Introduction
Thrombocytopenia is defined as a low number of
platelets. There are multiple mechanisms and differential diagnosis
includes: artifactual thrombocytopenia, accelerated platelet
destruction (intra- or extra-corpuscular anomalies), deficient
production (bone marrow failure, disordered proliferation or
thrombopoietin deficiency), and abnormal distribution (disorders
associated with splenomegaly or dilution in massive transfusions)
(1).
Immune thrombocytopenia (ITP) is an autoimmune
disorder characterized by immune-mediated destruction and impaired
production of platelets, with isolated thrombocytopenia. Primary
ITP remains a diagnosis of exclusion, in contrast to ITP which is
secondary to various conditions: infections are among common
causes, mostly viral ones (hepatitis C, HIV infection, CMV, EBV)
(2).
Some of the many underlying disorders that are
associated with immune thrombocytopenia are also
lymphoproliferative disorders. The prevalence of ITP in
non-Hodgkin's lymphoma patients is lower compared to autoimmune
hemolytic anemia, occurring in only up to 1.8% of cases (3).
In the present study, the case of a patient with
diffuse large B-cell lymphoma evolving from grade 3a follicular
lymphoma, who maintained a complete response for 4-years, and then
presented with severe isolated thrombocytopenia, is reported.
Case presentation
Ethics approval for the study was obtained from the
Emergency University Hospital, Bucharest, Romania. The patient
included in the study provided written informed consent.
The 62-year-old male was admitted to the Hematology
Clinic with generalized purpura and atraumatic ecchymoses. The
patient had been diagnosed 5 years prior to this presentation with
diffuse large B-cell lymphoma evolving from grade 3a follicular
lymphoma. He was treated with chemotherapy (8 R-CHOP protocols)
with partial response, followed by high-dose chemotherapy and
autologous hematopoietic stem cell transplantation, with a complete
response, and was stable for the previous 4 years. The patient also
had a history of poorly controlled diabetes mellitus type 2,
arterial hypertension, and depressive disorder. The lymphoma and
its treatment, and also the poorly controlled diabetes contributed
to the immune deficiency of our patient.
The patient reported that purpura and ecchymoses had
appeared two days prior to presentation. He denied use of new
medication, including vaccines, recent international travels,
weight loss, fever, or other symptoms. The physical examination was
normal except for the cutaneous hemorrhagic syndrome.
Investigations at admission revealed severe
thrombocytopenia (PLT=3x109/l), normal white blood cell
count and hemoglobin, without any significant changes in hepatic
and renal function tests, electrolyte levels, or coagulation.
Screening tests for infectious diseases (HBV, HCV, HIV,
Helicobacter pylori and stool antigen test), tumors
(α-fetoprotein, carcinoembryonic antigen, prostate-specific
antigen, CA19-9, imaging studies), and immune-mediated disorders
(C3 complement, serum cryoglobulins, rheumatoid factor, antinuclear
antibodies, anticardiolipin antibodies, lupus anticoagulant) were
negative. No pathogenic bacteria were found in blood cultures.
Tests for CMV infection and thrombopoietin levels were not
available in our center. The bone marrow biopsy was not noteworthy,
normal and active megakaryocytes were observed, and negative for
malignant lymphoid infiltration. The CT scan of the thorax,
abdomen, and pelvis showed no other modifications compared to the
previous examinations. Previously, the patient was periodically
examined by a CT scan every 6 months. He had small (<1 cm)
metabolically inactive mediastinal lymphadenopathies, unchanged for
4 years.
