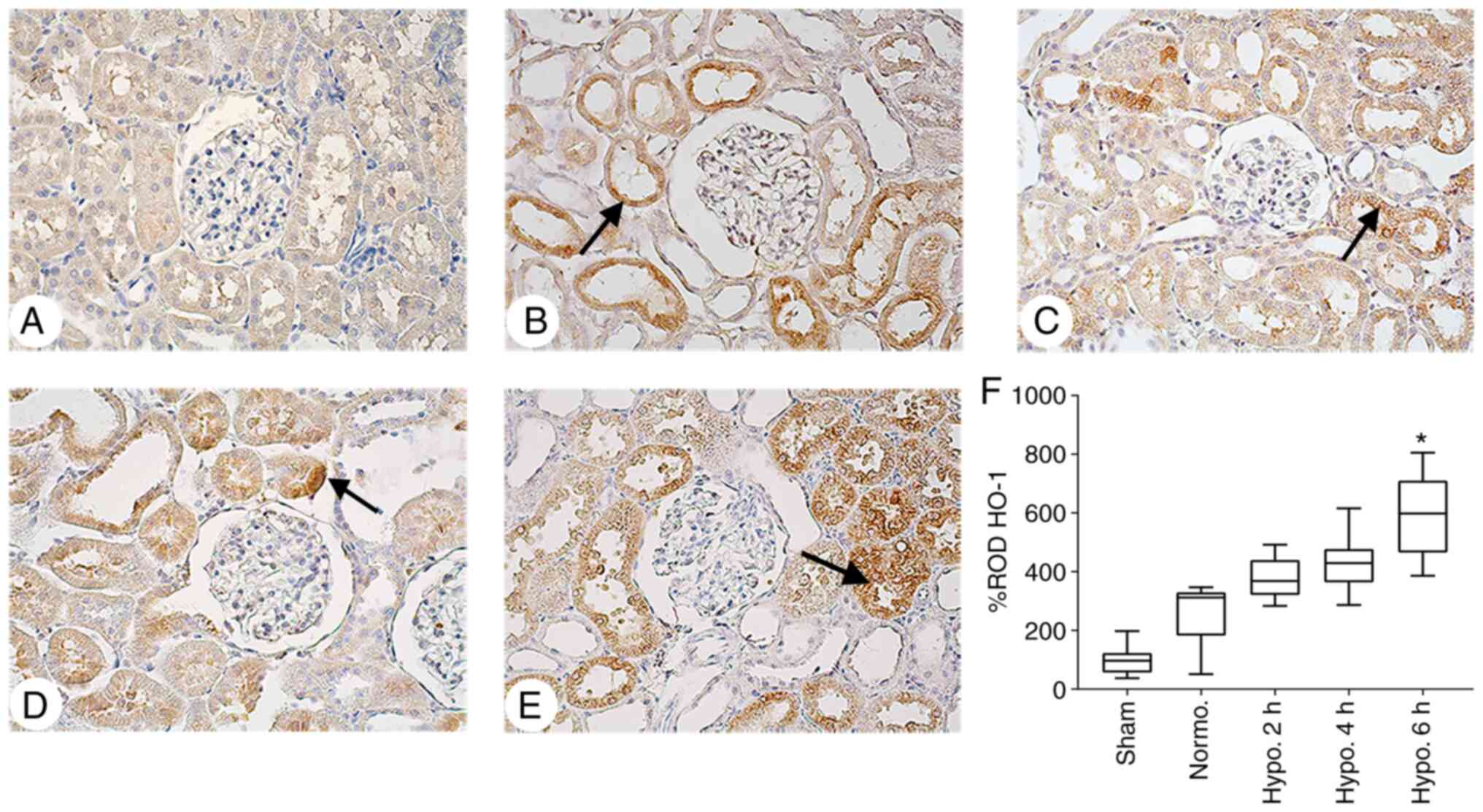 | Figure 6IHC analysis of HO-1 expression in
renal cortical tissues. Representative IHC staining images for HO-1
in the kidney tissues of (A) the Sham group, (B) Normo. group, (C)
2 h TH group, (D) 4 h TH group and (E) 6 h TH group (positive
staining indicated by arrows; magnification, x400). It was
indicated that TH increased HO-1 expression in the renal cortical
tissues. (F) The ROD% of HO-1 staining was elevated in the Normo.
