Introduction
With the development of therapeutic strategies and
technologies, the dental retention rates are increasing every year;
however, certain non-reversible diseases, such as caries, can cause
tooth loss, periodontal diseases and tooth trauma (1,2).
Although numerous novel materials for replacing lost teeth have
been identified, various deficiencies in the reliability and
tolerance of these materials exist (3).
Stem cells have been extensively applied in the
treatment of tooth loss, and this method has been demonstrated to
be a novel strategy (4),
particularly in tissue engineering technology, which can induce
stem cell differentiation into bioactive teeth. Teeth
generation-associated stem cells mainly include dental stem cells
and non-dental stem cells. The unavailability of dental stem cells,
including dental pulp stem cells, dental follicle stem cells and
periodontal ligament stem cells, limit their wide clinical
application (5,6). Non-dental stem cells are characterized
by weak differentiation and inability to convert into bioactive
stem cells (5,6). Despite the fact that embryonic stem
cells have an infinite proliferative ability and high self-renewal
capability, they are affected by immune rejection and ethical
controversies (7). In previous
years, pluripotent stem cells (PSCs) have been prepared by
transplanting nuclei separated from mother cells into somatic
cells; however, the obtained PSCs exhibit decreased synthesis
efficacy (8). In 2006, Japanese
scientists successfully synthesized a category of multipotent cells
with characteristics of embryonic stem cells, named induced PSCs
(iPSCs) (9). A recent study has
indicated that iPSCs can differentiate into several functional
cells, including nerve, islet secretory, hematopoietic, liver and
renal cells (10). Additionally, a
previous study has revealed that co-culturing iPSCs with
odontogenic cells in microenvironments containing specific growth
factors can differentiate them into ameloblasts and odontoblasts
(11). Therefore, iPSCs are
considered to be the main source of seed cells for inducing new
teeth and triggering dental replacement therapy.
In mature teeth, the pulp-dentin complex has
powerful regenerative functions (9,10).
However, when dental caries or injuries occur, the specific
pathological environment induces the release of bioactive
extra-cellular matrix (ECM) and further initiates dentin repair
(12). The ECM is composed of
growth factors and bioactive factors, such as TGF-β, insulin-like
growth factor-1, fibroblast growth factor (FGF) and vascular
endothelial growth factor, and serves critical roles in cell
adhesion, proliferation, differentiation and angiogenesis (13,14).
Additionally, the ECM contains collagen I and various non-collagen
components, such as dentine sialoprotein, dentine phosphoprotein,
dentin matrix protein 1 (DMP-1), osteopontin and bone sialoprotein,
which are beneficial for the formation of the main part of teeth
(15,16). Furthermore, dentin non-collagen
proteins (DNCPs), which consist of sialoproteins, glycoproteins,
proteoglycans, phosphoproteins and growth factors, are involved in
the modulation of dentin mineralization and the promotion of cell
differentiation (17,18).
Stem cell-associated tissue regeneration mainly
involves three aspects, including seed cells, growth factors and
scaffold materials. Among these factors, the appropriate growth
factor [such as transforming growth factor β (TGF-β)] is considered
to be the most important one for simulating the growth processes of
natural teeth. Bone morphogenetic protein (BMP), a type of
multifunctional glycoprotein, belongs to the TGF-β protein family,
and serves crucial roles in treating bone defects, osteoporosis and
periodontal diseases (19,20). BMPs includes >20 subtypes, of
which BMP-4 and BMP-2 are the proteins most associated with dental
development (19). Previous studies
have reported that targeted inactivation of BMP-2 and BMP-4 causes
damage of mature odontoblasts and root development defects
(20,21). The BMP signaling pathway can
regulate dentin sialophosphoprotein (DSPP) gene expression, which
is the specific terminal differentiation gene with the highest
expression in the odontoblasts (22). It has been previously reported that
transfection of plasmids containing BMP-4 and paired
box-containing-9 induces iPSC differentiation into dentin-like
cells (23,24). Therefore, the present study
investigated the roles of DNCP-associated microenvironment
(containing specific growth factors) in the differentiation of
iPSCs into dentin cells.
Materials and methods
iPSC culture
Mouse iPSCs (OSKM strain, a type of iPSCs) were
purchased from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences, and the cells were maintained
according to a previous study (25). The OSKM strain of iPSCs was
established from adult human somatic stem cells through the
transient expression of c-Myc (OSKM) transcription factor
reprogramming technology (26). The
iPSCs were cultured by seeding a layer of feeder cells, which could
not secrete various growth factors and promote the proliferation
and differentiation of iPSCs, keeping their pluripotency (27). The seeded layer of feeder cells was
prepared and treated as previously described (28). In brief, iPSCs were cultured in the
presence of mitomycin C (Thermo Fisher Scientific, Inc.)-arrested
mouse embryonic fibroblasts (MEFs; The Cell Bank of Type Culture
Collection of The Chinese Academy of Sciences) and human iPSCs
medium consisting of DMEM (Gibco; Thermo Fisher Scientific, Inc.),
10% FBS (Gibco; Thermo Fisher Scientific, Inc.), L-glutamine (EMD
Millipore), 2-mercaptoethanol (Sigma-Aldrich; Merck KGaA), leukemia
inhibitory factor (LIF; AmyJet Scientific, Inc.) and non-essential
amino acids (Gibco; Thermo Fisher Scientific, Inc.) at 37˚C in 5%
CO2, and were passaged every 36 h using Tryp LE Express
(Invitrogen; Thermo Fisher Scientific, Inc.). In order to exclude
differentiated iPSCs, puromycin (1 µg/ml) was added to DMEM, and
the undifferentiated iPSCs were monitored by detecting Nanog-GFP
expression using a FACS Calibur flow cytometer (BD Biosciences)
equipped with Cell Quest software 6.0 (BD Biosciences), as
previously described (29).
Embryoid bodies (EBs) were generated and cultured using the
hanging-drop approach, according to a previous study (30). The formed EBs were observed by the
light microscopy (ECLIPSE Ti-S; Nikon Corporation) at a
magnification of x400.