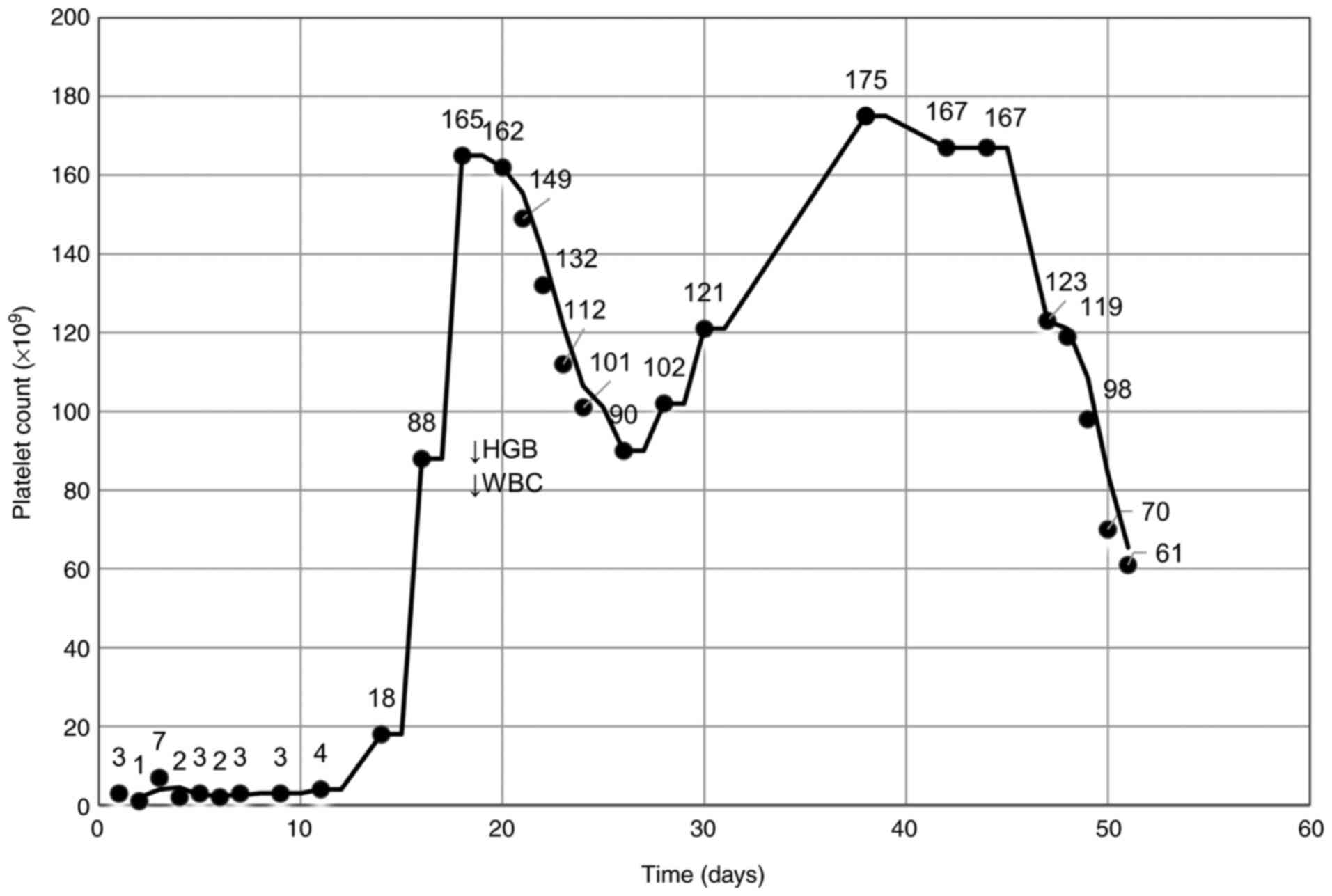
A diagnosis of primary immune thrombocytopenia was
established and treatment was started with intravenous
corticosteroids. The platelet count remained low for about 14 days,
and then began to progressively increase up to normal values on day
18 of treatment (Fig. 1).
Since day 11-12, progressive modifications of the
patient's mental status, including poor eye-contact, inattention to
personal appearance, sad mood, monosyllabic speech with long
pauses, negative self-view and negative view of the future,
hypersomnia, and appetite loss, was observed. Considering his
history of depressive disorder, which may have been aggravated
under corticosteroids, prolonged hospitalization, and the initial
lack of response, the psychiatrist recommended treatment with
anxiolytics and antidepressants.
On day 16, the patient developed two episodes of low
fever; blood cultures were collected, and empirical broad-spectrum
antibiotics were started.
On day 18 of treatment, the patient's condition
suddenly worsened: he became noncompliant, agitated, aphasic,
dyspneic with low blood oxygen saturation (86%), later followed by
fever (39˚C). Laboratory findings revealed leukocytosis with
neutrophilia and lymphopenia (which had been present for a few
days, possibly also due to the corticosteroid treatment), followed
by leukopenia, moderate anemia (without any bleeding, also observed
progressively in the previous days), slightly increased
aminotransferase and indirect bilirubin, severe hyponatremia,
normal C-reactive protein and coagulation. Chest X-ray was negative
for infection.
