Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most
common chronic liver disease in China (incidence 15%). Improved
quality of life has altered the diet of the Chinese. The total
calorie intake has increased greatly, and a lack of effective
exercise has led to an increase in the prevalence of NAFLD
(1). NAFLD is a metabolic syndrome
characterized by excessive accumulation of fat in hepatocytes and
by hepatic parenchymal cell degeneration as a major characteristic
of liver damage, which may progress to liver fibrosis, cirrhosis,
and liver cancer (2). NAFLD is
prone to insulin resistance and is closely related to type 2
diabetes (3,4). Regarding the pathogenesis of NAFLD,
the secondary percussion hypothesis is accepted. The primary cause
of NAFLD is excessive deposition of triglycerides in the liver
because of decreased very-low-density lipoprotein (VLDL) output and
increased fatty acid oxidation (5,6). NAFLD
is treated by changing lifestyle factors, such as diet and exercise
(7,8). However, patients with poor compliance
show no significant improvement, and there is no drug for NAFLD.
The drugs commonly used for NAFLD include insulin sensitizers and
antioxidants; however, their long-term efficacy is unclear, and new
interventions for NAFLD are needed.
Berberine (BBR) has a phenyltetrahydroxyquinoline
structure. Berberine bioactive alkali has the effect of improving
fatty liver in mice (9). Berberine
is an important component of the biologically active alkali of
Coptidis Rhizoma. Previous studies have found that BBR also lowers
blood sugar, corrects blood lipid disorders, reduces the production
of inflammatory factors, and inhibits the release of endotoxin. It
has certain pharmacological effects on metabolic syndrome,
digestive-system diseases, cardiovascular diseases, tumors, and
mental disorders (10). BBR has
been used to treat type 2 diabetes (11). Because there is no drug for NAFLD,
and BBR has hypoglycemic, lipid-lowering, and antioxidant effects,
it has potential for the treatment of NAFLD. Xing et al
(12) and Yang et al
(13) reported that BBR has a
protective effect on NAFLD. Other previous studies also
demonstrated that BBR had an important role in NAFLD and hepatic
inflammations, such as hepatic steatosis, through regulating
insulin receptor substrate 2, triggering AMP-activated protein
kinase and inhibiting oxidative stress (14-16).
In liver lipid metabolism, the key factors for VLDL transport are
microsomal triglyceride transfer protein (MTTP), apolipoprotein B
(ApoB) and low-density lipoprotein receptor (LDLR), and their
expression levels affect the assembly and secretion of VLDL. Pan
and Hussain (17) showed that the
MTTP gene was associated with the risk of NAFLD; therefore,
expression of these key genes is important for BBR intervention in
NAFLD. The present study established a NAFLD animal model, observed
changes in liver tissue by hematoxylin and eosin (H&E)
staining, and assayed biochemical markers to evaluate the effect of
berberine on NAFLD. Reverse transcription-quantitative PCR and
western blotting were performed to assess the mRNA and protein
expression levels of MTTP, ApoB and LDLR in liver tissue, and to
explore the mechanism underlying the effect of BBR on NAFLD.
Materials and methods
Animal model of NAFLD
A rat model of NAFLD was established as described
previously (18). Thirty-five
specific-pathogen-free 8-week-old male SD rats (weight range,
310-322 g) were purchased from the Laboratory Animal Center of
Guangzhou University of Chinese Medicine [approval no. SYXK (Yue)
2013-0034] and adaptively fed for 1 week. The rats were then
randomly divided into a control group which was fed with a normal
diet (ND, n=12; diet purchased from the Laboratory Animal Center of
Jinan University) and an experimental group which was fed a
high-fat diet (HFD, n=23; diet composed of 80% regular chow, 8%
yolk powder, 10% lard oil, 1.5% cholesterol, and 0.5% bile salt;
purchased from Guangdong Medical Laboratory Animal Center) for 8
weeks. Both groups had free access to the specified diet and water.
The body weight of the rats was measured once weekly, and their
hair color, appetite, action, feces, and response to external
stimuli were observed. After 8 weeks, two rats in the ND group and
three in the HFD group were euthanized by cervical dislocation
under deep anesthesia (3% pentobarbital sodium; 30 mg/kg), and
liver specimens were stained with H&E to confirm the
establishment of the model. The standard for success was liver
cells with particle infiltration, and abnormal liver-function
indicators. The formula for both diets is listed in Table I. All experimental procedures were
approved by the Animal Experimental Ethics Committee of the
Affiliated Hospital of Shandong Medical College (permit no.
AEECAHSMC20205059).
 | Table IFeed composition. |
Table I
Feed composition.
| A, Normal diet |
|---|
| Ingredient | Content (%) |
|---|
| Nitrogen-free
extract | 44.72 |
| Fat | 5.34 |
| Crude protein | 23.82 |
| Crude ash | 6.18 |
| Crude fiber | 2.82 |
| Crude fat | 6.16 |
| Water | 8.96 |
| Calcium | 1.17 |
| Phosphorus | 0.83 |
| B, High-fat
diet |
| Ingredient | Content (%) |
| Normal diet | 80 |
| Yolk powder | 8 |
| Lard oil | 10 |
| Cholesterol | 1.5 |
| Sodium cholate | 0.5 |
BBR treatment
The remaining NAFLD rats (n=20) were randomly
divided into a BBR intervention group (BBR, n=10) and a solvent
control group (HFD, n=10). The BBR group was intragastrically
administered with 100 mg/kg/day of BBR (Mysun Pharma Co., Ltd.) in
0.5% carmellose sodium (CS) as solvent, once daily for 8 weeks. The
ND and HFD groups were only administered with the vehicle control,
4 ml/kg/day of 0.5% CS. Previous studies have used 100 and 300
mg/kg/day of BBR; preliminary experiments were performed with both
doses and no significant difference was observed, therefore the 100
mg/kg/day dose of BBR was selected for the present study (19-21).
