Introduction
Cardiovascular disease is currently the leading
cause of mortality in humans (1).
Cellular senescence is defined as a state of cell cycle arrest that
has the potential to promote tissue remodeling, and is involved in
developmental and injury responses (2). Cellular senescence decreases tissue
regenerative capacity and function, and induces inflammation and
pathological remodeling of aged organs (3). Cardiomyocytes are particularly
vulnerable to oxidative damage due to the lack of biochemical
reserves required for successful antioxidant action (4).
Doxorubicin (DOX) is one of the most widely and
successfully used antitumor agents in the clinical setting;
however, its cumulative and dose-dependent cardiotoxicity has been
a major concern over the previous decades and limits its use as a
therapeutic agent (5). Despite the
progress made over the past few decades in elucidating DOX-induced
cardiomyopathy, the underlying mechanisms remain poorly understood.
Oxidative stress, inflammation and apoptosis were found to be
responsible for the induction of cardiotoxicity by DOX and
contributed to increased levels of cardiomyocyte senescence, which
led, at least in part, to heart remodeling and impairment of
cardiac function (6). Therefore,
identifying more specific strategies for protect patients against
DOX-induced cardiotoxicity remains a priority. DOX-induced
oxidative stress in H9c2 cardiomyocytes represents a senescent
phenotype similar to the cardiomyocyte characteristics observed in
senescent rats (7).
The complement system is a central component that
participates in the activation of innate immunity, inflammation and
tissue remodeling. The complement activation product complement
component 5a (C5a) is a potent chemoattractant involved in
recruiting inflammatory cells, such as neutrophils, eosinophils,
monocytes and T lymphocytes, inactivating phagocytes, promoting the
release of granulocytes and generating oxidants (8). C5a can bind to the C5a receptor
(C5aR), which has been reported to be an essential modulator of the
inflammatory response (9). The
C5a/C5aR signaling pathway can activate inflammatory nuclear
factor-κB, which may lead to the direct release of proinflammatory
cytokines and chemokines (10,11).
C5aR signaling was discovered to play a key role in certain
inflammation-related diseases, including acute kidney injury
(12), adipose tissue inflammation
(13) and cardiovascular fibrosis
(14), by inducing inflammatory
responses and increasing the release of inflammation-associated
cytokines. The C5a/C5aR signaling pathway was also demonstrated to
play an important role as a mediator of a wide range of other
inflammation-related diseases, such as spinal cord injury (15), asthma (16) and myocardial ischemic injury
(17). However, the role of C5a and
C5aR in DOX-induced cardiomyocyte senescence remains unclear;
therefore, the present study was undertaken to investigate the
effects of C5a and C5aR on DOX-induced cardiomyocyte
senescence.
Materials and methods
Cell culture and treatments
The H9c2 rat embryonic cardiac cell line and AC16
human cardiomyocyte-like cells were purchased from The Cell Bank of
Type Culture Collection of The Chinese Academy of Sciences. The
cells were cultured in DMEM supplemented with 10% FBS (both from
Sigma-Aldrich; Merck KGaA) and 100 mg/ml penicillin/streptomycin,
and maintained in a humidified atmosphere of 5% CO2 at
37˚C.
DOX (Sangon Biotech Co., Ltd.) was dissolved in DMSO
and diluted with cell culture serum-free medium (Sigma-Aldrich;
Merck KGaA) to achieve a final concentration of 0.1 µM (18). The C5aR antagonist (C5aRA; Shanghai
Jier Biochemistry Inc.) was dissolved in DMSO and diluted with cell
culture serum-free medium (Sigma-Aldrich; Merck KGaA) to a final
concentration of 2.5 µg/ml.
Reverse transcription-quantitative
(RT-q) PCR analysis
Total RNA was extracted from H9c2 and AC16 cells
using a RNeasy Fibrous Tissue Mini kit (Qiagen, Inc.). Total RNA (1
µg) was reverse-transcribed into cDNA using random hexamers and
SuperScript III Reverse Transcriptase (Invitrogen; Thermo Fisher
Scientific, Inc.) under the manufacturer's protocol. qPCR was
subsequently performed on a LightCycler® instrument
(Roche Diagnostics) using a QuantiTect SYBR-Green PCR kit (Qiagen,
Inc.) by pre-denaturation at 95˚C for 3 min, denaturation at 95˚C
for 40 cycles of 15 sec, annealing at 58˚C for 1 min and extension
at 72˚C for 30 sec. Relative gene expression levels were quantified
using the 2-ΔΔCq method (19) and were normalized to GAPDH. The
following primers were used for the qPCR: C5a forward,
5'-ATTGGGAAGGCTACACATGA-3' and reverse, 5'-TGCCTTGACAGTATCAGCAA-3';
C5aR forward, 5'-GAGCCCAGGAGACCAGAACATG-3' and reverse,
5'-TACATGTTGAGCAGGATGAGGGA-3'; and GAPDH forward,
5'-TGTGTCCGTCGTGGATCTGA-3' and reverse,
5'-CCTGCTTCACCACCTTCTTGA-3'.