Cerebral-computed tomography was normal, and
repeated lumbar punctures revealed hyperglycorrhachia, normal
protein and albumin levels; CSF cellularity was low (21
elements/µl). Magnetic resonance imaging could not be performed at
that time due to the presence of a metallic object in the patient's
thumb.
We started correction of hyponatremia, but without
significant improvements in symptomatology, corticosteroids
(de-escalation of doses), and prophylactic broad-spectrum
antibiotics. Blood cultures proved to be negative for aerobic and
anaerobic bacteria and fungi.
Given the neurological symptoms and the fever, we
considered that the most likely cause was infection. As the patient
was admitted to the hospital in the fall and Romania is an endemic
area, we considered it opportune to perform serologic testing for
West-Nile virus; serum immunoglobulin M antibodies in the blood and
cerebrospinal fluid were positive. The final diagnosis was
West-Nile encephalitis. CBC also showed transient pancytopenia for
72 h.
Antibiotics were stopped and corticosteroids were
continued as per the Infectious Disease specialist's
recommendation. The clinical evolution was favorable, although the
patient developed progressive muscle weakness. He became compliant,
and the hematological and biochemical tests improved.
After stabilization of clinical and biological
parameters, the patient was transferred to the Department of
Neurology for additional investigations regarding his muscle
weakness, and for neurological rehabilitation. Although there was
improvement, a week after admission, the patient's state worsened
with fever, dyspnea, and productive cough. Thoracic imaging was
suggestive for bronchopneumonia; aerobic and anaerobic blood
cultures were positive for Klebsiella pneumoniae. The patient
developed septic shock in spite of broad spectrum-specific
antibiotherapy and intensive care, and subsequent multiple organ
failure. He died 72 h later.
Discussion
West-Nile virus is a member of the Flaviviridae
family transmitted mostly by mosquito bite, but also through blood
transfusions, organ transplantation, breastfeeding, or percutaneous
inoculation. The usual incubation period is 3-14 days (4). Most of the infected patients (75%) are
asymptomatic, while the remaining patients may experience
influenza-like symptoms (25%), and only a minority develop
neuroinvasive disease (<1%) (5,6).
Patient risk-factors for developing central nervous system
involvement are advanced age, a history of cardiovascular disease,
chronic renal disease, hepatitis C virus infection, and
immunosuppression (7). Furthermore,
a study reported that defects in draining lymph nodes result in
increased susceptibility for West-Nile infection (8).
It is widely known that viral infections are
associated with various degrees of thrombocytopenia. Several cases
of West-Nile-related thrombocytopenia have been reported, but the
severe ones have been quite rare (9-11).
The underlying mechanisms are not yet completely understood;
studies regarding another virus from the same family (Dengue virus)
suggest complex pathogenesis of thrombocytopenia, including the
formation of platelet-leukocyte aggregates, sequestration in the
reticuloendothelial system, followed by phagocytosis, production of
cross-reactive antibodies for platelet antigens, and suppression of
hematopoiesis (12).
Determining the cause of thrombocytopenia can be
challenging. Underlying diseases that are associated with
thrombocytopenia include lymphoproliferative disorders (e.g.,
chronic lymphocytic leukemia, non-Hodgkin's lymphoma, and Hodgkin
lymphoma), bone-marrow diseases (e.g., myelodysplastic syndromes,
leukemias, metastatic disease), post-transplantation (allogeneic or
autologous stem cell transplantation), microangiopathic processes
(thrombotic thrombocytopenic purpura, hemolytic uremic syndrome),
infectious diseases (e.g., Helicobacter pylori, hepatitis C,
HIV infection, CMV, EBV), autoimmune disorders (e.g., systemic
lupus erythematosus), liver diseases (cirrhosis, portal
hypertension), splenomegaly, and drugs (2,13-20).
Failure in demonstrating the presence of an underlying pathology
conducts towards the diagnosis of primary immune
thrombocytopenia.
We performed an extensive search for secondary ITP.
At presentation, from anamnesis and physical examination, no
underlying condition or risk factors were identified: No fever or
other signs of infection, no lymphadenopathy or hepatosplenomegaly,
and the patient denied exposure to new medication, vaccination or
other toxins. Thrombocytopenia secondary to a microangiopathic
process (thrombotic thrombocytopenic purpura or hemolytic uremic
syndrome) was also excluded: absence of renal, neurological,
gastrointestinal or other typical symptoms, lack of anemia,
reticulocytosis or schistocytes, and otherwise normal organ
functions (15).
