Introduction
Sepsis is a systemic inflammatory response syndrome
that is caused by pathogenic infection (1). Sepsis is one of the leading causes of
mortality in patients in intensive care units, with a rate of
30-50% worldwide (2). Pathogenic
invasion triggers the release of substantial amounts of
inflammatory factors that results in a systemic inflammatory
response (3,4). In particular, macrophages have been
previously reported to serve as a cell type of the innate immune
response that is indispensable for the process of sepsis (5).
Lipopolysaccharide (LPS) is a major component of the
outer membrane of pathogenic Gram-negative bacteria that can induce
cytotoxicity by activating the innate immune system, leading to the
production of inflammatory mediators associated with septic shock
(6-8).
LPS is recognized by toll-like receptor 4 on macrophages which
induces the triggering of downstream signaling cascades and the
production of cytokines, including tumor necrosis factor (TNF)-β
and interleukin (IL)-10 (9,10). These cytokines in turn bind to their
respective receptors and promote the constitutive activation of the
Janus kinase (JAK)/STAT pathway during inflammation and sepsis
(11,12). However, the detailed mechanism
underlying inflammation following LPS exposure remain to be fully
elucidated.
MicroRNAs (miRNA or miR) are noncoding,
single-stranded RNA molecules that participate in the regulation of
gene expression by reducing mRNA stability and inhibiting mRNA
translation (13). miRNAs serve as
key regulators in a variety of biological processes, including
development, differentiation, homeostasis and elements during
immune responses (14,15). Although previous studies have
demonstrated associations of a large number of miRNAs with the
inflammatory response mediated by macrophages, including
miR-709(16), miR-1224(17), miR-203(18), miR-125b (19) and miR-149(20), functional studies in the potential
relationship between miR-150 and LPS challenge in macrophages
remain insufficient.
In the present study, an LPS-treated macrophage cell
line THP-1 was used to mimic sepsis in vitro, where the
relationship between miR-150 and the secretion of inflammatory
cytokines IL-1β, IL-6 and TNF-α in LPS-treated THP-1 cells was
subsequently examined. Dual-luciferase assays revealed that miR-150
could regulate STAT1 expression by direct binding, whilst
functional studies demonstrated that miR-150/STAT1 was involved in
the sepsis progression in vitro.
Materials and methods
Cell culture
Human monocytic cell line THP-1 was purchased from
American Type Culture Collection. The cells were maintained in DMEM
(Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS (Invitrogen; Thermo Fisher Scientific, Inc.) in a humidified
incubator under 37˚C and 5% CO2.
To establish the sepsis model in vitro, THP-1
cells were treated with LPS (Sigma-Aldrich; Merck KGaA) at
concentrations of 0, 0.5, 1 or 2 µg/ml for 0, 12, 24 or 48 h at
37˚C. The untreated THP-1 cells were considered as the control. In
transfected cells, after 24 h, 1 µg/ml LPS were used to treat the
cells for another 24 h, following which the levels of cytokines in
the medium were measured by ELISA.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total mRNA was isolated using an RNAiso Plus kit
(cat. no. 9109; Takara Bio, Inc.) according to manufacturer's
protocol. In total, 5 µg RNA was used to synthesize cDNA using the
PrimeScript™ RT reagent kit according to manufacturer's protocol
(Takara Bio, Inc.). Briefly, the transcription was conducted in a
10 µl reaction mixture, including polyadenylated RNA (100 ng), 5X
PrimeScript buffer (2 µl), PrimeScript RT Enzyme Mix I (0.5 µl), RT
primer mixure (1 µl) and RNase-free water. Then, total reaction
mixture was incubated at 50˚C for 15 min and 85˚C for 5 sec. qPCR
was performed using SYBR® Premix Ex Taq™ kit (Takara
Bio, Inc.) in 15 µl final volume containing 1.5 µl template cDNA
mixed with 7.5 µl 2X SYBR Green PCR master mix and 3 µl of each
forward and reverse primers. The amplification parameters were as
follows: Denaturation at 95˚C for 10 min, followed by 40 cycles of
denaturation at 95˚C for 30 sec, annealing at 60˚C for 30 sec and
extension at 72˚C for 1 min.
miRNA was isolated from cells using the miRCURY™ RNA
Isolation kit (cat. no. 300110; Exiqon; Qiagen China Co., Ltd.)
according to the manufacturer's protocols, which was reverse
transcribed in cDNA using Reverse Transcriptase M-MLV (RNase H;
cat. no. 2641A; Takara Bio, Inc.). First, 5 µl of RNA, 2 µl
miR39-specific primer (10 µM), 2 µl miR150-specific primer (10 µM)
and 1 µl of 10 mM dNTP mix, were heated to 65˚C for 5 min and
incubated on ice for 2 min. Next, 2 µl 10X first-strand buffer, 4
µl 25 mM MgCl2, 2 µl 0.1 M DTT, 1 µl RNaseOUT™
Recombinant RNase Inhibitor, and 1 µl of SuperScript™ III RT (200
U/µl) are added to the mixture. The 10 µl mixtures were incubated
in a thermocycler for 30 min at 16˚C, 30˚C for 30 sec, 42˚C for 30
sec, and 50˚C for 1 sec and finally heated at 70˚C for 15 min. The
20 µl PCR included 2.0 µl RT product, 1X Premix ExTaq buffer for
qPCR, 0.2 µM TaqMan probe, 0.02 µM forward primer 1, 0.6 µM forward
primer 2 and 0.4 µM reverse primer. The reaction mixtures were
incubated in a 96-well plate at 95˚C for 30 sec, followed by 55
cycles of 95˚C for 15 sec and 60˚C for 30 sec.
Quantity and quality of the RNA obtained were
determined using a Quant-iT™ RNA Assay kit according to
manufacturer's protocol (Invitrogen; Thermo Fisher Scientific,
Inc.). U6 was used as the reference gene for miR-150 expression
whilst β-actin was used as the reference gene for STAT1 mRNA
expression. Relative quantification of target genes was performed
using the 2-ΔΔCq method (21). The primer sequences were as follows:
β-actin forward, 5'-AGAGCTACGAGCTGCCTGAC-3' and reverse,
5'-AGCACTGTGTTGGCGTACAG-3'; miR-150 forward,
5'-GGGTCTCCCAACCCTTGTA-3' and reverse, 5'-CAGTGCGTGTCGTGGAGT-3'
(22); STAT1 forward,
5'-GTGAAGTTGAGAGATGTGAATGAG-3' and reverse,
5'-GATCACCACAACGGGCAGA-3' and U6 forward,
5'-AACGCTTCACGAATTTGCGT-3' and reverse,
5'-CTCGCTTCGGCAGCACA-3'.
Cell transfection
STAT1 overexpression vector was obtained by cloning
the sequences of STAT1 (Shanghai GenePharma Co., Ltd.) into
pcDNA3.1 (Invitrogen; Thermo Fisher Scientific, Inc.) and termed
pcDNA3.1-STAT1 (STAT1). Before transfection, THP-1 cells
(4x105 cells per well) were seeded into 12-well plate.