Western blotting
Cells were digested with trypsin and total protein
was extracted from cells using RIPA lysis buffer (Beyotime
Institute of Biotechnology). The protein concentration was measured
with a bicinchoninic acid protein (BCA) assay kit (Beyotime
Institute of Biotechnology). Total protein was adjusted to a
concentration of 1 mg/ml and 10 µl protein/lane was separated via
SDS-PAGE on 12% gel for 90 min, then transferred to PVDF membranes
for 90 min. The membranes were subsequently blocked with 5% skimmed
milk for 2 h at room temperature, and after washing thrice with
Tris-buffered saline Tween-20 (0.1%, TBST), the membranes were
incubated with the following antibodies at 4˚C overnight: Anti-C5a
(1:1,000; cat. no. sc-52636; Santa Cruz Biotechnology, Inc.),
anti-C5aR (1:1,000; cat. no. sc-53797; Santa Cruz Biotechnology,
Inc.), anti-p53 (1:1,000; cat. no. ab26; Abcam), anti-p21 (1:1,000;
cat. no. ab109520; Abcam), anti-p16 (1:1,000; cat. no. ab51243;
Abcam), anti-insulin-like growth factor-binding protein 3 (IGFBP3;
1:1,000; cat. no. ab220429; Abcam), anti-IFN-γ (1:1,000; cat. no.
sc-12755; Santa Cruz Biotechnology, Inc.), anti-TNF-α (1:1,000;
cat. no. sc-12744; Santa Cruz Biotechnology, Inc.) and anti-GAPDH
(1:1,000; cat. no. sc-47724; Santa Cruz Biotechnology, Inc.).
Following incubation with the primary antibody, the membranes were
washed thrice with TBST and incubated with horseradish
peroxidase-conjugated anti-mouse IgG (1:2,000; cat. no. 7076; Cell
Signaling Technology, Inc.) or anti-rabbit IgG (1:2,000; cat. no.
7074; Cell Signaling Technology, Inc.) secondary antibodies for 1 h
at room temperature. Protein bands were visualized using a
FluorChem E system (Hot Technology Co., Ltd.) after the membranes
were washed thrice with TBST. The protein bands were visualized by
luminescent reagents (Santa Cruz Biotechnology, Inc.) and analyzed
using ImageJ software (version 1.43; National Institutes of
Health).
Reactive oxygen species (ROS)
assay
ROS levels were analyzed using a ROS Assay kit
(Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. Briefly, a total of H9c2 or AC16
1x104 cells/well were seeded into 24-well sterile
culture plates and treated with 0.1 µM DOX and/or 2.5 µg/ml C5aRA
for 24 h at 37˚C. Cells were subsequently washed thrice with
phosphate buffered saline (PBS; cat no. C0221A; Beyotime Institute
of Biotechnology) to remove any drug that did not enter the cells.
Finally, a total of 1x103 cells per three fields of view
were imaged using a fluorescence microscope under x20
magnification.
ELISA
The concentrations of TNF-α and IFN-γ secreted into
the cell culture media were measured using ELISA kits (cat nos.
PT518 and PI511; Beyotime Institute of Biotechnology), according to
the manufacturer's protocols. The absorbance was measured at a
wavelength of 450 nm on a FlexStation 3 Multi-Mode microplate
reader (Molecular Devices, LLC). Experiments were repeated in
triplicate.
Senescence-associated β-galactosidase
(SA-β-Gal) staining
SA-β-Gal staining was performed using a SA-β-Gal
Staining kit (cat no. C0602; Beyotime Institute of Biotechnology)
according to the manufacturer's protocol. Briefly, H9c2 and AC16
cardiomyocytes were fixed with 4% formaldehyde at room temperature
for 15 min and then washed three times with PBS for 3 mins each
time. Subsequently, the cells were incubated with freshly prepared
SA-β-gal staining solution overnight at 37˚C without
CO2. Stained cells were visualized using an inverted
microscope (magnification, x200; Olympus Corporation).
Measurement of relative telomere
length
Quantification of the relevant telomere lengths in
H9c2 and AC16 cardiomyocytes was performed using qPCR according to
a previously described method (20). GAPDH was used as the control gene
for normalization. The primer pairs used to measure the telomere
length were as follows: Forward
5'-GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGA-3' and reverse
5'-TCCCGACTATCCCTATCCCTATCCCTATCCCTATCC-3'.
Measurement of relative telomerase
activity
The telomerase activity in H9c2 and AC16
cardiomyocytes was analyzed using the TeloTAGGG™ Telomerase PCR
ELISA PLUS kit (cat no. 11854666910; Roche Applied Science),
according to the manufacturer's protocol. Briefly, cell lysates
were centrifuged at 4˚C and 12,000 x g for 20 min, then 3 µl
inactivated cell lysate was used to amplify each telomere
replicate. Inactivated cell lysates were used for the telomeric
repeat amplification protocol (TRAP) reaction, according to the
manufacturer's protocol (Roche Applied Science). An internal
control from the kit was used along with each TRAP reaction to
verify the lack of the presence of any PCR inhibitors.
