Introduction
Melanins are dark-brown to black pigments found in
animals, plants and microorganisms (1,2). The
presence of melanin has been associated with various immune
responses in animals, plants and invertebrates (3-6).
Nigella sativa L. is an herbaceous plant, traditionally used
in folk medicine in the Middle East and South East Asia. Various
research/clinical groups studied the effect of the total extracts
of Nigella sativa on the immune system (7,8).
Melanin extracted from the seed coats of Nigella sativa have
been chemically described and characterized (9,10).
Previously, a similarity was reported between the biological
effects of herbal melanin (HM) extract and bacterial
endotoxin-lipopolysaccharide (LPS) in modulating the production of
interleukin (IL)-6, tumor necrosis factor (TNF)α and vascular
endothelial growth factor (VEGF) through Toll-like receptor (TLR)4
and nuclear factor (NF)-κB activation, the main pathway for
cytokine production (10-12).
As a potential alternative medicinal plant-based drug for the
treatment of inflammatory-associated diseases, the assessment of
the potential immunoregulatory effects of HM on the production of
major pro- and anti-inflammatory cytokines such as IL-1β is
required.
IL-1, an important inflammatory and immunoregulatory
cytokine, is usually expressed by activated monocytes and
macrophages (13,14). IL-1 is composed of two distinct
proteins, IL-1α and IL-1β (14).
IL-1β is a 35-kDa secreted protein induced in response to a variety
of stimuli including LPS, phorbol myristate acetate and IL-1β
itself (14,15). IL-1β possesses a broad range of
biological activities, such as the resolution of inflammation
through the induction of apoptosis in active immune cells (16). The role of IL-1β varies greatly,
depending on the tissues and organs involved and the stage of
inflammation (17). Elevated and
decreased levels of IL-1β have been implicated in various acute
inflammatory-associated diseases, such as bacterial meningitis and
human immunodeficiency virus type 1-seropositive hemophiliacs
(18,19).
TLRs are a family of evolutionarily conserved
receptors with a crucial role in early host defense against
pathogens, through the regulation of both the innate and adaptive
immune responses. TLRs are capable of recognizing
pathogen-associated molecule patterns. Thirteen TLRs and their
respective ligands have been identified in mammals, including TLR2,
TLR4 and LPS, the main ligand (20-22).
The TLR signaling pathway plays a role in regulating cytokine
production, including the production of IL-1β (19), through the activation of the NF-κB
pathway (23,24) and the p38 mitogen-activated protein
kinase (MAPK) pathway (25).
In the present study, the effect of HM on IL-1β
secretion and production was investigated in human monocytes and
THP-1 cells. The requirement of TLR4, TLR2 receptor and the p38
MAPK pathway activation for HM-induced IL-1β production was also
demonstrated in THP-1 cells, after using an anti-TLR neutralizing
antibody and the p38 MAPK pharmacological inhibitor,
respectively.
Materials and methods
Herbal melanin preparation
The detailed extraction, characterization and stock
solution preparation for the experimental use of the melanin from
Nigella sativa L. seed coats were conducted according to the
methods previously described (10).
Human monocyte isolation
Peripheral blood mononuclear cells (PBMC) were
obtained and cultured from blood collected from healthy donors who
voluntarily consented, as previously described (11). Briefly, cells were separated by a
Ficoll-Paque® (GE Healthcare) density gradient
centrifugation at 400 x g for 30 min at 4˚C. The monocytes-enriched
layer was collected, plated for 2 h and non-adherent cells were
removed with PBS. The pure monocytes were positively selected by an
anti-CD14-coated microbead (MiniMACS separation column; Milteny
Biotec, Inc.) as previously described (11). The cells were >95% viable, as
assessed by the Trypan blue exclusion method, and consisted of
>90% monocytes, as determined by a flow cytometry analysis. Flow
cytometry (Coulter® Epics® XL-MCL™ flow
cytometer including System II™ software; Beckman Coulter, Inc.) was
based on CD14 and CD45 antigen expression after cell incubation for
30 min at 4˚C in PBS containing 2% FBS (Gibco®; Thermo
Fisher Scientific, Inc.) with mouse monoclonal anti-CD14
(phycoerythrin-cyanin 5.5; clone RMO52; IgG2a; cat. no. A70204;
Beckman Coulter, Inc.) and anti-CD45 (fluorescein-5-isothiocyanate;
clone 30-F11; IgG2b; cat. no. 103107; BioLegend, Inc.) antibodies
(data not shown).
Cell culture and treatment
The human monocytic cell line THP-1 was obtained
from the American Type Culture Collection. Both the isolated human
monocytes and THP-1 cells were maintained in suspension in complete
medium, composed of RPMI-1640 medium, supplemented with 10% fetal
bovine serum (FBS) and 1% antibiotics (100 IU/ml penicillin and 100
µg/ml streptomycin), provided by Gibco® (Thermo Fisher
Scientific, Inc.). The cells were maintained in a 37˚C humidified,
5% CO2 incubator. The THP-1 cells and human monocytes
(1x106/ml) were separately treated with HM (5, 10 and 50
µg/ml) and lipopolysaccharides (LPS; 10 µg/ml; E. coli,
0.26:B6; Sigma-Aldrich; Merck KGaA). Untreated THP-1 cells and
monocytes were used as controls.
RNA extraction and reverse
transcription (RT)-PCR
The expression of IL-1β mRNA was assessed in
the THP-1 cells, in the presence or absence of either HM or LPS.
After 3-h incubation, the total RNA was extracted from the THP-1
cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), following the manufacturer's instructions. The total extract
(2 µg) was transcribed into a single strand cDNA in a reaction
mixture (30 µl) containing 1X reaction buffer (75 mM KCl, 3 mM
MgCl2 and 50 mM Tris-HCl pH 8.3), 0.5 mM deoxynucleoside
triphosphate mixture, 1.5 mM oligo(dT)primer, 1 U RNasin and 10 U
Moloney murine leukemia virus reverse transcriptase (Clontech
Laboratories, Inc.), as described previously (10). Amplification of the IL-1β
cDNA, along with the amplification of β-actin cDNA (used as
a housekeeping gene), was performed on a ThermoHybrid thermocycler
(Thermo Fisher Scientific, Inc.) using Taq DNA polymerase (Roche
Molecular Diagnostics) and the following PCR primers (Invitrogen;
Thermo Fisher Scientific, Inc.): IL-1β sense,
5'-AAACAGATGAAGTGCTCCTTCCAGG-3' and antisense,
5'-TGGAGAACACCACTTGTTGCTCCA-3'; and β-actin sense,
5'-ATCTGGCACCACACCTTCTACAATGAGCTGCG-3' and antisense,
5'-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3'. The PCR thermocycling
conditions for IL-1β cDNA amplification consisted if an
initialization step for 10 min at 95˚C, followed by 30 cycles of
denaturation at 94˚C for 30 sec, annealing at 65˚C for 1 min and
extension at 72˚C for 2 min, while the PCR conditions for
β-actin cDNA amplification consisted of an initialization
step for 10 min at 95˚C, followed by 40 cycles of denaturation at
94˚C for 45 sec, annealing at 61˚C for 45 sec and extension at 72˚C
for 2 min. The cDNA products were separated on 2% agarose gel with
electrophoresis, and visualized through ethidium bromide
staining.