Based on laboratory and imaging results, the
following conditions seemed improbable: infectious diseases
(negative for HIV, HCV, HBV, Helicobacter pylori),
autoimmune disorders (normal values for C3 complement, serum
cryoglobulins, rheumatoid factor, autoimmune panel), and solid
tumors (normal values for the tested tumoral markers, normal CT
scan). Testing for CMV was not available in our center.
In our case, given the prior medical history of
non-Hodgkin's lymphoma (NHL), we considered vital to exclude a
relapse or a secondary malignancy, both hematological and
solid.
Lymphoproliferative disorders are occasionally
associated with hematological autoimmune phenomena, mostly
autoimmune anemia, but also ITP, the latest occurring in 0-1.8% of
patients with NHL (3). Several
case-reports and meta-analyses described cases of ITP occurring
prior or synchronous to the diagnosis of lymphoma, or after the
completion of treatment (3,21-27).
In addition, immune thrombocytopenia occurring after autologous
stem cell transplantation has been mentioned, with an onset period
of 1-31 months (3).
We found no evidence suggestive for relapsed
lymphoma or secondary malignancy, both solid and hematological
(e.g., acute leukemia, myelodysplastic syndrome): no clinical
signs, no adenopathy or hepatosplenomegaly, no weight loss or fever
or night sweats, no other CBC anomalies, normocellular bone marrow,
without malignant lymphoid infiltration, and unremarkable CT
scan.
After the exclusion of all the previously mentioned
causes of thrombocytopenia, we established the diagnosis of ITP and
started intravenous corticosteroid treatment. The platelet counts
gradually increased to normal values, while the neutrophil count
began to rise, and the hemoglobin level to decrease (Table I). Furthermore, the patient's
condition worsened and he developed first psychiatric and then
neurological symptoms. Considering the clinical evolution, we
performed several investigations that conducted to the unexpected
diagnosis of West-Nile encephalitis.
 | Table ILaboratory findings. |
Table I
Laboratory findings.
| Parameter (normal
value) | Days 1-2 Admission in
the Hematology Clinic | Days 18-19 Onset of
neurological symptoms | Day 23 | Day 38 Admission in
the Neurology Clinic | Day 51 Deceased |
|---|
| CBC | | | | | |
|
WBC
(3.6-10.2x109/l) |
10.5x109/l |
17.4x109/l |
2.5x109/l |
8.3x109/l |
2.7x109/l |
|
Neutrophils
(1.7-7.6x109/l) |
6.2x109/l |
15.3x109/l |
1.7x109/l |
6.6x109/l |
2.0x109/l |
|
Lymphocytes
(1.0-3.2x109/l) |
3.2x109/l |
1.4x109/l |
0.7x109/l |
1.5x109/l |
0.6x109/l |
|
HGB
(12.5-16.3 g/dl) | 13.2 g/dl | 8.2 g/dl | 7.5 g/dl | 10.9 g/dl | 9.7 g/dl |
|
PLT
(150-400x109/l) |
3x109/l |
165x109/l |
112x109/l |
175x109/l |
61x109/l |
| Biochemistry | | | | | |
|
ALT (3-65
U/l) | 47 U/l | 87 U/l | 60 U/l | 86 U/l | 592 U/l |
|
AST (2-40
U/l) | 31 U/l | 38 U/l | 28 U/l | 26 U/l | 853 U/l |
|
Total
bilirubin (0-1.2 mg/dl) | 0.66 mg/dl | 1.16 mg/dl | 0.8 mg/dl | 0.64 mg/dl | 0.88 mg/dl |
|
Direct
bilirubin (0-0.3 mg/dl) | 0.23 mg/dl | 0.45 mg/dl | 0.3 mg/dl | 0.18 mg/dl | 0.13 mg/dl |
|
Sodium
(135-150 mmol/l) | 136 mmol/l | 123 mmol/l | 136 mmol/l | 127 mmol/l | 138 mmol/dl |
|
LDH (125-220
U/l) | 204 U/l | - | 372 U/l | 245 U/l | - |
|
Urea (12-45
mg/dl) | 33 mg/dl | 50 mg/dl | 66 mg/dl | 44 mg/dl | 128 mg/dl |
|
Creatinine
(0.5-1.5 mg/dl) | 1.41 mg/dl | 1.27 mg/dl | 1.16 mg/dl | 1.07 mg/dl | 2.27 mg/dl |
|
CRP (0-5
mg/l) | 17.77 mg/l | 0.5 mg/l | 5 mg/l | 11.38 mg/l | - |
| Coagulation | | | | | |
|
PT (9-13.5
sec) | 11.3 sec | 11.7 sec | 11.7 sec | 11.1 sec | 17.7 sec |
|
APTT (22-36
sec) | 26.8 sec | 24.8 sec | 24.9 sec | 24.1 sec | 66.0 sec |
|
Fibrinogen
(238-498 mg/dl) | 369.33 mg/dl | 368 mg/dl | 280 mg/dl | 291 mg/dl | 385 mg/dl |
| CSF exam | | | | | |
|
Glucose
(40-70 mg/dl) | - | 82 mg/dl | 110 mg/dl | 97 mg/dl | - |