Amplification products were fixed in microtiter plates labeled with
streptavidin via biotin-streptavidin interactions, and ELISA was
performed by incubation with peroxidase-conjugated anti-dioxin
antibodies for 30 min at 25˚C (100 µl working solution; provided in
the kit). After addition of the peroxidase substrate
(3,3',5,5'-tetramethylbenzidine), the amount of TRAP product was
determined by measuring the absorbance at a wavelength of 450 nm
using a microplate reader.
Statistical analysis
Data are presented as the mean ± standard deviation
from three independent experiments. Statistical differences between
more than two groups were analyzed using one-way ANOVA followed by
a Tukey's multiple comparisons test. Comparisons between two groups
were performed using a paired Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Statistical analysis was performed using GraphPad Prism 6.0
software (GraphPad Software, Inc.).
Results
C5a and C5aR expression levels are
upregulated in DOX-induced H9c2 and AC16 cardiomyocytes
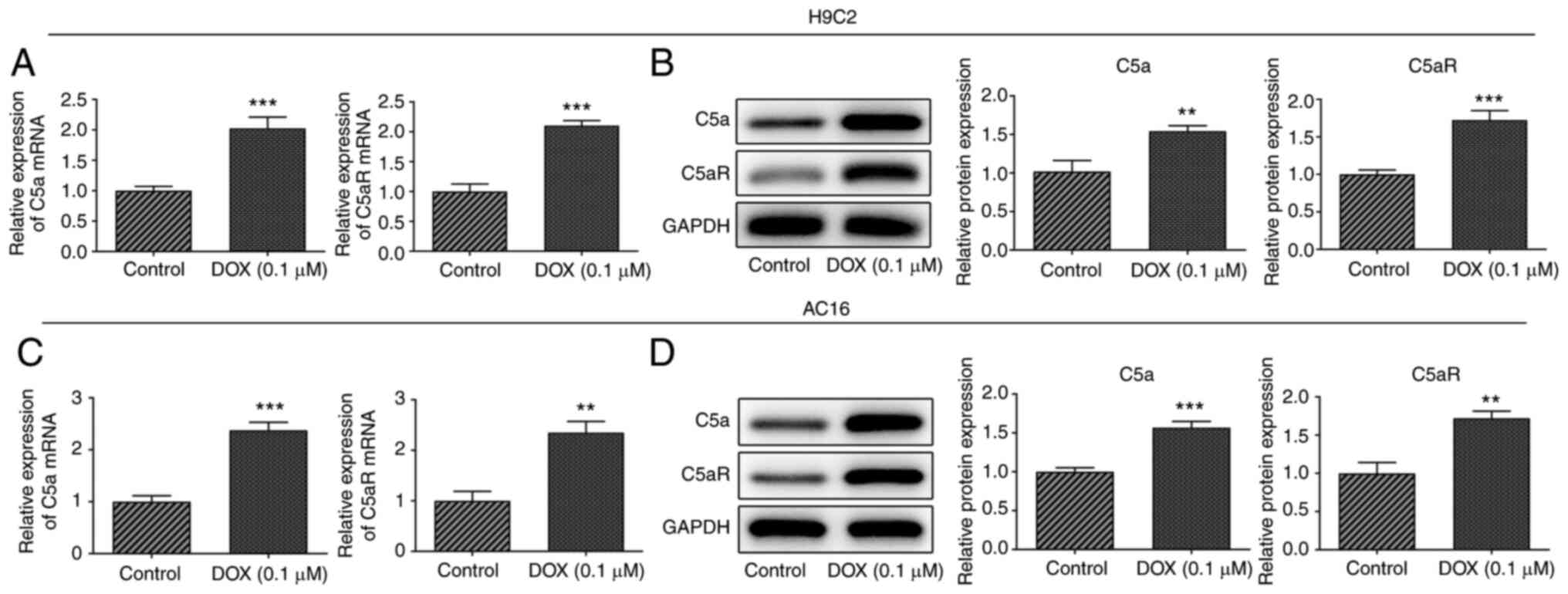
The expression levels of C5a and C5aR in H9c2 rat
embryonic cardiomyocytes and AC16 human cardiomyocyte-like cells
following exposure to 0.1 µmol/l DOX were analyzed using RT-qPCR
analysis and western blotting. The results revealed that DOX
treatment significantly upregulated the mRNA and protein expression
levels of C5a and C5aR in both H9c2 and AC16 cardiomyocytes
(Fig. 1). These results indicated
that C5a and C5aR may play a role in DOX-induced
cardiomyocytes.
