Introduction
Laryngeal cancer is a common tumour type with the
highest degree of malignancy of head and neck tumours and is
associated with high mortality and morbidity rates (1). The occurrence and development of
laryngeal cancer is a multifactorial and multistep complex process,
and may be associated with several complications, including
dyspnoea, coughing and dysphagia, which severely affect the quality
of life of the patients and render perioperative intervention
difficult (2,3). At present, surgery and radiotherapy
are the main treatment strategies for laryngeal cancer, despite
major advances in therapeutic techniques. However, as the early
symptoms of laryngeal cancer are not obvious, early diagnosis and
treatment remain a challenge (4,5).
Therefore, elucidating the molecular pathogenesis of laryngeal
cancer is of utmost importance for the development of novel
treatment strategies.
Studying the genome of laryngeal cancer is helpful
for identifying novel therapeutic targets and understanding the
molecular pathology of the disease. MicroRNAs (miRNAs/miRs) are a
type of small single-stranded non-coding RNA molecules that
regulate the proliferation, differentiation and apoptosis of
multiple types of cells, and play an important role in the
development of multiple tumours, such as esophageal squamous cell
carcinoma, breast cancer, pancreatic cancer and laryngeal cancer
(6-9).
It has been reported that a variety of miRNAs are abnormally
expressed in laryngeal cancer cells and serve as important
biomarkers in laryngeal cancer and other head and neck tumours
(10-12).
For example, the expression of miR-21-5p is upregulated in
laryngeal cancer cells, and promotes the proliferation, migration
and invasion of laryngeal cancer cells by inhibiting the expression
of laryngeal oncogene laryngeal carcinoma-related gene 1(13). Moreover, miR-34a and miR-34c have
been shown to inhibit the proliferation of laryngeal cancer cells
by negatively regulating polypeptide
N-acetylgalactosaminyltransferase 7 in HEp-2 cells (14). In addition, the upregulation of
miR-375 can suppress the proliferation and invasion of squamous
cell laryngeal carcinoma (15).
miR-195 is one of the most important members of the miR-15/16
family and is widely considered to exert antitumor effects. Studies
have demonstrated that miR-195-5p is expressed at low levels in
several types of cancer, such as melanoma, cervical cancer and
colorectal cancer, which may be associated with tumour resistance,
metabolism and proliferation (16-18).
However, the role and mechanisms of miR-195-5p in laryngeal cancer
remain unclear.
The present study confirmed the role of miR-195-5p
in laryngeal cancer by detecting the expression of miR-195-5p in
laryngeal cancer cell lines, as well as its effects on the
proliferation, migration and invasion of laryngeal cancer cells. In
addition, through database search, it was found that E2F3 may be a
potential target gene of miR-195-5p and the effects of miR-195-5p
on the biological function of laryngeal cancer cells via the
regulation of E2F3 were analysed. The results may provide a
theoretical basis for the important role of miR-195-5p/E2F3 in the
diagnosis and treatment of laryngeal cancer.
Materials and methods
Cells and cell culture
A normal immortalized nasopharyngeal epithelial cell
line (NP-69), laryngeal carcinoma cell lines (AMC-HN-8 and SNU-899)
and oropharyngeal squamous cell line (M4E) were purchased from the
The Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences. NP-69, AMC-HN-8 and M4E cells were cultured in DMEM
(HyClone; Cytiva) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.). SNU-899 cells were cultured
in RPMI-1640 medium containing 10% FBS and 2% glutamine (Gibco;
Thermo Fisher Scientific, Inc.). All cells were cultured in a 37˚C
humidified incubator with 5% CO2. Cells in the
logarithmic growth phase were used in subsequent experiments.
Reverse transcription-quantitative PCR
(RT-qPCR)
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract total RNA from the
cells according to the manufacturer's instructions. RNA (2 µg) was
transcribed into cDNA using the PrimeScript RT Reagent kit with
gDNA Eraser (Takara Biotechnology Co., Ltd.). The RT conditions
were as follows: 70˚C for 5 min, 37˚C for 5 min and 42˚C for 1 h.
qPCR was conducted using an ABI 7300 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The miRNA expression
levels were quantified using the miScript SYBR-Green PCR kit
(Toyobo Life Science). The qPCR reaction conditions were as
follows: Pre-denaturation at 95˚C for 5 min, followed by 36 cycles
of denaturation at 95˚C for 15 sec, annealing at 60˚C for 30 sec
and extension 72˚C for 30 sec. Relative expression was calculated
using the 2-ΔΔCq method (19). U6 or GAPDH were used as the internal
references. The primer sequences were: miR-195-5p forward,
5'-GAATTCGCCTCAAGAGAACAAAGTGGAG-3'; miR-195-5p reverse,
5'-AGATCTCCCATGGGGGCTCAGCCCCT-3'; E2F3 forward,
5'-CCCTAAACCCGCTTCC-3'; E2F3 reverse, 5'-GTTCACAAACGGTCCTTCTA-3';
E-cadherin forward 5'-CGAGAGCTACACGTTCACGG-3'; E-cadherin reverse
5'-GGGTGTCGAGGGAAAAATAGG-3'; vimentin forward,
5'-GACGCCATCAACACCGAGTT-3'; vimentin reverse,
5'-CTTTGTCGTTGGTTAGCTGGT-3'; N-cadherin forward,
5'-AGCTCCATTCCGACTTAGACA-3'; N-cadherin reverse,
5'-CAGCCTGAGCACGAAGAGTG-3'; matrix metalloproteinase (MMP)2
forward, 5'-GGCCAGGTGGTATCTTAGGC-3'; MMP2 reverse,
5'-AGCTGACCAGTGTTCATTCTTG-3'; MMP9 forward,
5'-GGGACGCAGACATCGTCATC-3'; MMP9 reverse,
5'-TCGTCATCGTCGAAATGGGC-3'; snail forward,
5'-TCGGAAGCCTAACTACAGCGA-3'; snail reverse,
5'-AGATGAGCATTGGCAGCGAG-3'; GAPDH forward,
5'-AGGTGGTCTCCTCTGACTTCAA-3'; GAPDH reverse,
5'-TTCGTTGTCATACCAGGAAATG-3'; U6 forward,
5'-GCTTCGGCAGCACATATACTAAAAT-3'; and U6 reverse,
5'-CGCTTCACGAATTTGCGTGTCAT-3'.