Sample collection
The rats were fasted for 12 h prior to specimen
collection. Sample collection was performed prior to the
measurement detections. After anesthesia with 3% pentobarbital
sodium (30 mg/kg), the rats were dissected under aseptic
conditions, and 10 ml of fresh blood was taken from the abdominal
aorta (the rats that were not involved with sample collections were
not anesthetized). Full anesthesia was confirmed by corneal or
pedal reflex (firm toe pinch). The serum was separated at room
temperature for 20 min, followed by ultra-low-temperature
centrifugation (4˚C, 1,006 x g, 15 min). The supernatant was stored
at -80˚C for later use. The liver was quickly dissected and rinsed
with physiological saline. Surface moisture was blotted with filter
paper, and the liver was weighed. A small piece of tissue from the
right hepatic liver tip was placed in 10% formalin and fixed at
room temperature for 24 h, for pathological analysis. Sample
collection was performed after rats were anaesthetized and before
they were euthanized. The rats were then sacrificed by cervical
dislocation at the same time. Animal death was defined as
mydriasis, respiratory arrest and cardiac arrest for a period of
>5 min.
Serum biochemical indicator
detection
The biochemical indicators serum alanine
aminotransferase (ALT), aspartate aminotransferase (AST), total
cholesterol (TC), triglyceride (TG), fasting blood glucose (FBG),
low-density lipoprotein (LDL) and high-density lipoprotein (HDL)
were assayed using a 7180 model automatic biochemical analyzer
(Hitachi, Ltd.).
Assessment of pathological changes in
the liver by H&E staining
The specimens were fixed in 4% paraformaldehyde and
embedded in paraffin. Sections were cut at 5 µm thickness in the
coronal plane through the repaired tendon-bone interface. The
sections were stained with H&E and observed under a light
microscope (Zeiss AG). Liver histopathological changes were scored
by the NAFLD activity score (NAS) (22): steatosis (0-3 points), hepatic
lobular inflammation (0-3 points), and vacuolar-like degeneration
(0-2 points). Higher scores mean increased severity.
Determination of liver TG levels
Liver tissue (100 mg) was ground in chloroform:
methanol (2:1 volume ratio) solution and vigorously shaken at room
temperature for 2 h. Then, 0.5 mg of 0.1 M NaCl was added, and the
mixture was centrifuged at 1530 x g at 37˚C for 10 min. The organic
lipid oil phase was removed, and the TG levels were measured using
an automatic biochemical analyzer (Hitachi, Ltd.).
Reverse transcription-quantitative PCR
(RT-qPCR)
qPCR analysis was performed to determine the MTTP,
ApoB and LDLR mRNA expression levels. Total RNA from liver tissue
was extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
RNA (1 µg) was subjected to reverse transcription using a
PrimeScript RT Reagent kit (Takara Bio, Inc.). The reverse
transcription conditions were: one cycle at 37˚C for 15 min and one
cycle at 85˚C for 5 sec. Next, the diluted cDNA (2 µl) was
subjected to qPCR using SYBR-Green I (Takara Bio, Inc.) with
primers synthesized by Nanjing GenScript Co., Ltd. The mRNA
expression levels were normalized to the housekeeping gene β-actin.
The primer sequences were: MTTP, forward
5'-TCGGGTGGCTGTGGTAATAAC-3' and reverse
5'-AACTGCACTGTGGAGATGAAC-3'; ApoB, forward,
5'-GAGCCTCTAATTTTGCTGGG-3' and reverse 5'-TGTTCCCATGTGCCATAGAT-3';
LDLR, forward 5'-AAGGCTGTGGGTTCCATAGG-3'and reverse
5'-TGGACCCTTTCTCTCGGAAC-3'; and β-actin, forward 5'-
GCTAACAGTCCGCCTAGAAGCA-3' and reverse 5'-GTCATCACCATCGGCAATGAG-3'.
The qPCR conditions were: one cycle at 95˚C for 5 min; 40 cycles at
95˚C for 15 sec and 60˚C for 60 sec; one cycle at 95˚C for 15 sec,
60˚C for 1 min, and 95˚C for 15 sec. Thermal cycling and real-time
detection were conducted on a StepOnePlus Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Relative fold
changes in mRNA expression were calculated using the formula
2-ΔΔCq (23).
Western blot analysis
A bicinchoninic acid kit (Sigma-Aldrich; Merck KGaA)
was used to determine the protein concentration of liver samples
from five rats per group. Sample buffer (SDS-PAGE sample buffer;
Beyotime Institute of Biotechnology) was added, and the samples
were boiled at 95˚C for 10 min. Next, proteins (30 µg per sample,
per well) were separated by 10% polyacrylamide gel electrophoresis.
The proteins were transferred to polyvinylidene fluoride membranes
by 100 V transfer-molded voltage for 45 to 70 min. The samples were
incubated at room temperature for 1 h with 5% bovine serum albumin,
and subsequently with rabbit anti-MTTP (cat. no. PA5-42391), rabbit
anti-ApoB (cat. no. PA5-114864), rabbit anti-LDLR (cat. no.
PA5-82385) and mouse anti-β-actin primary antibodies (cat. no.
MA5-15739-D550; all used at 1:1,000 dilution; all supplied from
Thermo Fisher Scientific, Inc.), at 4˚C overnight. Next, the
samples were washed three times in Tris-buffered saline/0.05%
Tween-20 (5 min each wash). The corresponding HRP-conjugated
secondary antibody (cat. no. 21130; 1:20,000 dilution; Thermo
Fisher Scientific, Inc.) was added and incubated at room
temperature for 1 h. The membranes were washed three times (5 min
each wash). Bands were developed using chemiluminescence reagents;
β-actin was used as the internal reference. Bands were visualized
with a Bio-Rad Gel Doc EZ Imager (Bio-Rad Laboratories, Inc.) and
band intensity was analyzed using ImageJ software (Version 1.53;
National Institutes of Health).
Statistical analysis
Data were analyzed using Prism version 6 (GraphPad
Software, Inc.) statistical software. Each experiment was repeated
three times. Data are expressed as means ± standard deviation.
Kruskal-Wallis followed by Dunn's test was used to assess NAS
scores. One-way analysis of variance followed by LSD post hoc test
was applied for comparisons of multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
General observation
The rats in the ND group were active, responsive,
had a good appetite, and were clean and shiny, without hair loss.
Their stools were granular, yellow, and hard. In the HFD group, the
rats were obese, unresponsive, and their hair was yellow and dull.