Ca5RA inhibits the DOX-induced
upregulation of TNF-α and IFN-γ expression and increases ROS levels
in H9c2 and AC16 cardiomyocytes
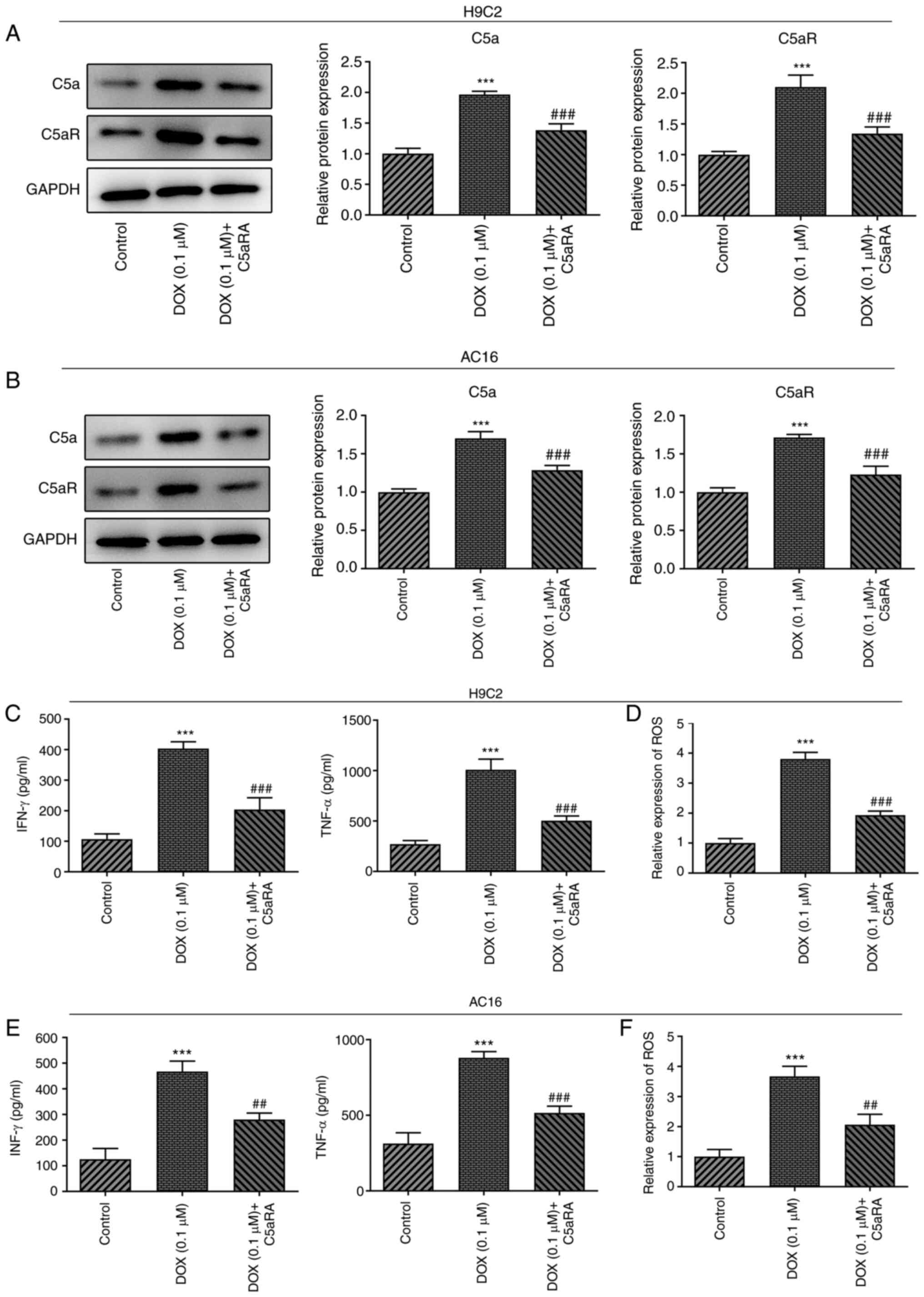
To determine the function of C5a and C5aR in
DOX-induced cardiomyocytes, H9c2 and AC16 cardiomyocytes were
treated with C5aRA and DOX. The protein and mRNA expression levels
of C5a and C5aR were downregulated following co-treatment with
C5aRA and DOX compared with the DOX group (Fig. 2A and B). Furthermore, the expression levels of
TNF-α and IFN-γ and the levels of ROS were significantly increased
in DOX-treated H9c2 and AC16 cardiomyocytes compared with the
control group, whereas treatment with C5aRA effectively reduced the
DOX-induced effects (Fig. 2C-F).
These results suggested that C5aRA may inhibit inflammation in
DOX-induced cardiomyocytes.