After incubation for 24 h, 0.2 µg of STAT1 overexpression vector
(STAT1) and the control pcDNA were transfected into THP-1 cells
using 0.5 µl of Lipofectamine 3000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). Furthermore, the oligonucleotide
sequences were as follows: miR-150 mimic
(5'-UCUCCCAACCCUUGUACCAGUG-3') and negative control (miR-NC:
5'-GCCUCCGUACCGAUCCUACUUA-3'); miR-150 inhibitor
(5'-CACUGGUACAAGGGUUGGGAGA-3') and the negative control
(anti-miR-NC: 5'-GGACAGGAUGGUCGAAACUGGU-3'); and small interfering
RNA for STAT1 (si-STAT1:
5'-CCGGCTGGAAGATTTACAAGATGAACTCGAGTTCATCTTGTAAATCTTCCAGTTTTTG-3')
and the negative control (si-NC:
5'-CCGGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTG-3').
These were purchased from Guangzhou RiboBio Co., Ltd. THP-1 cells
were transfected with 0.5 µg of the aforementioned oligonucleotides
using 0.6 µl of Lipofectamine 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After 24 h, THP-1 cells treated with 1 µg/ml LPS
and untreated cells were incubated for a further 24 h at 37˚C prior
to further experimentation.
ELISA
THP-1 cells (2x105 cells/well) were grown
in 12-well plates, and supernatants were collected by centrifuging
at 4,000 x g for 10 min at room temperature. ELISA assay kits for
IL-1β (cat. no. 437004), IL-6 (cat. no. 430501) and TNF-α (cat. no.
430201) were obtained from Biolegend, Inc. The levels of these
cytokines were measured using their respective ELISA kits,
according to the manufacturer's protocol.
Western blotting
Total proteins were isolated from THP-1 cells using
RIPA buffer (Pierce; Thermo Fisher Scientific, Inc.). In total, 20
µg proteins were separated by 10% SDS-PAGE and subsequently
transferred onto PVDF membranes (EMD Millipore). After blocking
with 5% dried skimmed milk at room temperature for 2 h, the
membranes were incubated with anti-STAT1 (1:1,000; cat. no. sc-464,
Santa Cruz Biotechnology Inc.) or anti-β-actin (1:5,000; cat. no.
sc-47778, Santa Cruz Biotechnology Inc.) primary antibodies
overnight at 4˚C. After washing in TBS supplemented with 1.5%
Tween-20, the membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies (1:1,000, cat. no.
sc-516102, Santa Cruz Biotechnology Inc.) at room temperature for
1.5 h. The blots were visualized using the ECL detection kit
(Thermo Fisher Scientific, Inc.) and imaged using the ChemiDoc XRS
System (Bio-Rad Laboratories, Inc.). Protein bands were analyzed by
ImageJ v1.8.0 software (National Institutes of Health).
Dual-luciferase reporter assay
StarBase v2.0 (http://starbase.sysu.edu.cn/starbase2/) (23) predicted that STAT1 was a target gene
of miR-150. Based on these prediction results, wild-type (WT) or
mutant-type (with a mutation in the region of the predicted miR-150
binding site) STAT1 3'UTR sequence was amplified from DNA prepared
from THP-1 cells using PCR. Then, these wild-type (WT) or mutant
(MUT) 3'-unstranslated regions (3'-UTR) of STAT1 (STAT1-WT1,
STAT1-MUT1, STAT1-WT2 or STAT1-MUT2) were synthesized and cloned
into the pmirGLO vector (Promega Corporation). The vectors were
then co-transfected into 293T cells alongside the miR-150 mimic or
corresponding negative control (miR-NC). DNA sequencing was used to
detect the reporters. Then, 400 ng of the constructed plasmids, 50
ng of renilla luciferase reporter plasmid (pRL-TK) and 50 nM of
miR-NC or miR-150 were transfected into THP-1 cells using
Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.).
Relative luciferase activities were measured using the
Dual-Luciferase® Reporter Assay kit, according to
manufacturer's protocols (Promega Corporation), 48 h after
transfection. Renilla luciferase activity was regarded as
the internal control for the normalization of firefly luciferase
activity.
RNA immunoprecipitation (RIP)
assay
THP-1 cells were transfected with either miR-NC or
miR-150 mimics, which were used for RIP assays 48 h following
transfection. RIP assay was performed using the Magna RIP™
RNA-Binding Protein Immunoprecipitation kit (EMD Millipore)
according to manufacturer's protocols. 2x105 THP-1 cells
were lysed by RIPA buffer. The samples (200 µl) were incubated with
5 µg anti-Ago2 antibody (cat. no. 04-642, EMD Millipore) or
negative control IgG (cat. no. AC111J, EMD Millipore). Magnetic
beads conjugated to protein A/G were then used to pull down the
Ago2-Ab complexes. Then, these complexes were then digested,
following which the RNA sequences were isolated for RT-qPCR.
Statistical analysis
Data were presented as the means ± standard
deviation. Every assay was repeated independently at least three
times. Unpaired student's t-test and one-way ANOVA followed by
Tukey's test were applied to assess the data between two groups and
among multiple groups, respectively. The results were analyzed
using the GraphPad Prism 7.0 software (GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
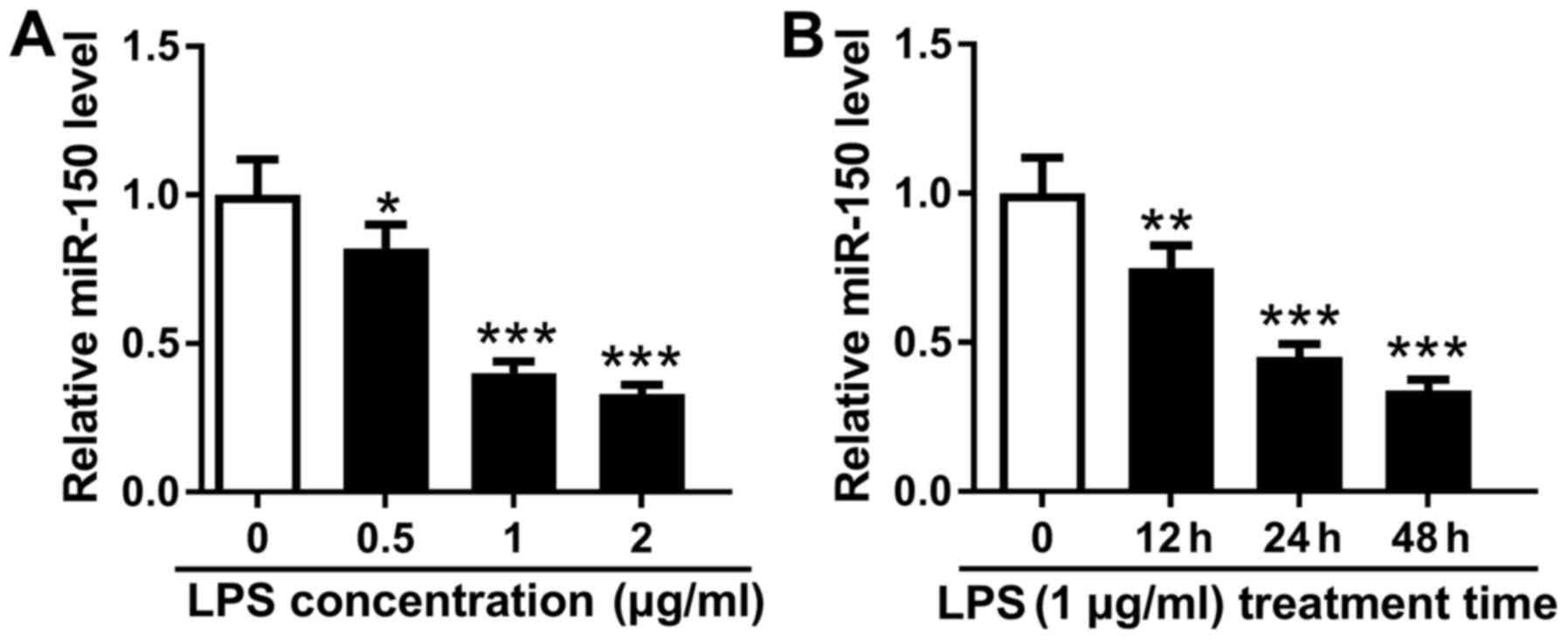
miR-150 expression is downregulated in
THP-1 cells following LPS treatment
LPS was first used to treat THP-1 cells to mimic
sepsis in vitro. THP-1 cells were first treated with 0, 0.5,
1 and 2 µg/ml LPS for 48 h, where it was found that miR-150
expression reduced in a dose-dependent manner (Fig. 1A). To optimize treatment time, a
dose of 1 µg/ml LPS was used to treat THP-1 cells for 0, 12, 24 and
48 h. The expression of miR-150 was found to be downregulated by
LPS treatment in a time-dependent manner from 0 to 48 h (Fig. 1B). A dose of 1 µg/ml LPS and
treatment time of 24 h was determined to be has the optimal
treatment strategy for subsequent experiments. These data indicate
that miR-150 expression in THP-1 cells was reduced by LPS treatment
in dose- and time-dependent manners.
