Introduction
In the early 1960s, three French scientists
(Lejeune, Gautier and Turpin) demonstrated the presence of an
additional copy of Hsa21 (human chromosome 21) in the cells of
patients with Down syndrome (1).
Despite the small number of genes on this chromosome compared to
other human chromosomes in the same category, it has not been
possible to link the function of specific genes with the defining
characteristics of individuals with Down syndrome and intellectual
disability, along with other signs and symptoms of this pathology
(2,3). There is a network of registries at the
European level that aims to assess and epidemiologically monitor
congenital anomalies that in the early 2000s covered approximately
25% of births in the European Union. Trisomy 21 (Down syndrome)
accounted for about 24 cases for every 10,000 births in Europe, for
the years 2011-2018(4). Since the
1980s, the proportion of births to mothers over the age of 35 has
increased alarmingly, from 8 to 14%, and by the end of the 1990s
this proportion varied between 10 and 25%, depending on the country
and region (5). Cases of pregnancy
interruption due to the prenatal diagnosis of Down syndrome, in the
member countries of the European Union, for the years 1995-1999,
ranged from 0% (Ireland, Malta; interruption of pregnancy is
illegal) to less than 50% in 14 other regions, with a percentage of
77% in Paris. With the increase in the average maternal age in
Europe there has been an increase in the number of pregnancies
affected by Down syndrome and prenatal screening has become a
public health issue in these cases, especially due to significant
differences in percentages based on geographical regions (5).
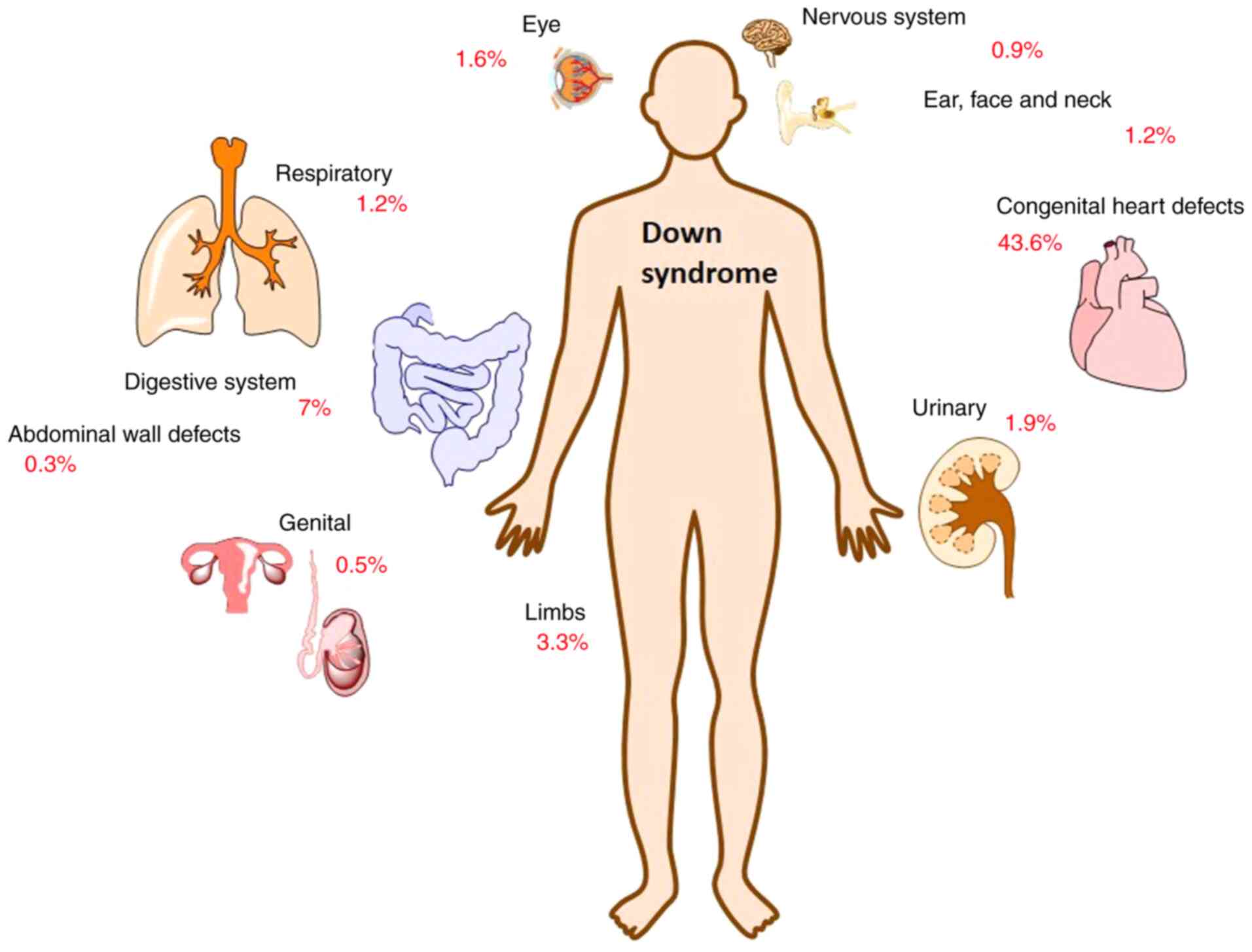
The disorder caused by chromosome 21 trisomy is also
associated with a number of physical abnormalities and intellectual
disabilities. More than 40% of babies diagnosed with Down syndrome
have a major heart abnormality, while other major birth defects
without heart damage are also common, as is evident in Fig. 1, as presented in EUROCAT registries
(6).