Enzyme-linked immunosorbent assay
(ELISA)
The THP-1 cells and isolated monocytes
(1x106/ml) were treated with different concentrations of
HM or LPS at 10 µg/ml, for various incubation times (1, 3, 6 and 24
h). The cell-free supernatants were recovered through
centrifugation at 10,000 x g for 10 min at 4˚C and stored at -20˚C
until assayed. Complete medium was used as a negative control. The
concentration of IL-1β was determined using an IL-1β ELISA kit
(cat. no. HSLB00D; R&D Systems, Inc.), following the
manufacturer's instructions. To verify that the HM did not
interfere with the measurements, an additional control was obtained
by incubating 100 µg/ml HM in complete medium for 24 h at 37˚C. Of
note, repeated assays showed that the HM solution did not affect
the cytokine measurements (data not shown). The sensitivity of the
assay for IL-1β detection was >4 pg/ml.
Protein extraction and western
blotting analysis
The THP-1 cells (1x106/ml) were seeded in
a 24-well plate (Nunc™), in 0.5 ml complete medium. The untreated
cells (control) and cells treated with 10 µg/ml of HM or LPS were
incubated for 1, 6 and 24 h. After each incubation period, the
medium was discarded, and each well was rinsed with 500 µl cold
PBS. Then, ice-cold NP40 lysis buffer (80 µl; Invitrogen; Thermo
Fisher Scientific, Inc.) were added to the wells and the plate was
kept on ice and shaken gently for 25 min. The proteins were
extracted from the fully lysed cells and protein concentration was
estimated using the fluorescence-based Qubit™ Protein quantitation
assay kit (Thermo Fisher Scientific, Inc.). The cell lysate
preparation, protein separation by 12% SDS-PAGE, and the transfer
of the separated proteins to polyvinylidene difluoride (PVDF)
membranes (EMD Millipore) were performed as previously described
(26). The PVDF membranes (Thermo
Fisher Scientific, Inc.) were stained with primary antibodies
diluted (1:1,000) in TBS-0.1% Tween-20 containing 1% BSA (Thermo
Fisher Scientific, Inc.), overnight at 4˚C on a rotating shaker.
The primary antibodies included mouse monoclonal [T2.5] anti-TLR2
antibody (cat. no. ab16894), rabbit polyclonal anti-TLR4 antibody
(cat. no. ab13867), mouse monoclonal [OTI3E1] anti-IL-1β antibody
(cat. no. ab156791), rabbit monoclonal anti-phospho-p38 (Y182)
antibody (cat. no. ab47363), mouse monoclonal [M138] anti-total-p38
antibody (cat. no. ab31828) and mouse monoclonal [6C5] anti-GAPDH
antibody (cat. no. ab8245), all from Abcam. The primary antibodies
were detected with an infrared fluorescent IRDye® 680RD
(red)-conjugated goat anti-rabbit (cat. no. 926-68071) or
IRDye® 800RD (green)-conjugated goat anti-mouse
secondary antibody (cat. no. 926-32210) (both LI-COR Biosciences)
diluted in TBS-0.1% Tween-20 containing 3% BSA (1:5,000) for 1 h at
room temperature with continuous shaking. After five additional
washes in TBS-0.1% Tween-20, the proteins were visualized using the
LI-COR Odyssey CLx Scanner (LI-COR Biosciences) and analyzed using
ImageJ software v.1.46r (http://rsbweb.nih.gov/ij/index.html).
TLR neutralization and p38 MAPK
pathway blockade
To investigate whether the HM-induced IL-1β
production functions through the TLR and p38 MAPK pathway, the
cells were treated with anti-TLR2 (cat. no. ab16894) and anti-TLR4
(cat. no. ab13867) antibodies (both Abcam), or the p38 MAPK
pharmacological inhibitor SB202190 (cat no. sc-222294; Santa Cruz
Biotechnology, Inc.) to neutralize the TLR receptors and block the
p38 MAPK pathway. Briefly, the THP-1 cells (1x106/ml)
were seeded in complete medium in each well of a 24-well plate. The
medium was renewed with 20 µg/ml mouse monoclonal anti-TLR2 or
polyclonal anti-TLR4 antibodies (concentration fixed from pilot
studies), and 20 µg/ml IgG1 was used as an isotype
control or the medium was renewed with 20 µM SB202190 or with DMSO,
used as a negative control. After 2 h of incubation, the cells were
treated with either 10 µg/ml of HM or LPS for 1 h stimulation at
37˚C, followed by protein extraction for western blotting.
Statistical analysis
All the experimental data are expressed as means +
standard deviation (SD). A generalized linear mixed model (GLMM)
procedure from SAS system software (version 9.2; SAS Institute
Inc.) was used to compare the protein expression level between and
within each treatment at different exposure times. Pairwise
comparisons were performed using Tukey's studentized range (HSD)
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Herbal melanin (HM) increases
monocytic IL-1β gene expression and secretion from THP-1 cells and
isolated human monocytes
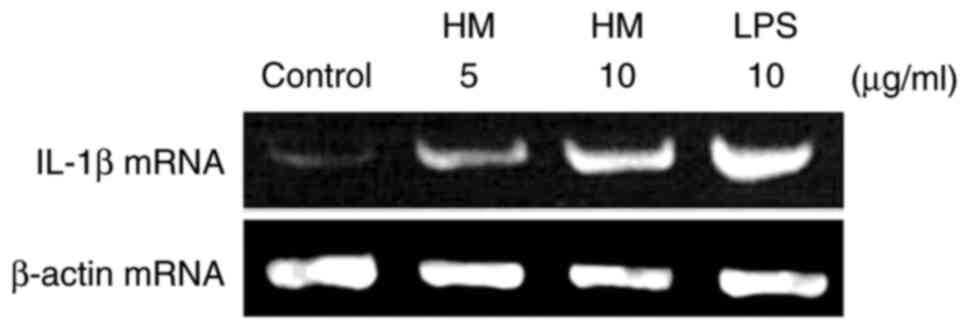
IL-1β gene expression was monitored by the mRNA
expression level, analyzed by RT-PCR after extraction of the total
RNA from the cultured human THP-1 cells incubated with and without
HM (5 and 10 µg/ml) and LPS (10 µg/ml) for 3 h. As shown in
Fig. 1, the untreated THP-1 cells
(the control) expressed very low levels of IL-1β mRNA, which was
concomitantly induced in response to LPS (10 µg/ml). The addition
of different concentrations of HM (5 and 10 µg/ml) increased the
IL-1β mRNA expression level in a dose-dependent manner compared
with the basal level of IL-1β mRNA expression detected in the
untreated THP-1 cells (Fig. 1).