Cell transfection
miR-195-5p mimic and the negative control miRNA
(miRNA-NC) were obtained from Guangzhou RiboBio Co., Ltd. The E2F3
overexpression vector pcDNA-E2F3 and empty control vector pcDNA-NC
were constructed by Shanghai GenePharma Co., Ltd. The sequences
were as follows: miR-195-5p mimic sense,
5'-UAGCAGCACAGAAAUAUUGGC-3'; miR-195-5p mimic antisense,
5'-CAAUAUUUCUGUGCUGCUAUU-3'; mimics negative control (miR-NC)
sense, 5'-UUCUCCGAACGUGUCACGUTT-3'; miR-NC antisense,
5'-ACGUGACACGUUCGGAGAATT-3'. Cells were plated into 6-well plates
(1x106 cells per well), and transfection was performed
when cells at the logarithmic growth phase reached 80% confluence.
According to the product instructions, AMC-HN-8 cells were
transfected with miR-195-5p mimic (50 nM), miR-NC (50 nM),
pcDNA-E2F3 (100 nM) and pcDNA-NC (100 nM) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The transfection efficiencies were assessed by
RT-qPCR at 48 h after transfection.
Cell counting kit-8 (CCK-8) assay
Cell proliferation was detected using a CCK-8 assay
(Dojindo Molecular Technologies, Inc.) according to the
manufacturer's instructions. AMC-HN-8 cells in each group were
inoculated into the 96-well plate at a density of 2x104
cells/ml and then cultured for 24, 48 and 72 h. Subsequently, 10 µl
CCK-8 solution were added to each well followed by routine culture
for 2 h at 37˚C. The optical density at a 480 nm wavelength was
measured using a microplate reader (BioTek Instruments, Inc.).
Wound-healing assay
AMC-HN-8 cells were inoculated in 6-well plates at a
density of 2x105 cells/well. A linear scratch was
created on the surface of the cell monolayer using a sterile 20-µl
pipette tip perpendicular to the 6-well plate after the cell growth
density had reached ~100%. The surface of cell culture plate was
washed twice with phosphate buffered solution (PBS). The cells were
then routinely cultured in serum-free DMEM. After 24 h, the cell
migration rate was calculated under an inverted light microscope
(magnification, x100; Olympus Corporation). Quantitative analysis
of the wound healing area was performed using ImageJ software
(version 1.52r; National Institutes of Health). The relative cell
migration rate of each group (distance at 24 h-initial distance)
was normalized according to the average migrated distance of the
control.
Transwell assay
A Transwell chamber (8 µm; BD Biosciences) was used
to detect the invasive ability of the AMC-HN-8 cells. Cells
(1x105 cells/ml) were added to the upper Transwell
chamber coated with Matrigel (BD Biosciences) and cultured in
serum-free DMEM. DMEM (800 µl) containing 10% FBS was added to the
lower chamber. Following culture at room temperature for 24 h, the
cells were fixed with 4% formaldehyde for 40 min at room
temperature and then stained with 0.5% crystal violet for 1 h at
room temperature. The number of invading cells were observed and
counted in four representative fields using an inverted light
microscope (magnification, x100; Olympus Corporation).
Luciferase reporter assay
E2F3 was predicted to be the target gene of
miR-195-5p by TargetScan (www.targetscan.org) and ENCORI (http://starbase.sysu.edu.cn/), which was in accordance
with the previous study (20).
Firstly, wild-type (WT) and mutant (MUT) E2F3 3'untranslated region
(UTR) luciferase reporter vectors were constructed by Shanghai
GenePharma Co., Ltd.. The AMC-HN-8 cells were inoculated in 24-well
plates at a density of 2x105 cells/well. miR-195-5p
mimics or negative control and the WT and MUT 3'UTR of E2F3 were
co-transfected into the AMC-HN-8 cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). At 48 h following transfection, the luciferase
activity was detected strictly according to the instructions
provided with the Dual-Luciferase reporter assay kit (Promega
Corporation). Firefly luciferase activity was normalized to
Renilla luciferase activity.
Western blot analysis
Cells were lysed with RIPA lysis buffer (Beyotime
Institute of Biotechnology) and the protein concentration was
determined using a BCA protein assay kit (Thermo Fisher Scientific,
Inc.). The proteins (40 µg/lane) were separated by 10% SDS-PAGE and
then transferred to PVDF membranes (EMD Millipore). Skimmed milk
(5%, dissolved in PBS) was used to block the PVDF membranes for 2 h
at 37˚C. The membranes were then incubated with primary antibodies
against E-cadherin (1:1,000; cat. no. 14472), vimentin (1:1,000;
cat. no. 5741), N-cadherin (1:1,000; cat. no. 13116), snail
(1:1,000; cat. no. 3879T), MMP-2 (1:1,000; cat. no. 40994), MMP-9
(1:1,000; cat. no. 13667) and GAPDH (1:1,000; cat. no. 5174) (all
from Cell Signaling Technology, Inc.) at 4˚C overnight. After
washing the membranes twice with PBS for 10 min each time, they
were incubated with HRP-conjugated secondary antibody (1:5,000;
cat. no. sc-2357; Santa Cruz Biotechnology, Inc.) for 1.5 h at room
temperature. The protein bands were detected using ECL detection
reagent (Cytiva). GAPDH was used as an internal reference. The
intensity of the bands was semi-quantified using ImageJ software
(version 1.52r; National Institutes of Health). Experiments were
performed in triplicate followed by statistical analysis.