Efficient evaluation for trisomy 21 can be made
using a combination of maternal age, fetal nuchal translucency
(FNT), serum markers such as β-human chorionic gonadotropin (β-hCG)
and pregnancy-associated plasma protein-A (PAPP-A) during gestation
of 11+0-13+6 weeks with a detection rate of
fetuses with trisomy 21 of over 90%, taking into account the
false-positive rate of 5% (7,8). In
the case of risk detection, a solution to increase screening
accuracy is to include the evaluation of the nasal bone and blood
flow in the ductus venosus and on the tricuspid valve, leading to
an improvement of the screening efficiency, towards a detection
rate of 93-96% and a false-positive rate of 2.5% (9).
The aim of the present study was to screen for a
period of four years, pregnant women in south-western Romania from
rural areas, through a combination of maternal age, measurement of
fetal NT thickness and evaluation of biochemical markers in order
to evaluate the incidence of the risk associated with trisomy 21,
while emphasizing the need to update databases to highlight cases
of trisomy 21. The percentage variations related to the incidence
of Down syndrome from country to country and from area to area are
significant. In rural areas from Romania, access to medical
services is more limited and the application of screening programs
is a solution to contribute to a realistic assessment of the
incidence of fetal abnormalities associated with Down syndrome.
Materials and methods
General
The present study included all determinations
obtained from women in a given population who had undergone one or
more index tests compared to a reference standard. At the same
time, only pregnant women at a gestational age of less than 14
weeks confirmed by ultrasound and who have completed an informed
consent form were eligible. Patients who were not in the first
trimester of pregnancy and did not reside in rural areas were not
eligible for inclusion in the study. Individual ultrasound markers
or combinations thereof with one or more tests of serum markers
were examined in 269 patients, aged between 16 and 45 years
(average age, 30 years) between January, 2015 and December, 2018.
None of the patients reported diabetes. Informed consent was
obtained from all the patients prior to evaluations and analysis
and all data were collected according to the principles of The
Declaration of Helsinki. In the case of minor patients, informed
consent was obtained from parents or guardians. The study received
the approval No. 36/2017 from the Ethics Committee of the ‘Victor
Babes’ University of Medicine and Pharmacy Timisoara, Romania,
respecting the standards of personal data protection.
Method
Fetal nuchal translucency (FNT) was examined
primarily as an ultrasound marker and serum markers were evaluated
as free human chorionic gonadotropin beta (β-hCG) and
pregnancy-associated plasma protein A (PAPP-A). The current study
was based on data from single fetal pregnancies from prospective
combined screening in the first trimester for trisomy 21 in the
Caransebeș area, Banat region in south-western Romania. Serum
samples collected at gestational age between 11+0 and
13+6 weeks were analyzed in order to determine free
β-hCG and PAPP-A concentrations using the Time Resolved Amplified
Cryptate Emission (TRACE) technology, an immunochemical method
based on fluorescence that allows the determination of homogeneous
phase analytes, with the help of the KRYPTOR analyser (Brahms,
Thermo Scientific). Transabdominal and, when necessary due to
visualization problems, transvaginal ultrasound measurements were
performed in order to diagnose possible major fetal defects and
make specific measurements (e.g., fetal crown length and nuchal
translucent thickness). Gestational age was calculated based on CRL
(crown-rump length) at the time of FNT measurement. Maternal
weight, measured at the time of serum screening, ultrasonographic
measurements and biochemical results were recorded in a database.
The associated risks largely depended on the accuracy of the data
provided by patients, and smoking status and the existence of
diabetes, were reported by each patient on a completed personal
questionnaire.
The analysis and processing models included the
terms from the screening center, gestational age, type of pregnancy
(monofetal or not), maternal weight, smoking-related condition and
the existence or not of diabetes. Following the results of the
analyzes, the patients were divided into two main groups: i) Low
risk-a situation in which the value of the calculated risk was
below the established cut-offs, but which did not necessarily
guarantee the absence of Trisomy 21 (Down syndrome); ii) increased
risk-a situation in which the value of the calculated risk exceeded
the established cut-offs, did not establish the diagnosis of
Trisomy 21 (Down syndrome) but required further investigations.