Immunofluorescence staining for
identifying iPSCs
iPSCs were fixed using 4% paraformaldehyde (Beyotime
Institute of Biotechnology) for 15 min, treated with 0.1% Triton
X-100 (Beyotime Institute of Biotechnology) for 10 min and then
blocked using 5% FBS (Gibco; Thermo Fisher Scientific, Inc.) for 20
min, all at 37˚C. Subsequently, iPSCs were treated with mouse
anti-mouse Oct-4 monoclonal antibody (cat. no. ab184665; 1:3,000;
Abcam) and mouse anti-mouse Sox-2 monoclonal antibody (cat. no.
ab171380; 1:3,000; Abcam) at 4˚C overnight. The primary antibodies
were detected using Alexa Fluor 488 (green fluorescence)-labeled
goat anti-mouse IgG (cat. no. A-10684, 1:1,000; Thermo Fisher
Scientific, Inc.) at room temperature for 2 h. iPSCs were washed
with PBS and mounted in DMEM containing DAPI (Sigma-Aldrich; Merck
KGaA) for 5 min for nuclei visualization, and then washed three
times with PBS (5 min each wash). Finally, the stained iPSCs were
visualized by laser-scanning confocal microscopy (magnification,
x400; FV1000; Olympus Corporation).
Differentiation of iPSCs and trial
grouping
For odontoblastic generation, iPSCs were seeded at a
density of 5×104 cells/well in 60-mm culture plates (Corning,
Inc.). When the iPSCs reached 50-60% confluence, DMEM was
substituted by induction medium (DMEM containing DNCP solution at
dosage of 500 ng/ml). In this study, the DNCP was prepared
according to a previous study (31). iPSCs were cultured for 48 h at 37˚C,
and the optimal concentration of DNCP (including 10, 100, 500,
1,000 and 10,000 ng/ml) was evaluated using an MTT assay. For the
MTT assay, iPSCs were treated with MTT (Amresco Inc.) at dose of 5
mg/ml and the purple formazan was dissolved with 150 µl dimethyl
sulfoxide (DMSO, Amresco Inc.) at 37˚C. Finally, the absorbance was
measured at wavelength of 570 nm. In order to determine effects of
BMP and/or Noggin (an inhibitor of BMP) on iPSCs, the
differentiated iPSCs were sub-divided into control (treated without
any reagents), DNCP (treated with 500 ng/ml DNCP), DNCP+BMPs group
(co-treated with 500 ng/ml DNCP, 25 ng/ml BMP-2 and 25 ng/ml BMP-4)
and DNCP+Noggin [co-treated with 500 ng/ml DNCP and 25 ng/ml Noggin
(an inhibitor of BMP)] groups. In the above groups, the iPSCs were
treated with the DNCP, BMPs and/or Noggin and cultured 37˚C for 10
continuous days, with change of medium every day. BMP-2 and BMP-4
were purchased from R&D Systems, Inc., while Noggin was
purchased from PeproTech, Inc.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from iPSCs using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.).
cDNA was generated using the cDNA Synthesis kit (cat. no. 18080200;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. The mRNA expression levels of Msh homeobox 1 (Msx-1),
DSPP and DMP-1 were evaluated using a SYBR Green I PCR kit (Thermo
Fisher Scientific, Inc.) with specific primers (Table I). qPCR was conducted using a Master
cycler Gradient PCR system (Eppendorf). The thermal cycles for the
PCR assay were listed as the following conditions: 95˚C for 2 min,
40 cycles at 95˚C for 30 sec, 56˚C for 45 sec, 72 ˚C for 45 sec and
followed with 95˚C for 15 sec, 60˚C for 15 sec. The relative gene
transcription of targeting genes were normalized to the control
gene GAPDH and analyzed with a professional gel scanning system
(GDS8000; Analytik Jena AG). The gene expression levels were
analyzed using the 2-∆∆Cq method (32).
 | Table IPrimers used for reverse
transcription-quantitative PCR. |
Table I
Primers used for reverse
transcription-quantitative PCR.
| Genes | Primers |
|---|
| Msx-1 | F:
5'-GAGACCAGAGGCCAAAAGG-3' |
| | R:
5'-GGACCGGAAGCAGCTGAT-3' |
| DSPP | F:
5'-GTGAGGACAAGGACGAATCTGA-3' |
| | R:
5'-CACTACTGTCACTGCTGTCACT-3' |
| DMP-1 | F:
5'-CATTCTCCTTGTGTTCCTTTGGG-3' |
| | R:
5'-TGTGGTCACTATTTGCCTGTG-3' |
| GAPDH | F:
5'-GCTGGCGCTGAGTACGTCGT-3' |
| | R:
5'-ACGTTGGCAGTGGGGACACG-3' |
Western blotting
iPSCs were lysed using Cell Lysis Buffer (cat. no.
P0013; Beyotime Institute of Biotechnology), and total protein was
extracted using a Protein Extraction kit (cat. no. P0033; Beyotime
Institute of Biotechnology). The proteins in each group (20 µg per
sample) were separated by 12% SDS-PAGE and then electro-transferred
onto nitrocellulose (NC) membranes (Bio-Rad Laboratories, Inc.)
with Bio-Rad170-3940 Semi-Dry Electrophoretic Transfer (Bio-Rad
Laboratories, Inc.). NC membranes were then incubated with rabbit
anti-mouse p38 polyclonal antibody (pAb) (cat. no. sc-728;
1:2,000), anti-phosphorylated (p)-p38 pAb (cat. no. sc-17852;
1:2,000), anti-Smad pAb (cat. no. sc-6031; 1:2,000), anti-p-Smad
pAb (cat. no. sc-517575; 1:2,000) and anti-GAPDH pAb (cat. no.
sc-25778; 1:2,000) at 4˚C overnight. Next, the NC membranes were
incubated with HRP-labeled goat anti-rabbit IgG secondary
antibodies (cat. no. sc-2004; 1:2,000) at room temperature for 2 h.