miR-150 negatively regulates the
secretion of inflammatory factors in LPS-treated THP-1 cells
Sepsis is an inflammatory syndrome that is typically
characterized by the secretion of cytokines including IL-1, IL-6
and TNF-α (24,25). To investigate the role of miR-150 in
sepsis, miR-150 mimics were transfected into THP-1 cells for 24 h
before treatment with 1 µg/ml LPS for a further 24 h. Transfection
efficiency of miR-150 mimics and miR-150 inhibitors into THP-1
cells were first confirmed in comparison with their respective
negative controls (Fig. S1A and
B), following which cytokine
secretion into the cell supernatant was measured by ELISA after LPS
challenge. The levels of IL-1β, IL-6 and TNF-α secretion were
revealed to be significantly higher in the media of THP-1 cells
treated with LPS compared with control THP-1 cells (Fig. 2). In the presence of LPS, miR-150
overexpression in THP-1 cells resulted in significant reductions in
IL-1β, IL-6 and TNF-α secretion compared with THP-1 cells
transfected with miR-NC (Fig.
2A-C). By contrast, transfection of THP-1 cells with the
miR-150 inhibitor lead to significantly higher IL-1β, IL-6 and
TNF-α secretion compared with cells transfected with the
corresponding miR inhibitor negative control (Fig. 2D-F). These findings suggest that in
THP-1 cells, LPS can stimulate the secretion of IL-1β, IL-6 and
TNF-α in a manner that can be reversed by miR-150 overexpression
but enhanced by transfection with the miR-150 inhibitor. Therefore,
miR-150 may function as a negative regulator of the
macrophage-mediated inflammatory response by suppressing the
production of pro-inflammatory cytokines IL-1β, IL-6 and TNF-α.
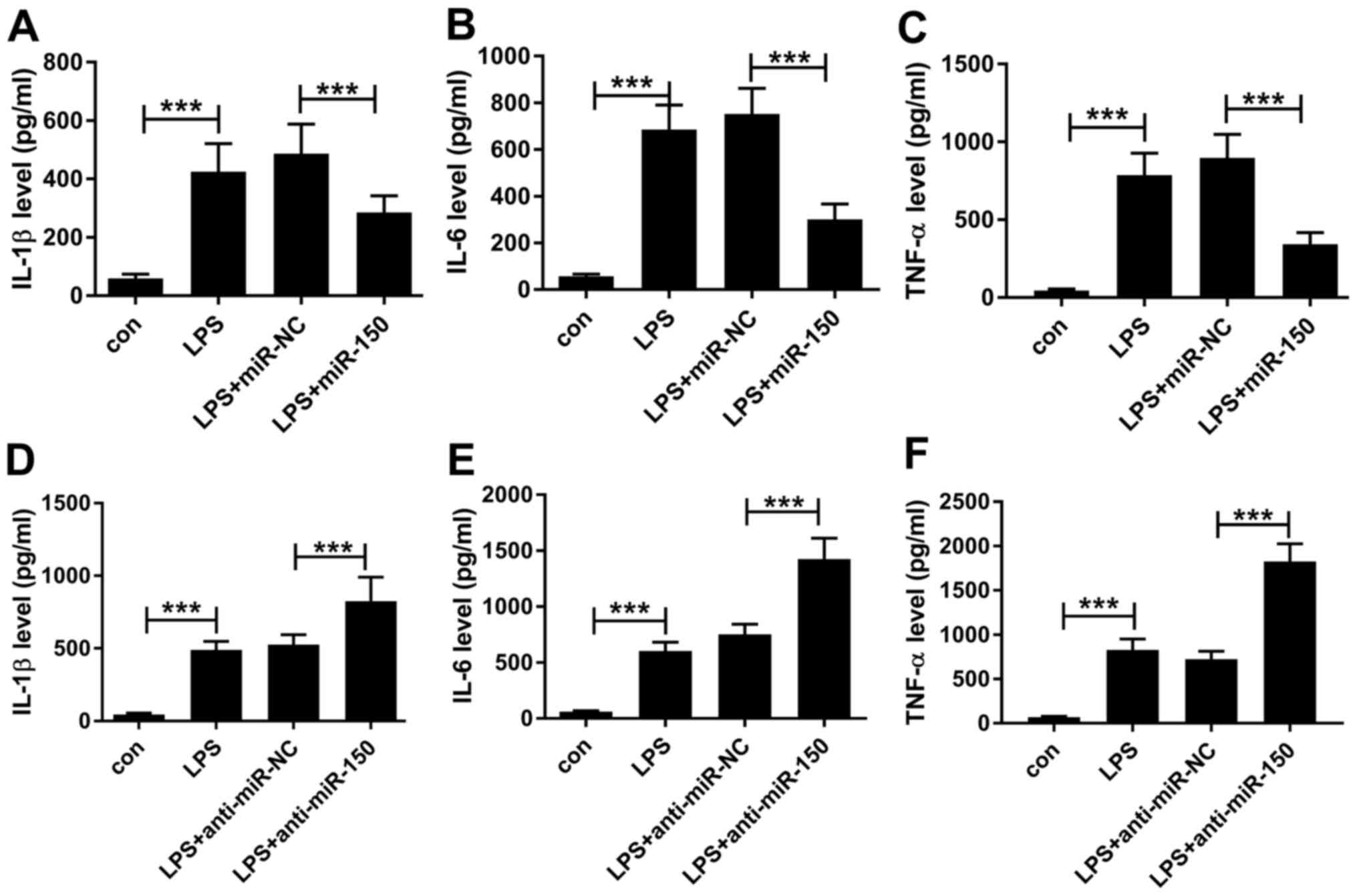 | Figure 2miR-150 regulate the secretion of
inflammatory cytokines in LPS-treated THP-1 cells. THP-1 cells
transfected with miR-150 mimics were treated with 1 µg/ml LPS for
24 h, following which (A) IL-1β, (B) IL-6 and (C) TNF-α levels in
the cell supernatant were measured by ELISA. THP-1 cells
transfected with the miR-150 inhibitor were treated with 1 µg/ml
LPS for 24 h, following which (D) IL-1β (E) IL-6 and (F) TNF-α
levels in the cell supernatant were measured by ELISA.