Statistical analysis
Statistical analyses were performed using IBM SPSS
v.27 software and descriptive statistics using Excel from MS Office
Pro Plus 2019, respectively. Data are expressed as mean ± SD. The
normality of distributions of biochemical markers was ascertained
using the Kolgomorov-Smirnov test; the differences in age, weight,
between the other parameters, were assessed by one-way ANOVA
followed by Bonferonni's post-hoc comparisons test. Correlations
between the studied parameters were examined using Pearson's
coefficient. P<0.05 was considered statistically
significant.
Results
Patient characteristics
To obtain an improved diagnostic performance a
combination of tests was used including, maternal age and
assessment of specific serum markers, or combinations of maternal
age, specific serum markers, and sonographic measurements.
Individual ultrasound markers or combinations thereof with one or
more tests of serum markers were examined in the first trimester,
with or without adjustment for maternal age. In the present study,
269 patients were subjected to investigations (by ultrasound and
quantification of serum markers-double test), between January 2015
and December 2018 (Table I). The
investigation of the correlations between the studied parameters is
presented as values of Pearson' coefficient in Table II.
 | Table IDescription of the studied group from
the rural areas of southwestern Romania. |
Table I
Description of the studied group from
the rural areas of southwestern Romania.
| Parameter | Min. value | Max. value | Mean ± SD | Median | SE | CI 95% |
|---|
| Age, years | 16 | 41 | 27.55±5.09 | 27 | 0.31 | 26.94-28.16 |
| Weight, kg | 43 | 122 | 65.75±12.97 | 63 | 0.79 | 64.20-67.30 |
| FNT, mm | 0.70 | 2.90 | 1.57±0.29 | 1.60 | 0.02 | 1.54-1.61 |
| PAPP-A, UI/l | 0.61 | 14.65 | 3.91±2.65 | 3.12 | 0.16 | 3.59-4.23 |
| Free β-hCG,
ng/ml | 9.72 | 284.00 | 44.79±27.30 | 38.03 | 1.66 | 41.53-48.05 |
 | Table IIClassification of correlations between
fetal nuchal translucency, free human chorionic gonadotropin beta
and pregnancy- associated plasma protein A (using multiple median
approaches), based on the criteria of Colton (10). |
Table II
Classification of correlations between
fetal nuchal translucency, free human chorionic gonadotropin beta
and pregnancy- associated plasma protein A (using multiple median
approaches), based on the criteria of Colton (10).
| Correlation type | Direct
dependence | Inverse
dependence |
|---|
| Strong
correlations | NT vs. its MoM
(r=0.900, P<0.01) | |
| | PAPP-A vs. its MoM
(r=0.778, P<0.01) | |
| | Free β-hCG vs. its
MoM (r=877, P<0.01) | - |
| Medium
correlations | - | - |
| Weak
correlations | - | PAPP-A vs. weight
(r=-347, P<0.01) |
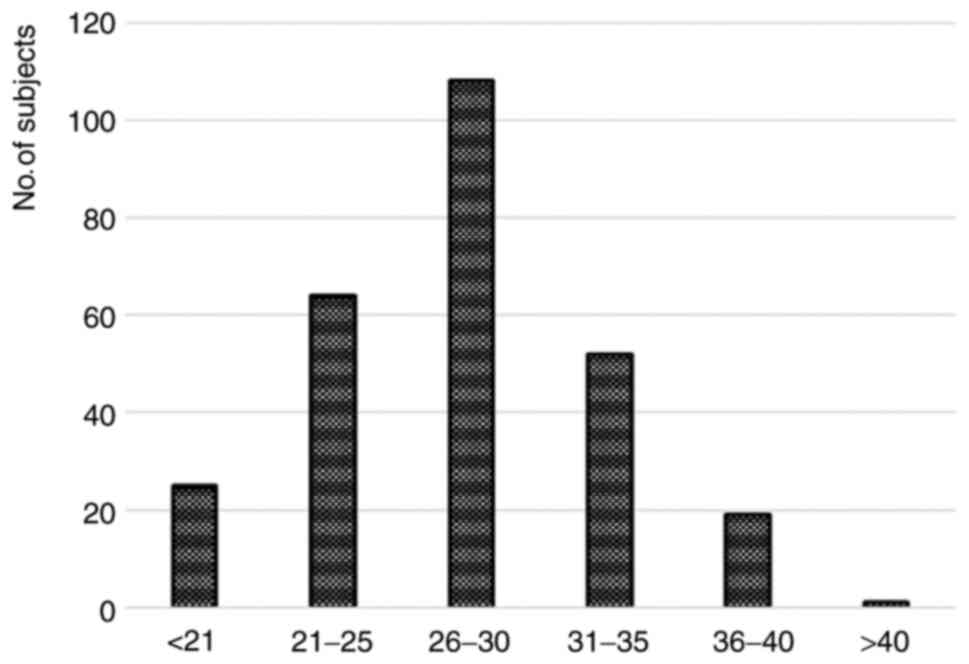
Distribution of maternal age
Fig. 2 presents the
distribution of patients based on their age; the group has been
divided in 6 equal sub-groups in order to observe the
presence/absence of a normal (gaussian) distribution. Patients
under 21 years represent almost 10%, while the age group 21-25
years almost a quarter of the total. The age group 26-30 years are
the predominant group (more than 40%), 31-35 years almost 20%,
36-40 years around 7% and the last group, 40-45 years is
represented by a percentage of 1.5% (Fig. 2).