Hair loss occurred with time, appetite decreased, and stools were
granular, black, and hard. Following administration of BBR, the
rats exhibited fur that was less yellow, reduced hair loss, they
were more responsive, and their feces were watery.
Body weight and liver wet weight
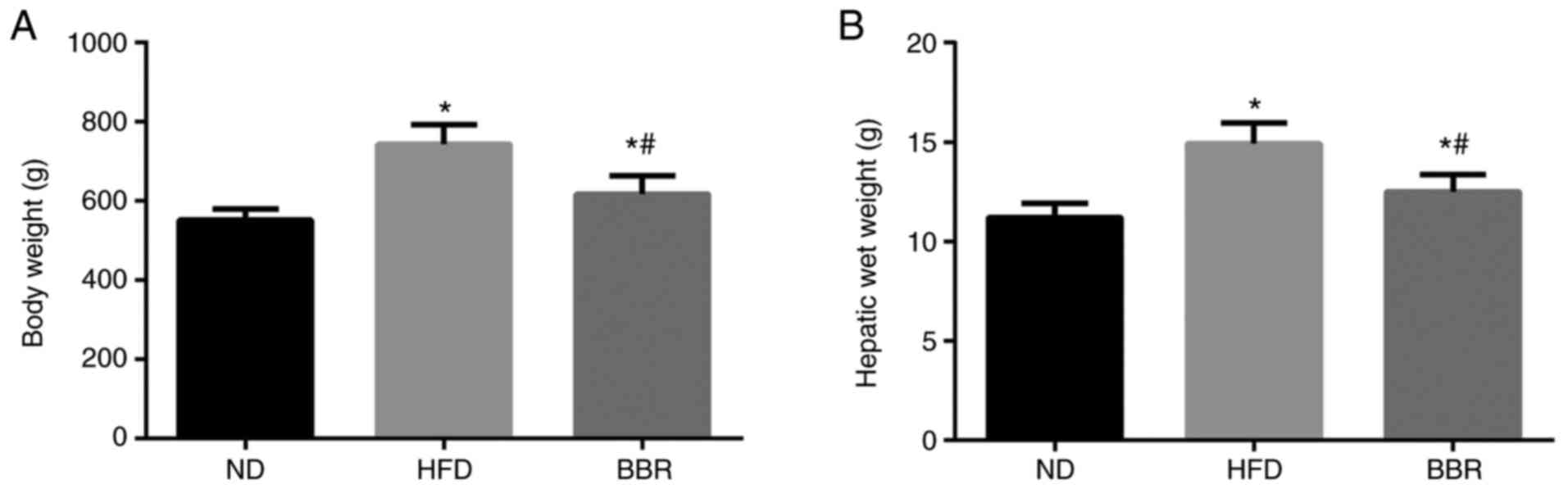
As shown in Fig. 1,
the body weight and liver wet weight of HFD rats increased
significantly compared with the ND group (P<0.05). The body
weight and liver wet weight of the BBR group were significantly
lower compared with the HFD group (P<0.05; Fig. 1).
Serum biochemical indices
Long-term feeding of a high-fat diet resulted in
higher levels of ALT, AST, TG, TC, FBG, and LDL in the HFD group
compared with the ND group, and significantly lower HDL
concentrations (all P<0.05; Table
II). After BBR intervention, serum ALT, AST, TG, TC and LDL
decreased significantly compared with the HDF group (P<0.05;
Table II). Notably, the serum
levels of ALT, TG, TC and LDL were similar to those of the ND
group, suggesting that BBR treatment successfully reversed these
biochemical indices (P>0.05; Table
II). No effect was observed after BBR administration for the
serum levels of FBG and HDL, with their levels being similar in the
BBR and HFD groups (Table II).
 | Table IISerum biochemical parameters. |
Table II
Serum biochemical parameters.
| Group | ALT (U/l) | AST (U/l) | TG (mol/l) | TC (mmol/l) | FBG (mmol/l) | LDL (mmol/l) | HDL (mmol/l) |
|---|
| ND | 49.63±4.21 | 120.19±10.36 | 1.57±0.16 | 1.74±0.09 | 5.19±0.34 | 0.63±0.08 | 1.19±0.08 |