All the primary and secondary antibodies were purchased from Santa
Cruz Biotechnology, Inc. Finally, the NC membranes were incubated
with the components of an ECL kit (EMD Millipore) for 2 min in the
dark. The western blotting bands were analyzed with Labworks™
Analysis Software 4.0 (Analytik Jena AG).
Statistical analysis
The data are represented as the mean ± SD. The data
were analyzed with SPSS software 23.0 (IBM Corp.). The one-way
ANOVA followed by Tukey's post-hoc test was used to compare the
differences among multiple groups. P<0.05 was considered to
indicate a statistically significant difference. All experiments or
tests were conducted for at least 6 repeats.
Results
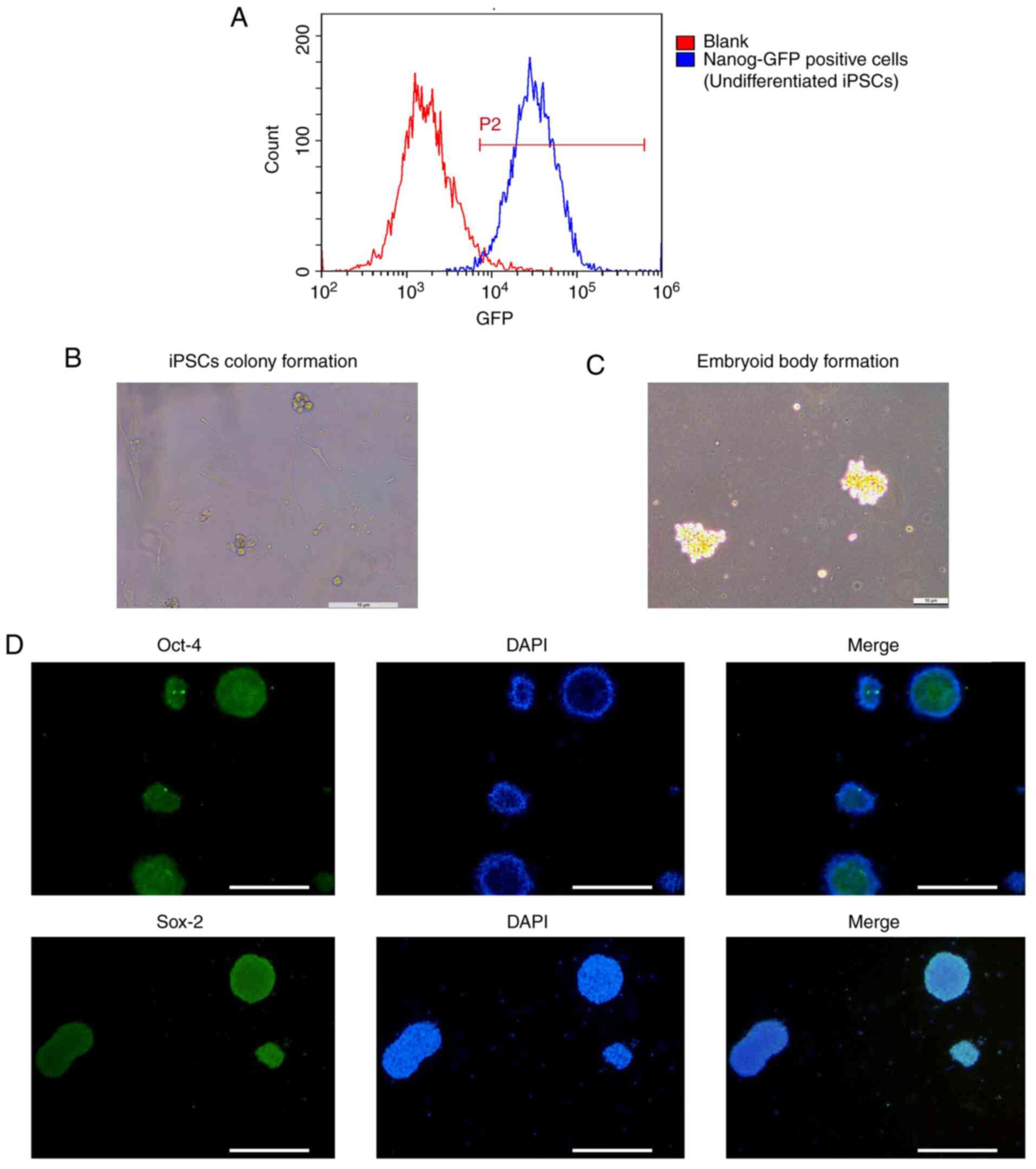
Undifferentiated state of iPSCs
Undifferentiated iPSCs were monitored by detecting
Nanog-GFP expression via flow cytometry. The flow cytometry results
indicated that the population of Nanog-GFP-positive iPSCs reached
97.88% (Fig. 1A), suggesting that
the iPSCs were in an undifferentiated state.
Induction of iPSCs and EBs
At ~5 days post co-culture, iPSCs appeared
surrounded by MEFs (Fig. 1B). Clone
clusters of iPSCs exhibited a small and irregular spherical shape
with clear borders. EBs were also generated successfully and had a
spherical-shape morphology (Fig.
1C).
Identification of iPSCs
In the present study, iPSCs were identified using an
immunofluorescence staining method. The results indicated that the
multifunctional genes Oct-4 and Sox-2 were positively expressed in
iPSCs (Fig. 1D), suggesting that
iPSCs exhibited increased pluripotency at the primary stage of
EBs.