***P<0.001. miR, microRNA, LPS, lipopolysaccharide;
IL, interleukin; TNF-α, tumor necrosis factor-α; miR-NC, negative
control; anti-miR-NC, miR inhibitor negative control. |
STAT1 is a target of miR-150
A number of studies have previously demonstrated
miRNA to be involved in sepsis by regulating the transcription of a
number of genes downstream (26,27).
Therefore, the potential downstream targets of miR-150 were
investigated using Starbase2.0 software. It was found that the
3'UTR of STAT1 shared two complementary binding sites with miR-150
(Fig. 3A). To verify the predicted
result, dual-luciferase reporter assay was performed in 293T cells.
Co-transfection with the miR-150 mimic significantly inhibited the
luciferase activities of STAT1-WT plasmids in 293T cells but did
not affect those of STAT1-MUT plasmids (Fig. 3B and C). In addition, overexpression of miR-150
significantly increased the association between Ago2 and STAT1 in
THP-1 cells compared with cells transfected with the miR mimic
control, supporting the notion of a direct interaction between
STAT1 and miR-150 (Fig. 3D).
miR-150 overexpression significantly reduced STAT1 protein
expression in THP-1 cells, whilst transfection with the miR-150
inhibitor significantly increased STAT1 expression (Fig. 3E). In conclusion, these results
suggest that STAT1 is a downstream target of miR-150.
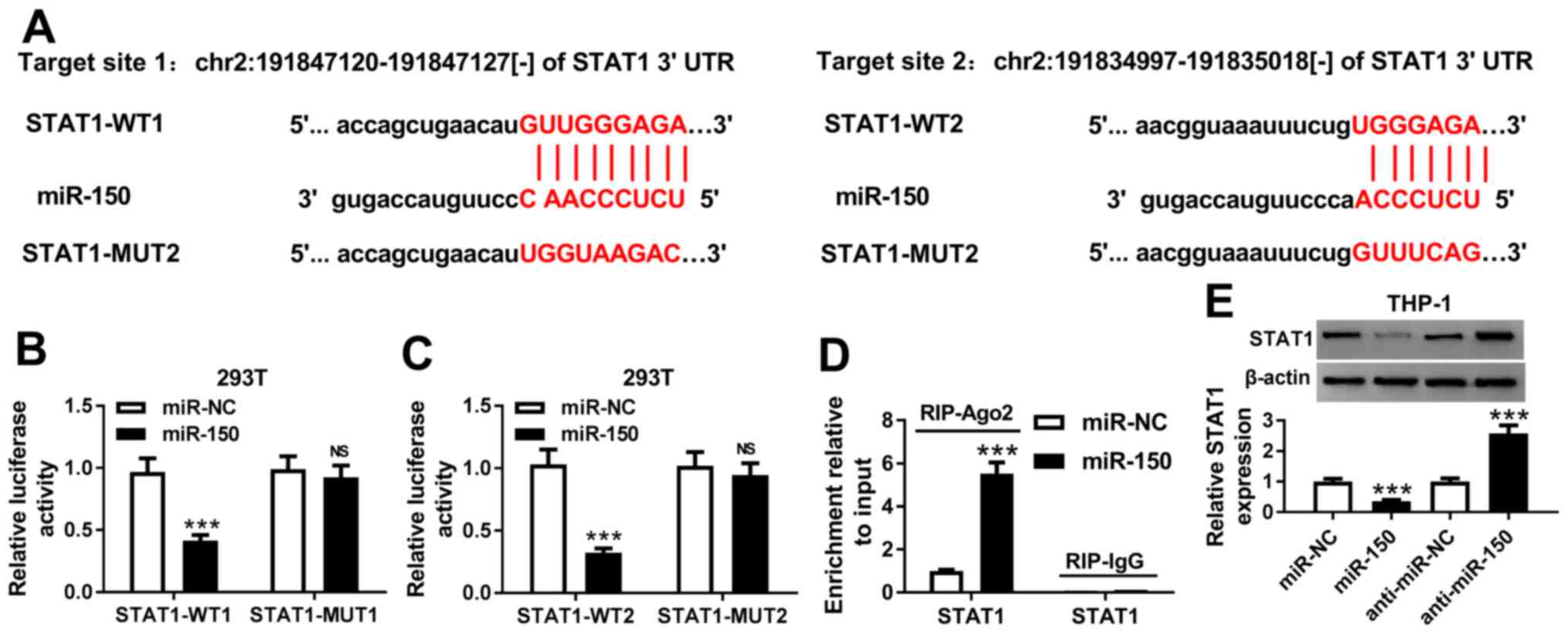 | Figure 3STAT1 is a direct target of miR-150.
(A) Two complementary miR-150 binding sites on the 3'-UTR of STAT1
as predicted using the Starbase 2.0 software. (B) Overexpression of
miR-150 reduced the luciferase activity of STAT1-WT1, but not that
of STAT1-MUT1 in 293T cells. (C) Overexpression of miR-150 reduced
the luciferase activity of STAT1-WT2 in 293T cells, but not that of
STAT1-MUT2 in 293T cells. (D) Anti-Ago2 RIP assays were performed
in THP-1 cells transfected with the miR-150 mimic or miR mimic NC.
STAT1 mRNA levels in the precipitates were measured by reverse
transcription-quantitative PCR. (E) Protein expression of STAT1 was
measured by western blotting in THP-1 cells following transfection
with miR-mimic NC, miR-150 mimic, miR-inhibitor NC or miR-150
inhibitor. ***P<0.001. miR, microRNA; 3'UTR,
3'untranslated region; WT, wild-type; MUT, mutant; Ago2, protein
argonaute 2; miR-NC, miR mimic negative control; anti-miR-NC, miR
inhibitor negative control; RIP, RNA immunoprecipitation assay. |
STAT1 expression is upregulated in
LPS-treated THP-1 cells
To investigate the relationship between STAT1 and
sepsis, THP-1 cells were stimulated with 0, 0.5, 1 and 2 µg/ml LPS
for 24 h. LPS treatment increased STAT1 mRNA expression in a
dose-dependent manner (Fig. 4A). In
addition, stimulation with 1 µg/ml LPS upregulated the expression
of STAT1 mRNA in a time-dependent manner from 0 to 48 h (Fig. 4B). This was subsequently confirmed
on a protein level, which exhibited a similar trend compared with
the data obtained using RT-qPCR (Fig.