| HFD |
96.24±10.11a |
173.20±15.46a |
2.10±0.19a |
2.33±0.18a |
6.30±0.61a |
1.97±0.15a |
0.57±0.05a |
| BBR |
53.17±5.35b |
136.51±15.34a,b |
1.69±0.09b |
1.85±0.14b |
5.78±0.50a |
0.73±0.13b |
0.64±0.08a |
BBR alleviates liver damage induced by
a high-fat diet
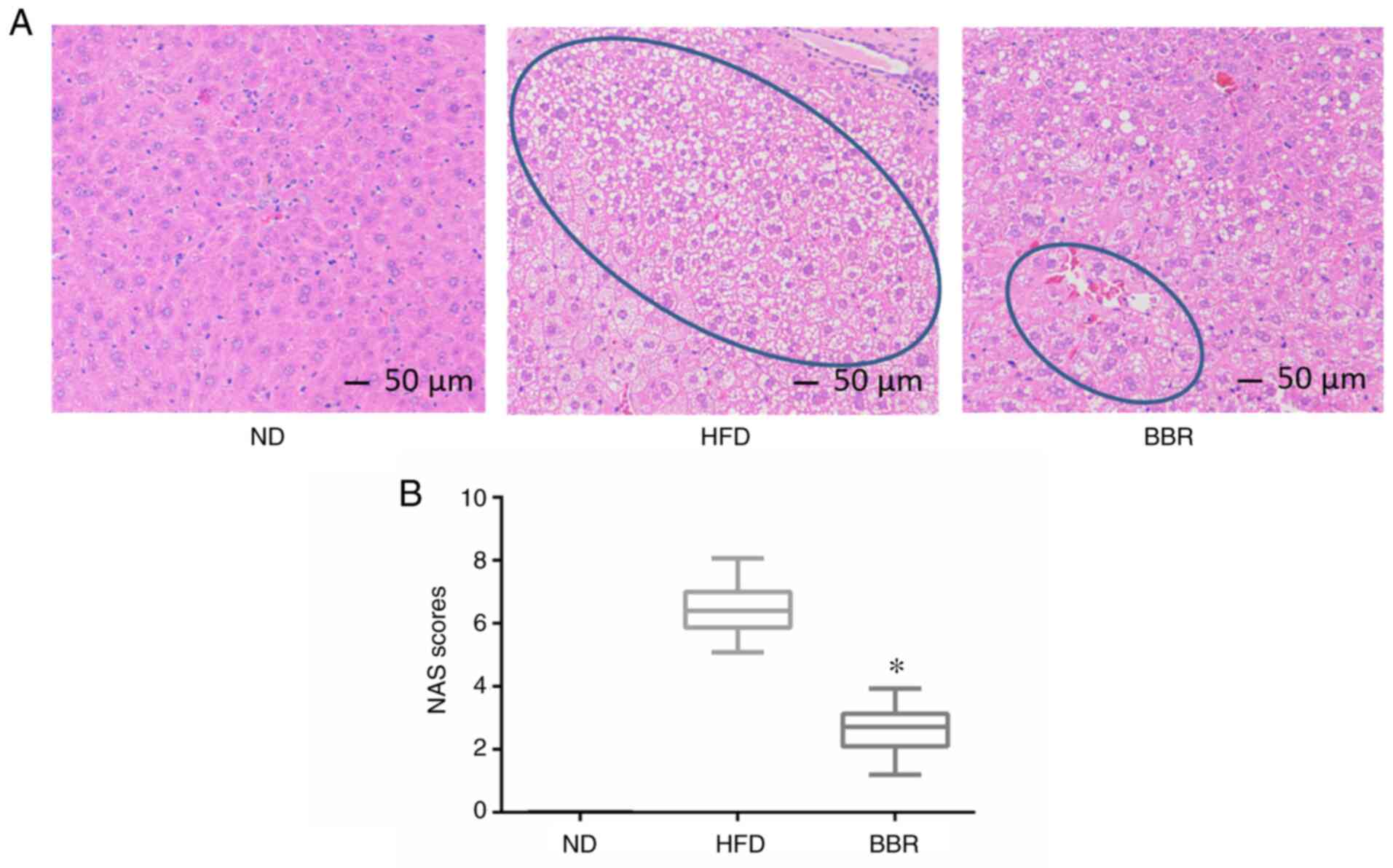
H&E staining revealed that the liver cells of
the ND group were arranged neatly and tightly, the hepatic lobule
structure was intact, the nucleus was located in the center of
hepatocytes, the cytoplasm was uniform, and no lipid droplets were
deposited (Fig. 2A). In the HFD
group, the hepatocyte volume was increased, the structure of the
hepatic lobule was incomplete, and there was a large amount of
vacuolar-like steatosis (Fig. 2A).
In severe cases, the cytoplasm exhibited a fishnet-like change.
Following BBR treatment, vacuolar-like steatosis was reduced
compared with the HFD group, and hepatocytes were arranged neatly
(Fig. 2A). The NAS of the ND, HFD
and BBR groups was 0, 657 ±0.87, and 2.55±0.56, respectively
(Fig. 2B), indicating that BBR
significantly reduced NAS. These findings suggested that BBR
administration alleviated liver damage.
A high-fat diet results in hepatic
lipid deposition
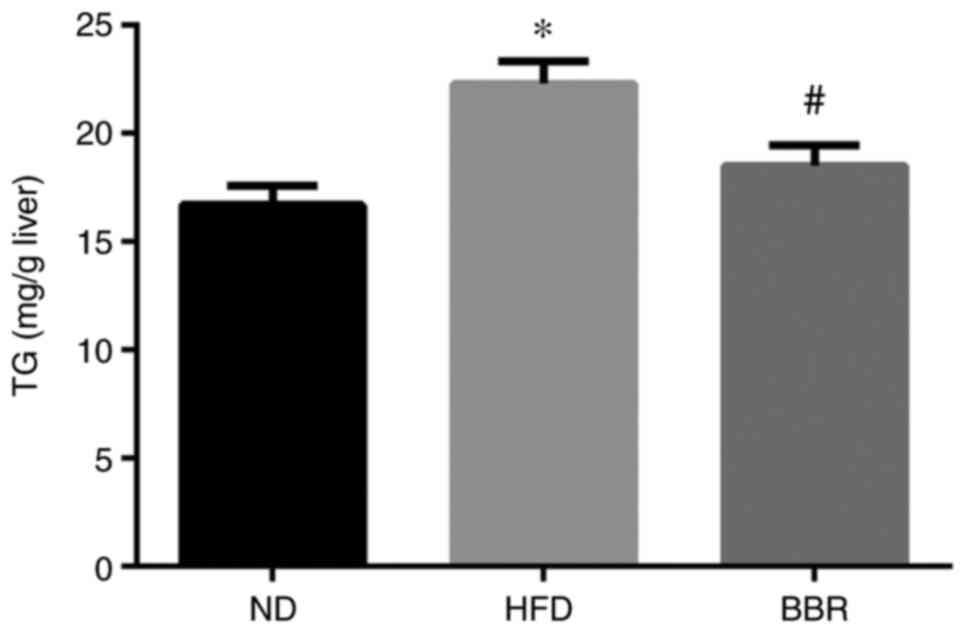
The liver TG content was significantly higher in the
HFD group compared with the ND group (Fig. 3). BBR administration significantly
reduced the liver TG content (P<0.05; Fig. 3), consistent with the liver
pathology results.