vs. Sham group and increased significantly at 6 h of TH in
comparison with the Normo. group. ROD% of HO-1 data are expressed
as the median with interquartile range. *P<0.05
compared with the Normo. group. Groups: Normo., Normothermia + CA;
Hypo., TH after return of spontaneous circulation following CA. TH,
therapeutic hypothermia; CA, cardiac arrest; IHC,
immunohistochemical; ROD, relative optical density; HO-1, heme
oxygenase. |
Discussion
The present study demonstrated that TH decreased the
levels of BUN, serum Cr and MDA in renal tissues and increased the
expression of Nrf2 and HO-1 in a TH time-dependent manner. The
results suggested that TH ameliorated asphyxial CA-induced
ischemia/reperfusion (I/R) renal dysfunction and oxidative stress.
In the present asphyxial CA model, TH improved the survival rate
and attenuated histopathological damage following ROSC after CA
compared to those in the Normo. group. It is thus indicated that TH
effectively mitigates oxidative stress markers in renal injury and
increases the survival rate in a TH treatment time-dependent
manner.
I/R injury is defined as paradoxical aggravation of
cellular damage and death after the restoration of blood flow to
previously viable ischemic tissue (35). CA is a case of whole-body I/R injury
following ROSC characterized by multi-organ dysfunction (3) and acute kidney failure is an outcome
of whole-body I/R injury (24). In
the present study, acute renal tubular necrosis, proximal
convoluted tubule, brush border erosion and dilatation of renal
glomerular capillaries were more severe in the Normo. group
compared to those receiving 2, 4 and 6 h of TH after CA. Thus, it
was indicated that TH treatment decreased renal injury and
dysfunction in a TH treatment time-dependent manner.
The application of hypothermia treatment is a
controversial topic. In one study, it achieved no beneficial
effects on the survival of patients with CA following ROSC
(23). Previous studies
demonstrated that the survival rate of rats reached up to 7-8% at 2
days following ROSC after CPR in an asphyxial CA model (33,36). A
study on ventricular fibrillation CA also revealed a low survival
rate at 72 h following ROSC in rats (37). In the present study, the survival
rate of the rats in the Normo. group also decreased, which was
similar to previous results obtained with the asphyxial CA model
(33,36,37).
However, the present study reported that TH treatment increased the
survival rate of the rats in a TH treatment time-dependent manner
(23). Roberts et al
(3) demonstrated that TH
ameliorated kidney damage following ROSC; however, the injury
mechanism and attenuation via TH remained elusive. In the present
study, renal injury was attenuated with TH treatment in a
time-dependent manner. The previous and present results suggested
that TH treatment for various durations after ROSC is a favorable
factor for patients with CA and renal injury and may result in
improved outcomes (3).
Atypical or unnecessary ROS is involved in the
pathogenesis of tissue damage and injury (38). Guidet and Shah (39) reported that the capacity of the
kidney to produce ROS is not accompanied by a related defense
system against the resulting harm. A marked level of the
pro-oxidation production MDA is generated due to reaction of ROS
with components of cell membranes, and Grekas et al
(40) suggested that I/R injury
significantly elevated MDA production in renal tissues.
Furthermore, Xia et al (41)
reported that mild hypothermia decreased the MDA level in the
kidneys of RI/RI mice. Hackenhaar et al (42) reported that TH reduces serum MDA
concentration following ROSC after CA. In addition, Islam et
al (25) reported that TH
decreased the level of MDA in the renal tissues of their asphyxial
CA rat model. In the present study, MDA levels in the renal
cortical tissues following ROSC after asphyxial CA in rats were
investigated, revealing that TH ameliorated MDA levels in the
kidneys in a TH treatment time-dependent manner, which was in
agreement with other studies. In the present study, TH ameliorated
renal injury time-dependently and may be associated with an
increased survival rate.
Previous studies demonstrated that the levels of
kidney injury markers, MDA levels, renal histopathological ailments
and Nrf2/HO-1 expression levels increased at 24 h following 45 min
of RI/RI; however, in the present study, asphyxial CA induced 5 min
of whole-body I/R injury (13,31).