4C and D). Collectively, these
observations suggest that STAT1 expression is upregulated by LPS
treatment in THP-1 cells.
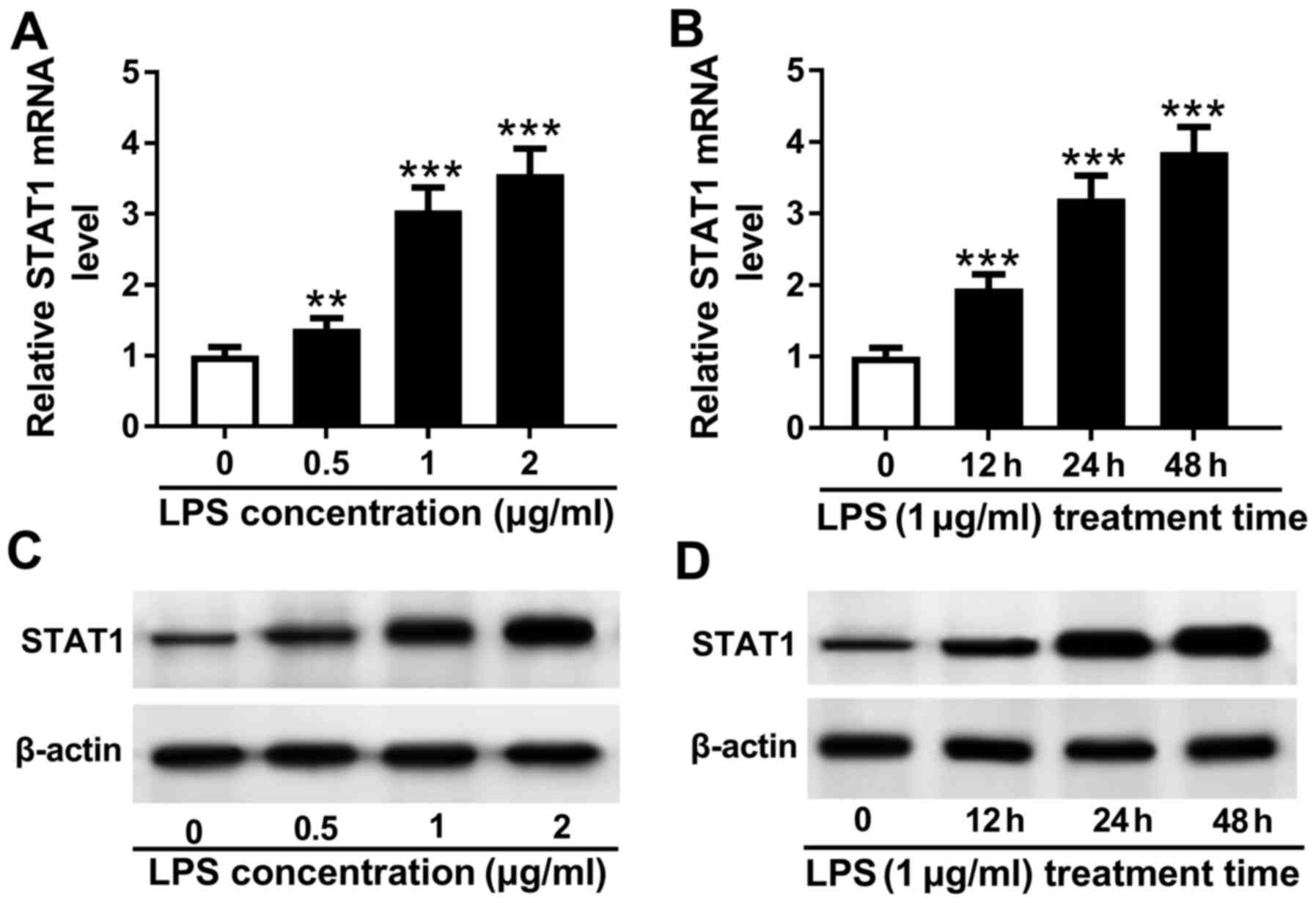 | Figure 4STAT1 expression is upregulated by
LPS treatment in THP-1 cells. (A) THP-1 cells were treated with 0,
0.5, 1 and 2 µg/ml LPS for 48 h, following which STAT1 mRNA
expression was measured by reverse transcription-quantitative PCR.
(B) THP-1 cells were treated with l µg/ml LPS for 0, 12, 24 and 48
h, following which STAT1 mRNA expression was measured by reverse
transcription-quantitative PCR. (C) THP-1 cells were treated with
0, 0.5, 1 and 2 µg/ml LPS for 48 h, following which STAT1 protein
expression was measured by western blotting. (D) THP-1 cells were
treated with l µg/ml LPS for 0, 12, 24 and 48 h, following which
STAT1 protein expression was measured by western blotting.
Significant differences between groups were shown as
**P<0.01 and ***P<0.001 vs. 0 µg/ml.
LPS, lipopolysaccharide. |
miR-150 is involved in LPS-induced
inflammatory cytokine secretion by regulating STAT1
miR-150 overexpression inhibited the secretion of
inflammatory cytokines induced by LPS whilst transfection with the
miR-150 inhibitor promoted secretion as aforementioned. To
investigate whether STAT1 is involved in this inflammatory process
and explore the functional relationship between miR-150 and STAT1,
THP-1 cells were treated with LPS following co-transfection with
both miR-150 mimics and plasmids encoding STAT1. Western blot
analysis data showed that transfection with the pcDNA-STAT1 plasmid
significantly increased STAT1 expression in THP-1 cells compared
with cells transfected with the blank plasmid (Fig. S1C). In the presence of LPS and
miR-150 overexpression, the levels of IL-1β, IL-6 and TNF-α
secretion were revealed to be significantly increased following
transfection with the pcDNA-STAT1 plasmid compared with those
following transfection with the blank pcDNA plasmid (Fig. 5A-C). Following confirmation that
transfection of THP-1 cells with the si-STAT1 significantly reduced
the expression of STAT1 compared with cells transfected with the
(Fig. S1D). STAT1 knockdown was
found to significantly suppress IL-1β, IL-6 and TNF-α secretion
compared with corresponding siRNA control in the presence of LPS
and miR-150 overexpression (Fig.
5D-F). These results suggest that miR-150 modulated the
inflammatory response following LPS exposure by regulating STAT1
expression in THP-1 cells.