Expression levels of MTTP, ApoB, and
LDLR
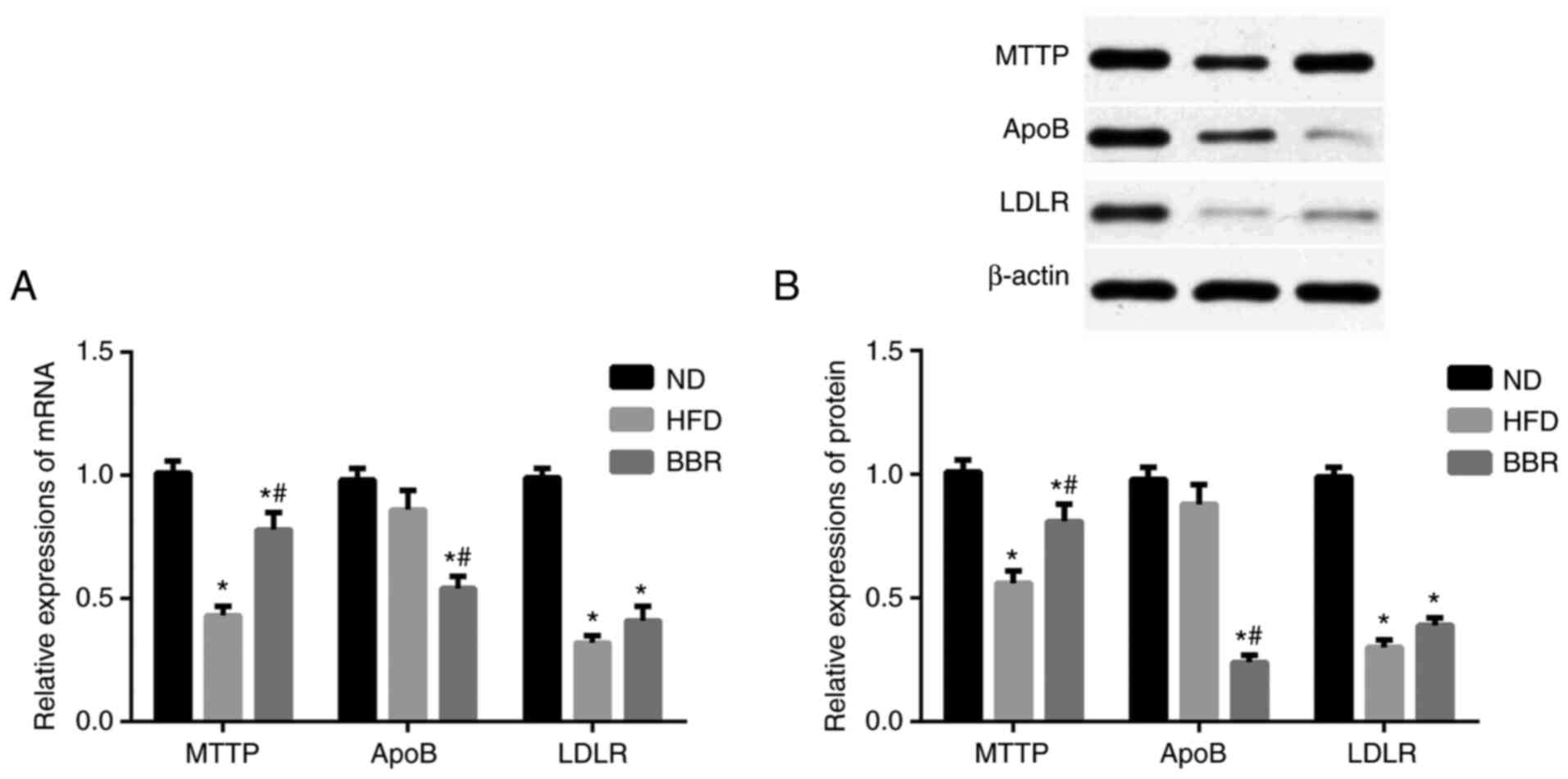
Next, the mRNA and protein expression levels of
MTTP, ApoB, and LDLR were determined in the liver. As shown in
Fig. 4A, the mRNA expression levels
of MTTP and LDLR in the HFD group decreased significantly compared
with the ND group (P<0.05), while the mRNA expression levels of
ApoB did not significantly change (P>0.05). Following BBR
administration, the mRNA expression levels of MTTP and LDLR were
upregulated, albeit the change in LDLR levels was not significant
(P>0.05; Fig. 4A). The mRNA
expression levels of ApoB were significantly decreased in the BBR
group compared with the ND and HFD groups (P<0.05; Fig. 4A). Western blot analysis revealed
similar trends at the protein level. The protein expression levels
of MTTP and LDLR were significantly decreased in the HFD group
compared with the ND group (P<0.05; Fig. 4B), but there was no significant
difference in ApoB protein expression levels (P>0.05; Fig. 4B). BBR administration significantly
increased MTTP protein expression levels compared with those in the
HFD group (P<0.05; Fig. 4B), but
the increase in LDLR protein levels was not significant (P>0.05;
Fig. 4B). Finally, ApoB protein
expression levels were significantly decreased in the BBR group
compared with the ND and HFD groups (P<0.05; Fig. 4A).
Discussion
The global incidence of NAFLD is ~20%. Lifestyle
changes have led to a growing incidence of NAFLD (24-27).
NAFLD is a metabolic syndrome that is often accompanied by diabetes
and hyperlipidemia. These diseases are closely related to lipid
metabolism disorders and abnormal lipid deposition, and their
prevention and treatment are a focus of interest. The pathogenesis
of NAFLD has been explained by first and second-strike theories.
The second-strike theory proposed by Day and James (28) is generally accepted by the
scientific community. The first strike refers to the rapid
production of free fatty acids in the case of insulin resistance.
The liver's ability to β-oxidize fatty acids and to synthesize VLDL
decreases, leading to fat deposition in liver cells. The resulting
imbalance of lipid metabolism triggers development of fatty liver.
In the second strike, damaged hepatocytes result in increased
levels of inflammatory factors and a reduced antioxidant capacity,
which further aggravates fatty liver disease. In summary, the
occurrence and development of NAFLD are closely related to lipid
metabolism.
BBR has an anti-arrhythmia effect, dilates coronary
arteries, induces hypoglycemia, and regulates blood lipids. BBR has
produced good results in clinical trials in type 2 diabetes with
hyperlipidemia (29). Currently,
there is no drug for NAFLD; because BBR has hypoglycemic,
lipid-lowering, and antioxidant activities, the present study
evaluated its effect on NAFLD. Yang et al (13) showed that BBR had a protective
effect on NAFLD in animals. Kim et al (8) reported that BBR ameliorated lipid
metabolism disorders in obese rats by interfering with the
metabolism of surrounding tissues, thereby reducing liver wet
weight and blood TG and TC levels. In the present study, BBR was
administered to NAFLD rats to investigate its effect on NAFLD
(30,31). The mean body weight of the rats in
the HFD group was significantly higher compared with that of rats
in the ND group, and significantly reduced following BBR
administration. Total visceral fat was increased in NAFLD rats. The
mean liver wet weight was significantly higher in rats in the HFD
group compared with those in the ND group. After 8 weeks of BBR
administration, the liver wet weight of rats in the BBR group
decreased significantly to a level similar to that of the ND group.