Statistical analysis
All experiments were repeated at least three times.
SPSS software 20.0 (IMB Corp.) was used for the statistical
analysis of data and all the experimental data are expressed as the
mean ± standard deviation (SD). An unpaired Student's t-test was
used for comparison between two groups, and comparisons among more
than two groups were analyzed using the one-way analysis of
variance followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-195-5p expression is downregulated
in laryngeal cancer cell lines
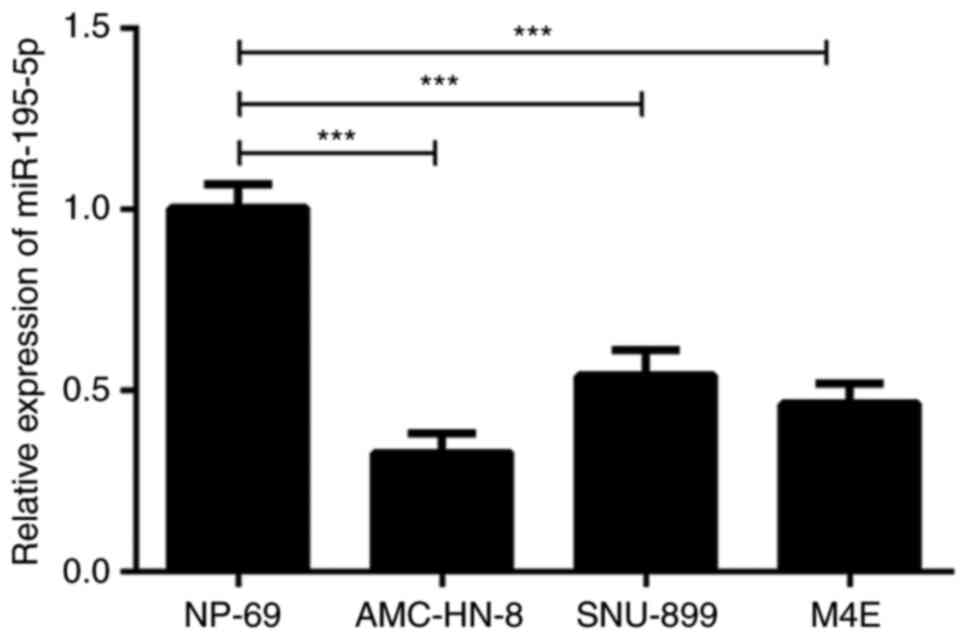
Firstly, the expression of miR-195-5p in laryngeal
carcinoma cell lines was detected by RT-qPCR. As shown in Fig. 1, the expression of miR-195-5p in the
laryngeal cancer cell lines was significantly lower compared with
that in the normal cell line. Of note, the expression of miR-195-5p
was the lowest in the AMC-HN-8 cells. Therefore, the AMC-HN-8 cells
were used in the subsequent experiments. These results indicated
that miR-195-5p was expressed at low levels in laryngeal cancer
cell lines.
miR-195-5p overexpression inhibits the
proliferation, migration and invasion of AMC-HN-8 cells
Due to the low expression of miR-195-5p in the
AMC-HN-8 laryngeal cancer cell line, the expression of miR-195-5p
was modified by transfection with miR-195-5p overexpression
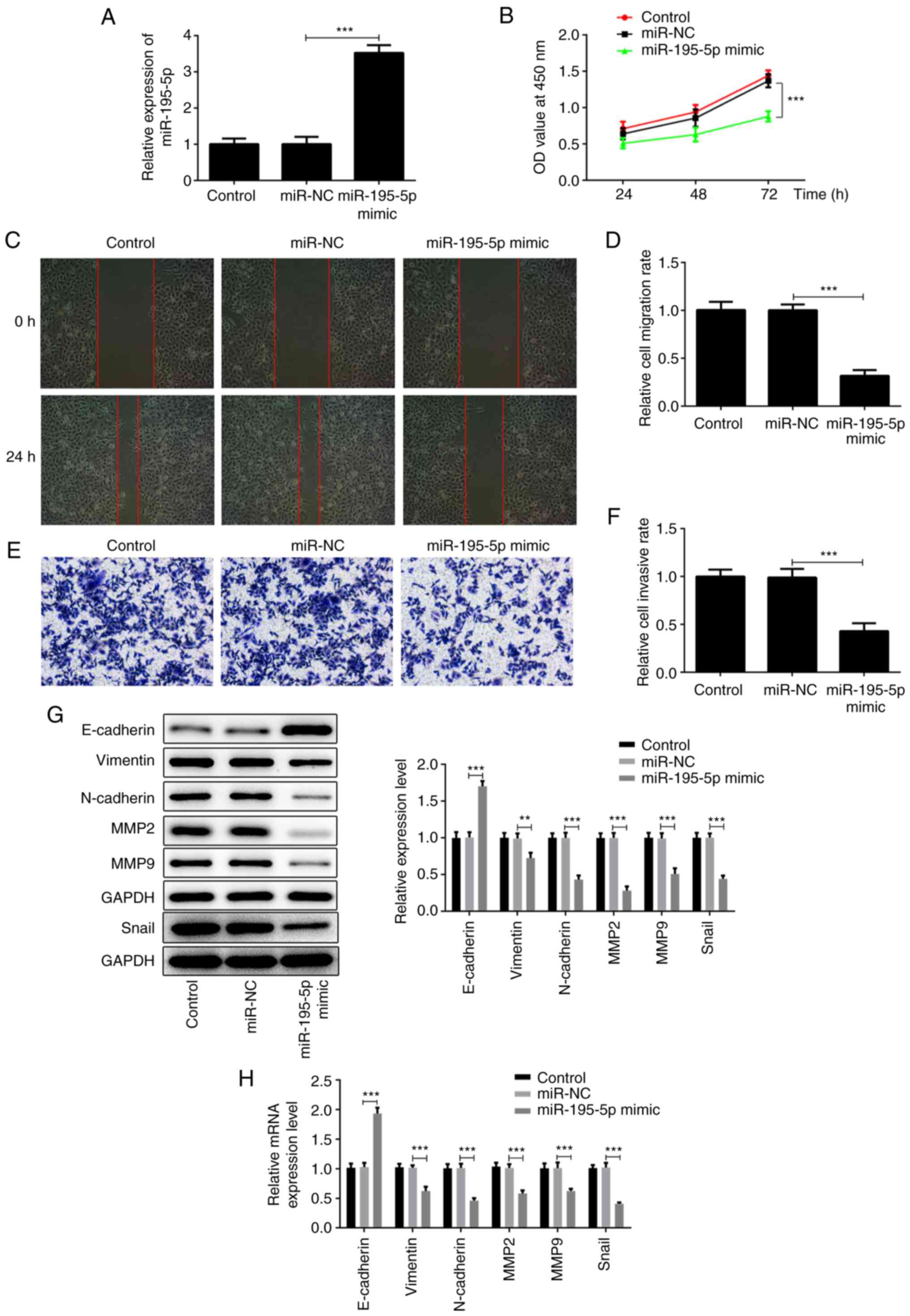
plasmid. As shown in Fig. 2A, the
expression of miR-195-5p in the miR-195-5p mimic group was
significantly higher compared with that of the miR-NC group. The
aforementioned results indicated that the miR-195-5p overexpression
plasmid was successfully constructed. Subsequently, CCK-8, wound
healing and Transwell assays, and western blot analysis, were used