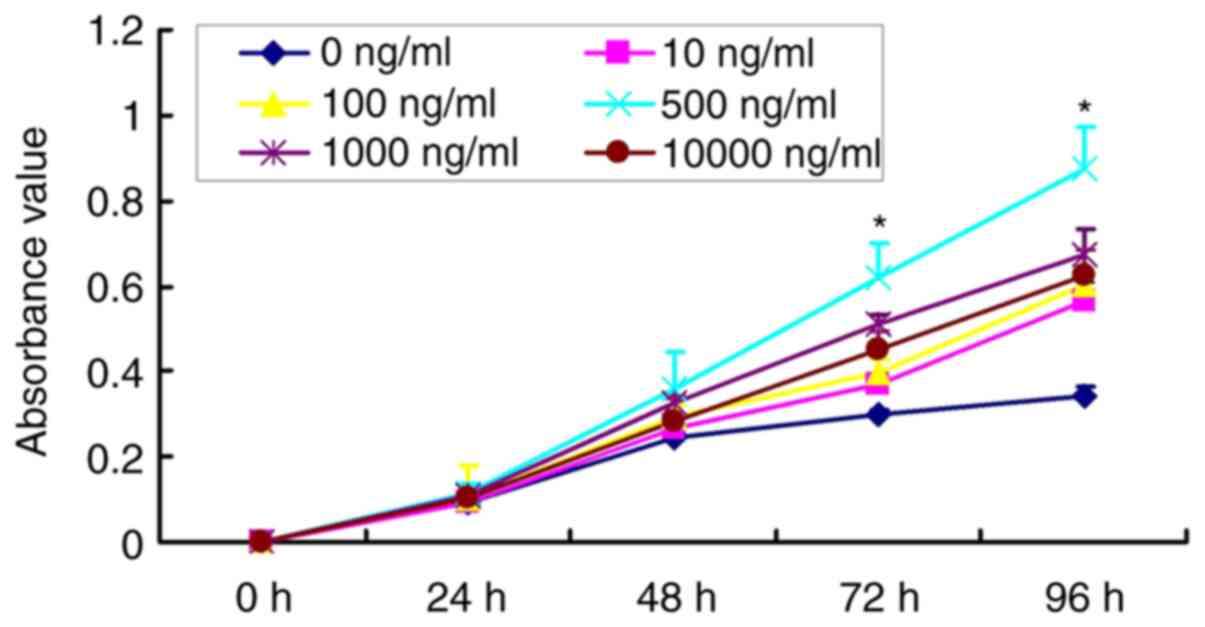
Optimal concentration of DNCP for
iPSCs differentiation
The results revealed that the proliferative rates of
iPSCs were increased after treatment with DNCP for a prolonged time
(Fig. 2). However, the
proliferative rate in iPSCs treated with 500 ng/ml DNCP for 72 and
96 h was significantly higher compared with that in the other DNCP
groups at the respective time points (P<0.05; Fig. 2). This result suggested that 500
ng/ml DNCP was the optimal concentration for iPSC differentiation
and was therefore employed in subsequent experiments.
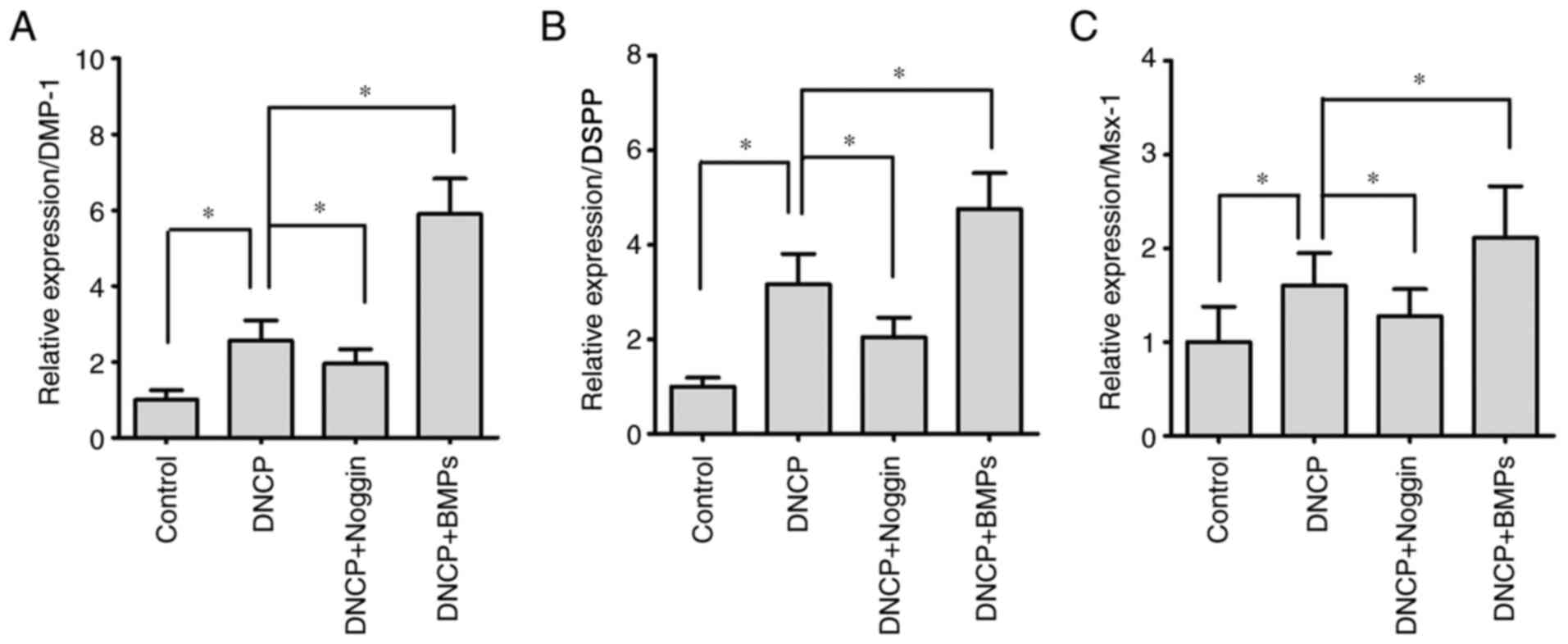
Differentiated iPSCs exhibit higher
levels of odontoblastic biomarkers
In the present study, the odontoblastic biomarkers
DSPP and DMP-1(33) were examined
by RT-qPCR. The results revealed that the expression levels of
DMP-1 (Fig. 3A) and DSPP (Fig. 3B) were significantly higher in the
DNCP group compared with those in the Control group (P<0.05).
For iPSCs undergoing DNCP and Noggin treatment, the levels of DMP-1
(Fig. 3A) and DSPP (Fig. 3B) were significantly downregulated
compared with those of iPSCs treated only with DNPC (P<0.05).