AST and ALT are important functional enzymes in hepatocytes and are
used as indicators of liver function. AST is mainly present in the
myocardium, followed by the liver mitochondria. Under normal
circumstances, serum ALT and AST levels are low. Damage to
hepatocytes increases their membrane permeability, resulting in
release of ALT and AST into plasma. In patients with hepatocyte
injury, changes in ALT and AST levels reflect disease progression,
thus facilitating clinical diagnosis. ALT and AST levels were
significantly higher in the serum of the rats in the HFD group
compared with the normal rats. The ALT and AST levels in those two
groups were significantly lower than those in the HFD group after
BBR administration. BBR may protect against liver damage caused by
non-alcoholic factors by regulating ALT and AST, possibly by
improving the function of the liver cell membrane. NAFLD is
associated with dyslipidemias such as hypertriglyceridemia,
elevated LDL cholesterol, and decreased HDL cholesterol (32). HDL and LDL can be used to measure
cholesterol carrying capacity. LDL is positively correlated with
serum total cholesterol levels and it transports liver cholesterol
to extrahepatic tissue cells. When the LDL concentration increases,
LDL is deposited on arterial walls, forming an atherosclerotic
plaque and blocking blood vessels. HDL is negatively correlated
with total cholesterol levels. Its main role is to transport of
extrahepatic cholesterol into liver cells for degradation and
decomposition. The LDL and HDL levels reflect liver function.
Accordingly, the present study demonstrated that rats fed a
high-fat diet showed elevated serum TC and LDL levels and decreased
HDL levels. BBR administration reduced serum TC and LDL levels and
increased HDL levels. Continuous administration of BBR to rats for
8 weeks reduced liver TG content, significantly reversed hepatic
steatosis, and enhanced liver function.
NAFLD features accumulation of large amounts of TG
in the liver; this can be caused by diverse factors. Under normal
circumstances, TG is excreted from the liver in the form of VLDL.
Therefore, timely synthesis and secretion of VLDL is important for
relieving TG deposition. The assembly and maturation of VLDL occur
as follows (33-36):
in the endoplasmic reticulum, ApoB forms under the action of MTTP,
resulting in immature VLDL particles with lower precursor fat
content, smaller particle size and higher density. The lipid
droplets transfer large amounts of fat to immature VLDL particles,
transforming them into higher fat, less dense, mature VLDL
particles. An adequate fat supply and MTTP activity are necessary
for VLDL synthesis. In the absence of sufficient fat supply or
MTTP, ApoB cannot obtain fat, causing it to be degraded by the
proteasome during or after translation (31). This prevents the production of VLDL
particles to excrete TG. Wang et al (37) found that inhibition of MTTP activity
impeded assembly of VLDL, leading to increased intrahepatic TG
levels. It was hypothesized that the decreased expression of the
MTTP gene in NAFLD rats may reduce TG excretion from the liver in
VLDL, promoting hepatic fat deposition. BBR administration
upregulated the expression and activity of MTTP, thereby enhancing
the assembly of VLDL and reducing the liver TG content.
LDLR is a cell-surface glycoprotein that regulates
plasma cholesterol levels by mediating LDL transport. Defects in
LDLR function are one of the main causes of hypercholesterolemia,
atherosclerosis and fatty liver (38). Current understanding of the role of
LDLR in fatty liver has increased, and drugs that enhance LDLR
expression to lower blood cholesterol levels have been developed.
In the present study, BBR administration upregulated the expression
of LDLR in NAFLD rats, suggesting that BBR may prevent fatty liver
by regulating the LDLR content of hepatocytes and increasing the
LDL clearance rate.
The second-strike is damage to cells by oxygen free
radicals, causing lipid peroxidation, protein denaturation, damage
to the cell membrane, and release of inflammatory mediators. These
changes result in an impaired inflammatory response. BBR exerts an
anti-inflammatory effect by inhibiting neutrophil chemotaxis,
generation of oxygen free radicals, the activity of phospholipase
A2 and reducing the tissue prostaglandin E2 content, thus reducing
inflammatory damage. The anti-inflammatory effect of BBR also
enhances superoxide dismutase (SOD) activity, inhibits the
production of TNF-α, improves ischemia reperfusion and reduces
liver damage (39-42).
However, further research into the mechanism by which BBR enhances
SOD activity and inhibits TNF-α production is needed. As a
bacteriostatic drug, BBR is used clinically mainly for intestinal
infectious diseases and diarrhea. In vitro, BBR enhances the
phagocytic capacity of leukocytes and the hepatic
reticuloendothelial system, inhibits the growth of intestinal
bacteria, reduces the release of bacterial endotoxins, and enhances
the removal of endotoxin from serum. This decreases the intestinal
inflammatory response, as well as the release of inflammatory
mediators, such as TNF-α and IL-6. These effects reduce
inflammatory damage to the liver and insulin resistance, thereby
protecting the liver (43-46).
While displaying anti-inflammatory effects, BBR
treatment also increases liver AMP-activated protein kinase (AMPK)
phosphorylation (which leads to its activation). When activated,
AMPK is capable of suppressing lipogenesis through phosphorylating
and inactivating the lipogenic enzyme acetyl-CoA carboxylase (ACC).
In addition, a decrease in the production of malonyl-CoA due to
AMPK inhibition of ACC results in an increase in fatty acid
oxidation via releasing the inhibitory effect of malonyl-CoA on
carnitine palmitoyltransferase 1A. These combined effects of AMPK
activation are considered to largely account for BBR actions on
reducing hepatic steatosis as described by previous studies
(8,47-49).