C5aRA protects H9c2 and AC16
cardiomyocytes against DOX-induced senescence
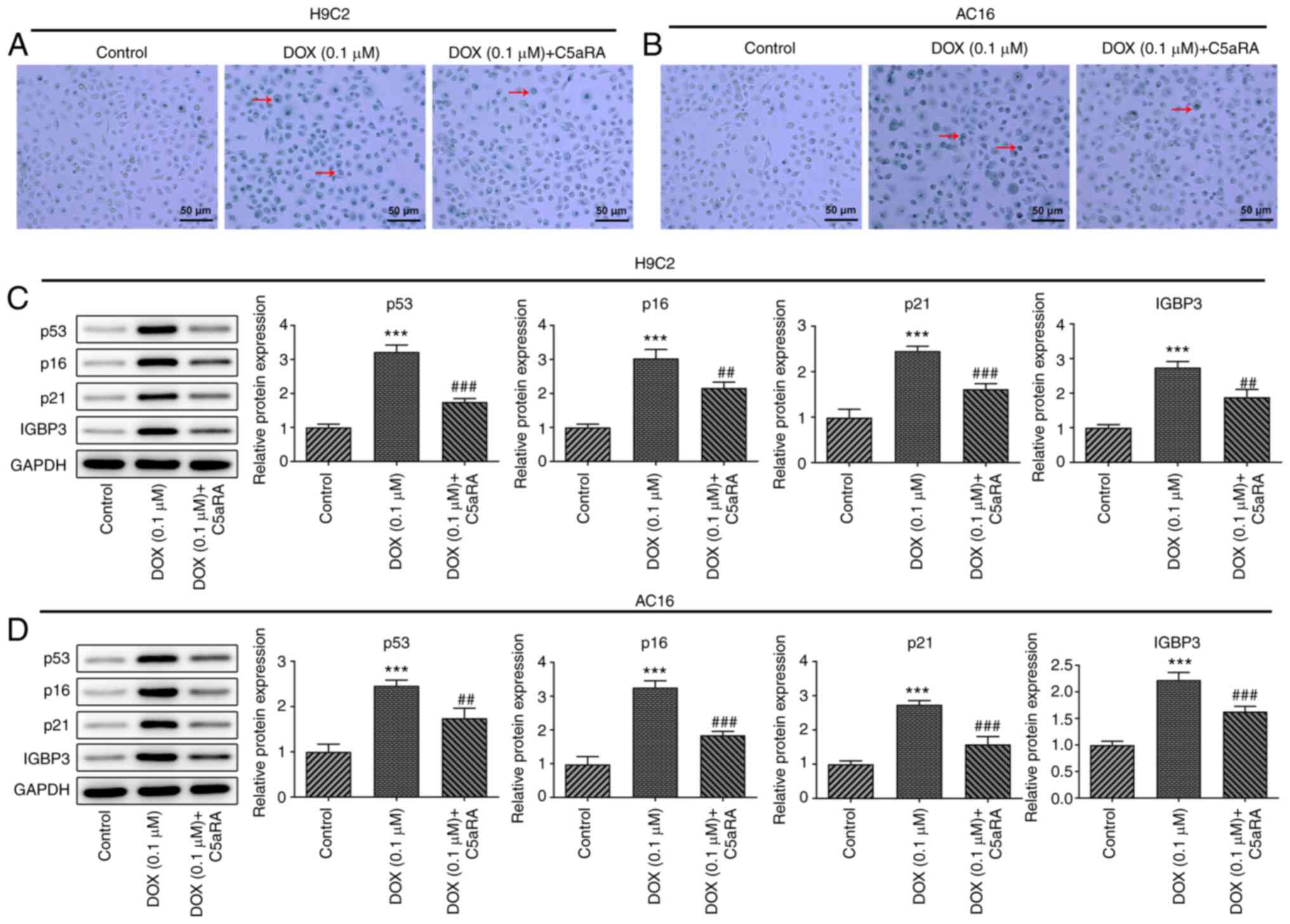
To determine the roles of C5a and C5aR in
DOX-induced cardiomyocyte senescence, SA-β-gal staining was
performed to assess cell aging. The treatment of H9c2 and AC16
cardiomyocytes with DOX significantly increased the percentage of
senescent cells, while C5aRA treatment significantly reversed this
effect (Fig. 3A and B). In addition, the protein expression
levels of p53, p16, p21 and IGFBP3 were upregulated in DOX-treated
H9c2 and AC16 cardiomyocytes compared with the control group, while
pretreatment with C5aRA antagonized the effects induced by DOX
(Fig. 3C and D). These results suggested that C5aRA may
protect H9c2 and AC16 cardiomyocytes against DOX-induced
senescence.
C5aRA inhibits the DOX-induced
reduction in telomere length and telomerase activity in H9c2 and
AC16 cardiomyocytes
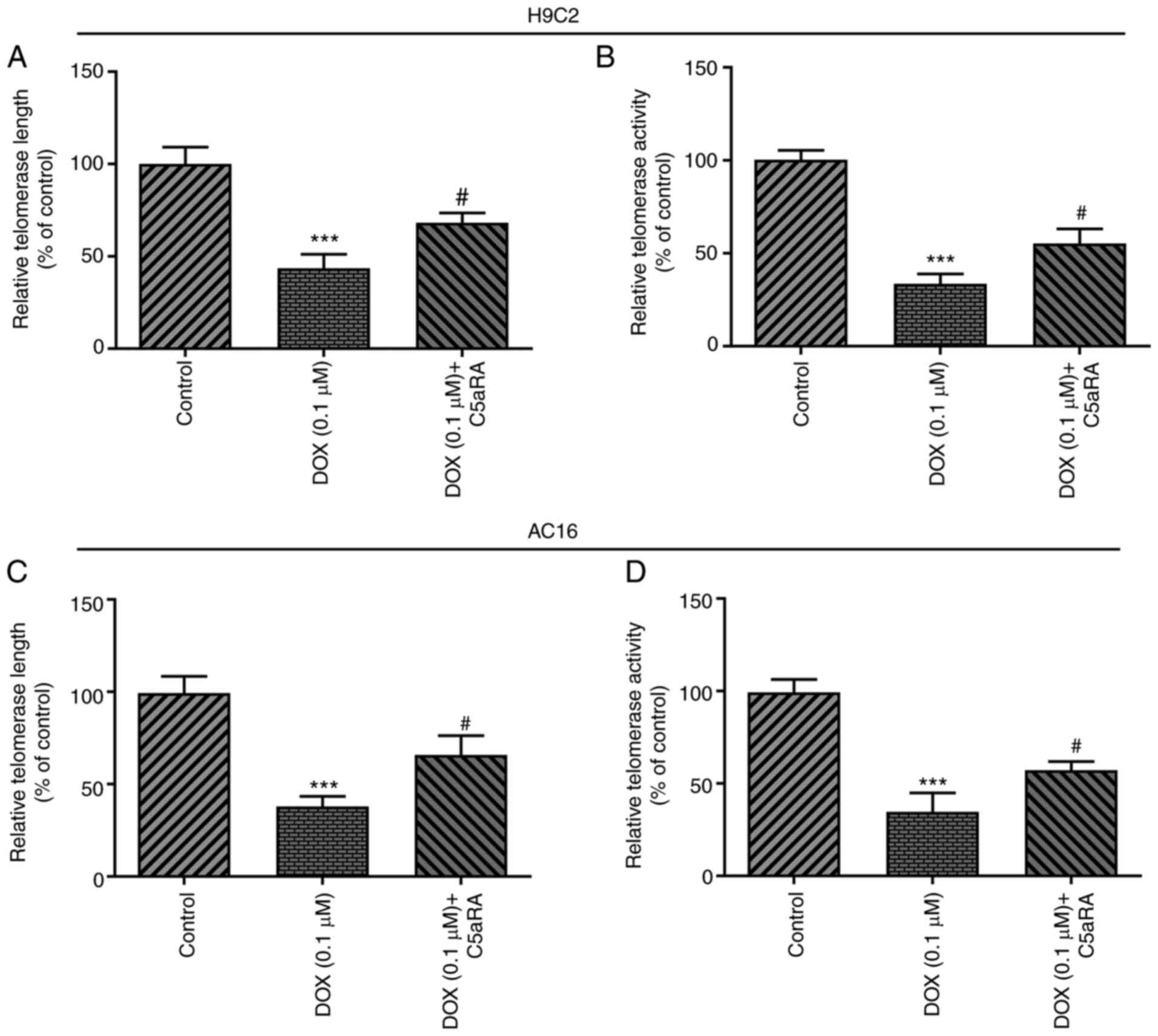
To further validate the role of C5a and C5aR in
DOX-induced cardiomyocyte senescence, both the telomere length and
telomerase activity were measured in cardiomyocytes. Changes in
telomere length and telomerase activity have been suggested to be
potential biomarkers of aging (21). As shown in Fig. 4, C5aRA treatment markedly increased
the telomere length and telomerase activity, whereas the telomerase
activity was decreased in both H9c2 and AC16 cardiomyocytes
following exposure to DOX. These results indicated that DOX
treatment may induce telomere shortening and decrease telomerase
activity, which may be alleviated by treatment with C5aRA.
Discussion
DOX is a widely used and potent anticancer agent;
however, dose-dependent DOX-induced cardiotoxicity significantly
limits its use in clinical practice (22). DOX was discovered to induce a
senescent-like phenotype in cardiomyocytes and DOX-induced
cytotoxicity may promote the progression of cellular senescence
(23). Thus, there is great value