to examine the effects of miR-195-5p overexpression on the
proliferation, migration and invasion of laryngeal cancer cells.
Pang et al (21) reported
that miR-195 is associated with the cell viability of the AMC-HN-8
cell line. In the present study, the results of CCK-8 assay
revealed that miR-195-5p overexpression significantly inhibited the
proliferation of AMC-HN-8 cells compared with the control group
(Fig. 2B). The wound-healing assay
revealed that miR-195-5p overexpression significantly inhibited the
migration of the AMC-HN-8 cells compared with the control group
(Fig. 2C and D). Similar results were obtained by
Transwell assay; miR-195-5p overexpression significantly lowered
the invasive ability of the AMC-HN-8 cells (Fig. 2E and F). In addition, the results of western
blot analysis and RT-qPCR assay demonstrated that, compared with
the control group, miR-195-5p overexpression significantly
increased the expression of E-cadherin, while it inhibited the
expression levels of vimentin, N-cadherin, snail, MMP-2 and MMP-9,
indicating that miR-195-5p overexpression can significantly
suppress the epithelial-mesenchymal transition (EMT) of AMC-HN-8
cells (Fig. 2G and H). Taken together, these results suggest
that miR-195-5p overexpression inhibits the proliferation,
migration and invasion of laryngeal cancer cells.
E2F3 is the direct target gene of
miR-195-5p
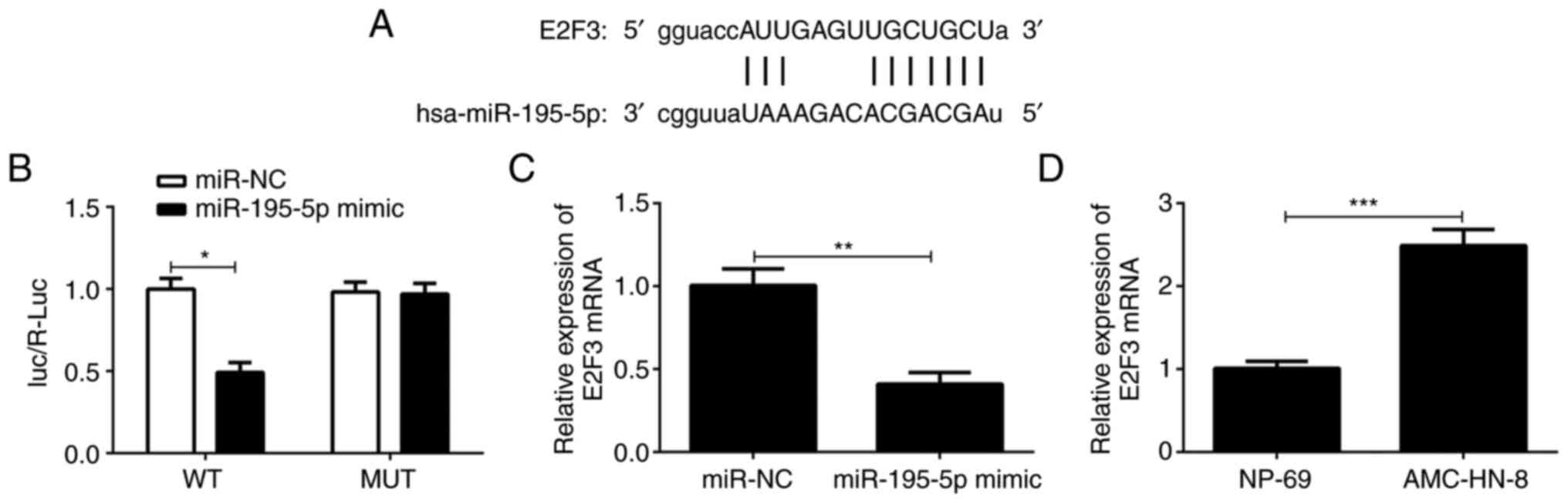
Subsequently, the target gene of miR-195-5p was
predicted through the TargetScan and ENCORI website. As shown in
Fig. 3A, E2F3 has potential sites
for binding to miR-195-5p. Therefore, the association between
miR-195-5p and E2F3 was determined using the dual-luciferase
reporter assay. As shown in Fig.