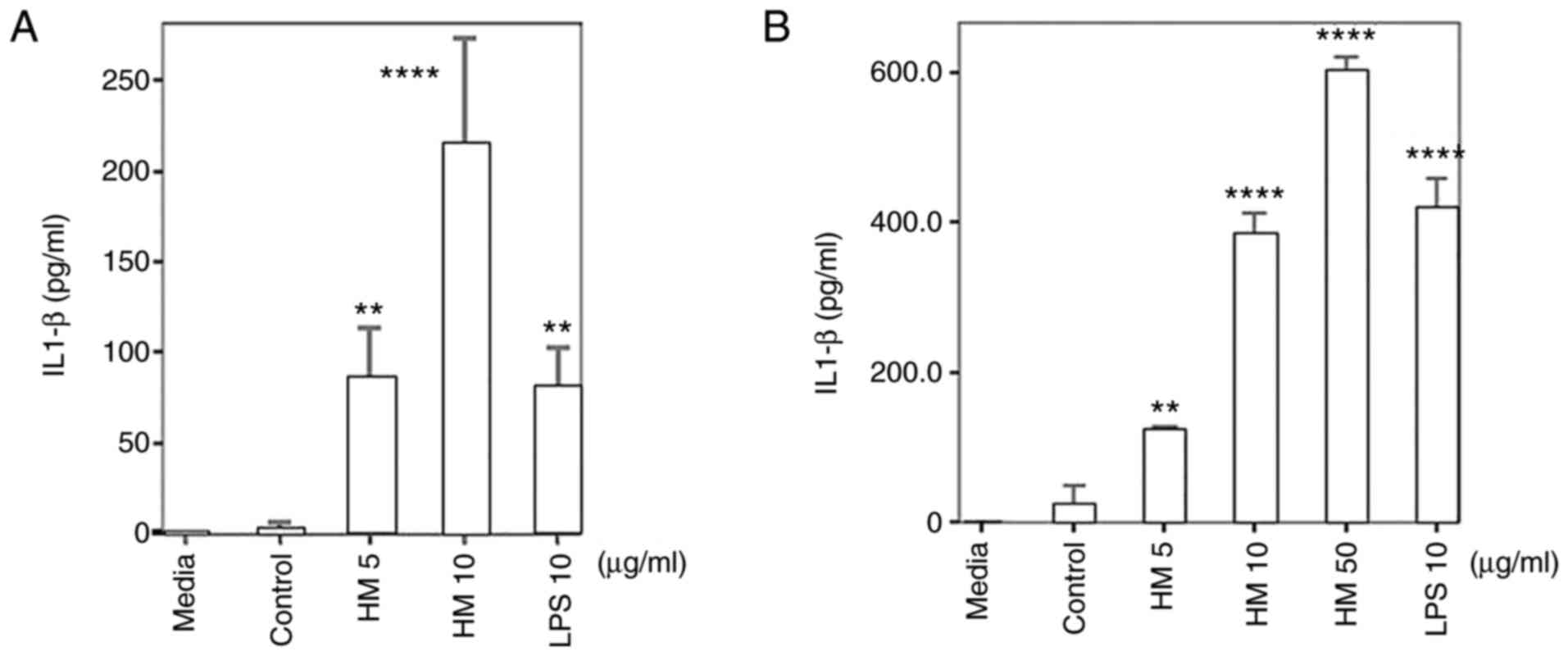
Using ELISA, very low levels of IL-1β protein were detected in the
supernatant of the primary culture of untreated monocytes, with a
mean of 1.9 pg/ml (Fig. 2A). Both
the LPS (10 µg/ml) and HM (5 and 10 µg/ml) significantly enhanced
IL-1β secretion from the monocytes compared with the primary
culture of the untreated monocytes (Fig. 2A; P<0.01). Using equal
concentrations of HM and LPS (10 µg/ml), the HM was more effective
in inducing the IL-1β secretion compared with LPS (215.6 and 81.5
pg/ml, respectively; Fig. 2A).
Fig. 2B displays detectable amounts
of the IL-1β protein in the supernatants of the untreated THP-1
cells (24.5 pg/ml). Both the LPS and HM significantly induced IL-1β
secretion (reaching ~400 pg/ml) from the THP-1 cells, when tested
at 10 µg/ml (P<0.0001; Fig. 2B).
The THP-1 cell treatment with 50 µg/ml HM, resulted in the highest
stimulatory effect on IL-1β secretion, (mean, 6037.7 pg/ml;
P<0.0001; Fig. 2B). At the
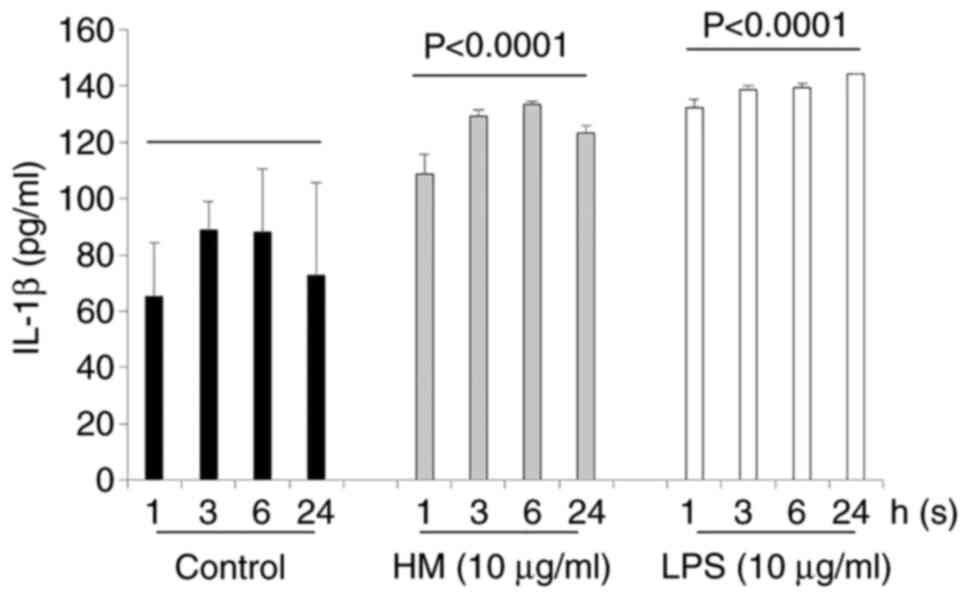
incubation time points (1, 3, 6 and 24 h), both the HM and LPS
increased IL-1β secretion by the THP-1 cells in a time-dependent
manner compared with the untreated cells (Fig. 3). As previously observed, when using
equal concentrations (10 µg/ml), the HM was as effective as the LPS
in inducing IL-1β secretion by the THP-1 cells, after 3 and even 6
h of incubation (Fig. 3). After 24
h of THP-1 cell treatment, a decrease in IL-1β secretion was
detected in the supernatant of HM-treated THP-1 cells compared with
the quantity of IL-1β released by the LPS-treated THP-1 cells
(Fig. 3).