in identifying effective therapies for suppressing cardiomyocyte
senescence in order to prevent DOX-induced cardiotoxicity.
Components of the complement system have emerged as
crucial mediators of tumor progression (24). Products of complement activation are
known to maintain long-term inflammation, facilitate an
immunosuppressive microenvironment, induce angiogenesis and enhance
the migratory and metastatic potential of cancer cells (25). Recent in-depth studies on the role
of C5a in cancer have provided novel insight into the potential
development of biomarkers and therapeutic approaches targeting C5a.
C5a was found to play a crucial role in the suppression of joint
and skin autoimmune inflammation mediated by neutrophils (26). The inhibition of C5aR was also
reported to be a substitute for the use of oral glucocorticoids, as
C5a was identified to be an important inflammatory mediator in
anti-neutrophil cytoplasm antibody-associated vascular inflammation
(27). Thus, the role of C5a and
C5aR in cardiomyocyte senescence was further investigated in the
present study. The findings demonstrated that the expression levels
of C5a and C5aR were upregulated in both H9c2 and AC16
cardiomyocytes following DOX stimulation. Subsequently, the effects
of C5a and C5aR on cardiomyocyte senescence were determined.
C5aRA blocks the binding of the C5a antitoxin with
its receptor, C5aR, and was discovered to be one of the most
effective agents in the treatment of various autoimmune diseases
and acute inflammatory conditions (28,29). A
previous study suggested that inhibition of C5aR may hold promise
as a therapeutic strategy for preventing organ injury in
angiotensin II-induced hypertension (30). The results of the present study
revealed that treatment of cardiomyocytes with C5aRA downregulated
the expression levels of C5a and C5aR following DOX treatment. In
addition, the increased levels of inflammatory factors and ROS
induced by DOX stimulation were also reduced following treatment
with C5aRA.