During the four years, correlated with age, eight
minors also presented themselves for investigations: Two aged 17
during 2015, three aged 17 during 2016, two aged 16 during 2017 and
a 16-year-old during 2018. Regarding the smoking status, out of the
269 patients included in the present study for the years 2015-2018,
31 of them declared themselves smokers while the remaining 238 were
non-smokers. None of the patients reported diabetes.
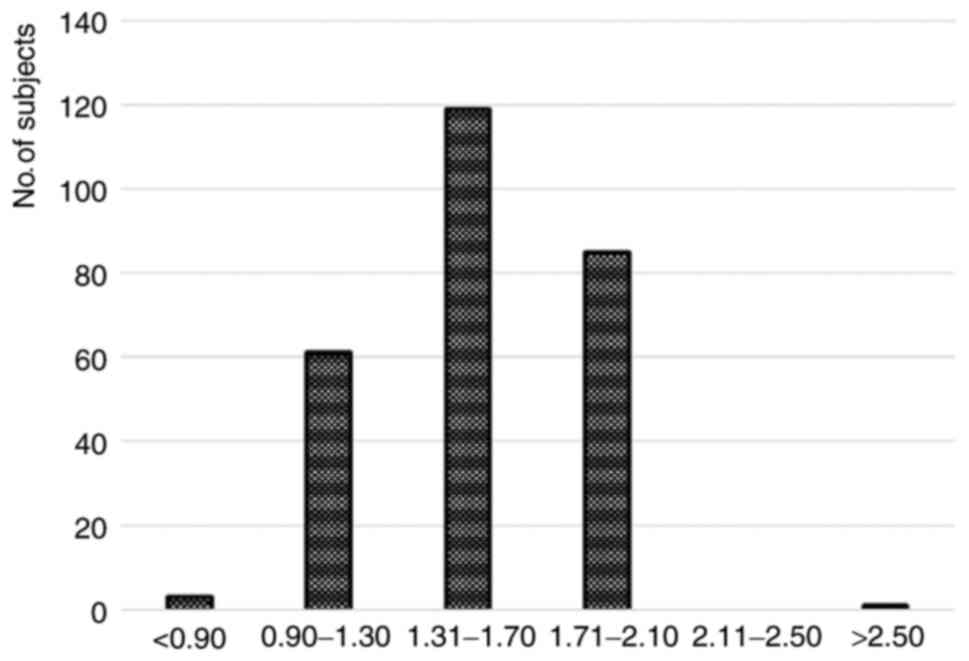
Distribution of FNT
Of the 269 cases studied, a percentage of 5.6% were
included in the risk group (≥1:250) and a percentage of 1.5% was at
the limit (in the risk range 1:251-1:300).
NT was initially implemented to predict the
likelihood of a fetus with Down syndrome. Maternal age can be
combined with fetal NT and maternal serum biochemistry (free βHCG
and PAPP-A) at 11+0 to 13+6 weeks to identify
approximately 90% of affected fetuses. Establishing FNT screening
programs requires licensed physicians and certified sonographers.
In the present study, no values of NT ≥3 were recorded. A single
value of 2.9 was recorded during 2015, the patient was included in
a risk group after association with serum markers, age and other
factors was taken into consideration. Fig. 3 shows the distribution of fetal
nuchal translucency values in the studied group of patients from
the rural areas of southwestern Romania.