HM modulates IL-1β, TLR4 and TLR2
protein expression in THP-1 cells
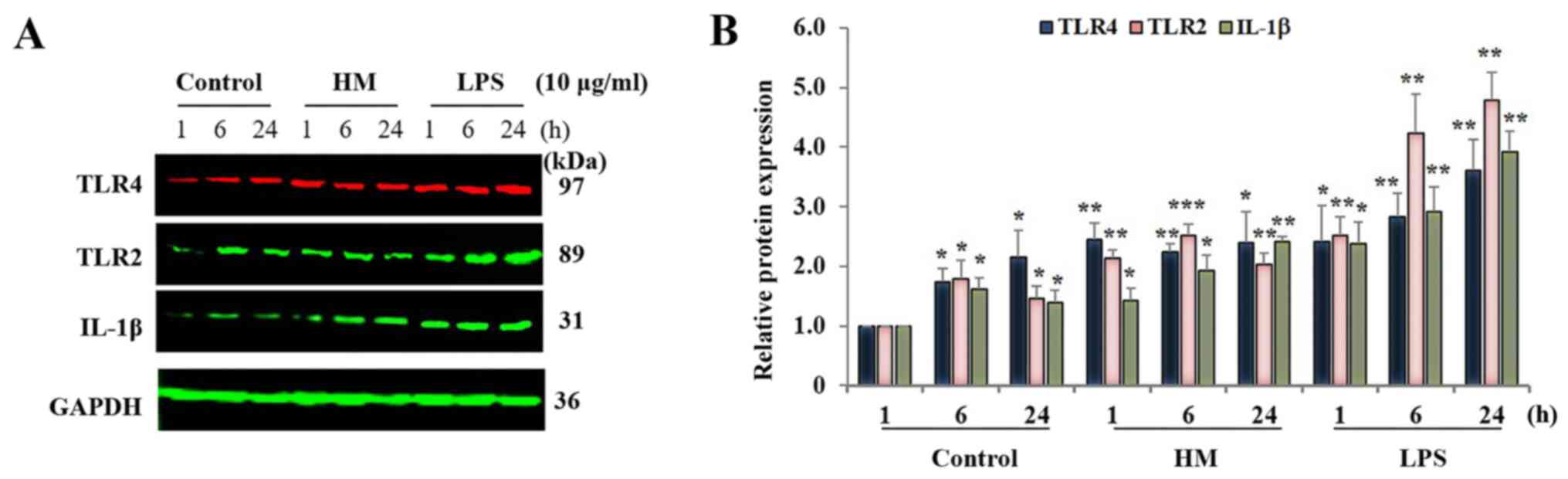
Western blot analysis revealed that the IL-1β
protein expression level in the untreated THP-1 cells fluctuated
over time (1, 6 and 24 h), with a peak expression after 6 h
incubation compared with the IL-1β basal level detected in the
untreated cells after 1-h incubation (Fig. 4A). Both the 10 µg/ml HM and LPS
significantly (P<0.01) increased IL-1β protein expression at the
6-h incubation with HM, and at 1-h incubation with LPS (Fig. 4B). No significant difference in the
IL-1β expression levels was detected in the HM- and LPS-treated
cells between 6 and 24 h of incubation (Fig. 4).
As the main receptor for HM (11), a time course of the TLR4 protein
expression was assessed in 10 µg/ml HM- and LPS-treated THP-1
cells. In addition, as the TLR2 is mostly involved in LPS-induced
IL-1β production and secretion by THP-1 cells (27), the time course of the TLR2 protein
expression was also monitored. In the untreated cells, significant
increases in the TLR4 and TLR2 protein expression were observed at
6 and 24 h od incubation compared to the basal level of the TLR4
and TLR2 expression detected in the untreated cells after 1 h
incubation (Fig. 4; P<0.05). The
addition of HM induced a significant increase in TLR4 (2.2-fold;
P<0.01) at all incubation times, and the TLR2 expression
increased by 2.51-fold (P<0.01) at 6 h incubation, followed by a
decrease at 24 h incubation, reaching a level of expression similar
to the level detected in the HM-treated cells after 1 h of
incubation (Fig. 4). The THP-1 cell
treatment with LPS resulted in a higher increase in both the TLR4
(P<0.0001) and TLR2 (P<0.001) expression levels by 2.41- and
2.52-folds after 1-h incubation and by 3.3- and 4.5-fold after 6-24
h incubation compared with the basal level of the TLR4 and TLR2
protein expression detected in untreated cells, after 1 h
incubation, respectively (Fig.
4).