Despite the difference in ischemia duration and experimental
models, the present study demonstrated consistency with the
previous RI/RI rat models (13,31).
Thus, it is suggested that renal injury markers, MDA and renal
histopathological changes were attenuated and high expression level
of Nrf2/HO-1 was achieved at 24 h after return of spontaneous
circulation. Signaling pathways associated with asphyxial
CA-induced RI/RI remain to be fully elucidated. In the Nrf2
signaling pathway, under normal physiological conditions, Nrf2
binds with Kelch-like ECH-associated protein in the cytoplasm
(43). However, the oxidative
stress in pathological conditions may lead to dissociation of the
Nrf2-Keap1 complex (12,44), allowing Nrf2 to translocate into the
nucleus, where it binds with ARE and HO-1 and results in an offset
of cellular oxidative stress (12,44).
The Nrf2/HO-1 expression level was correlated with scavenging and
amelioration of ROS during the oxidative stress process (45). Therefore, Nrf2/HO-1 expression was
considered beneficial in the case of RI/RI (45). Xia and Zhang (46) demonstrated that mild hypothermia
significantly upregulated the expression of Nrf2/HO-1 in the
cerebral cortex and hippocampus following asphyxial CA of rats;
however, the role of mild TH on Nrf2 and HO-1 expression in
asphyxial CA-induced-renal ischemia has remained elusive. In the
present study, Nrf2 and HO-1 expression increased in the Normo.
group compared with that in the sham group; however, application of
TH for 2, 4 and 6 h increased the expression of Nrf2/HO-1 in the
renal cortical tissues in a TH treatment time-dependent manner.
Thus, it was indicated that immediate TH after CPR decreased the
renal oxidative stress effects of post cardiac arrest syndrome,
which is associated with post cardiac arrest myocardial
dysfunction, brain injury and systemic ischemia/reperfusion
response.
However, the present study still has certain
limitations. TH treatment was followed by gradual rewarming in
previous studies on the asphyxial CA and CPR model, in which the
focus was on the brain and myocardium and the results demonstrated
improved neurological outcome and myocardial function (29,47).
By contrast, rapid rewarming after TH treatment was reported to
improve the survival rate, histopathological ailments and renal
injury markers in the asphyxial CA rat model (29,47).
In the present study rapid rewarming was performed after
therapeutic hypothermia (2, 4 and 6 h) which was one of the
potential limitations and requires further investigation.
Furthermore, western blot analysis of Nrf2 and HO-1 expression, use
of Nrf2 and HO-1 inhibitors/agonists, the lack of an alternative
method for oxidative stress measurement and the lack of a variety
of different experiments to verify the results are potential
limitations of the present study and an objective of future studies
by our group.
In conclusion, in the present study, immediate TH
exhibited renoprotective effects against asphyxial CA-induced RI/RI
by inhibiting oxidative stress and increased the survival rate of
rats in a TH treatment time-dependent manner. It was indicated that
TH confers protection by decreasing ROS levels, increasing the
expression levels of Nrf2 and HO-1 in renal tissues and improving
the survival rate in a TH treatment time-dependent manner. In
short, immediate TH may be a favorable protective method for
asphyxial CA-induced RI/RI; however, its role in the relationship
among the renal system, heart and brain in CA remains elusive.
Further study is required to evaluate the underlying protective
mechanisms of TH in the kidneys via asphyxial CA-induced RI/RI in
rats.
Supplementary Material
Temperature diagram. Recording of
changes in rectal temperature during hypothermic treatment and
rewarming. Values are expressed as the mean ± standard error of the
mean. Groups: Normo., Normothermia + CA; Hypo., TH after return of
spontaneous circulation following CA. TH, therapeutic hypothermia;
CA, cardiac arrest.
Acknowledgements
Not applicable.
Funding
Funding: This research was supported by the Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education (grant nos.