The presence of low-grade inflammation has been
recognized as a defining characteristic of aging (31). In the present study, two different
characteristics of aging (telomere shortening and reduced
telomerase activity) were further analyzed. The results
demonstrated that DOX treatment upregulated the levels of SA-β-gal,
the expression levels of the cell cycle inhibitor proteins, p53,
p16 and p21, and the expression levels of IGFBP3. These effects
were reversed following the co-culture with C5aRA. Telomere
shortening and reduced telomerase activity in humans were also
identified to be predictive markers of aging and disease (32-34).
Thus, the telomere length and telomerase activity in cardiomyocytes
were analyzed in the present study. The results revealed that
treatment with Ca5RA suppressed the DOX-induced decrease in
telomere length and telomerase activity in cardiomyocytes.
In conclusion, the findings of the present study
revealed that the expression levels of C5a and C5aR were
upregulated in DOX-induced cardiomyocytes, while treatment with
C5aRA inhibited DOX-induced cardiomyocyte senescence. These
findings suggest that C5a and C5aR may play a key role in
DOX-induced cardiomyocyte senescence and provide a theoretical
basis for future clinical applications. C5aRA treatment may be an
effective method for protecting cardiomyocytes against DOX-induced
senescence and may prove to be of value as a cardioprotective
agent. However, a limitation of the present study is the lack of
further validation in animal experiments and clinical samples,
which will addressed in future studies.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Key Discipline
Construction Project of Shenzhen Municipal Health Commission (grant
no. SZXJ2018007).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW designed the research study. YW and FS performed
the research and analyzed the data. FS wrote the main manuscript
text. All authors contributed to revising the manuscript. All
authors have read and approved the final manuscript. All authors
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of
interest.
References
|
1
|
Xu T, Ding W, Ji X, Ao X, Liu Y, Yu W and
Wang J: Oxidative stress in cell death and cardiovascular diseases.
Oxid Med Cell Longev. 2019(9030563)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tang X, Li PH and Chen HZ: Cardiomyocyte
senescence and cellular communications within myocardial
microenvironments. Front Endocrinol (Lausanne).
11(280)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hernandez-Segura A, Nehme J and Demaria M:
Hallmarks of cellular senescence. Trends Cell Biol. 28:436–453.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu D, Ma Z, Di S, Yang Y, Yang J, Xu L,
Reiter RJ, Qiao S and Yuan J: AMPK/PGC1α activation by melatonin
attenuates acute doxorubicin cardiotoxicity via alleviating
mitochondrial oxidative damage and apoptosis. Free Radic Biol Med.
129:59–72. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang YW, Shi J, Li YJ and Wei L:
Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch
Immunol Ther Exp (Warsz). 57:435–445. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Singh P, Sharma R, McElhanon K, Allen CD,
Megyesi JK, Beneš H and Singh SP: Sulforaphane protects the heart
from doxorubicin-induced toxicity. Free Radic Biol Med. 86:90–101.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Maejima Y, Adachi S, Ito H, Hirao K and
Isobe M: Induction of premature senescence in cardiomyocytes by
doxorubicin as a novel mechanism of myocardial damage. Aging Cell.
7:125–136. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Guo RF and Ward PA: Role of C5a in
inflammatory responses. Annu Rev Immunol. 23:821–852.
2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Phieler J, Chung KJ, Chatzigeorgiou A,
Klotzsche-von Ameln A, Garcia-Martin R, Sprott D, Moisidou M,
Tzanavari T, Ludwig B, Baraban E, et al: The complement
anaphylatoxin C5a receptor contributes to obese adipose tissue
inflammation and insulin resistance. J Immunol. 191:4367–4374.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jiang Y, Zhao G, Song N, Li P, Chen Y, Guo
Y, Li J, Du L, Jiang S, Guo R, et al: Blockade of the C5a-C5aR axis
alleviates lung damage in hDPP4-transgenic mice infected with
MERS-CoV. Emerg Microbes Infect. 7(77)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yu S, Wang D, Huang L, Zhang Y, Luo R,
Adah D, Tang Y, Zhao K and Lu B: The complement receptor C5aR2
promotes protein kinase R expression and contributes to NLRP3
inflammasome activation and HMGB1 release from macrophages. J Biol
Chem. 294:8384–8394. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Merle NS, Grunenwald A, Rajaratnam H,
Gnemmi V, Frimat M, Figueres ML, Knockaert S, Bouzekri S, Charue D,
Noe R, et al: Intravascular hemolysis activates complement via
cell-free heme and heme-loaded microvesicles. JCI Insight.
3(e96910)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vlaicu SI, Tatomir A, Boodhoo D, Vesa S,
Mircea PA and Rus H: The role of complement system in adipose
tissue-related inflammation. Immunol Res. 64:653–664.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Martin IV, Bohner A, Boor P, Shagdarsuren
E, Raffetseder U, Lammert F, Floege J, Ostendorf T and Weber SN:
Complement C5a receptors C5L2 and C5aR in renal fibrosis. Am J
Physiol Renal Physiol. 314:F35–F46. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Brennan FH, Gordon R, Lao HW, Biggins PJ,
Taylor SM, Franklin RJ, Woodruff TM and Ruitenberg MJ: The
Complement receptor C5aR controls acute inflammation and
astrogliosis following spinal cord injury. J Neurosci.