|
CSF/serum
glucose ratio | - | 0.68 | 0.81 | 0.94 | - |
|
Protein
(15-45 mg/dl) | - | 31.1 mg/dl | 23.6 mg/dl | 65.4 mg/dl | - |
|
Albumin
(5-20 mg/dl) | - | - | 14.5 mg/dl | 38.7 mg/dl | - |
|
Nucleated
elements | - |
21/mm3 |
5/mm3 |
6/mm3 | - |
|
Erythrocytes | - |
224/mm3 |
29/mm3 |
74/mm3 | - |
| CSF-WNV IgM
(<0.8) | - | 6.27-Positive | - | - | - |
| Serum-WNV IgM
(<0.8) | - | 8.24-Positive | - | - | - |
Regarding our patient, we evaluated two hypotheses.
First, considering the onset of neurological symptoms at about 18
days since admission, one might think that the patient presented
with primary immune thrombocytopenia and developed West-Nile
infection while hospitalized. This hypothesis seems improbable.
Furthermore, no other patients in the hospital experienced similar
symptoms or were diagnosed with West-Nile virus infection in that
period, although there is a high number of immunodeficient patients
in our care.
It is more attractive to think that the patient
developed immune thrombocytopenia secondary to West-Nile infection.
He was asymptomatic at presentation, but he already had a degree of
immunodeficiency (history of lymphoma treated with chemotherapy,
followed by autologous stem cell transplantation) supplementary
aggravated by corticotherapy, which could be a risk factor for the
development of neuroinvasive disease. The downside of this
hypothesis is related to the rarity of severe thrombocytopenia in
West-Nile-infected patients and to the long period of time between
onset of thrombocytopenia and neurological impairment. Concerning
this point, we would like to mention the case of a patient with
West-Nile infection who first developed severe thrombocytopenia and
then symptoms, including high fever, generalized weakness, and
altered mental status (11).
Furthermore, neurological impairment can have a delayed onset due
to delayed neuroinvasion (28).
Moreover, our patient developed psychiatric symptoms at about 11-12
days after admission (within cited incubation period), followed by
severe neurological abnormalities at about 18 days.
Therefore, we reason that the patient presented
immune thrombocytopenia triggered by exposure to West-Nile virus.
We argue that the viral infection was not symptomatic at admission,
as it generally is, but later became so, due to immunodeficiency
induced by intense corticotherapy.
The clinical evolution of patients with West-Nile
neuroinvasive disease is variable. Some may develop long-term
neurological sequelae, including muscle weakness, myalgia, fatigue,
memory loss, and depression (5,7).
In general, the prognosis in West-Nile infection is
favorable, except for the elderly and patients with multiple
medical complications who have a poorer prognosis. Biological
predictors of fatal outcome are C-reactive protein >100 mg/l,
CSF protein >100 mg/dl, and West-Nile encephalitis (9).
Concerning our patient, he had a history of
immunosuppression due to lymphoma, chemotherapy and autologous stem
cell transplantation, and especially recent steroid treatment, as
possible risk factors for developing a more severe form of
West-Nile infection. He also had multiple comorbidities, such as
poorly controlled diabetes mellitus type 2, arterial hypertension,
and depressive disorder. He did not have any of the previously
mentioned biological predictors of fatal outcome, but he did in
fact have an unfavorable progression. However, the direct cause of
death was not encephalitis per se, but further complications. The
patient had multiple risk factors for developing severe infections,
such as prolonged hospitalization in multiple clinics (almost two
months), prolonged corticotherapy (for immune thrombocytopenia and
West-Nile encephalitis) and antibiotherapy, and immobilization
secondary to neurological complications.