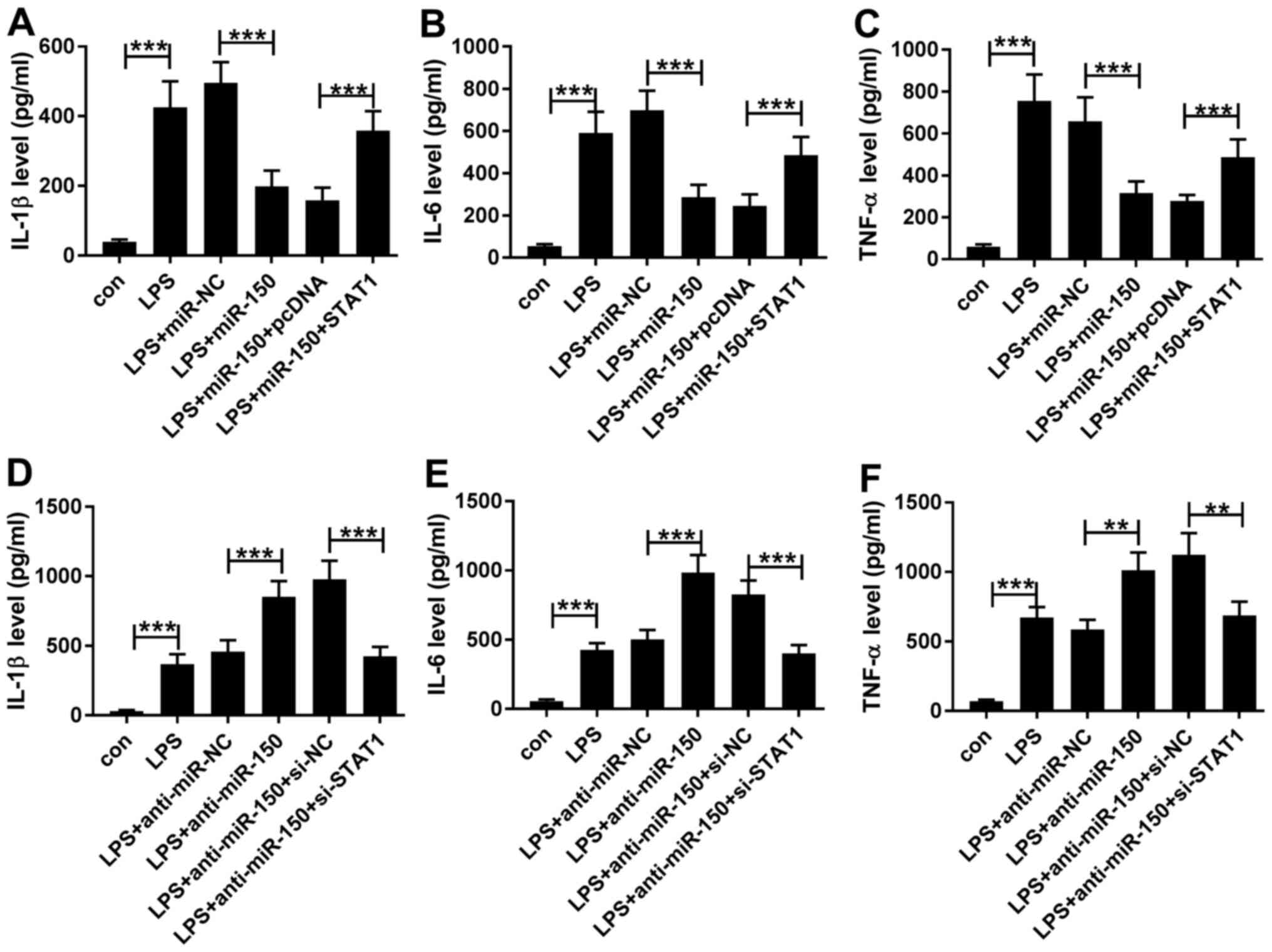 | Figure 5miR-150 regulates the secretion of
inflammatory cytokines by modulating STAT1 expression. (A-C) THP-1
cells were first transfected with either miR-NC or miR-150 mimics
alone, or co-transfected with miR-NC or miR-150 mimics and pcDNA or
pcDNA-STAT1 before being treated with 1 µg/ml LPS for 24 h. THP-1
cells in the con and LPS groups were not transfected whilst cells
in the con group were not treated with LPS. (A) IL-1β, (B) IL-6 and
(C) and TNF-α secretion into the media supernatant by THP-1 cells
were measured by ELISA. (D-F) THP-1 cells were first transfected
with either miR inhibitor NC or miR-150 inhibitor alone, or
co-transfected with miR inhibitor NC or miR-150 inhibitor and pcDNA
or pcDNA-STAT1 before being treated with 1 µg/ml LPS for 24 h.
THP-1 cells in the con and LPS groups were not transfected whilst
cells in the con group were not treated with LPS. (D) IL-1β, (E)
IL-6 and (F) and TNF-α secretion into the media supernatant by
THP-1 cells were measured by ELISA. **P<0.01 and
***P<0.001. miR, microRNA; LPS, lipopolysaccharide;
miR-NC, miR mimic negative control; anti-miR-NC, miR inhibitor
control; IL, interleukin; TNF-α, tumor necrosis factor-α; si, small
interfering RNA; si-NC, si-negative control. |
Discussion
The STAT family of proteins are major downstream
signaling proteins of growth factors and cytokines in mammalian
cells (28). STATs serve important
roles in regulating immune cell homeostasis, differentiation and
function (29). Significant
progress has been made over the last number of decades in the
characterization of the JAK/STAT signaling cascade, including the
identification of multiple STATs and associated regulatory proteins
(30). Seven STAT proteins,
including STAT1, STAT2, STAT3, STAT4, STAT6 and the STAT5a and
STAT5b isoforms, have been identified in mammals, each with their
own distinct established roles in the immune response (31). The predominant mode of STAT
regulation is by phosphorylation, mainly by JAKs (32). STAT1 and STAT2 have been
demonstrated to promote gene transcription downstream of interferon
stimulation and anti-viral immunity (33), whereas STAT3 responds to factors
including IL-6, IL-10 and vascular endothelial growth factor and
has been previously associated with tumorigenesis, T helper cell
(Th)17 and regulatory T cell development (34). STAT4 and STAT6 regulate Th1 and Th2
cell differentiation in response to IL-12 and IL-4/13(35), respectively. As a result, stringent
regulation of cellular STAT activity is of great importance due to
their involvement in such a wide variety of physiological
processes.
Sepsis is a systemic inflammatory response and
remains to be a major cause of morbidity and mortality in patients
that are critically ill (36),
which is associated with tissue damage and organ failure (37). In particular, following the failure
of one organ as a result of sepsis, others typically follow, in a
process known as organ failure amplification (38). Therefore, clinical intervention of
sepsis remains to be a great challenge. The pathophysiological
process of sepsis involves a series of complex reactions, one of
which is the activation of numerous inflammatory responses. A
number of studies have demonstrated that the inflammatory response
can be regulated via the JAK/STAT pathway. Chitnis et al
(35) found that STAT4 and STAT6
genes exerted a vital role in regulating the autoimmune response in
experimental autoimmune encephalomyelitis. Wang et al
(11) demonstrated that miR-30a
could inhibit MD-2 expression by targeting STAT1 in human monocytes
of sepsis, whilst Song et al (39) revealed that IL-4-induced activation
of the STAT6 signaling pathway contributes to the suppression of
cell-mediated immunity and death in sepsis.