Furthermore, BMPs treatment significantly enhanced the expression
levels of DMP-1 (Fig. 3A) and DSPP
(Fig. 3B) in DNCP-treated iPSCs
(P<0.05).
Differentiated iPSCs exhibit higher
Msx-1 expression
Msx-1, a key molecule of the BMP/Smad signaling
pathway, is closely associated with bone and teeth formation. The
present results demonstrated that DNCP treatment significantly
enhanced Msx-1 expression compared with the control group
(P<0.05; Fig. 3C). However,
Noggin treatment significantly decreased Msx-1 expression
(P<0.05), while BMPs treatment significantly increased Msx-1
expression in DNCP-treated iPSCs (P<0.05; Fig. 3C).
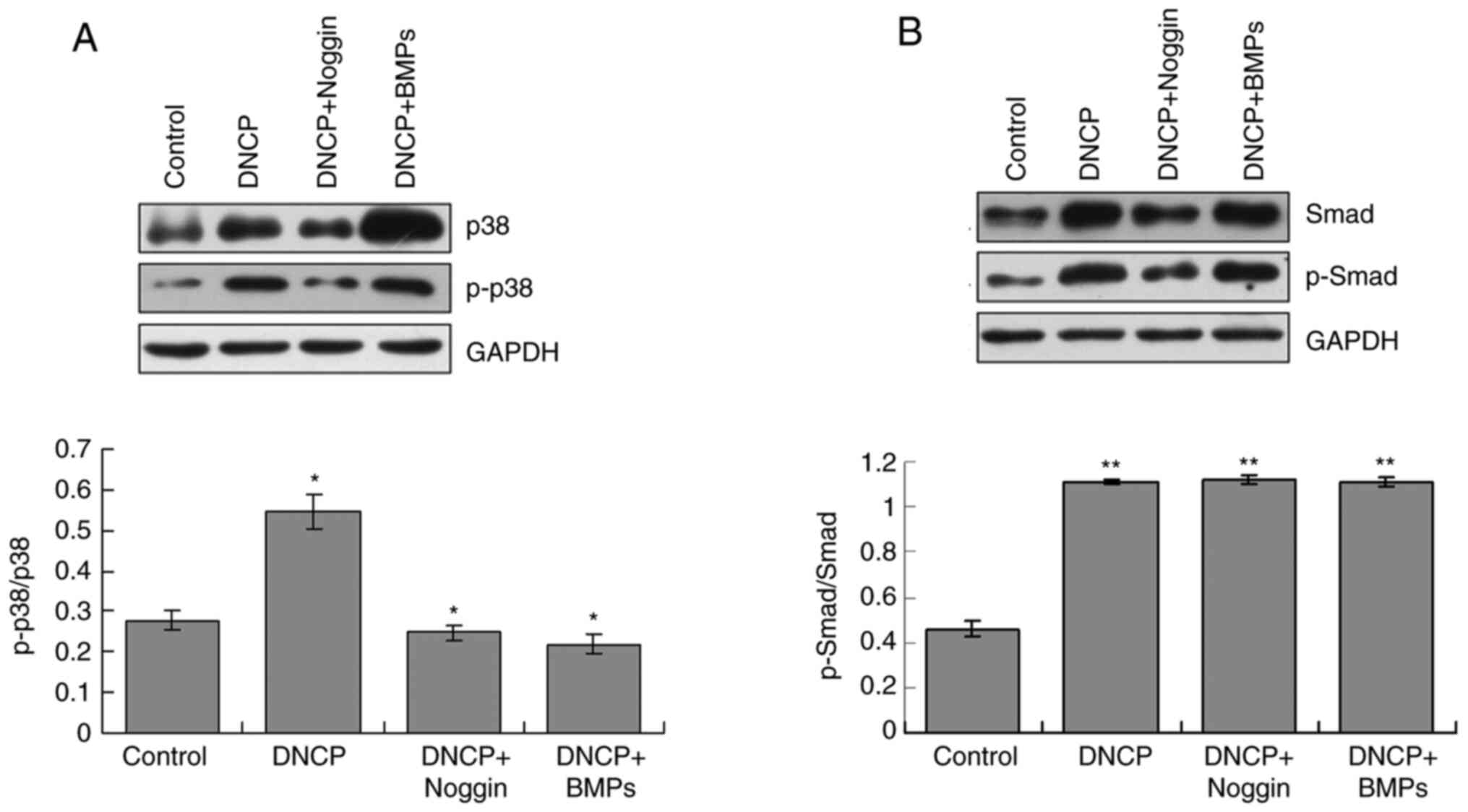
p38/p-p38 signaling pathway serves
critical roles in iPSCs differentiation
The western blotting results indicated that ratio of
p-p38/p38 was significantly increased in the DNCP group compared
with those of the Control group (P<0.05; Fig. 4A). Noggin treatment significantly
decreased ratio of p-p38/p-38 in DNCP-treated iPSCs compared with
DNCP treatment alone (P<0.05; Fig.
4A). Co-treatment with DNCP and BMPs significantly decreased
the ratio of p-p38/p38 compared with that of the DNCP group
(P<0.05; Fig. 4A). However, when
compared with the DNCP group, there were no specific effects of
Noggin and BMPs on the ratios of p-p38/p38 (Fig. 4A).
Smad/p-Smad signaling pathway is
involved in iPSCs differentiation
The western blotting findings revealed that the
ratios of p-Smad/Smad were significantly higher in the DNCP group
compared with those in the Control group (P<0.05; Fig. 4B). Noggin treatment significantly
decreased ratio of p-Smad/Smad in DNCP-treated iPSCs (P<0.05;
Fig. 4B). However, no significant
differences for ratios of p-Smad/Smad were observed between the
DNCP and DNCP+BMPs groups (P>0.05; Fig. 4B). Furthermore, DNCP treatment
significantly decreased the ratio of p-Smad/Smad compared with that
of the Control group (P<0.05), and no other effects of Noggin or
BMPSs on p-Smad/Smad were observed (Fig. 4B).