Thrombocytopenia is known to be associated with
numerous disorders, therefore determining the cause is a major
challenge. A careful physical examination, a complete medical
history review, and a laboratory investigation can orientate
towards the etiology.
Regarding our case, the etiology of thrombocytopenia
remains incompletely clarified. Out of the many possible causes,
although uncommon, the apparent etiology would be the West-Nile
virus, which most likely triggered severe immune thrombocytopenia.
The viral infection was initially asymptomatic, but shortly
thereafter induced neuroinvasive disease which eventually became
lethal by secondary complications.
Therefore, we recommend considering West-Nile
infection as a potential etiology of thrombocytopenia, especially
in endemic regions during West Nile activity season and for cases
where more common causes have been excluded.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
Conceptualization was done by MO, AMV, HB, MG
according to ICMJE guidelines. Authenticity of all the raw data was
assessed by MO, AMV, MG, AS, AA. Methodology was established by MO,
AMV, II, HB, MG, CC, IV, DC, DV, CM, AN, AS, RN, AA. Validation was
done by MO, AMV, HB, IV, DC. MO, II, MG, IV, DC performed the
formal analysis. Investigation was performed by MO, AMV, II, HB,
MG, CC, IV, DC, DV, CM, AN, AS, RN, and AA. Resources were
established by MO, II, CC, IV, DC, DV, CM, AN, AS. Data curation
was carried out by MO, CC, DV, AS, AA. Writing and original draft
preparation was done by MO, II, CC, IV, DC, DV, CM, AN, AS.
Visualization was accomplished by MO, AMV, HB, MG, RN, AA. MO, AMV,
MG, IV, DC, AA supervised the project. Project Administrators were
MO, AMV. All authors have read and agreed to the published version
of the manuscript.
Ethics approval and consent to
participate
Ethics approval for the study was obtained from the
Emergency University Hospital Bucharest.
Patient consent for publication
The patient signed the informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Greer JP, Rodgers GM, Glader B, Arber DA,
Means RT, List AF, Appelbaum FR, Dispenzieri A and Fehninger TA:
Wintrobe's Clinical Hematology: Fourteenth edition. Philadelphia,
PA. Wolters Kluwer, 1069-1095, 2019.
|
|
2
|
Onisâi M, Vlădăreanu AM, Spînu A, Găman M
and Bumbea H: Idiopathic thrombocytopenic purpura (ITP)-new era for
an old disease. Rom J Intern Med. 57:273–283. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hauswirth AW, Skrabs C, Schutzinger C,
Raderer M, Chott A, Valent P, Lechner K and Jager U: Autoimmune
thrombocytopenia in non-Hodgkin's lymphomas. Haematologica.
93:447–450. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rudolph KE, Lessler J, Moloney RM, Kmush B
and Cummings DA: Incubation periods of mosquito-borne viral
infections: A systematic review. Am J Trop Med Hyg. 90:882–891.
2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Greco M, Cofano P and Lobreglio G:
Seropositivity for west Nile virus antibodies in patients affected
by myastenia gravis. J Clin Med Res. 8:196–201. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ferrarini I, Rigo A, Gandini A and Vinante
F: West Nile virus encephalitis in haematological setting: Report
of two cases and a brief review of the literature. Mediterr J
Hematol Infect Dis. 11(e2019033)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Montgomery RR and Murray KO: Risk factors
for West Nile virus infection and disease in populations and
individuals. Expert Rev Anti Infect Ther. 13:317–325.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Richner JM, Gmyrek GB, Govero J, Tu Y, van
der Windt GJ, Metcalf TU, Haddad EK, Textor J, Miller MJ and
Diamond MS: Age-dependent cell trafficking defects in draining
lymph nodes impair adaptive immunity and control of west Nile virus
infection. PLoS Pathog. 11(e1005027)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Urošević A, Dulović O, Milošević B, Maksić
N, Popović N, Milošević I, Delić D, Jevtović D, Poluga J, Jordović
J, et al: The importance of haematological and biochemical findings
in patients with West Nile virus neuroinvasive disease. J Med
Biochem. 35:451–457. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Uç D, Çelik T, Gönen D, Sucu A, Celiloglu
C, Tolunay O and Celik U: A factor that should raise awareness in
the practice of pediatric medicine: West Nile virus. J Pediatr Inf.