miRNAs are endogenous, non-coding single-stranded
RNAs that are typically 19-23 nucleotides in length (40). They have been previously
demonstrated to serve an important role in sepsis. Wu et al
(41) previously showed that
miR-23b serves a significant role in the pathogenesis and
progression of sepsis by inhibiting the expression of inflammatory
factors. Gao and Dong (42)
demonstrated that miR-146 regulates the expression of inflammatory
cytokines in vascular endothelial cells during sepsis. Wang et
al (43) previously found
miR-27a expression to be upregulated, which promotes inflammatory
responses in sepsis, whereas another study previously demonstrated
elevated miR-15a/16 levels in the serum of patients with neonatal
sepsis, which inhibited the LPS-induced inflammatory pathway
(44). These aforementioned studies
suggest that miRNAs serve key regulatory roles in the inflammatory
response during sepsis. Although miR-150 has been previously
revealed to serve key roles in diabetes and other autoimmune
diseases (45), the role of this
miRNA in sepsis remains unclear. In the present study, it was
confirmed that miR-150 expression was downregulated in
LPS-stimulated THP-1 cells, consistent with the reduced miR-150
expression observed in patients with sepsis (46). Results from the present study also
demonstrated that miR-150 functioned as a negative regulator of the
macrophage inflammatory response by suppressing the production of
pro-inflammatory cytokines IL-1β, IL-6 and TNF-α. miR-150 was found
to share two complementary binding sites with the 3'-UTR of STAT1
by Bioinformatics analysis, which was confirmed by dual-luciferase
reporter assay. In accordance with this finding, the expression
levels of STAT1 in THP-1 cells was measured in the present study,
which revealed that STAT1 was upregulated in LPS-treated THP-1
cells. It was also found that the secretion of IL-1β, IL-6 and
TNF-α were increased by STAT1 overexpression, whilst STAT1
knockdown resulted in the opposite effect.
In conclusion, the present study demonstrated that
miR-150 inhibits the LPS-induced THP-1 inflammatory response and
revealed a direct regulatory relationship between miR-150 and STAT1
in LPS-induced THP-1 cells. These results highlight the association
of miRNAs with sepsis and specifically suggest that miR-150 may
serve as a potential target for the development of future treatment
of sepsis.
Supplementary Material
Transfection efficiency of miR-150
mimic, miR-150 inhibitor, pcDNA-STAT1 and si-STAT1 into THP-1
cells. Expression of miR-150 in THP-1 cells as measured by reverse
transcription-quantitative PCR following transfection with (A)
miR-150 mimics and (B) miR-150 inhibitor in comparison with their
corresponding negative controls. ***P<0.001 vs.
miR-NC or anti-miR-NC. The expression levels of STAT1 protein in
THP-1 cells measured by western blotting following transfection
with (C) pcDNA-STAT1 and (D) si-STAT1 in comparison with their
corresponding negative controls. ***P<0.001 vs. pcDNA
or si-NC. miR, microRNA; miR-NC, miR mimic negative control;
anti-miR-NC, miR inhibitor negative control; si, small-interfering
RNA; si-NC, si negative control.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Project of
Clinical Outstanding Clinical Discipline Construction in Shanghai
Pudong New Area (No. PWYgy2018-07). Project of Shanghai
Municipality Key Medical Specialties Construction (No. ZK2019C08).
Key Clinical Medical Specialties Project in Shanghai Pudong New
Area No. PWZzk2017-22). Leading Talent Training in Shanghai Pudong
New Area Health System No. PWRl2018-08).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SC and JW conceived and designed the study,
collected the clinical data and drafted the manuscript. HZ and JS
performed the cellular experiments. LZ and LQ interpreted the data
and revised the manuscript. SC and JW confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Csoka B, Nemeth ZH, Toro G, Idzko M, Zech
A, Koscso B, Spolarics Z, Antonioli L, Cseri K, Erdelyi K, et al:
Extracellular ATP protects against sepsis through macrophage P2X7
purinergic receptors by enhancing intracellular bacterial killing.
FASEB J. 29:3626–3637. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rudd KE, Johnson SC, Agesa KM, Shackelford
KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer
S, et al: Global, regional, and national sepsis incidence and
mortality, 1990-2017: Analysis for the Global Burden of Disease
Study. Lancet. 395:200–211. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Stearns-Kurosawa DJ, Osuchowski MF,
Valentine C, Kurosawa S and Remick DG: The pathogenesis of sepsis.
Annu Rev Pathol. 6:19–48. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yao YM, Luan YY, Zhang QH and Sheng ZY:
Pathophysiological aspects of sepsis: An overview. Methods Mol
Biol. 1237:5–15. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
He Q, Zhao L, Liu Y, Liu X, Zheng J, Yu H,
Cai H, Ma J, Liu L, Wang P, et al: circ-SHKBP1 regulates the
angiogenesis of U87 Glioma-exposed endothelial cells through
miR-544a/FOXP1 and miR-379/FOXP2 pathways. Mol Ther Nucleic Acids.
10:331–348. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Owens TW, Taylor RJ, Pahil KS, Bertani BR,
Ruiz N, Kruse AC and Kahne D: Structural basis of unidirectional
export of lipopolysaccharide to the cell surface. Nature.
567:550–553. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li Y, Orlando BJ and Liao M: Structural
basis of lipopolysaccharide extraction by the LptB2FGC complex.
Nature. 567:486–490. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Beutler B and Rietschel ET: Innate immune
sensing and its roots: The story of endotoxin. Nat Rev Immunol.
3:169–176. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Wang Y, Zhang MX, Meng X, Liu FQ, Yu GS,
Zhang C, Sun T, Wang XP, Li L, Wang YY, et al: Atorvastatin
suppresses LPS-induced rapid upregulation of Toll-like receptor 4
and its signaling pathway in endothelial cells. Am J Physiol Heart
Circ Physiol. 300:H1743–H1752. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ma Y, Liu Y, Hou H, Yao Y and Meng H:
miR-150 predicts survival in patients with sepsis and inhibits
LPS-induced inflammatory factors and apoptosis by targeting
NF-kappaB1 in human umbilical vein endothelial cells. Biochem
Biophys Res Commun. 500:828–837. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang Y, Li T, Wu B, Liu H, Luo J, Feng D
and Shi Y: STAT1 regulates MD-2 expression in monocytes of sepsis
via miR-30a. Inflammation. 37:1903–1911. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lv X, Zhang Y, Cui Y, Ren Y, Li R and Rong
Q: Inhibition of microRNA155 relieves sepsisinduced liver injury
through inactivating the JAK/STAT pathway. Mol Med Rep.
12:6013–6018. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Seven M, Karatas OF, Duz MB and Ozen M:
The role of miRNAs in cancer: From pathogenesis to therapeutic
implications. Future Oncol. 10:1027–1048. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
van Rooij E and Olson EN: MicroRNA
therapeutics for cardiovascular disease: Opportunities and
obstacles. Nat Rev Drug Discov. 11:860–872. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Liu YP, Gruber J, Haasnoot J,
Konstantinova P and Berkhout B: RNAi-mediated inhibition of HIV-1
by targeting partially complementary viral sequences. Nucleic Acids
Res. 37:6194–6204. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li M, Chen H, Chen L, Chen Y, Liu X and Mo
D: miR-709 modulates LPS-induced inflammatory response through
targeting GSK-3β. Int Immunopharmacol. 36:333–338. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Alipoor SD, Mortaz E, Tabarsi P, Marjani
M, Varahram M, Folkerts G, Garssen J and Adcock IM: miR-1224
expression is increased in human macrophages after infection with
bacillus Calmette-Guerin (BCG). Iran J Allergy Asthma Immunol.