3B, compared with the miR-NC group, the luciferase activity of
the cells co-transfected with MUT E2F3 3'UTR reporter plasmid and
miR-195-5p mimic was not altered; however, that of the cells
transfected with the WT E2F3 3'UTR reporter plasmid and miR-195-5p
mimic was significantly decreased. In addition, as exhibited in
Fig. 3C, E2F3 expression was
notably downregulated in AMC-HN-8 cells after transfection with
miR-195-5p mimic compared with the miR-NC group. The results of
Fig. 3D indicated that E2F3 level
was markedly enhanced in AMC-HN-8 cells compared with that in the
NP-69 cells. Collectively, the results indicate that E2F3 is a
direct target of miR-195-5p.
miR-195-5p suppresses the
proliferation, migration and invasion of AMC-HN-8 cells by
regulating E2F3 expression
Based on the aforementioned results, it was
suggested that miR-195-5p can affect the biological function of
AMC-HN-8 cells by regulating E2F3. As shown in Fig. 4A, compared with the pcDNA-NC group,
the expression of E2F3 in the cells transfected with pcDNA-E2F3 was
significantly increased. Remarkably, cells co-transfected with
miR-195-5p mimic and pcDNA-E2F3 displayed upregulated E2F3
expression compared with the miR-195-5p mimic + pcDNA-NC group. The
results of the CCK-8 assay revealed that the overexpression of E2F3
in AMC-HN-8 cells transfected with miR-195-5p mimic reversed the
inhibitory effect of miR-195-5p overexpression on the proliferation
of AMC-HN-8 cells (Fig. 4B). The
results of wound healing and Transwell assays demonstrated that the
co-transfection with pcDNA-E2F3 and miR-195-5p mimic reversed the
blocking effects of miR-195-5p overexpression alone on the
migration and invasion of AMC-HN-8 cells (Fig. 4C-F). Similarly, the E2F3
upregulation attenuated the inhibitory effects of miR-195-5p
overexpression on EMT of AMC-HN-8 cells transfected with miR-195-5p
mimic, obtained by downregulated expression of E-cadherin,
upregulated expression of vimentin, N-cadherin, snail, MMP-2 and
MMP-9 (Fig. 4G and H). In general, the results suggest that
miR-195-5p may suppress the progression of laryngeal cancer by
downregulating E2F3.
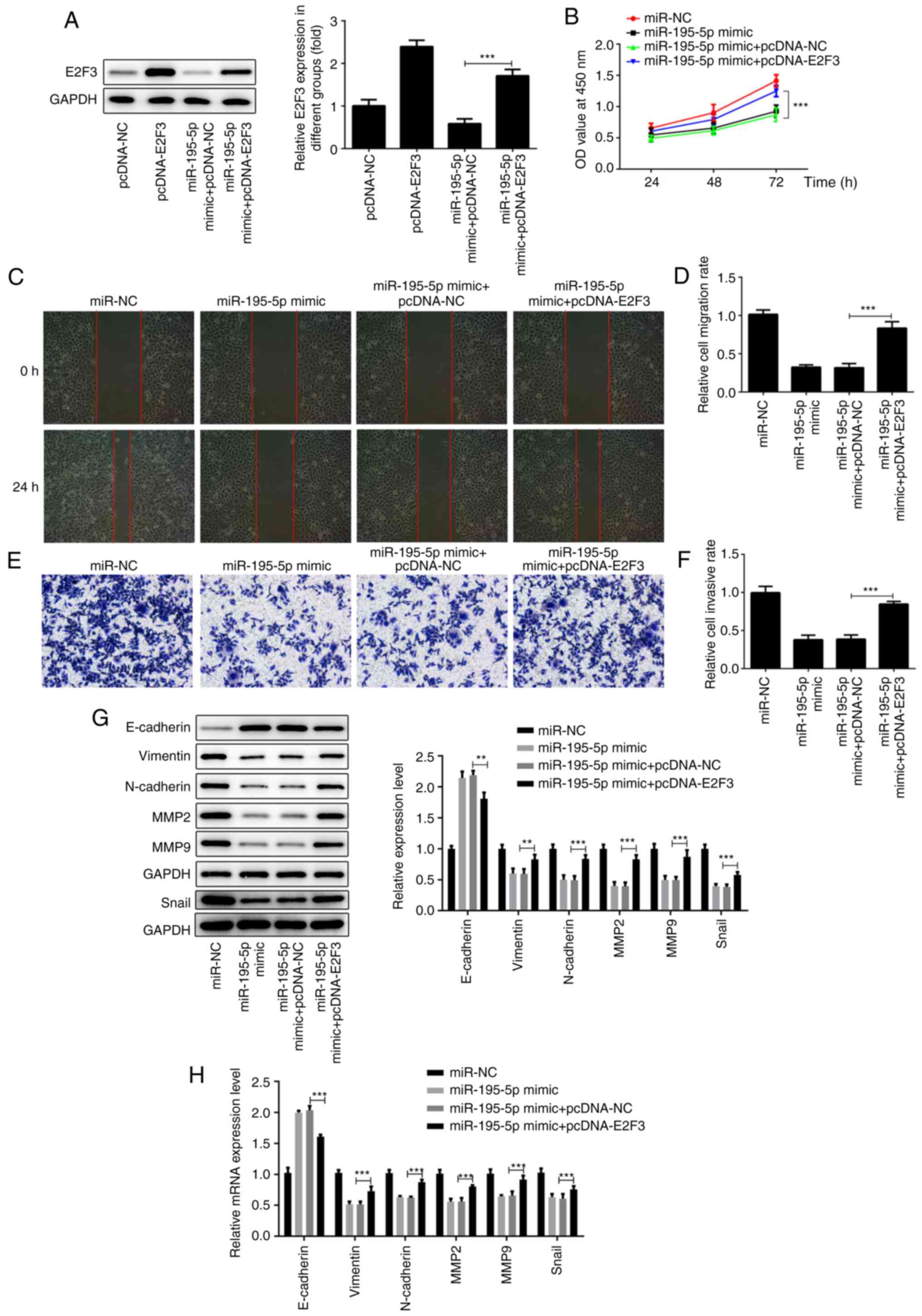 | Figure 4miR-195-5p suppresses the
proliferation, migration and invasion of AMC-HN-8 cells by
regulating E2F3 expression. (A) E2F3 overexpression efficiency was
detected by western blot analysis. (B) miR-195-p mimic and
pcDNA-E2F3 were co-transfected into AMC-HN-8 cells and cell
proliferation was detected by Cell Counting Kit-8 assays. (C and D)
Cell migration in each group following transfection with miR-195-5p
and pcDNA-E2F3 was detected by wound-healing assays. Magnification,
x100. (E and F) Cell invasion in each group following transfection
with miR-195-5p and pcDNA-E2F3 was detected by Transwell assays.