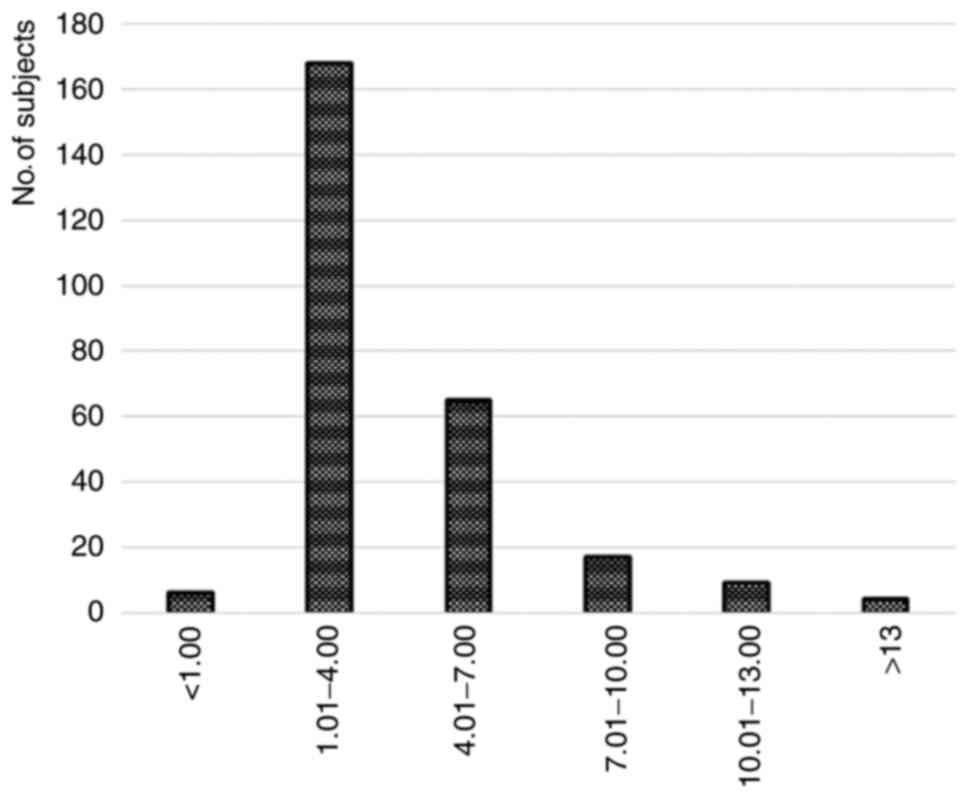
Distribution of PAPP-A values
PAPP-A values were determined by well-known
fluorescence immunochemical methods. There were only 20 MoM values
≤0.5 for PAPP-A which could theoretically signal some associated
risks (intrauterine growth restriction, premature birth,
preeclampsia, miscarriage or fetal death at ≥24 weeks), as follows:
i) During 2015 three values of 0.37, 0.48, 0.41 and a value of 0.36
for a 33-year-old patient who was classified after taking into
account all the criteria selected in the risk category; ii) during
2016 two values of 0.4 and 0.36, but not included in the risk
category of trisomy 21; iii) during 2017 six values of 0.41, 0.49,
0.31, 0.29, 0.47, and 0.46, and two included after the analysis of
all parameters in the risk group, 0.35 and 0.48; and iv) during
2018 six values of 0.31, 0.4, 0.49, 0.4, 0.49 and 0.43. The
graphical representation of the values of pregnancy-associated
plasma protein A values in the studied group of patients from the
rural areas of southwestern Romania, is shown in Fig. 4.
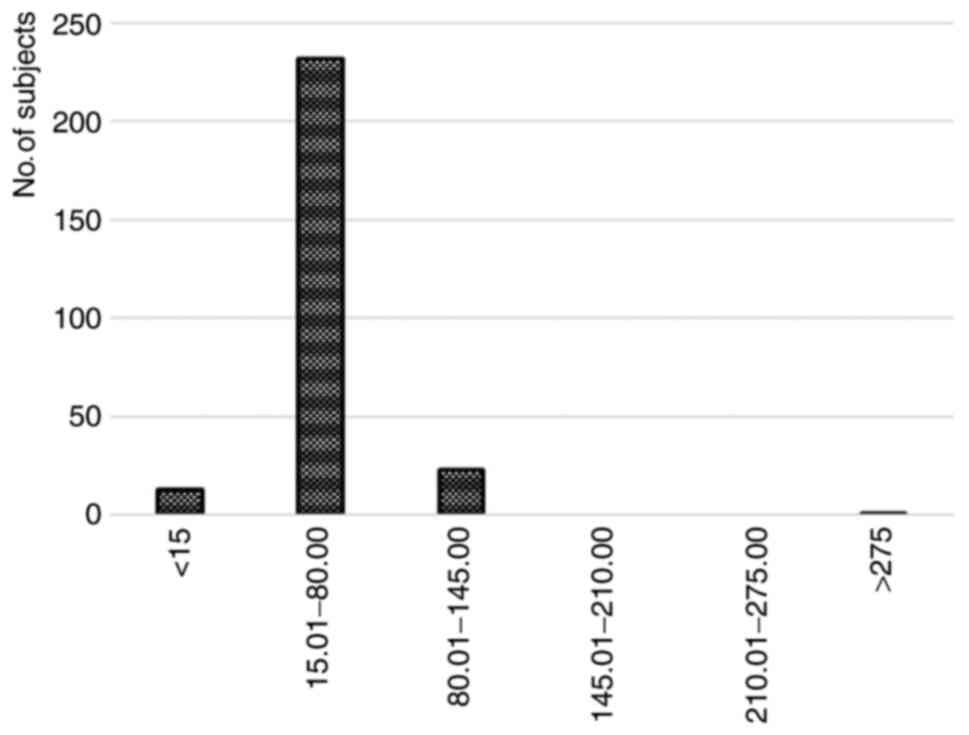
Distribution of β-hCG values
β-hCG values were also determined by well-known
fluorescence immunochemical methods. There were 23% values ≥1.5 and
of the 269 patients included in the risk group 60% had values ≥1.5
of β-hCG-associated MoM, as follows: i) During 2015 two values of
1.71, 1.64; ii) during 2016 one value of 3.67; iii) during 2017
five values of 1.89, 1.65, 1.84, 1.95 and 3.33; and iv) during 2018
one value of 4.17. The graphical representation of the values of
β-hCG registered in the period January, 2015-December, 2018, is
presented in Fig. 5.