NRF-2020R1I1A3070874, NRF-2019R1C1C1002564, NRF-2019R1F1A1062696
and 2019R1A6A1A03033084) and the Biomedical Research Institute of
Jeonbuk National University Hospital.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AJ, YJY, JHL and HJT were responsible for the
experimental design, data acquisition, data analysis and manuscript
writing. JHC, WST, HYS, SEK, MSI, EYL and KHK performed the
experiments and data analysis. DCA, BYP, JCY and ISK performed data
analyses and made critical comments on the entire process of the
study. All of the authors read and approved the final version of
manuscript. JHL and HJT confirm the authenticity of the raw
data.
Ethics approval and consent to
participate
All the procedures were performed in full compliance
with the recommendations in the guidelines of the Institutional
Animal Care and Use Committee of Jeonbuk National University
(approval no. JBNU 2019-005).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Girotra S, Chan PS and Bradley SM:
Post-resuscitation care following out-of-hospital and in-hospital
cardiac arrest. Heart. 101:1943–1949. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Forman-Hoffman VL, Ault KL, Anderson WL,
Weiner JM, Stevens A, Campbell VA and Armour BS: Disability status,
mortality, and leading causes of death in the United States
community population. Med Care. 53:346–354. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Roberts BW, Kilgannon JH, Chansky ME,
Mittal N, Wooden J, Parrillo JE and Trzeciak S: Multiple organ
dysfunction after return of spontaneous circulation in postcardiac
arrest syndrome. Crit Care Med. 41:1492–1501. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Damman K, Valente MA, Voors AA, O'Connor
CM, van Veldhuisen DJ and Hillege HL: Renal impairment, worsening
renal function, and outcome in patients with heart failure: An
updated meta-analysis. Eur Heart J. 35:455–469. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hasper D, von Haehling S, Storm C, Jörres
A and Schefold JC: Changes in serum creatinine in the first 24
hours after cardiac arrest indicate prognosis: An observational
cohort study. Crit Care. 13(R168)2009.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Geri G, Guillemet L, Dumas F, Charpentier
J, Antona M, Lemiale V, Bougouin W, Lamhaut L, Mira JP, Vinsonneau
C and Cariou A: Acute kidney injury after out-of-hospital cardiac
arrest: Risk factors and prognosis in a large cohort. Intensive
Care Med. 41:1273–1280. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Laurent I, Monchi M, Chiche JD, Joly LM,
Spaulding C, Bourgeois B, Cariou A, Rozenberg A, Carli P, Weber S
and Dhainaut JF: Reversible myocardial dysfunction in survivors of
out-of-hospital cardiac arrest. J Am Coll Cardiol. 40:2110–2116.
2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Madl C and Holzer M: Brain function after
resuscitation from cardiac arrest. Curr Opin Crit Care. 10:213–217.
2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nath KA and Norby SM: Reactive oxygen
species and acute renal failure. Am J Med. 109:665–678.
2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tsuda H, Kawada N, Kaimori JY, Kitamura H,
Moriyama T, Rakugi H, Takahara S and Isaka Y: Febuxostat suppressed
renal ischemia-reperfusion injury via reduced oxidative stress.
Biochem Biophys Res Commun. 427:266–272. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Feng L, Ke N, Cheng F, Guo Y, Li S, Li Q
and Li Y: The protective mechanism of ligustrazine against renal
ischemia/reperfusion injury. J Surg Res. 166:298–305.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jaiswal AK: Nrf2 signaling in coordinated
activation of antioxidant gene expression. Free Radic Biol Med.
36:1199–1207. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jiang G, Liu X, Wang M, Chen H, Chen Z and
Qiu T: Oxymatrine ameliorates renal ischemia-reperfusion injury
from oxidative stress through Nrf2/HO-1 pathway. Acta Cir Bras.
30:422–429. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang Y, Rong S, Feng Y, Zhao L, Hong J,
Wang R and Yuan W: Simvastatin attenuates renal
ischemia/reperfusion injury from oxidative stress via targeting
Nrf2/HO-1 pathway. Exp Ther Med. 14:4460–4466. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Williams GR Jr and Spencer FC: The
clinical use of hypothermia following cardiac arrest. Ann Surg.
148:462–468. 1958.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Palmers PJ, Hiltrop N, Ameloot K,
Timmermans P, Ferdinande B, Sinnaeve P, Nieuwendijk R and Malbrain
ML: From therapeutic hypothermia towards targeted temperature
management: A decade of evolution. Anaesthesiol Intensive Ther.