Magnification, x100. (G) Western blot analysis and (H) RT-qPCR
assay were respectively used to measure the protein and mRNA levels
of the epithelial-mesenchymal transition-associated proteins
(E-cadherin, vimentin, N-cadherin and snail) and MMP-2 and -9.
**P<0.01; ***P<0.001. miR, microRNA;
E2F3, E2F transcription factor 3; miR, microRNA; NC, negative
control; MMP, matrix metalloprotease. |
Discussion
It is well known that the occurrence and development
of laryngeal cancer is closely associated with a number of factors,
and its pathogenesis is complex, affected and restricted by a
variety of regulatory networks (22-25).
miRNAs are a type of gene expression regulators in laryngeal cancer
cells and play an important role in the occurrence and development
of laryngeal cancer (26).
Therefore, the study of miRNAs may provide new insights into the
research and development of drugs for the targeted treatment of
laryngeal cancer. The results of the present study revealed that
the expression of miR-195-5p in laryngeal cancer cells was lower
compared with that in normal cells. In addition, miR-195-5p
overexpression significantly inhibited the proliferation, migration
and invasion of AMC-HN-8 cells, suggesting that miR-195-5p may be a
potential target for the diagnosis and treatment of laryngeal
cancer.
miR-195, located on chromosome 17, is unanimously
considered as an inhibitor of tumour proliferation and has broad
prospects as a tumour suppressor (27). It has been demonstrated that
miR-195-5p is downregulated in breast, ovarian, colorectal and
liver cancer, indicating that miR-195-5p is associated with tumour
cell invasion and metastasis (28).
In non-small cell lung cancer, the low expression of miR-195-5p
inhibited cell migration and invasion (29). Moreover, miR-195-5p overexpression
has been shown to suppress the proliferation of lung cancer cells,
and to induce apoptosis and G0/G1 phase
arrest (30). Importantly, miR-195
is reported to be associated with regulating the pathophysiologic
process of human laryngeal squamous cell carcinoma (21). In the present study, the expression
of miR-195-5p in laryngeal cancer cells was found to be
significantly lower compared with that in normal cells, which was
consistent with the expression of miR-195-5p in other types of
tumours. The proliferation, migration and invasion of AMC-HN-8
cells were significantly suppressed upon miR-195-5p mimic
transfection. It is thus suggested that miR-195-5p mainly functions
as a tumour suppressor, and can inhibit the proliferation,
migration and invasion of laryngeal cancer cells.
EMT is the process through which the cell phenotype
changes from an epithelial to a stromal phenotype, accompanied by
the decreased expression of epithelial cell markers (E-cadherin)
and the increased expression of stromal cell markers (N-cadherin,
vimentin and snail) (31). EMT
decreases cell-cell and cell-matrix adhesion, so as to enhance the
ability of cell migration, invasion and metastasis (32). MMP-2 and MMP-9 are the most
extensively investigated members of the MMP family, which can
degrade the basement membrane and the extracellular matrix, and
promote tumour cell migration and invasion (33-35).
In the present study, it was demonstrated that miR-195-5p
overexpression significantly promoted the expression of E-cadherin,
and inhibited the expression of N-cadherin, vimentin and snail,
while MMP-2 and MMP-9 were also suppressed, suggesting that
miR-195-5p overexpression inhibited the EMT process in laryngeal
cancer cells.
E2F3 was predicted to be the target gene of
miR-195-5p by the bioinformatics algorithms miRTarBase, DIANA-tools
and miRDB in the previous study (20). The transcription factor E2F3 plays
an important role in cell cycle progression, tumorigenesis and
cancer development. E2F3 is involved in the regulation of the cell
cycle by forming dimers with cyclin D1, and is associated with a
variety of carcinogenic and tumour suppressor genes (36,37).