Discussion
Tooth development undergoes a series of stages,
among which differentiation and generation of odontoblasts serve
critical roles in forming crowns (34). Usually, dental mesenchymal cells can
differentiate into odontoblasts (35), although with lower differentiating
efficacy compared with other stem cells. iPSCs are considered to be
characterized by their ability to differentiate into numerous types
of cells, including odontoblast-like cells, neural cells,
adipocytes and osteoblasts (36).
Therefore, the present study established a DNCP-induced strategy to
induce the differentiation of iPSCs into odontoblast-like cells,
which may be useful for developing novel therapeutic methods.
In the present study, iPSCs were cultured by seeding
a layer of feeder cells, which could not secrete various growth
factors and promote the proliferation and differentiation of iPSCs
(27). The formation of EBs is a
critical stage of PSCs, such as their differentiation into
embryonic stem cells (37).
Therefore, EBs were generated in the present study using the
hanging-drop culture method to obtain large quantities of EBs
(27,28,37).
The undifferentiation and pluripotency of iPSCs must
be maintained by exposure to exogenous cytokines (such as LIF and
BMP-4) (38) and intracellular
transcription factors (such as Oct-4, Sox-2, Kruppel-like factor-4
and c-Myc) (39). Therefore, in
order to verify the pluripotency of iPSCs at EBs stage, the
pluripotent cell biomarkers Oct-4 and Sox-2 were examined using
immunofluorescence staining. The present results indicated that
iPSCs exhibited higher Oct-4 and Sox-2 expression after 5 days of
culturing in differentiated medium. These results suggested that
iPSCs may differentiate into odontoblast-like cells.
Normally, epithelial and mesenchymal cells can
secrete associated signaling molecules, including BMPs, FGFs, Sonic
hedgehog and Wnt molecules, all of which can induce cell
differentiation and the formation of odontogenic cells, such as
odontoblasts, ameloblasts and dentin cells (37-40).
Therefore, both epithelial and mesenchymal cells appear to be
necessary for iPSC differentiation. However, the present study
employed a more simple and direct method to differentiate iPSCs.
The current results indicated that iPSCs could be differentiated
into odontogenic cells without induction by other cytokines or
epithelial cells. In the present study, the in vivo
microenvironment was simulated using 500 ng/ml DNCP, which promotes
the proliferation of dental pulp, embryoid and epithelial cells
(40). A total of 500 ng/ml DNCP
was employed as the optimal concentration for treating iPSCs,
according to the MTT results. However, the proliferation of cells
cannot accurately reflect cell differentiation, particularly in the
case of stem cells, which is a limitation of the present study. In
future studies, the optimal concentration of DNCP in iPSCs should
be determined by examining biomarkers for iPSCs. Furthermore, the
present findings revealed that DNCP-induced differentiated iPSCs
exhibited higher levels of the odontoblastic biomarkers DMP-1 and
DSPP (41,42). In addition, the expression levels of
a bone formation-associated protein, Msx-1(43), were significantly increased in
DNCP-induced iPSCs. These data suggested that iPSCs were
successfully differentiated into odontoblast-like cells. However,
due to limited time and funding, the secretory functions of
differentiated odontoblasts were not evaluated in the present
study. In future studies, the optimal therapeutic effects of
differentiated odontoblasts on tooth repair should be evaluated
using animal models. Furthermore, the present study only examined
the expression levels of odontoblastic biomarkers, including DMP-1,
DSPP and Msx-1, which is not sufficient for determining the
differentiation from iPSCs into odontoblasts. In future studies,
the expression levels of odontogenic biomarkers should be detected,
and the mineralization of the iPSCs should be determined.
According to the crucial roles of BMPs in dental
germ development and dentin formation (44), and the fact that DNCP contained
BMPs, differentiated odontoblasts supplemented with BMPs were
incubated together. The results indicated that exogenous BMPs
treatment markedly enhanced DMP-1 and DSPP expression in
differentiated odontoblasts. Furthermore, the BMP inhibitor,
Noggin, was used to suppress BMP expression in differentiated
odontoblasts. It was found that Noggin treatment significantly
decreased the BMP production efficiency of dentin cells. The
aforementioned results suggested that blocking or enhancing BMP
signals induced the differentiation of iPSCs. Therefore, BMPs may
be critical for iPSCs differentiation-associated
microenvironments.
A previous study reported that BMPs mainly serve
Smad-dependent roles in the development of teeth (44). In the cytoplasm, Smad
phosphorylation leads to its translocation to the nucleus where it
regulates Msx-2 gene expression (45). The present results indicated that
DNCP treatment significantly increased ratio of p-Smad/Smad, while
Noggin significantly decreased ratio of p-Smad/Smad in DNCP-treated
iPSCs. Exogenous BMPs treatment increased ratio of p-Smad/p-Smad in
DNCP-treated iPSCs. Therefore, the Smad/p-Smad signaling pathway
may be involved in iPSCs differentiation, which is consistent with
the results of a previous study (46). Another study has revealed that the
BMP-2/p38 MAPK/Wnt/β-catenin signaling pathway participates in the
induction of odontoblast differentiation (47). The present results indicated that
DNCP treatment significantly enhanced ratio of p-p38/p38, while
Noggin treatment decreased ratio of p-p38/p38; however, there were
no additional effects of exogenous BMPs on ratio of p-p38/p38
expression. These results suggested that the DNCP-mediated
differentiation of iPSCs maybe initiated through the p38/p-p38 MAPK
signaling pathway. However, Noggin and BMP treatments led to the
inconsistent changes of p-p38/p38 and p-Smad/Smad ratios in
DNCP-treated iPSCs. Therefore, there may be other molecules
involved in the differentiation of iPSCs into odontoblast-like
cells or odontoblasts, which require to be clarified in future
studies.
However, the present study presents a limitation.
Whether the effect of DNCP on iPSCs is BMP-dependent has not been
clarified, which is a promising field to further explore the effect
of DNCP on iPSCs.