12:e70–e72. 2018.
|
|
11
|
Armstrong WS, Bashour CA, Smedira NG,
Heupler FA, Haeltge GA, Mawhorter SD, Sudheendra V and Gordon SM: A
case of fatal west Nile virus meningoencephalitis associated with
receipt of blood transfusions after open heart surgery. Ann Thorac
Surg. 76:605–607. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hottz ED, Bozza FA and Bozza PT: Platelets
in immune response to virus and immunopathology of viral
infections. Front Med. 5(121)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cooper N and Bussel J: The pathogenesis of
immune thrombocytopenia. Br J Haematol. 133:364–374.
2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cines DB, Bussel J, Liebman HA and Luning
Prak ET: The ITP syndrome: Pathogenesis and clinical diversity.
Blood. 113:6511–6521. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
George JN: How I treat patients with
thrombocytopenic purpura: 2010. Blood. 116:4060–4069.
2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Voican I, Onisâi M, Nicolescu A,
Vlădăreanu AM and Vlădăreanu R: Heparin induced thrombocytopenia: A
review. Farmacia. 60:773–784. 2012.
|
|
17
|
Onisâi M, Vlădăreanu AM, Delcea C,
Ciorăscu M, Bumbea H, Nicolescu A, Voican I, Filipescu A, Rotaru O
and Vlădăreanu R: Perinatal outcome for pregnancies complicated
with thrombocytopenia. J Matern Fetal Neonatal Med. 25:1622–1626.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Brooks WH: Viral impact in autoimmune
diseases: Expanding the ‘X chromosome-nucleolus nexus’ hypothesis.
Front Immunol. 8(1657)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Găman M, Vlădăreanu AM, Dobrea C, Onisâi
M, Marinescu C, Voican I, Vasile D, Bumbea H and Cîșleanu D: A
challenging case of Kikuchi-Fujimoto disease associated with
systemic lupus erythematosus and review of the literature. Case Rep
Hematol. 2018(1791627)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Provan D, Arnold DM, Bussel JB, Chong BH,
Cooper N, Gernsheimer T, Ghanima W, Godeanu B, Gonzalezz-Lopez TJ,
Grainger J, et al: Updated international consensus report on the
investigation and management of primary immune thrombocytopenia.
Blood Adv. 22:3780–3817. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ono K, Onishi Y, Kobayashi M, Ichikawa S,
Hatta S, Watanabe S, Okitsu Y, Fukuhara N, Ichinohasama R and
Harigae H: Successful treatment of aggressive mature B-cell
lymphoma mimicking immune thrombocytopenic purpura. Intern Med.
57:2573–2579. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Poponea N, Suede M and Muhsim Christi M:
Idiopathic thrombocytopenia purpura masking Hodgkin disease: A
paraneoplastic syndrome or simply a mere association? Case Rep
Oncol. 10:1116–1120. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Park SY, Kim S, Kim ES, Choi SU, Hyun HJ,
Ahn JY, Lee JH, Ryu SH, Park JH, Park JH, et al: A case of
Non-Hodgkin's lymphoma in patient with Coombs' negative hemolytic
Anemia and idiopathic thrombocytopenic purpura. Cancer Res Treat.
44:69–72. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Takahashi T, Maruyama Y, Saitoh M, Itoh H,
Yosimoto M, Tsujisaki M and Nakayama M: Synchronous occurrence of
diffuse large B-cell lymphoma of the duodenum and gastrointestinal
stromal tumor of the Ileum in a patient with immune
thrombocytopenic purpura. Intern Med. 55:2951–2956. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Uchiyama M, Sato K and Ikeda T: Diffuse
large B-cell lymphoma complicated with autoimmune thrombocytopenia.
Intern Med. 50:1215–1218. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Watanabe T, Kurihara H, Magarisawa S,
Shimoda S, Yoshida K and Ishiuchi S: Resolution of immune
thrombocytopenic purpura associated with extranodal B-cell lymphoma
of the petroclival region after radiotherapy. Surg Neurol Int.
1(76)2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Karami H, Naderisorski M, Ghasemi M and
Sakhaei SM: Immune thrombocytopenia as a primary sign of relapse in
Hodgkin lymphoma: A case report. IJBC. 11:72–74. 2019.
|
|
28
|
Sejvar JJ, Davis LE, Szabados E and
Jackson AC: Delayed-onset and recurrent limb weakness associated
with West Nile virus infection. J Neurovirol. 16:93–100.
2010.PubMed/NCBI View Article : Google Scholar
|