Previous studies have found that miR-449a and miR-125b decrease
cell proliferation and induce apoptosis by inhibiting E2F3
(38,39). Although E2F3 showed no differential
expression in colorectal cancer cells after transfection with
miR-195-5p mimic (20), E2F3 was
the downstream target gene of miR-195-5p and the functional
mediator of miR-195-5p in laryngeal cancer cells in the present
study. Further analysis indicated that miR-195-5p overexpression
inhibited the proliferation, migration and invasion of laryngeal
cancer cells by downregulating E2F3. The mechanism of miR-195-5p
downregulation in laryngeal cancer might be associated with the
regulatory effects of competing endogenous RNAs.
miR-195-5p is a type of inhibitory miRNA of
laryngeal cancer. The overexpression of miR-195-5p can inhibit the
proliferation, migration and invasion of laryngeal cancer cells.
Further analyses revealed that miR-195-5p suppressed the activity
of laryngeal cancer cells by negatively regulating E2F3. However,
the present study had certain limitations. miRNAs can regulate
multiple genes and one gene can also be regulated by multiple
miRNAs. Therefore, there may be other genes regulated by miR-195-5p
that are involved in the proliferation, migration and invasion of
laryngeal cancer cells. Thus, these issues need to be resolved
progressively in future research, in order to provide further
in-depth knowledge of the regulatory pathway(s) of miR-195-5p.
Moreover, the effect of miR-195-5p on apoptosis and cell cycle of
laryngeal cancer cells will be investigated in the following
studies. Furthermore, whether miR-195-5p can regulate the
progression of laryngeal cancer in vivo remains to be
elucidated in the next investigation.
In conclusion, the findings of the present study
demonstrated that the upregulation of miR-195-5p decreases the
expression of E2F3, and inhibits the proliferation, migration and
invasion of laryngeal cancer cells, suggesting that the
miR-195-5p/E2F3 axis may be used as a potential target for the
treatment of laryngeal cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MZ and XX made substantial contributions to the
conception and design of the present study. MZ and YW designed the
study; MZ, YW, CZ, MQ, MY and LS performed the experiments; MZ and
YW analyzed the data and wrote the manuscript. MZ and XW confirm
the authenticity of all the raw dat. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thompson LD: Laryngeal dysplasia, squamous
cell carcinoma, and variants. Surg Pathol Clin. 10:15–33.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Echanique KA, Desai SV, Marchiano E,
Spinazzi EF, Strojan P, Baredes S and Eloy JA: Laryngeal verrucous
carcinoma: A systematic review. Otolaryngol Head Neck Surg.
156:38–45. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kolator M, Kolator P and Zatoński T:
Assessment of quality of life in patients with laryngeal cancer: A
review of articles. Adv Clin Exp Med. 27:711–715. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Feng W, Yang Y, Xin ZH and Ni XG:
Misdiagnosis experience of laryngeal carcinoma with acute
laryngitis as the first symptom. Zhonghua Er Bi Yan Hou Tou Jing
Wai Ke Za Zhi. 53(640)2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
5
|
Obid R, Redlich M and Tomeh C: The
treatment of laryngeal cancer. Oral Maxillofac Surg Clin North Am.
31:1–11. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chang HY, Lee CH, Li YS, Huang JT, Lan SH,
Wang YF, Lai WW, Wang YC, Lin YJ, Liu HS and Cheng HC:
MicroRNA-146a suppresses tumor malignancy via targeting vimentin in
esophageal squamous cell carcinoma cells with lower fibronectin
membrane assembly. J Biomed Sci. 27(102)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Escuin D, López-Vilaró L, Bell O, Mora J,
Moral A, Pérez JI, Arqueros C, Ramón Y, Cajal T, Lerma E and
Barnadas A: MicroRNA-1291 is associated with locoregional
metastases in patients with early-stage breast cancer. Front Genet.
11(562114)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xu WG, Kuang YM, Wang D, Li Z and Xia RP:
Effect of miR-210 on proliferation and migration of pancreatic
cancer cells through regulating Runx3 level. J Biomater Tissue Eng.
10:1827–1831. 2020.
|
|
9
|
Chen L, Sun DZ, Fu YG, Yang PZ, Lv HQ, Gao
Y and Zhang XY: Upregulation of microRNA-141 suppresses
epithelial-mesenchymal transition and lymph node metastasis in
laryngeal cancer through HOXC6-dependent TGF-β signaling pathway.
Cell Signal. 66(109444)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yang B, Zang J, Yuan W, Jiang X and Zhang
F: The miR-136-5p/ROCK1 axis suppresses invasion and migration, and
enhances cisplatin sensitivity in head and neck cancer cells. Exp
Ther Med. 21(317)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu YB, Wang Y, Zhang MD, Yue W and Sun
CN: MicroRNA-29a functions as a tumor suppressor through targeting
STAT3 in laryngeal squamous cell carcinoma. Exp Mol Pathol.
116(104521)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cossu AM, Mosca L, Zappavigna S, Misso G,
Bocchetti M, De Micco F, Quagliuolo L, Porcelli M, Caraglia M and
Boccellino M: Long non-coding RNAs as important biomarkers in
laryngeal cancer and other head and neck tumours. Int J Mol Sci.
20(3444)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Erkul E, Yilmaz I, Gungor A, Kurt O and
Babayigit MA: MicroRNA-21 in laryngeal squamous cell carcinoma:
Diagnostic and prognostic features. Laryngoscope. 127:E62–E66.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li W, Ma HP and Sun J: MicroRNA-34a/c
function as tumor suppressors in Hep-2 laryngeal carcinoma cells
and may reduce GALNT7 expression. Mol Med Rep. 9:1293–1298.