In summary, the present study suggested that BBR may
increase VLDL production by reversing the abnormal expression of
key genes in lipid metabolism (MTTP and LDLR), thereby improving
the symptoms of NAFLD. A limitation of the current results is that
only one dose of BBR was used based on previous literature; future
studies further investigating additional doses and their effects
will be required to fully elucidate the role of BBR in ameliorating
NAFLD.
Acknowledgements
We thank Dr Wenxia Bai from Jiangsu Drug Safety
Evaluation Center for performing the histopathological observation
and assessment.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
PC, YL and LX performed the experiments, analyzed
the data and wrote the paper. PC and LX designed the study and
provided experimental materials, PC and LX confirmed the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All experimental procedures involving animals were
approved by the Animal Experimental Ethics Committee of the
Affiliated Hospital of Shandong Medical College (permit no.
AEECAHSMC20205059).
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Perumpail BJ, Khan MA, Yoo ER, Cholankeril
G, Kim D and Ahmed A: Clinical epidemiology and disease burden of
nonalcoholic fatty liver disease. World J Gastroenterol.
23:8263–8276. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Forbes S, Taylor-Robinson SD, Patel N,
Allan P, Walker BR and Johnston DG: Increased prevalence of
non-alcoholic fatty liver disease in European women with a history
of gestational diabetes. Diabetologia. 54:641–647. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chang Y, Jung HS, Yun KE, Cho J, Cho YK
and Ryu S: Cohort study of non-alcoholic fatty liver disease, NAFLD
fibrosis score, and the risk of incident diabetes in a Korean
population. Am J Gastroenterol. 108:1861–1868. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yamada T, Fukatsu M, Suzuki S, Wada T,
Yoshida T and Joh T: Fatty liver predicts impaired fasting glucose
and type 2 diabetes mellitus in Japanese undergoing a health
checkup. J Gastroenterol Hepatol. 25:352–356. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cohen JC, Horton JD and Hobbs HH: Human
fatty liver disease: Old questions and new insights. Science.
332:1519–1523. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fabbrini E, Sullivan S and Klein S:
Obesity and nonalcoholic fatty liver disease: Biochemical,
metabolic, and clinical implications. Hepatology. 51:679–689.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sreenivasa Baba C, Alexander G, Kalyani B,
Pandey R, Rastogi S, Pandey A and Choudhuri G: Effect of exercise
and dietary modification on serum aminotransferase levels in
patients with nonalcoholic steatohepatitis. J Gastroenterol
Hepatol. 21:191–198. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim WS, Lee YS, Cha SH, Jeong HW, Choe SS,
Lee MR, Oh GT, Park HS, Lee KU, Lane MD, et al: Berberine improves
lipid dysregulation in obesity by controlling central and
peripheral AMPK activity. Am J Physiol Endocrinol Metab.
296:E812–E819. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Qian S, Ma L, Peng S, Xu Y, Wu K, Shen S,
Zhang X, Sun Y and Ye J: ATP reduces mitochondrial MECR protein in
liver of diet-induced obese mice in mechanism of insulin
resistance. Biosci Rep. 40(40)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Qin S, Tang H, Li W, Gong Y, Li S, Huang
J, Fang Y, Yuan W, Liu Y, Wang S, et al: AMPK and its activator
berberine in the treatment of neurodegenerative diseases. Curr
Pharm Des. 26:5054–5066. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li Q, Zhao C, Zhang Y, Du H, Xu T, Xu X,
Zhang J, Kuang T, Lai X, Fan G, et al: 1H NMR-based metabolomics
coupled with molecular docking reveal the anti-diabetic effects and
potential active components of berberis vernae on type 2 diabetic
rats. Front Pharmacol. 11(932)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xing LJ, Zhang L, Liu T, Hua YQ, Zheng PY
and Ji G: Berberine reducing insulin resistance by up-regulating
IRS-2 mRNA expression in nonalcoholic fatty liver disease (NAFLD)
rat liver. Eur J Pharmacol. 668:467–471. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yang QH, Hu SP, Zhang YP, Xie WN, Li N, Ji
GY, Qiao NL, Lin XF, Chen TY and Liu HT: Effect of berberine on
expressions of uncoupling protein-2 mRNA and protein in hepatic
tissue of non-alcoholic fatty liver disease in rats. Chin J Integr
Med. 17:205–211. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li S, Xu Y, Guo W, Chen F, Zhang C, Tan
HY, Wang N and Feng Y: The impacts of herbal medicines and natural
products on regulating the hepatic lipid metabolism. Front
Pharmacol. 11(351)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu R, Wu K, Li Y, Sun R and Li X: Human
antigen R: A potential therapeutic target for liver diseases.
Pharmacol Res. 155(104684)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li CH, Tang SC, Wong CH, Wang Y, Jiang JD
and Chen Y: Berberine induces miR-373 expression in hepatocytes to
inactivate hepatic steatosis associated AKT-S6 kinase pathway. Eur
J Pharmacol. 825:107–118. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pan X and Hussain MM: Diurnal regulation
of microsomal triglyceride transfer protein and plasma lipid
levels. J Biol Chem. 282:24707–24719. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yuan F, Wang H, Tian Y, Li Q, He L, Li N
and Liu Z: Fish oil alleviated high-fat diet-induced non-alcoholic
fatty liver disease via regulating hepatic lipids metabolism and
metaflammation: A transcriptomic study. Lipids Health Dis.
15(20)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lu Z, He B, Chen Z, Yan M and Wu L:
Anti-inflammatory activity of berberine in non-alcoholic fatty
liver disease via the Angptl2 pathway. BMC Immunol.
21(28)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mai W, Xu Y, Xu J, Zhao D, Ye L, Yu G,
Wang Z, Lu Q, Lin J, Yang T, et al: Berberine inhibits nod-like
receptor family pyrin domain containing 3 inflammasome activation
and pyroptosis in nonalcoholic steatohepatitis via the ROS/TXNIP
axis. Front Pharmacol. 11(185)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhu X, Bian H, Wang L, Sun X, Xu X, Yan H,
Xia M, Chang X, Lu Y, Li Y, et al: Berberine attenuates
nonalcoholic hepatic steatosis through the AMPK-SREBP-1c-SCD1
pathway. Free Radic Biol Med. 141:192–204. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bedossa P: Pathology of non-alcoholic
fatty liver disease. Liver Int. 37 (Suppl 1):85–89. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kojima S, Watanabe N, Numata M, Ogawa T
and Matsuzaki S: Increase in the prevalence of fatty liver in Japan
over the past 12 years: Analysis of clinical background. J
Gastroenterol. 38:954–961. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Adams LA, Angulo P and Lindor KD:
Nonalcoholic fatty liver disease. CMAJ. 172:899–905.