Screening based on maternal age, fetal NT and the
two additional serum markers resulted in a detection rate ≥85%, and
a false-positive rate of 3%.
Discussion
Screening programs must be implemented through
effective health policies and testing of patients in rural areas
must be carried out especially taking into account the limited
access of this set of individuals to medical services. In the
present study, the patients from a rural area from the
south-western part of Romania were tested, and of 269 patients, a
percentage of 5.6% were included in the risk group for trisomy 21.
Down syndrome is an abnormality that does not take into account
race and socio-economic status and can occur in any woman, albeit
the probability increases in direct proportion to age, especially
over 35 years. In modern times, the decision to become a parent has
been postponed in favor of a career and increasingly couples are
finding themselves at an advanced age when they become parents.
Therefore, compared to the 1990s there is an increase in the
prevalence of Down syndrome from an average of 16% to one of 23% in
2014. The variation in percentages is significant in different
regions of the European Union (11). Initially, the inclusion of
pregnancies in the risk category for Down syndrome was realized
exclusively according to maternal age (high risk at the age of over
35 years); in 1987, β-hCG was introduced to detect chromosomal
abnormalities; in 1994, PAPP-A was introduced as the second serum
marker to detect such abnormalities (β-hCG increases significantly
and PAPP-A decreases significantly in pregnancies with trisomy 21)
while measurement of NT was introduced as the first sonographic
marker in 1992(12). Since 2007
most revised guidelines have proposed that screening for Down
syndrome be conducted regardless of age (12,13).
Sonographic measurements should be made by an
experienced person taking into account well-established rules
(e.g., median sagittal fetal scan, by appropriate zoom denoting the
fetus on three quarters of the screen image showing the profile and
upper chest) with differentiation of fetal skin and amniotic
membrane and a ‘neutral’ position of the fetus. Any deviation from
these rules translates into erroneous measurements and
false-positive results (e.g., in a hyper-extended position NT
detection can be overestimated) (14). During the examination, several
measurements are performed and the risk calculation is made with
the measurement that meets all the mentioned criteria. In most
cases (over 95%) NT measurement is conducted by transabdominal
scan; however, in particular instances (≤5% of cases) NT
measurement must be realized by transvaginal scan (14). NT thickness denotes an increase
directly proportional to gestational age, after 14 weeks it is no
longer relevant, and the normal range of changes is variable in the
literature: A fixed cut off (2.5, 3.0 or 3.5 mm), a cut-off
correlated with fetal CRL (95-99%) or by using multiple median
approaches (MoM) (15).
A number of clinical and biological factors,
including low plasma protein levels associated with plasma
pregnancy-A and elevated free human chorionic gonadotropin values,
can significantly influence pregnancy outcome. The PAPP-A
glycoprotein, one of the four placental antigens identified in the
mid-1970s and associated with unknown function, found in high
plasma concentrations in pregnant women, which was the name given
to pregnancy-associated plasma protein, is produced by the
trophoblast sinus that is present in the maternal blood in low
concentrations at the beginning of pregnancy and its value
increases with gestational age (14). This glycoprotein has an essential
role in the development of the placenta, values <5‰ are
associated with various obstetric complications, premature labor
and low birth weight, and vascular failure, especially in fetuses
where intrauterine growth restriction is also correlated with low
values (16). PAPP-A is also
responsible for IGFBP-4-dependent proteolysis of IGF (insulin-like
growth factor) in environments conditioned by normal human
fibroblasts, its mRNA is expressed in a significant number of
non-pregnancy-related cell and tissue types (17). Currently, protein has attracted
attention due to its possible involvement in certain types of
cancer, as it improves the action of IGF by specific cleavage of
inhibitory insulin-like growth factor binding proteins (IGFBPs),
mainly IGFBP-4 which leads to loss of binding affinity for IGF,
resulting in significant bioavailability of IGF for IGF-IR-mediated
proliferation, survival, and migration, considering the fact that
enhanced IGF-IR signaling is associated with tumor growth and
metastasis (18,19). The first link between breast cancer
and PAPP-A was made in the mid-1980s, and at that time it was
considered an independent predictor of early recurrence of stage I
breast cancer, but by using a specific monoclonal antibody, the
PAPP-A antigen has been shown to be expressed in human breast
cancer associated with an aggressive phenotype (20). The β subunit of human chorionic
gonadotropin (β-hCG) is detectable in the plasma of pregnant women
from day eight after ovulation, and plasma levels may provide
useful information for the following values: In patients with
viable intrauterine pregnancy lower β-hCG levels of 1,500 mIU/ml,
1,500-3,000 mIU/ml or more than 3,000 mIU/ml will increase after 48
h by at least 49, 40 and 33%, respectively, and a lower growth rate
indicates early pregnancy loss or ectopic pregnancy. From the
10th week of gestation, the level of β-hCG reaches a
plateau or decreases (21).