2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Guo Y, An R, Zhao R, Sun Y, Liu M and Tian
L: miR-375 exhibits a more effective tumor-suppressor function in
laryngeal squamous carcinoma cells by regulating KLF4 expression
compared with simple co-transfection of miR-375 and miR-206. Oncol
Rep. 36:952–960. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chai L, Kang XJ, Sun ZZ, Zeng MF, Yu SR,
Ding Y, Liang JQ, Li TT and Zhao J: MiR-497-5p, miR-195-5p and
miR-455-3p function as tumor suppressors by targeting hTERT in
melanoma A375 cells. Cancer Manag Res. 10:989–1003. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Feng C, Zhang L, Sun Y, Li X, Zhan L, Lou
Y, Wang Y, Liu L and Zhang Y: GDPD5, a target of miR-195-5p, is
associated with metastasis and chemoresistance in colorectal
cancer. Biomed Pharmacother. 101:945–952. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu X, Zhou Y, Ning YE, Gu H, Tong Y and
Wang N: MiR-195-5p inhibits malignant progression of cervical
cancer by targeting YAP1. OncoTargets Ther. 13:931–944.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Poel D, Boyd LNC, Beekhof R, Schelfhorst
T, Pham TV, Piersma SR, Knol JC, Jimenez CR, Verheul HMW and
Buffart TE: Proteomic analysis of miR-195 and miR-497 replacement
reveals potential candidates that increase sensitivity to
oxaliplatin in MSI/P53wt colorectal cancer cells. Cells.
8(1111)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pang H, Xu X, Dai L, Wang K and Yao X:
MicroRNA-195 is associated with regulating the pathophysiologic
process of human laryngeal squamous cell carcinoma. Mol Med Rep.
17:5283–5291. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cheng JZ, Chen JJ, Wang ZG, Yu D and Zu
YZ: The functional role of microRNAs in laryngeal carcinoma. Open
Life Sci. 12:460–464. 2017.
|
|
23
|
Wang PP, Ding SY, Sun YY, Li YH and Fu WN:
MYCT1 inhibits the adhesion and migration of laryngeal cancer cells
potentially through repressing collagen VI. Front Oncol.
10(564733)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Song Y, Yang M, Zhang H, Sun Y, Tao Y, Li
H, Zhang J, Li Y and Yang J: IL-17 affects the progression,
metastasis, and recurrence of laryngeal cancer via the inhibition
of apoptosis through activation of the PI3K/AKT/FAS/F pathways. J
Immunol Res. 2020(2953191)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li Y, Li D, Wang P, Zhu W and Yin W:
Tetrandrine partially reverses multidrug resistance of human
laryngeal cancer cells. J Int Med Res.
48(300060520944706)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shang Y, Wang LQ, Guo QY and Shi TL:
MicroRNA-196a overexpression promotes cell proliferation and
inhibits cell apoptosis through PTEN/Akt/FOXO1 pathway. Int J Clin
Exp Pathol. 8:2461–2472. 2015.PubMed/NCBI
|
|
27
|
Jin Y, Wang M, Hu H, Huang Q, Chen Y and
Wang G: Overcoming stemness and chemoresistance in colorectal
cancer through miR-195-5p-modulated inhibition of notch signaling.
Int J Biol Macromol. 117:445–453. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li Y, Di C, Li W, Cai W, Tan X, Xu L, Yang
L, Lou G and Yan Y: Oncomirs miRNA-221/222 and tumor suppressors
miRNA-199a/195 are crucial miRNAs in liver cancer: A systematic
analysis. Dig Dis Sci. 61:2315–2327. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yu X, Zhang Y, Ma X and Pertsemlidis A:
miR-195 potentiates the efficacy of microtubule-targeting agents in
non-small cell lung cancer. Cancer Lett. 427:85–93. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zheng J, Xu T, Chen F and Zhang Y:
MiRNA-195-5p functions as a tumor suppressor and a predictive of
poor prognosis in non-small cell lung cancer by directly targeting
CIAPIN1. Pathol Oncol Res. 25:1181–1190. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kalluri R and Neilson EG:
Epithelial-mesenchymal transition and its implications for
fibrosis. J Clin Invest. 112:1776–1784. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Wang Q, Wang F, Zhong W, Ling H, Wang J,
Cui J, Xie T, Wen S and Chen J: RNA-binding protein RBM6 as a tumor
suppressor gene represses the growth and progression in
laryngocarcinoma. Gene. 697:26–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hwang KE, Kim HJ, Song IS, Park C, Jung
JW, Park DS, Oh SH, Kim YS and Kim HR: Salinomycin suppresses
TGF-β1-induced EMT by down-regulating MMP-2 and MMP-9 via the
AMPK/SIRT1 pathway in non-small cell lung cancer. Int J Med Sci.
18:715–726. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Karmakar D, Maity J, Mondal P, Shyam
Chowdhury P, Sikdar N, Karmakar P, Das C and Sengupta S: E2F5
promotes prostate cancer cell migration and invasion through
regulation of TFPI2, MMP-2 and MMP-9. Carcinogenesis. 41:1767–1780.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Miles WO, Tschöp K, Herr A, Ji JY and
Dyson NJ: Pumilio facilitates miRNA regulation of the E2F3
oncogene. Genes Dev. 26:356–368. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tang W, Tang J, Qin J, Geng Q, Zhou Z, Li
B, Zhang J, Chen H, Xia Y and Wang X: Involvement of down-regulated
E2F3 in Hirschsprung's disease. J Pediatr Surg. 48:813–817.
2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Huang L, Luo J, Cai Q, Pan Q, Zeng H, Guo
Z, Dong W, Huang J and Lin T: MicroRNA-125b suppresses the
development of bladder cancer by targeting E2F3. Int J Cancer.
128:1758–1769. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ren XS, Yin MH, Zhang X, Wang Z, Feng SP,
Wang GX, Luo YJ, Liang PZ, Yang XQ, He JX and Zhang BL:
Tumor-suppressive microRNA-449a induces growth arrest and
senescence by targeting E2F3 in human lung cancer cells. Cancer
Lett. 344:195–203. 2014.PubMed/NCBI View Article : Google Scholar
|