In conclusion, 500 ng/ml DNCP-associated
microenvironment may induce the differentiation of iPSCs into
odontoblast-like cells. The Smad/p-Smad and p38/p-p38 signaling
pathways may serve critical roles in the DNCP-mediated
differentiation of iPSCs into odontoblasts. The establishment of
the method described in the present study may provide further
insight into the differentiation of iPSCs and repair of tooth
damage and may benefit the transplantation of iPSCs into animal
models.
Acknowledgements
Not applicable.
Funding
The present study was funded by National Natural Science
Foundation of Jiangxi Province (grant no. S2018ZRZDB0255).
Availability of data and materials
All of the data generated or analyzed in this study
are included in this published article.
Authors' contributions
LL and WL conceived and designed experiments. ZL, AZ
and SF performed experiments and analyzed data. ZL contributed
reagents/materials/analysis tools and wrote this paper. LL and WL
made critical revisions. ZL, LL and WL confirmed the authenticity
of all raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ji M, Xiao L, Xu L, Huang S and Zhang D:
How pH is regulated during amelogenesis in dental fluorosis. Exp
Ther Med. 16:3759–3765. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Holan G and Needleman HL: Premature loss
of primary anterior teeth due to trauma - potential short- and
long-term sequelae. Dent Traumatol. 30:100–106. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gual-Vaqués P, Polis-Yanes C,
Estrugo-Devesa A, Ayuso-Montero R, Mari-Roig A and López-López J:
Autogenous teeth used for bone grafting: A systematic review. Med
Oral Patol Oral Cir Bucal. 23:e112–e119. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cai J, Zhang Y, Liu P, Chen S, Wu X, Sun
Y, Li A, Huang K, Luo R, Wang L, et al: Generation of tooth-like
structures from integration-free human urine induced pluripotent
stem cells. Cell Regen (Lond). 2(6)2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Obara N, Suzuki Y, Irie K and Shibata S:
Expression of planar cell polarity genes during mouse tooth
development. Arch Oral Biol. 83:85–91. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Miyazono K, Maeda S and Imamura T: BMP
receptor signaling: Transcriptional targets, regulation of signals,
and signaling cross-talk. Cytokine Growth Factor Rev. 16:251–263.
2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang XP, Suomalainen M, Jorgez CJ, Matzuk
MM, Werner S and Thesleff I: Follistatin regulates enamel
patterning in mouse incisors by asymmetrically inhibiting BMP
signaling and ameloblast differentiation. Dev Cell. 7:719–730.
2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yang G, Yuan G, Ye W, Cho KW and Chen Y:
An atypical canonical bone morphogenetic protein (BMP) signaling
pathway regulates Msh homeobox 1 (Msx1) expression during
odontogenesis. J Biol Chem. 289:31492–31502. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kriangkrai R, Iseki S, Eto K and
Chareonvit S: Dual odontogenic origins develop at the early stage
of rat maxillary incisor development. Anat Embryol (Berl).
211:101–108. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ko SF, Chen YT, Wallace CG, Chen KH, Sung
PH, Cheng BC, Huang TH, Chen YL, Li YC, Chang HW, et al: Inducible
pluripotent stem cell-derived mesenchymal stem cell therapy
effectively protected kidney from acute ischemia-reperfusion
injury. Am J Transl Res. 10:3053–3067. 2018.PubMed/NCBI
|
|
11
|
da Cunha JM, da Costa-Neves A, Kerkis I
and da Silva MC: Pluripotent stem cell transcription factors during
human odontogenesis. Cell Tissue Res. 353:435–441. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Taşlı PN, Aydın S, Yalvaç ME and Sahin F:
Bmp 2 and bmp 7 induce odonto- and osteogenesis of human tooth germ
stem cells. Appl Biochem Biotechnol. 172:3016–3025. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gao B, Zhou X, Zhou X, Pi C, Xu R, Wan M,
Yang J, Zhou Y, Liu C, Sun J, et al: BMP7 and EREG contribute to
the inductive potential of dental mesenchyme. Sci Rep.
5(9903)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
You WK and Stallcup WB: Localization of
VEGF to vascular ECM is an important aspect of tumor angiogenesis.
Cancers (Basel). 9(E97)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Murashima-Suginami A, Takahashi K, Sakata
T, Tsukamoto H, Sugai M, Yanagita M, Shimizu A, Sakurai T, Slavkin
HC and Bessho K: Enhanced BMP signaling results in supernumerary
tooth formation in USAG-1 deficient mouse. Biochem Biophys Res
Commun. 369:1012–1016. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Duan P and Bonewald LF: The role of the
wnt/β-catenin signaling pathway in formation and maintenance of
bone and teeth. Int J Biochem Cell Biol. 77 (Pt A):23–29.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Smith AJ and Lesot H: Induction and
regulation of crown dentinogenesis: Embryonic events as a template
for dental tissue repair? Crit Rev Oral Biol Med. 12:425–437.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tabatabaei FS and Torshabi M: Effects of
non-collagenous proteins, TGF-beta 1, and PDGF-BB on viability and
proliferation of dental pulp stem cells. J Oral Maxillofac Res.
7(e4)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ruan S, Deng J, Yan L and Huang W:
Evaluation of the effects of the combination of BMP-2-modified
BMSCs and PRP on cartilage defects. Exp Ther Med. 16:4569–4577.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lin M, Li L, Liu C, Liu H, He F, Yan F,
Zhang Y and Chen Y: Wnt5a regulates growth, patterning, and
odontoblast differentiation of developing mouse tooth. Dev Dyn.
240:432–440. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li J, Chatzeli L, Panousopoulou E, Tucker
AS and Green JB: Epithelial stratification and placode invagination
are separable functions in early morphogenesis of the molar tooth.