In addition to this, a lot of surveys have been
conducted as far as early diagnosis of trisomy disorders is
concerned. Tang et al suggested that the reliability of
tests is increased when nuchal translucency thickness and ductus
venosus blood flow is integrated for the early diagnosis of trisomy
21, 18 and 13 for the Western Chinese population (22).
In addition, previous non-invasive prenatal tests
(NIPT) have been evolved for the detection of trisomies 13, 18 and
21. Specifically, Zhang and Zhang (23) and Qiang et al (24) showed that the NIPT technique is
feasible for the prenatal screening of T18 and T21 with very high
sensitivity and specificity and it can be used as an important
alternative screening method for Down's syndrome in women (23,24).
In certain instances, the calculations in the
combined tests may reveal a low risk of developing fetal
abnormalities, but the numerical values of the serum markers
monitored may be suggestively altered compared to the values
corresponding to gestational age, in which case these details must
be specified. For example, values ≤0.5 MoM for PAPP-A may signal a
risk associated with intrauterine growth restriction, premature
birth, preeclampsia, miscarriage, or fetal death at ≥24 weeks
(25,26). At the same time, values ≤0.25 MoM in
the first trimester of pregnancy can signal a significant risk of
pregnancy loss of up to 24 weeks (27).
In the present study, a significant number of
patients from the rural area were screened. It is disconcering that
only two patients in the risk group were over 40 years of age.
Moreover, two patients were under 30 years of age. Data on the
incidence of Down syndrome are extremely varied and difficult to
access. Patients included in this risk group easily forego further
testing due to social status, religion or lack of information.
Therefore, the realization of screening programs correlated with
counseling and monitoring programs is mandatory. Eastern Europe
lacks a database of the incidence of fetal anomalies, probably due
to the governing regimes in the Balkan countries. In order to
ensure access to investigations for patients in rural areas, it is
necessary to implement screening and counseling programs for
patients.
In summary, the use of sonographic and serum
markers, correlated with maternal age are tools recognized for
decades in the early diagnosis of the risk of Down syndrome. In
rural areas, access to medical services is limited for a variety of
reasons (lack of qualified personnel, social condition, material
condition, level of awareness). Thus, screening programs are needed
to make an assessment of the incidence of risk of Down syndrome as
accurate as possible. In the present study, over 5% of patients at
risk were detected following the screening. Maternal age is a key
factor in determining this risk and the values recorded in the
study reveal an early age at which these fetal abnormalities can
develop. This requires further testing and the development of more
extensive screening programs.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Authors may make available to the publisher the data
and materials presented in the manuscript.
Authors' contributions
VBS, LB and MSF conceived and designed the study.
IE, DCM, MS, DR, CS and SLI were involved in data curation and
analysis. VBS, DR, MSF and LB contributed to conducting the
experiment, acquired data and confirmed the originality of raw
data. MVB, DR, MS, DMA, DCM and SLI were involved in project
administration, supervision, visualization and data analysis. DCM
and CS were responsible for setting up the software. VBS, CS, MSF
and IE wrote the original draft, which was reviewed and edited by
DCM, CS and DMA. All authors have read and agreed to the published
version of the manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from all the patients
prior to evaluations and analysis and all data were collected
according to the principles of The Declaration of Helsinki. In the
case of minor patients, the informed consent was obtained from
parents or guardians. The study was approved by the ‘Victor Babes’
University of Medicine and Pharmacy Timisoara, Doctoral School (no.
23/5.11.2018) and the Ethics Committee of ‘Victor Babes’ University
of Medicine and Pharmacy Timisoara (no. 36/2017).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Menghini D, Costanzo F and Vicari S:
Relationship between brain and cognitive processes in Down
syndrome. Behav Genet. 41:381–393. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Piovesan A, Caracausi M, Antonaros F,
Pelleri MC and Vitale L: GeneBase 1.1: A tool to summarize data
from NCBI gene datasets and its application to an update of human
gene statistics. Database (Oxford). 2016(baw153)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Letourneau A and Antonarakis SE: Genomic
determinants in the phenotypic variability of Down syndrome. Prog
Brain Res. 197:15–28. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
European Commission: EUROCAT
Data-Prevalence and Tables. Accessible at: https://eu-rd-platform.jrc.ec.europa.eu/eurocat/eurocat-data/prevalence_en.
|
|
5
|
Dolk H, Loane M, Garne E, De Walle H,
Queisser-Luft A, De Vigan C, Addor MC, Gener B, Haeusler M, Jordan
H, et al: Trends and geographic inequalities in the prevalence of
Down syndrome in Europe, 1980-1999. Rev Epidemiol Sante Publique 53
Spec No. 2:2S87–2S95. 2005.PubMed/NCBI
|
|
6
|
Morris JK, Garne E, Wellesley D, Addor MC,
Arriola L, Barisic I, Beres J, Bianchi F, Budd J, Dias CM, et al:
Major congenital anomalies in babies born with Down syndrome: A
EUROCAT population-based registry study. Am J Med Genet A.