17:250–257. 2018.PubMed/NCBI
|
|
18
|
Guo X, Zhang Z, Zeng T, Lim YC, Wang Y,
Xie X, Yang S, Huang C, Xu M, Tao L, et al: cAMP-MicroRNA-203-IFNγ
network regulates subcutaneous white fat browning and glucose
tolerance. Mol Metab. 28:36–47. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Murphy AJ, Guyre PM and Pioli PA:
Estradiol suppresses NF-kappa B activation through coordinated
regulation of let-7a and miR-125b in primary human macrophages. J
Immunol. 184:5029–5037. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xu G, Zhang Z, Xing Y, Wei J, Ge Z, Liu X,
Zhang Y and Huang X: MicroRNA-149 negatively regulates
TLR-triggered inflammatory response in macrophages by targeting
MyD88. J Cell Biochem. 115:919–927. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xiao C, Calado DP, Galler G, Thai TH,
Patterson HC, Wang J, Rajewsky N, Bender TP and Rajewsky K: miR-150
controls B cell differentiation by targeting the transcription
factor c-Myb. Cell. 131:146–159. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Luo JF, Xu J and Zheng JZ: Long non-coding
RNA TTN-AS1 promotes cell proliferation and inhibits cell apoptosis
in prostatic cancer by sponging miR-193a-5p. Eur Rev Med Pharmacol
Sci. 23:7816–7825. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tsimikas S, Duff GW, Berger PB, Rogus J,
Huttner K, Clopton P, Brilakis E, Kornman KS and Witztum JL:
Pro-inflammatory interleukin-1 genotypes potentiate the risk of
coronary artery disease and cardiovascular events mediated by
oxidized phospholipids and lipoprotein(a). J Am Coll Cardiol.
63:1724–1734. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chaves de Souza JA, Nogueira AV, Chaves de
Souza PP, Kim YJ, Silva Lobo C, Pimentel Lopes de Oliveira GJ,
Cirelli JA, Garlet GP and Rossa C Jr: SOCS3 expression correlates
with severity of inflammation, expression of proinflammatory
cytokines, and activation of STAT3 and p38 MAPK in LPS-induced
inflammation in vivo. Mediators Inflamm.
2013(650812)2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sheng B, Zhao L, Zang X, Zhen J and Chen
W: miR-375 ameliorates sepsis by downregulating miR-21 level via
inhibiting JAK2-STAT3 signaling. Biomed Pharmacother. 86:254–261.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu F, Li Y, Jiang R, Nie C, Zeng Z, Zhao
N, Huang C, Shao Q, Ding C, Qing C, et al: miR-132 inhibits
lipopolysaccharide-induced inflammation in alveolar macrophages by
the cholinergic anti-inflammatory pathway. Exp Lung Res.
41:261–269. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Carlesso N, Frank DA and Griffin JD:
Tyrosyl phosphorylation and DNA binding activity of signal
transducers and activators of transcription (STAT) proteins in
hematopoietic cell lines transformed by Bcr/Abl. J Exp Med.
183:811–820. 1996.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wex T, Grungreiff K, Schutte K, Stengritt
M and Reinhold D: Expression analysis of zinc transporters in
resting and stimulated human peripheral blood mononuclear cells.
Biomed Rep. 2:217–222. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Marengo E, Robotti E, Cecconi D, Hamdan M,
Scarpa A and Righetti PG: Identification of the regulatory proteins
in human pancreatic cancers treated with Trichostatin A by 2D-PAGE
maps and multivariate statistical analysis. Anal Bioanal Chem.
379:992–1003. 2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gao B, Wang H, Lafdil F and Feng D: STAT
proteins-key regulators of anti-viral responses, inflammation, and
tumorigenesis in the liver. J Hepatol. 57:430–441. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Seidel HM, Lamb P and Rosen J:
Pharmaceutical intervention in the JAK/STAT signaling pathway.
Oncogene. 19:2645–2656. 2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li X, Leung S, Qureshi S, Darnell JE Jr
and Stark GR: Formation of STAT1-STAT2 heterodimers and their role
in the activation of IRF-1 gene transcription by interferon-alpha.
J Biol Chem. 271:5790–5794. 1996.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li Y, Yu C, Zhu WM, Xie Y, Qi X, Li N and
Li JS: Triptolide ameliorates IL-10-deficient mice colitis by
mechanisms involving suppression of IL-6/STAT3 signaling pathway
and down-regulation of IL-17. Mol Immunol. 47:2467–2474.
2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chitnis T, Najafian N, Benou C, Salama AD,
Grusby MJ, Sayegh MH and Khoury SJ: Effect of targeted disruption
of STAT4 and STAT6 on the induction of experimental autoimmune
encephalomyelitis. J Clin Invest. 108:739–747. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
36
|
Hranjec T and Sawyer RG: Management of
infections in critically ill patients. Surg Infect (Larchmt).
15:474–478. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Weledji EP and Ngowe MN: The challenge of
intra-abdominal sepsis. Int J Surg. 11:290–295. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pearse RM, Moreno RP, Bauer P, Pelosi P,
Metnitz P, Spies C, Vallet B, Vincent JL, Hoeft A and Rhodes A:
European Surgical Outcomes Study (EuSOS) group for the Trials
groups of the European Society of Intensive Care Medicine and the
European Society of Anaesthesiology. Mortality after surgery in
Europe: A 7 day cohort study. Lancet. 380:1059–1065.
2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Song GY, Chung CS, Chaudry IH and Ayala A:
IL-4-induced activation of the Stat6 pathway contributes to the
suppression of cell-mediated immunity and death in sepsis. Surgery.
128:133–138. 2000.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Spiegel JC, Lorenzen JM and Thum T: Role
of microRNAs in immunity and organ transplantation. Expert Rev Mol
Med. 13(e37)2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wu M, Gu JT, Yi B, Tang ZZ and Tao GC:
microRNA-23b regulates the expression of inflammatory factors in
vascular endothelial cells during sepsis. Exp Ther Med.
9:1125–1132. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gao N and Dong L: MicroRNA-146 regulates
the inflammatory cytokines expression in vascular endothelial cells
during sepsis. Pharmazie. 72:700–704. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang Z, Ruan Z, Mao Y, Dong W, Zhang Y,
Yin N and Jiang L: miR-27a is up regulated and promotes
inflammatory response in sepsis. Cell Immunol. 290:190–195.
2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wang X, Wang X, Liu X, Wang X, Xu J, Hou
S, Zhang X and Ding Y: miR-15a/16 are upreuglated in the serum of
neonatal sepsis patients and inhibit the LPS-induced inflammatory
pathway. Int J Clin Exp Med. 8:5683–5690. 2015.PubMed/NCBI
|
|
45
|
Estrella S, Garcia-Diaz DF, Codner E,
Camacho-Guillen P and Perez-Bravo F: Expression of miR-22 and
miR-150 in type 1 diabetes mellitus: Possible relationship with
autoimmunity and clinical characteristics. Med Clin (Barc).
147:245–247. 2016.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
46
|
Hirschberger S, Hubner M, Strauss G,
Effinger D, Bauer M, Weis S, Giamarellos-Bourboulis EJ and Kreth S:
Identification of suitable controls for miRNA quantification in
T-cells and whole blood cells in sepsis. Sci Rep.
9(15735)2019.PubMed/NCBI View Article : Google Scholar
|