2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bedogni G, Miglioli L, Masutti F,
Tiribelli C, Marchesini G and Bellentani S: Prevalence of and risk
factors for nonalcoholic fatty liver disease: The Dionysos
nutrition and liver study. Hepatology. 42:44–52. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Browning JD, Szczepaniak LS, Dobbins R,
Nuremberg P, Horton JD, Cohen JC, Grundy SM and Hobbs HH:
Prevalence of hepatic steatosis in an urban population in the
United States: Impact of ethnicity. Hepatology. 40:1387–1395.
2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Day CP and James OF: Steatohepatitis: A
tale of two ‘hits’? Gastroenterology. 114:842–845. 1998.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kong W, Wei J, Abidi P, Lin M, Inaba S, Li
C, Wang Y, Wang Z, Si S, Pan H, et al: Berberine is a novel
cholesterol-lowering drug working through a unique mechanism
distinct from statins. Nat Med. 10:1344–1351. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Adams LA, Lymp JF, St Sauver J, Sanderson
SO, Lindor KD, Feldstein A and Angulo P: The natural history of
nonalcoholic fatty liver disease: A population-based cohort study.
Gastroenterology. 129:113–121. 2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gibbons GF: Assembly and secretion of
hepatic very-low-density lipoprotein. Biochem J. 268:1–13.
1990.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gibbons GF, Wiggins D, Brown AM and
Hebbachi AM: Synthesis and function of hepatic very-low-density
lipoprotein. Biochem Soc Trans. 32:59–64. 2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rutledge AC, Su Q and Adeli K:
Apolipoprotein B100 biogenesis: A complex array of intracellular
mechanisms regulating folding, stability, and lipoprotein assembly.
Biochem Cell Biol. 88:251–267. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Fisher EA and Ginsberg HN: Complexity in
the secretory pathway: The assembly and secretion of apolipoprotein
B-containing lipoproteins. J Biol Chem. 277:17377–17380.
2002.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Olofsson SO, Stillemark-Billton P and Asp
L: Intracellular assembly of VLDL: Two major steps in separate cell
compartments. Trends Cardiovasc Med. 10:338–345. 2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhou M, Fisher EA and Ginsberg HN:
Regulated Co-translational ubiquitination of apolipoprotein B100. A
new paradigm for proteasomal degradation of a secretory protein. J
Biol Chem. 273:24649–24653. 1998.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang Y, Tran K and Yao Z: The activity of
microsomal triglyceride transfer protein is essential for
accumulation of triglyceride within microsomes in McA-RH7777 cells.
A unified model for the assembly of very low density lipoproteins.
J Biol Chem. 274:27793–27800. 1999.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Furbee JW Jr, Francone O and Parks JS: In
vivo contribution of LCAT to apolipoprotein B lipoprotein
cholesteryl esters in LDL receptor and apolipoprotein E knockout
mice. J Lipid Res. 43:428–437. 2002.PubMed/NCBI
|
|
39
|
Zou K, Li Z, Zhang Y, Zhang HY, Li B, Zhu
WL, Shi JY, Jia Q and Li YM: Advances in the study of berberine and
its derivatives: A focus on anti-inflammatory and anti-tumor
effects in the digestive system. Acta Pharmacol Sin. 38:157–167.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yan YQ, Fu YJ, Wu S, Qin HQ, Zhen X, Song
BM, Weng YS, Wang PC, Chen XY and Jiang ZY: Anti-influenza activity
of berberine improves prognosis by reducing viral replication in
mice. Phytother Res. 32:2560–2567. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
41
|
Warowicka A, Nawrot R and
Goździcka-Józefiak A: Antiviral activity of berberine. Arch Virol.
165:1935–1945. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wu X, Li X, Dang Z and Jia Y: Berberine
demonstrates anti-inflammatory properties in Helicobacter
pylori-infected mice with chronic gastritis by attenuating the
Th17 response triggered by the B cell-activating factor. J Cell
Biochem. 119:5373–5381. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li CL, Tan LH, Wang YF, Luo CD, Chen HB,
Lu Q, Li YC, Yang XB, Chen JN, Liu YH, et al: Comparison of
anti-inflammatory effects of berberine, and its natural oxidative
and reduced derivatives from Rhizoma Coptidis in vitro and in vivo.
Phytomedicine. 52:272–283. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chao G, Ye F, Yuan Y and Zhang S:
Berberine ameliorates non-steroidal anti-inflammatory drugs-induced
intestinal injury by the repair of enteric nervous system. Fundam
Clin Pharmacol. 34:238–248. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Cicero AF and Baggioni A: Berberine and
its role in chronic disease. Adv Exp Med Biol. 928:27–45.
2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wang K, Feng X, Chai L, Cao S and Qiu F:
The metabolism of berberine and its contribution to the
pharmacological effects. Drug Metab Rev. 49:139–157.
2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zang M, Zuccollo A, Hou X, Nagata D, Walsh
K, Herscovitz H, Brecher P, Ruderman NB and Cohen RA: AMP-activated
protein kinase is required for the lipid-lowering effect of
metformin in insulin-resistant human HepG2 cells. J Biol Chem.
279:47898–47905. 2004.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Brusq J-M, Ancellin N, Grondin P, Guillard
R, Martin S, Saintillan Y and Issandou M: Inhibition of lipid
synthesis through activation of AMP kinase: An additional mechanism
for the hypolipidemic effects of berberine. J Lipid Res.
47:1281–1288. 2006.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Assifi MM, Suchankova G, Constant S,
Prentki M, Saha AK and Ruderman NB: AMP-activated protein kinase
and coordination of hepatic fatty acid metabolism of
starved/carbohydrate-refed rats. Am J Physiol Endocrinol Metab.
289:E794–E800. 2005.PubMed/NCBI View Article : Google Scholar
|