35:6517–6531. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Leaker BR, Malkov VA, Mogg R, Ruddy MK,
Nicholson GC, Tan AJ, Tribouley C, Chen G, De Lepeleire I, Calder
NA, et al: The nasal mucosal late allergic reaction to grass pollen
involves type 2 inflammation (IL-5 and IL-13), the inflammasome
(IL-1β), and complement. Mucosal Immunol. 10:408–420.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang B, Wang J, Zhao Y, Duan W, Dai C, Han
Z, Wang M, Zhang B, Wei L, Chen Z and Chen D: Attenuating
ischemia/reperfusion injury in rat cardiac transplantation by
intracoronary infusion with siRNA cocktail solution. Biosci Rep.
40(BSR20193937)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang XL, Ji XT, Sun B, Qian LL, Hu XL,
Lou HX and Yuan HQ: Anti-cancer effect of marchantin C via inducing
lung cancer cellular senescence associated with less secretory
phenotype. Biochim Biophys Acta Gen Subj. 1863:1443–1457.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cawthon RM: Telomere length measurement by
a novel monochrome multiplex quantitative PCR method. Nucleic Acids
Res. 37(e21)2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tedone E, Huang E, O'Hara R, Batten K,
Ludlow AT, Lai TP, Arosio B, Mari D, Wright WE and Shay JW:
Telomere length and telomerase activity in T cells are biomarkers
of high-performing centenarians. Aging Cell.
18(e12859)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gupta SK, Garg A, Bär C, Chatterjee S,
Foinquinos A, Milting H, Streckfuß-Bömeke K, Fiedler J and Thum T:
Quaking inhibits doxorubicin-mediated cardiotoxicity through
regulation of cardiac circular RNA expression. Circ Res.
122:246–254. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Huang PP, Fu J, Liu LH, Wu KF, Liu HX, Qi
BM, Liu Y and Qi BL: Honokiol antagonizes doxorubicin-induced
cardiomyocyte senescence by inhibiting TXNIP-mediated NLRP3
inflammasome activation. Int J Mol Med. 45:186–194. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Afshar-Kharghan V: The role of the
complement system in cancer. J Clin Invest. 127:780–789.
2017.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Ajona D, Ortiz-Espinosa S and Pio R:
Complement anaphylatoxins C3a and C5a: Emerging roles in cancer
progression and treatment. Semin Cell Dev Biol. 85:153–163.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sadik CD, Miyabe Y, Sezin T and Luster AD:
The critical role of C5a as an initiator of neutrophil-mediated
autoimmune inflammation of the joint and skin. Semin Immunol.
37:21–29. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jayne DRW, Bruchfeld AN, Harper L, Schaier
M, Venning MC, Hamilton P, Burst V, Grundmann F, Jadoul M, Szombati
I, et al: Randomized trial of C5a receptor inhibitor avacopan in
ANCA-associated vasculitis. J Am Soc Nephrol. 28:2756–2767.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Syed SN, Rau E, Ziegelmann M, Sogkas G,
Brune B and Schmidt RE: C5aR activation in the absence of C5a: A
new disease mechanism of autoimmune hemolytic anemia in mice. Eur J
Immunol. 48:696–704. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tang M, Zhang K, Li Y, He QH, Li GQ, Zheng
QY and Zhang KQ: Mesenchymal stem cells alleviate acute kidney
injury by down-regulating C5a/C5aR pathway activation. Int Urol
Nephrol. 50:1545–1553. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang C, Li Y, Wang C, Wu Y and Du J:
Antagonist of C5aR prevents cardiac remodeling in angiotensin
II-induced hypertension. Am J Hypertens. 27:857–864.
2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Minguzzi M, Cetrullo S, D'Adamo S,
Silvestri Y, Flamigni F and Borzi RM: Emerging players at the
intersection of chondrocyte loss of maturational arrest, oxidative
stress, senescence and low-grade inflammation in osteoarthritis.
Oxid Med Cell Longev. 2018(3075293)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ornish D, Lin J, Chan JM, Epel E, Kemp C,
Weidner G, Marlin R, Frenda SJ, Magbanua MJM, Daubenmier J, et al:
Effect of comprehensive lifestyle changes on telomerase activity
and telomere length in men with biopsy-proven low-risk prostate
cancer: 5-year follow-up of a descriptive pilot study. Lancet
Oncol. 14:1112–1120. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Louzon M, Coeurdassier M, Gimbert F,
Pauget B and de Vaufleury A: Telomere dynamic in humans and
animals: Review and perspectives in environmental toxicology.
Environ Int. 131(105025)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Victorelli S, Lagnado A, Halim J, Moore W,
Talbot D, Barrett K, Chapman J, Birch J, Ogrodnik M, Meves A, et
al: Senescent human melanocytes drive skin ageing via paracrine
telomere dysfunction. EMBO J. 38(e101982)2019.PubMed/NCBI View Article : Google Scholar
|