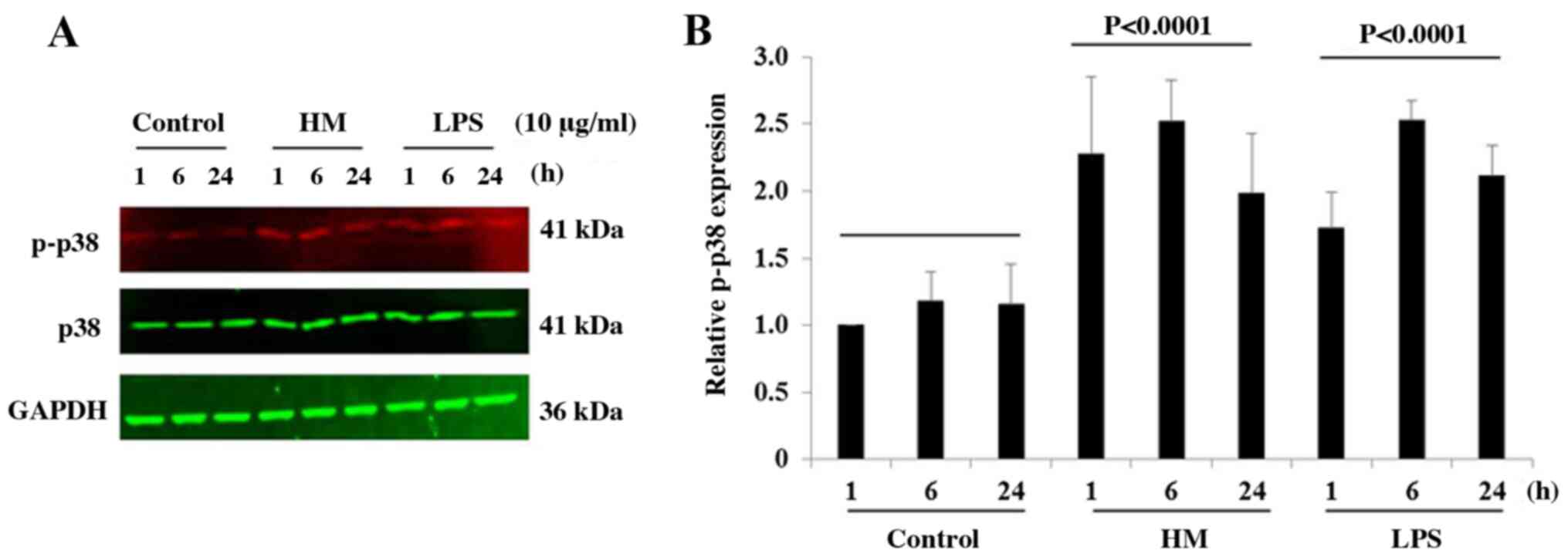
HM induces the phosphorylation of p38
MAPK in THP-1 cells
Different studies reported that the activation of
the TLR4 and TLR2 by LPS activates the MAPK signaling pathways,
including the p38 MAPK, which subsequently results in IL-1β
production (28,29). Over the time (1, 6 and 24 h), the
LPS and HM similarly increased the protein expression levels of
phospho-p38 in THP-1 cells with a peak p38 phosphorylation level
reached at 6-h incubation compared with the basal phopsho-p38
expression level detected in untreated cells after 1-h incubation
(Fig. 5).
HM mainly requires TLR2 and p38 MAPK
pathway activation for IL-1β production
To demonstrate the requirement of the TLR2, TLR4 and
p38 MAPK pathway in HM-induced IL-1β production, specific
inhibitors, anti-TLR2, anti-TLR4 neutralizing antibodies and p38
MAPK pharmacological inhibitor SB202190, were added to the cells
before cell exposure to 10 µg/ml HM or LPS for 1-h treatment.
Optimization of the respective concentrations of the anti-TLR2,
anti-TLR4 antibodies and SB202190 p38 MAPK pharmacological
inhibitor, were based on preliminary studies (data not shown). The
blockade of the p38 MAPK pathway resulted in a quasi-disappearance
of the p38 phosphorylation, detected in the untreated and
HM-treated cells, and a slight phosphorylation was still detected
in the LPS-treated THP-1 cells (Fig.
6A). The TLR4 receptor blockade did not completely inhibit the
HM- and LPS-induced p38 phosphorylation (Fig. 6A). In terms of the impact of the
TLR2/4 and p38 MAPK pathway blockade on the HM-induced IL-1β
production, both the TLR2 receptor neutralization and SB202190
pharmacological inhibitor highly suppressed the HM stimulatory
effect on the IL-1β protein expression and, to a lesser extent, the
LPS stimulatory effect (Fig. 6B and
C). However, the TLR4 receptor
blockade had a minor effect on the HM-induced IL-1β expression in
THP-1 cells (Fig. 6B).
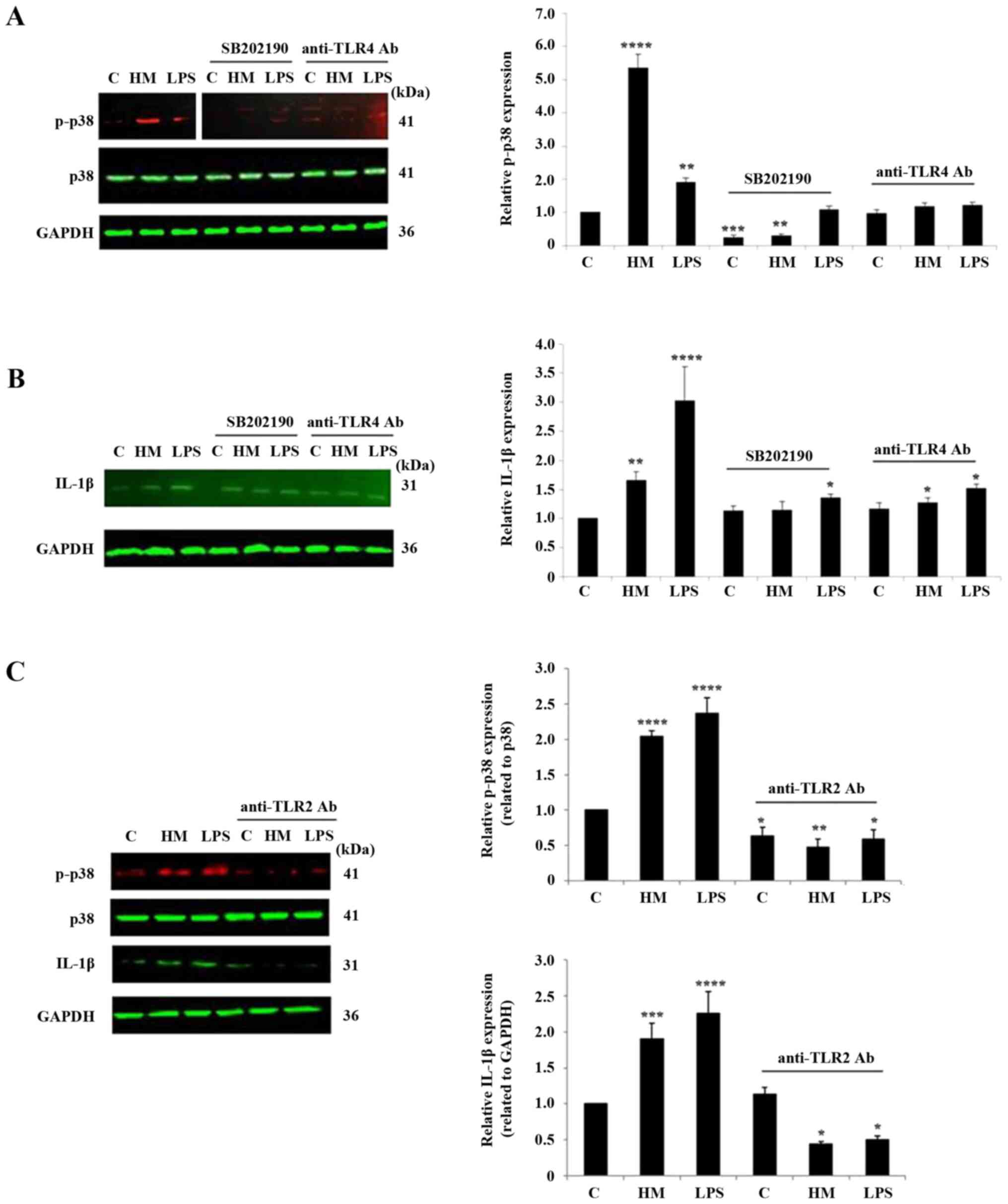 | Figure 6Impact of TLR2, TLR4 receptor
neutralization and of the p38 MAPK pathway pharmacological
inhibitor SB202190 on HM-induced p38 phosphorylation and IL-1β
protein expression. Representative western blot analysis showing
the impact of p38 MAPK pharmacological inhibitor SB202190 and TLR4
blockade using anti-TLR4 Ab on HM and LPS-induced. (A) p38 MAPK
phosphorylation and (B) IL-1β expression in THP-1 cells after 1-h
stimulation compared with the untreated cells, the control. (C)
Representative western blotting showing the impact of TLR2 blockade
using anti-TLR2 Ab on HM and LPS-induced p38 MAPK phosphorylation
and IL-1β expression in the THP-1 cells after 1-h stimulation
compared with the untreated cells, the control. The bar graphs show
the relative protein expression levels of (A and C) p-p38 and (B
and C) IL-1β calculated as a ratio of either total p38 expression
or GAPDH (the loading controls). The results are presented as the
mean ± SD of three separate experiments. *P<0.05,
**P<0.01, ***P<0.001 and
****P<0.0001 vs. control. TLR, Toll-like receptor;
HM, herbal melanin; C, control; LPS, lipopolysaccharide; IL-1β,
interleukin-1β; Ab, antibody; p-, phosphorylated. |
Discussion
The immunogenic properties of the HM extract from
Nigella sativa seeds have been reported, based on the
production and secretion of pro-inflammatory cytokines, such as
IL-6, TNF-α and VEGF, due to TLR4 activation and the NF-κB
signaling pathway (10-12).
However, no study reported the immunoregulatory potential of HM
through the pro- and anti-inflammatory cytokine IL-1β produced by
human monocytes and macrophages. In the current study, the effect
of HM was tested on the monocytic cell line THP-1, with isolated
human monocytes from healthy donors. The immunoregulatory potential
of HM was tested in parallel with LPS, used as a bacterial