164A:2979–2986. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kagan KO, Wright D, Baker A, Sahota D and
Nicolaides KH: Screening for trisomy 21 by maternal age fetal
nuchal translucency thickness, free beta-human chorionic
gonadotropin and pregnancy-associated plasma protein-A. Ultrasound
Obstet Gynecol. 31:618–624. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Nicolaides KH: Nuchal translucency and
other first-trimester sonographic markers of chromosomal
abnormalities. Am J Obstet Gynecol. 191:45–67. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kagan KO, Staboulidou I, Cruz J, Wright D
and Nicolaides KH: Two-stage first-trimester screening for trisomy
21 by ultrasound assessment and biochemical testing. Ultrasound
Obstet Gynecol. 36:542–547. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Colton T: Statistics in medicine. Little
Brown and Company, New York, pp19-20, 1974.
|
|
11
|
Lanzoni M, Kinsner-Ovaskainen A, Morris J
and Martin S: EUROCAT-Surveillance of Congenital Anomalies in
Europe: Epidemiology of Down syndrome 1990-2014. La Placa G and
Spirito L (eds). Publications Office of the European Union,
Luxembourg, 2019.
|
|
12
|
Heidari R, Akbariqomi M, Motevaseli E,
Omrani MD, Kooshki H, Shamshiri AR, Shafei S, Absalan M, Mazlomi
MA, Saleh Gargari S and Tavoosidana G: Performance and predictive
value of first Trimester screening markers for down syndrome in
Iranian pregnancies. J Family Reprod Health. 12:121–128.
2018.PubMed/NCBI
|
|
13
|
Royère D: Working Group Trisomy 21
Screening. The impact of introducing combined first-trimester
trisomy 21 screening in the French population. Eur J Public Health.
26:492–497. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cignini P, Maggio Savasta L, Gulino FA,
Vitale SG, Mangiafico L, Mesoraca A and Giorlandino C: Predictive
value of pregnancy-associated plasma protein-A (PAPP-A) and free
beta-hCG on fetal growth restriction: Results of a prospective
study. Arch Gynecol Obstet. 293:1227–1233. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Stefanovic V, Äyräs O, Eronen M, Paavonen
J and Tikkanen M: Clinical utility of nuchal translucency
screening. Res Rep Neonatol. 4:169–176. 2014.
|
|
16
|
Goetzinger KR, Singla A, Gerkowicz S,
Dicke JM, Gray DL and Odibo AO: Predicting the risk of
pre-eclampsia between 11 and 13 weeks' gestation by combining
maternal characteristics and serum analytes, PAPP-A and free β-hCG.
Prenat Diagn. 30:1138–1142. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Conover CA: Key questions and answers
about pregnancy- associated plasma protein-A. Trends Endrocrinol
Metab. 23:242–249. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Monget P and Oxvig C: PAPP-A and the IGF
system. Ann Endocrinol (Paris). 77:90–96. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pollak M: The insulin and insulin-like
growth factor receptor family in neoplasia: An update. Nat Rev
Cancer. 12:159–169. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Conover CA and Oxvig C: PAPP-A and cancer.
J Mol Endocrinol. 61:T1–T10. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hendriks E, MacNaughton H and MacKenzie
MC: First trimester bleeding: Evaluation and management. Am Fam
Physician. 99:166–174. 2019.PubMed/NCBI
|
|
22
|
Tang Y, Luo H, Mu D, Yang T, Zhu Q, Yang F
and Liu G: Early diagnosis of trisomy 21, trisomy 18 and trisomy 13
using nuchal translucency thickness and ductus venosus blood flow
waveform in West China. Mol Med Rep. 19:1349–1355. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang J and Zhang B: Second-generation
non-invasive high-throughput DNA sequencing technology in the
screening of Down's syndrome in advanced maternal age women. Biomed
Rep. 4:715–718. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Qiang R, Cai N, Wang X, Wang L, Cui K,
Wang W, Wang X and Li X: Detection of trisomies 13,18 and 21 using
non-invasive prenatal tasting. Exp Ther Med. 13:2304–2310.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Patil M, Panchanadikar TM and Wagh G:
Variation of PAPP-A level in the first trimester of pregnancy and
its clinical outcome. J Obstet Gynaecol India. 64:116–119.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lau H, Amarasekara C and Uppal T: Low
PAPP-A: What are the clinical implications? Australas J Ultrasound
Med. 15:26–28. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Antsaklis P, Fasoulakis Z, Theodora M,
Diakosavvas M and Kontomanolis EN: Association of low maternal
pregnancy-associated plasma protein a with adverse perinatal
outcome. Cureus. 11(e4912)2019.PubMed/NCBI View Article : Google Scholar
|