47:156–161. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hypothermia after Cardiac Arrest Study
Group. Mild therapeutic hypothermia to improve the neurologic
outcome after cardiac arrest. N Engl J Med. 346:549–556.
2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ostadal P, Mlcek M, Kruger A, Horakova S,
Skabradova M, Holy F, Svoboda T, Belohlavek J, Hrachovina V,
Taborsky L, et al: Mild therapeutic hypothermia is superior to
controlled normothermia for the maintenance of blood pressure and
cerebral oxygenation, prevention of organ damage and suppression of
oxidative stress after cardiac arrest in a porcine model. J Transl
Med. 11(124)2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gong P, Li CS, Hua R, Zhao H, Tang ZR, Mei
X, Zhang MY and Cui J: Mild hypothermia attenuates mitochondrial
oxidative stress by protecting respiratory enzymes and upregulating
MnSOD in a pig model of cardiac arrest. PLoS One.
7(e35313)2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dohi K, Miyamoto K, Fukuda K, Nakamura S,
Hayashi M, Ohtaki H, Shioda S and Aruga T: Status of systemic
oxidative stress during therapeutic hypothermia in patients with
post-cardiac arrest syndrome. Oxid Med Cell Longev.
2013(562429)2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Legriel S, Lemiale V, Schenck M, Chelly J,
Laurent V, Daviaud F, Srairi M, Hamdi A, Geri G, Rossignol T, et
al: Hypothermia for neuroprotection in convulsive status
epilepticus. N Engl J Med. 375:2457–2467. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Moler FW, Silverstein FS, Holubkov R,
Slomine BS, Christensen JR, Nadkarni VM, Meert KL, Browning B,
Pemberton VL, Page K, et al: Therapeutic hypothermia after
in-hospital cardiac arrest in children. N Engl J Med. 376:318–329.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nielsen N, Wetterslev J, Cronberg T,
Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J,
Kuiper M, et al: Targeted temperature management at 33˚C versus
36˚C after cardiac arrest. N Engl J Med. 369:2197–2206.
2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tujjar O, Mineo G, Dell'Anna A,
Poyatos-Robles B, Donadello K, Scolletta S, Vincent JL and Taccone
FS: Acute kidney injury after cardiac arrest. Crit Care.
19(169)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Islam A, Kim SE, Yoon JC, Jawad A, Tian W,
Yoo YJ, Kim IS, Ahn D, Park BY, Hwang Y, et al: Protective effects
of therapeutic hypothermia on renal injury in an asphyxial cardiac
arrest rat model. J Thermal Biol. 94(102761)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Drabek T, Foley LM, Janata A, Stezoski J,
Hitchens TK, Manole MD and Kochanek PM: Global and regional
differences in cerebral blood flow after asphyxial versus
ventricular fibrillation cardiac arrest in rats using ASL-MRI.
Resuscitation. 85:964–971. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Aoki T, Okuma Y, Becker LB, Hayashida K
and Shinozaki K: Methodological issue of mitochondrial isolation in
acute-injury rat model: Asphyxia cardiac arrest and resuscitation.
Front Med (Lausanne). 8(666735)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Junyun H, Hongyang L, Ruoxian D, Young L,
Shanbao T and Xiaofeng J: Real-time monitoring of cerebral blood
flow by laser speckle contrast imaging after cardiac arrest in rat.
Annu Int Conf IEEE Eng Med Biol Soc. 2015:6971–6974.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lu J, Qian HY, Liu LJ, Zhou BC, Xiao Y,
Mao JN, An GY, Rui MZ, Wang T and Zhu CL: Mild hypothermia
alleviates excessive autophagy and mitophagy in a rat model of
asphyxial cardiac arrest. Neurol Sci. 35:1691–1699. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Park Y, Ahn JH, Cho JH, Tae HJ, Lee TK,
Kim B, Lee JC, Park JH, Shin MC, Ohk TG, et al: Effects of
hypothermia on inflammatory cytokine expression in rat liver
following asphyxial cardiac arrest. Exp Ther Med.
21(626)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tong F and Zhou X: The Nrf2/HO-1 pathway
mediates the antagonist effect of L-arginine on renal
ischemia/reperfusion injury in rats. Kidney Blood Press Res.