stimulatory agent, based on the level of IL-1β production and
secretion from the primary culture of human monocytes and THP-1
cells. It was demonstrated that the HM upregulated the IL-1β gene
expression in the THP-1 cells, and induced the secretion of IL-1β
from isolated monocytes and cultured THP-1 cells compared with the
untreated cells. In addition, HM increased the protein expression
of IL-1β, TLR2 but not TLR4, and enhanced p38 MAPK phosphorylation
in the THP-1 cells. The blockade of the p38 MAPK pathway and the
TLR2 receptor decreased the HM-induced IL-1β upregulation in the
THP-1 cells. The TLR4 receptor blockade also decreased HM-induced
IL-1β expression, but to a lesser extent than the TLR2 blockade. In
conclusion, it was demonstrated that the HM mainly induces IL-1β
production by the monocytic cell line THP-1, in a TLR2/p38 MAPK
pathway-dependent manner. These findings suggest the potential
immunoregulation of the HM through the IL-1β is possibly of
interest for the treatment of inflammatory-related diseases.
The present study was conducted in vitro,
using the human monocytic cell line THP-1 and isolated monocytes,
as they represent the main source of cytokines including IL-1β. The
IL-1β gene is tightly regulated by positive and negative regulatory
elements in monocytes and macrophages (30). LPS is the best-characterized
stimulant that triggers IL-1β transcription and its release
(31,32). Consequently, in the present study
with the HM, LPS was used as a positive stimulant for promoting
IL-1β production in human monocytes and monocytic THP-1 cells
(33,34). Using a RT-PCR, ELISA and western
blotting, it was found that HM increased the IL-1β mRNA expression
level, secretion, and production of the protein by the THP-1 cells
and isolated monocytes. The effects of Nigella sativa total
extracts or melanins, other than Nigella sativa melanin on
IL-1β production were independently studied by various groups. Haq
et al (7) reported that the
whole Nigella sativa protein extract, containing a number of
proteins ranging from 94 Da to 10 kDa, induced IL-1β in PBMC.
However, literature associated with the effect of melanin on IL-1β
production are contradictory. Pugh et al (6) demonstrated that Echinacea
melanin increased the production of IL-1β in THP-1 cells, but
Mohagheghpour et al (35)
reported an inhibitory effect of the synthetic melanin on IL-1β
production. The present results regarding LPS/IL-1β production by
the THP-1 cells supported the results of Jablonska and Marcinczyk
(27), indicating that LPS
increased IL-1β production in THP-1 cells through TLR2
activation.
Due to the important role of TLR2 in IL-1β
production in monocytes and THP-1 cells, the protein expression
levels of TLR2 and TLR4, described as the main receptor for HM,
were monitored. The addition of 10 µg/ml HM or LPS increased the
TLR2 receptor, but no change in the TLR4 protein expression was
observed in the THP-1 cells. Literature demonstrated that a high
concentration of LPS upregulated both TLR4 and TLR2 in macrophages,
through the activation of the NF-κB pathway (36,37).
In addition, it was recently reported that a high concentration of
HM upregulated the TLR4 gene and protein expression in THP-1 cells
(38). Using an anti-inflammatory
peptide, such as the vasoactive intestinal peptide, LPS tested at
100 ng/ml increased the TLR2 gene expression in THP-1 cells after
the stimulation of the NF-κB and activator protein (AP) 1
transcription factors (39). After
reporting that HM induces the NF-kB pathway activation in THP-1
cells, an assessment of the NF-κB and AP1 transcription factors
could confirm the TLR2 gene upregulation in HM-treated THP-1
cells.
In addition to the NF-kB pathway and the MAPK
activated members stimulated by LPS, the p38 MAPK is primarily
involved in LPS-induced IL-1β production in THP-1 cells (25,40).