Development. 143:670–681. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jia J, Bian Z and Song Y: Dspp mutations
disrupt mineralization homeostasis during odontoblast
differentiation. Am J Transl Res. 7:2379–2396. 2015.PubMed/NCBI
|
|
23
|
Aurrekoetxea M, Irastorza I,
García-Gallastegui P, Jiménez-Rojo L, Nakamura T, Yamada Y,
Ibarretxe G and Unda FJ: Wnt/β-catenin regulates the activity of
epiprofin/Sp6, SHH, FGF, and BMP to coordinate the stages of
odontogenesis. Front Cell Dev Biol. 4(25)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fujimori S, Novak H, Weissenböck M,
Jussila M, Gonçalves A, Zeller R, Galloway J, Thesleff I and
Hartmann C: Wnt/β-catenin signaling in the dental mesenchyme
regulates incisor development by regulating Bmp4. Dev Biol.
348:97–106. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Okita K, Ichisaka T and Yamanaka S:
Generation of germline-competent induced pluripotent stem cells.
Nature. 448:313–317. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Llames S, García-Pérez E, Meana Á, Larcher
F and del Río M: Feeder layer cell actions and applications. Tissue
Eng Part B Rev. 21:345–353. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shao M, Liu C, Song Y, Ye W, He W, Yuan G,
Gu S, Lin C, Ma L, Zhang Y, et al: FGF8 signaling sustains
progenitor status and multipotency of cranial neural crest-derived
mesenchymal cells in vivo and in vitro. J Mol Cell Biol. 7:441–454.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yoshida S, Yasuda M, Miyashita H, Ogawa Y,
Yoshida T, Matsuzaki Y, Tsubota K, Okano H and Shimmura S:
Generation of stratified squamous epithelial progenitor cells from
mouse induced pluripotent stem cells. PLoS One.
6(e28856)2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Do EK, Park JK, Cheon HC, Kwon YW, Heo SC,
Choi EJ, Seo JK, Jang IH, Lee SC and Kim JH: Trib2 regulates the
pluripotency of embryonic stem cells and enhances reprogramming
efficiency. Exp Mol Med. 49(e401)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Onishi T, Umemura S, Shintani S and
Ooshima T: Phex mutation causes overexpression of FGF23 in teeth.
Arch Oral Biol. 53:99–104. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Martín-González J, Pérez-Pérez A,
Cabanillas-Balsera D, Vilariño-García T, Sánchez-Margalet V and
Segura-Egea JJ: Leptin stimulates DMP-1 and DSPP expression in
human dental pulp via MAPK 1/3 and PI3K signaling pathways. Arch
Oral Biol. 98:126–131. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Arakaki M, Ishikawa M, Nakamura T, Iwamoto
T, Yamada A, Fukumoto E, Saito M, Otsu K, Harada H, Yamada Y, et
al: Role of epithelial-stem cell interactions during dental cell
differentiation. J Biol Chem. 287:10590–10601. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Babb R, Chandrasekaran D, Carvalho Moreno
Neves V and Sharpe PT: Axin2-expressing cells differentiate into
reparative odontoblasts via autocrine Wnt/β-catenin signaling in
response to tooth damage. Sci Rep. 7(3102)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hazim RA, Karumbayaram S, Jiang M,
Dimashkie A, Lopes VS, Li D, Burgess BL, Vijayaraj P, Alva-Ornelas
JA, Zack JA, et al: Differentiation of RPE cells from
integration-free iPS cells and their cell biological
characterization. Stem Cell Res Ther. 8(217)2017.PubMed/NCBI View Article : Google Scholar : Erratum in: Stem
Cell Res Ther 10: 52, 2019.
|
|
37
|
Kasuda S, Kudo R, Yuui K, Sakurai Y and
Hatake K: Induced pluripotent stem cell-derived hematopoietic
embryoid bodies secrete sphingosine-1 phosphate and revert
endothelial injury. Bull Exp Biol Med. 164:775–779. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cortez J, Bahamonde J, De Los Reyes M,
Palomino J, Torres CG and Peralta OA: In vitro differentiation of
bovine bone marrow-derived mesenchymal stem cells into male germ
cells by exposure to exogenous bioactive factors. Reprod Domest
Anim. 53:700–709. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kuan II, Liang KH, Wang YP, Kuo TW, Meir
YJ, Wu SC, Yang SC, Lu J and Wu HC: EpEX/EpCAM and Oct4 or Klf4
alone are sufficient to generate induced pluripotent stem cells
through STAT3 and HIF2α. Sci Rep. 7(41852)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Naveau A, Zhang B, Meng B, Sutherland MT,
Prochazkova M, Wen T, Marangoni P, Jones KB, Cox TC, Ganss B, et
al: Isl1 controls patterning and mineralization of enamel in the
continuously renewing mouse incisor. J Bone Miner Res.
32:2219–2231. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yang Y, Zhao Y, Liu X, Chen Y, Liu P and
Zhao L: Effect of SOX2 on odontoblast differentiation of dental
pulp stem cells. Mol Med Rep. 16:9659–9663. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Patterson KI, Brummer T, O'Brien PM and
Daly RJ: Dual-specificity phosphatases: Critical regulators with
diverse cellular targets. Biochem J. 418:475–489. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Xuan B, Yang P, Wu S, Li L, Zhang J and
Zhang W: Expression of Dlx-5 and Msx-1 in craniofacial skeletons
and llia of rats treated with Zoledronate. J Oral Maxillofac Surg.
75:994.e1–994.e9. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yuan G, Yang G, Zheng Y, Zhu X, Chen Z,
Zhang Z and Chen Y: The non-canonical BMP and Wnt/β-catenin
signaling pathways orchestrate early tooth development.
Development. 142:128–139. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Miyazono K, Maeda S and Imamura T: BMP
receptor signaling: Transcriptional targets, regulation of signals,
and signaling cross-talk. . 6:251–63. 2005.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yun CY, Choi H, You YJ, Yang JY, Baek JA
and Cho ES: Requirement of Smad4-mediated signaling in odontoblast
differentiation and dentin matrix formation. Anat Cell Biol.
49:199–205. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yang J, Ye L, Hui TQ, Yang DM, Huang DM,
Zhou XD, Mao JJ and Wang CL: Bone morphogenetic protein 2-induced
human dental pulp cell differentiation involves p38
mitogen-activated protein kinase-activated canonical WNT pathway.
Int J Oral Sci. 7:95–102. 2015.PubMed/NCBI View Article : Google Scholar
|