42:519–529. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Akanda MR, Kim IS, Ahn D, Tae HJ, Nam HH,
Choo BK, Kim K and Park BY: Anti-inflammatory and gastroprotective
roles of rabdosia inflexa through downregulation of
pro-inflammatory cytokines and MAPK/NF-kappaB signaling pathways.
Int J Mol Sci. 19(584)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Park Y, Tae HJ, Cho JH, Kim IS, Ohk TG,
Park CW, Moon JB, Shin MC, Lee TK, Lee JC, et al: The relationship
between low survival and acute increase of tumor necrosis factor α
expression in the lung in a rat model of asphyxial cardiac arrest.
Anat Cell Biol. 51:128–135. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kocoglu H, Ozturk H, Ozturk H, Yilmaz F
and Gulcu N: Effect of dexmedetomidine on ischemia-reperfusion
injury in rat kidney: A histopathologic study. Renal Failure.
31:70–74. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Collard CD and Gelman S: Pathophysiology,
clinical manifestations, and prevention of ischemia-reperfusion
injury. Anesthesiology. 94:1133–1138. 2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tae HJ, Kang IJ, Lee TK, Cho JH, Lee JC,
Shin MC, Kim YS, Cho JH, Kim JD, Ahn JH, et al: Neuronal injury and
tumor necrosis factor-alpha immunoreactivity in the rat hippocampus
in the early period of asphyxia-induced cardiac arrest under
normothermia. Neural Regen Res. 12:2007–2013. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Janata A, Magnet IA, Schreiber KL, Wilson
CD, Stezoski JP, Janesko-Feldman K, Kochanek PM and Drabek T:
Minocycline fails to improve neurologic and histologic outcome
after ventricular fibrillation cardiac arrest in rats. World J Crit
Care Med. 8:106–119. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
McCord JM: Oxygen-derived free radicals in
postischemic tissue injury. N Engl J Med. 312:159–163.
1985.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Guidet BR and Shah SV: In vivo generation
of hydrogen peroxide by rat kidney cortex and glomeruli. Am J
Physiol. 256:F158–F164. 1989.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Grekas D, Dioudis C, Papageorgiou G,
Iliadis S, Zilidis C, Alivanis P, Dimitriadou A and Tourkantonis A:
Lipid peroxidation after acute renal ischemia and reperfusion in
rats: The effect of trimetazidine. Ren Fail. 18:545–552.
1996.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Xia Z, Wang W, Xiao Q, Ye Q, Zhang X and
Wang Y: Mild hypothermia protects renal function in
ischemia-reperfusion kidney: An experimental study in mice.
Transplant Proc. 50:3816–3821. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hackenhaar FS, Medeiros TM, Heemann FM,
Behling CS, Putti JS, Mahl CD, Verona C, da Silva ACA, Guerra MC,
Gonçalves CAS, et al: Therapeutic hypothermia reduces oxidative
damage and alters antioxidant defenses after cardiac arrest. Oxid
Med Cell Longev. 2017(8704352)2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kim HJ and Vaziri ND: Contribution of
impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in
chronic renal failure. Am J Physiol Renal Physiol. 298:F662–F671.
2010.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kobayashi M and Yamamoto M: Molecular
mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene
regulation. Antioxid Redox Signal. 7:385–394. 2005.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhang L, Zhu Z, Liu J, Zhu Z and Hu Z:
Protective effect of N-acetylcysteine (NAC) on renal
ischemia/reperfusion injury through Nrf2 signaling pathway. J
Recept Signal Transduct Res. 34:396–400. 2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Xia D and Zhang H: Effects of mild
hypothermia on expression of NF-E2-related factor 2 and
heme-oxygenase-1 in cerebral cortex and hippocampus after
cardiopulmonary resuscitation in rats. Iran J Basic Med Sci.
20:1002–1008. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lu X, Ma L, Sun S, Xu J, Zhu C and Tang W:
The effects of the rate of postresuscitation rewarming following
hypothermia on outcomes of cardiopulmonary resuscitation in a rat
model. Crit Care Med. 42:e106–113. 2014.PubMed/NCBI View Article : Google Scholar
|