In the present study, it was reported that both HM and LPS enhanced
p38 MAPK phosphorylation in the THP-1 cells. Zheng et al
(25) reported the important role
of the p38 MAPK in IL-1β transcription by acting through the
C/EBP/NFIL-6 transcription factors. A blockade of the p38 MAPK
pathway in the HM-treated THP-1 cells could result in the decrease
in IL-1β gene expression, and the inactivation of C/EBP/NFIL-6
transcription factors.
To expose the key role of the TLR2, TLR4 and the p38
MAPK pathway in HM-induced IL-1β production, respective receptors
were neutralized with antibodies, and the p38 MAPK was blocked with
a specific pharmacological inhibitor. It was shown that the
blockade of the p38 MAPK pathway and the TLR2 receptor decreased
HM-induced IL-1β production in THP-1 cells. The TLR4 receptor
blockade also decreased the HM-induced IL-1β expression, but to a
lesser extent compared with the TLR2 blockade. The detection of the
IL-1β protein following the TLR4 receptor blockade could be
attributed to the partial inhibitory effect observed on the HM- and
LPS-induced p38 MAPK phosphorylation and to the IL-1β protein
secreted via TLR2 activation. The blockade of the TLR2 or TLR4
receptor resulted in the inhibition of the p38 MAPK
phosphorylation, confirming subsequent shared downstream signaling
pathways, such as the IL-1 receptor associated kinase (IRAK)/tumor
necrosis factor receptor-associated factor 6/transforming growth
factor β-activated kinase, reported in the literature (41,42).
In conclusion, the present study provides evidence
that HM induces IL-1β production by human monocytes and THP-1 cells
expressing TLR2 and TLR4. Furthermore, HM upregulates TLR2 and
TLR4, and activates the p38 MAPK pathway. TLR2 blockade completely
inhibited HM-induced IL-1β production, and the TLR4 blockade had a
partially inhibitory effect. Overall, it is proposed that HM has
efficiently induced IL-1β production, primarily via the activation
of the TLR2/p38 MAPK signaling pathway. Future identification of
the signaling molecules involved in the observed HM stimulatory
effect of IL-1β production in monocytes, may assist in the
development of novel therapeutics to enhance or downregulate the
IL-1β signaling pathways in response to infections and other
inflammatory-associated diseases.
Acknowledgements
The authors would like to thank Ms. Nouf AlGaith
from Cell and Gene Therapy Group, King Abdullah International
Medical Research Center (Riyadh, Saudi Arabia), for her technical
assistance.
Funding
Funding: The experiments using THP-1 cells were financially
supported by King Abdullah International Medical Research Center
(grant no. #RC15/106).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AEO and SMN conceived the study. WBY, BA, AAT, MN,
AH performed the assays, collected the data, conducted the data
analysis, and reviewed the manuscript. SMN and AEO wrote the
manuscript. AEO and SMN confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Healthy control subject's blood samples were
processed by Dr Adila El-Obeid while she was affiliated with the
Department of Genetics and Pathology, Rudbeck Laboratory, Uppsala
University Hospital, Uppsala, Sweden, in 2005. The study was
approved by the institutional review board of Uppsala University
hospital and conducted in accordance with the Helsinki Declaration
of 1975. Written informed consents were obtained from all healthy
control subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Prota G: Regulatory mechanisms of
melanogenesis: Beyond the tyrosinase concept. J Invest Dermatol.
100 (Suppl 2):156S–161S. 1993.PubMed/NCBI
|
|
2
|
Riley P: Molecules in focus: Melanin. Int
J Biochem Cell Biol. 29:1235–1239. 1997.
|
|
3
|
Bell AA: Biochemical mechanisms of disease
resistance. Ann Rev Plant Physiol. 32:21–81. 1981.
|
|
4
|
Rosas ÁL, MacGill RS, Nosanchuk JD, Kozel
TR and Casadevall A: Activation of the alternative complement
pathway by fungal melanins. Clin Diagn Lab Immunol. 9:144–148.
2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Freitak D, Vanatoa A, Ots I and Rantala
MJ: Formation of melanin-based wing patterns is influenced by
condition and immune challenge in Pieris brassicae. Entomol
Exp et Applicata. 116:237–243. 2005.
|
|
6
|
Pugh ND, Balachandran P, Lata H, Dayan FE,
Joshi V, Bedir E, Makino T, Moraes R, Khan I and Pasco DS: Melanin:
Dietary mucosal immune modulator from Echinacea and other
botanical supplements. Int Immunopharmacol. 5:637–647.
2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Haq A, Lobo PI, Al-Tufail M, Rama NR and
Al-Sedairy ST: Immunomodulatory effect of Nigella sativa
proteins fractionated by ion exchange chromatography. Int J
Immunopharmacol. 21:283–295. 1999.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Islam SN, Begum P, Ahsan T, Huque S and
Ahsan M: Immunosuppressive and cytotoxic properties of Nigella
sativa. Phytother Res. 18:395–398. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Hassib A: Extraction of melanin from
Nigella sativa L. Patent no. 451. Khartoum, Sudan, 1998.
|
|
10
|
El-Obeid A, Al-Harbi S, Al-Jomah N and
Hassib A: Herbal melanin modulates tumor necrosis factor alpha
(TNF-alpha), interleukin 6 (IL-6. and vascular endothelial growth
factor (VEGF). production. Phytomedicine. 13:324–333.
2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
El-Obeid A, Hassib A, Pontén F and
Westermark B: Effect of herbal melanin on IL-8: A possible role of
Toll-like receptor 4 (TLR4). Biochem Biophys Res Commun.
344:1200–1206. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Oberg F, Haseeb A, Ahnfelt M, Pontén F,
Westermark B and El-Obeid A: Herbal melanin activates
TLR4/NF-kappaB signaling pathway. Phytomedicine. 16:477–484.
2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Madej MP, Töpfer E, Boraschi D and
Italiani P: Different regulation of interleukin-1 production and
activity in monocytes and macrophages: Innate memory as an
endogenous mechanism of IL-1 inhibition. Front Pharmacol.
8(335)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dinarello CA: Overview of the IL-1 family
in innate inflammation and acquired immunity. Immunol Rev.
281:8–27. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Semino C, Carta S, Gattorno M, Sitia R and
Rubartelli A: Progressive waves of IL-1b release by primary human
monocytes via sequential activation of vesicular and gasdermin
D-mediated secretory pathways. Cell Death Dis.
9(1088)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shen J, Xu S, Zhou H, Liu H, Jiang W, Hao
J and Hu Z: IL-1β induces apoptosis and autophagy via mitochondria
pathway in human degenerative nucleus pulposus cells. Sci Rep.
7(41067)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Baker KJ, Houston A and Brint E: IL-1
family members in cancer; two sides to every story. Front Immunol.
10(1197)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Coutinho LG, Grandgirard D, Leib SL and
Agnez-Lima LF: Cerebrospinal-fluid cytokine and chemokine profile
in patients with pneumococcal and meningococcal meningitis. BMC
Infect Dis. 13(326)2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Thobakgale C and Naidoo K, McKinnon LR,
Werner LR, Werner L, Samsunder N, Karim SA, Ndung'u T, Altfeld M
and Naidoo K: Interleukin 1-beta (IL-1β) production by innate cells
following TLR stimulation correlates with TB recurrence in
ART-treated HIV-infected patients. J Acquir Immune Defic Syndr.
74:213–220. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kawasaki T and Kawai T: Toll-like receptor
signaling pathways. Front Immunol. 5(461)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Molteni M, Bosi A and Rossetti C: Natural
products with Toll-like receptor 4 antagonist activity. Int J
Inflam. 2018(2859135)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Raby AC, González-Mateo GT, Williams A,
Topley N, Fraser D, López-Cabrera M and Labéta MO: Targeting
Toll-like receptors with soluble Toll-like receptor 2 prevents
peritoneal dialysis solution-induced fibrosis. Kidney Int.
94:346–362. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang G and Ghosh S: Toll-like
receptor-mediated NF-kappaB activation: A phylogenetically
conserved paradigm in innate immunity. J Clin Invest. 107:13–19.
2001.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Liu YX, Wang GD, Wang X, Zhang YL and
Zhang TL: Effects of TLR-2/NF-κB signaling pathway on the
occurrence of degenerative knee osteoarthritis: An in vivo and in
vitro study. Oncotarget. 8:38602–38617. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zheng DY, Zhou M, Jin J, He M, Wang Y, Du
J, Xiao XY, Li PY, Ye AZ, Liu J and Wang TH: Inhibition of P38 MAPK
downregulates the expression of IL-1β to protect lung from acute
injury in intestinal ischemia reperfusion rats. Mediators Inflamm.
2016(9348037)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Matou-Nasri S, Rhaban Z, Al-Baijan H,
Al-Eidi H, Yahya WB, Al Abdulrahman A, Almobadel N, Alsubeai M, Al
Ghamdi S, Alaskar A, et al: CD95-mediated apoptosis in Burkitt's
lymphoma B-cells is associated with Pim-1 down-regulation. Biochim
Biophys Acta Mol Basis Dis. 1863:239–252. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jablonska E and Marcinczyk M: TLR2
expression in relation to IL-6 and IL-1 beta and their natural
regulators production by PMN and PBMC in patients with Lyme
disease. Mediators Inflamm. 2006(32071)2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Goral J and Kovacs EJ: In vivo ethanol
exposure down-regulates TLR2-, TLR4-, and TLR9-mediated macrophage
inflammatory response by limiting p38 and ERK1/2 activation. J
Immunol. 174:456–463. 2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jin J, Samuvel DJ, Zhang X, Li Y, Lu Z,
Lopes-Virella MF and Huang Y: Coactivation of TLR4 and TLR2/6
coordinates an additive augmentation on IL-6 gene transcription via
p38MAPK pathway in U937 mononuclear cells. Mol Immunol. 49:423–432.
2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Auron PE and Webb AC: Interleukin-1: A
gene expression system regulated at multiple levels. Eur Cytokine
Netw. 5:573–592. 1994.PubMed/NCBI
|
|
31
|
Eggesbø JB, Hjermann I, Lund PK, Joø GB,
Ovstebø R and Kierulf P: LPS-induced release of IL-1 beta, IL-6,
IL-8, TNF-alpha and sCD14 in whole blood and PBMC from persons with
high or low levels of HDL-lipoprotein. Cytokine. 6:521–529.
1994.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Harrison LM, van den Hoogen C, van Haaften
WC and Tesh VL: Chemokine expression in the monocytic cell line
THP-1 in response to purified shiga toxin 1 and/or
lipopolysaccharides. Infect Immun. 73:403–412. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Murphy M, Xiong Y, Pattabiraman G, Qiu F
and Medvedev AE: Pellino-1 positively regulates Toll-like receptor
(TLR) 2 and TLR4 signaling and is suppressed upon induction of
endotoxin tolerance. J Biol Chem. 290:19218–19232. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Karwaciak I, Gorkiewicz M, Bartosz G and
Pulaski L: TLR2 activation induces antioxidant defence in human
monocyte-macrophage cell line models. Oncotarget. 8:54243–54264.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mohagheghpour N, Waleh N, Garger SJ,
Dousman L, Grill LK and Tusé D: Synthetic melanin suppresses
production of proinflammatory cytokines. Cell Immunol. 199:25–36.
2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu Y, Wang Y, Yamakuchi M, Isowaki S,
Nagata E, Kanmura Y, Kitajima I and Maruyama I: Upregulation of
Toll-like receptor 2 gene expression in macrophage response to
peptidoglycan and high concentration of lipopolysaccharide is
involved in NF-kappaB activation. Infect Immun. 69:2788–2796.
2001.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wan J, Shan Y, Fan Y, Fan C, Chen S, Sun
J, Zhu L, Qin L, Yu M and Lin Z: NF-κB inhibition attenuates
LPS-induced TLR4 activation in monocyte cells. Mol Med Rep.
14:4505–4510. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
El-Obeid A, Alajmi H, Harbi M, Yahya WB,
Al-Eidi H, Alaujan M, Haseeb A, Trivilegio T, Alhallaj A, Alghamdi
S, et al: Distinct anti-proliferative effects of herbal melanin on
human acute monocytic leukemia THP-1 cells and embryonic kidney
HEK293 cells. BMC Complement Med Ther. 20(154)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ibrahim H, Barrow P and Foster N:
Transcriptional modulation by VIP: A rational target against
inflammatory disease. Clin Epigenetics. 2:213–222. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Peroval MY, Boyd AC, Young JR and Smith
AL: A critical role for MAPK signalling pathways in the
transcriptional regulation of Toll like receptors. PLoS One.
8(e51243)2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li JY, Liu Y, Gao XX, Gao X and Cai H:
TLR2 and TLR4 signaling pathways are required for recombinant
Brucella abortus BCSP31-induced cytokine production, functional
upregulation of mouse macrophages, and the Th1 immune response in
vivo and in vitro. Cell Mol Immunol. 11:477–494. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Mukherjee S, Karmakar S and Babu SP: TLR2
and TLR4 mediated host immune responses in major infectious
diseases: A review. Braz J Infect Dis. 20:193–204. 2016.PubMed/NCBI View Article : Google Scholar
|