Introduction
Marked progress has been made in the management of
non-varicose upper gastrointestinal bleeding (UGIB) due to the
introduction of drugs that decrease gastric acid secretion,
Helicobacter pylori (H. pylori) eradication
therapies, and improved endoscopic hemostasis, yet, the mortality
rate has remained relatively constant (1). H. pylori induces chronic
superficial gastritis with neutrophil infiltration into the mucosa,
therefore, it has been speculated that H. pylori infection
underlies the bleeding lesion (2).
Gastric and duodenal ulcers are strongly related to H.
pylori (2). In initial reports
around the world, in the first decade after the discovery of H.
pylori, ~95% of duodenal ulcers and 85% of gastric ulcers were
associated with H. pylori infection (3), and eradication of H. pylori
changed the natural course of ulcer disease and almost completely
prevented the recurrence of the ulcer (4). Hemorrhage is the most common
complication of ulcer disease and is estimated to be present in
15-20% of ulcers. Approximately 40% of patients with UGIB have a
hemorrhagic ulcer, and ulcerative disease is therefore the leading
cause of upper gastrointestinal hemorrhage (1). Eradication of H. pylori greatly
reduces the risk of ulcer and also bleeding in those patients whose
hemorrhage was due to H. pylori infection (4). Mortality varies between 3 and 14% and
has not changed in the last 10 years (1,4).
Mortality increases with age and is significantly higher in
patients who are already hospitalized for associated comorbidities.
Risk factors for bleeding in peptic ulcer are the administration of
non-steroidal anti-inflammatory drugs (NSAIDs), oral anticoagulants
and H. pylori infection. H. pylori infection
increases the risk of UGIB by 1.7 times, but the exact influence of
H. pylori in the evolution of UGIB (spontaneous hemostasis
or to surgery) is not known (5,6).
The present study aimed to analyze the factors of
care influencing the evolution of non-variceal UGIB dating some
lesions caused by H. pylori infection, as well as to assess
the relationship between bacteria and alcohol consumption, NSAIDs
and oral antiplatelet/anticoagulated agents in triggering lesion
hemorrhage.
Patients and methods
The study included 166 patients with clinical signs
of UGIB, hospitalized in the ATI Clinic, Gastroenterology Clinic or
in the Surgery II Clinic of the County Emergency Clinical Hospital
(SCJU) in Craiova, Romania between 2017 and 2019 (3 years). The
diagnosis of non-variceal UGIB was established by objective
clinical examination and endoscopic examination, and the diagnosis
of H. pylori infection was made by noninvasive tests and by
histological and immunohistochemical examination (IHC). Testing for
H. pylori was conducted in most patients with signs of UGIB
after stopping the bleeding episode. Patients were tested for H.
pylori using fecal antigen detection (after melena termination,
normal-looking stool) and by highlighting this bacterium by
specific histological staining (performed from gastric endobiopsies
or surgery). Biopsy was collected during the first endoscopy, after
hemostasis therapy, in patients without massive bleeding and
without the diagnosis of H. pylori infection prior to the
hemorrhagic episode. The biopsy was obtained from the gastric
mucosa from 5 different sites, according to the Updated Sydney
System (7): 2 from the antrum, 2
from the mucosa of the gastric body and one from the gastric
incision. In the case of suspicious or certain lesions for
malignant lesions, several biopsies were taken from this level. Two
types of PENTAX EG-290 and EVIS EXERA III Olympus endoscopy devices
were used for endoscopic therapy and biopsy collection. The bioptic
samples were processed in the Pathological Anatomy Laboratory of
the SCJU in Craiova and in the Center for Studies of Microscopic
Morphology and Immunology within the University of Medicine and
Pharmacy in Craiova, Romania. The stains used to highlight the
bacterium were hematoxylin and eosin and Giemsa. IHC has superior
accuracy in highlighting H. pylori from gastric biopsy
collected from patients with non-variceal UGIB who were both
positive and negative on the noninvasive test.
Histological evaluation of endobiopsies using common
stains is considered the ‘gold standard’ in identifying this
bacterium. The samples obtained were collected in containers with
formalin solution and were processed according to the standard
technique of paraffin inclusion, following the following steps:
fixation in 10% buffered formalin, washing with water or 80%
alcohol, dehydration (by successive alcohol baths), clarification
(by baths of benzene, toluene, xylene and paraffin). The final
results were obtained after an interval of 20-25 days from the
sampling of the biopsy fragments. At 30 days after discharge,
patients were informed of the histological and IHC results
performed from biopsies collected at endoscopic evaluation or by
open surgery (in cases where hemostasis was obtained surgically).
Another purpose for the introduction of routine IHC was related to
the evaluation of the value of this protocol in the diagnosis of
subsequent or associated lesions: bleeding: acute/chronic
gastritis, metaplasia, cancer or gastric lymphoma. Although
histological examination is accurate in providing data on the
degree of atrophy, metaphase, or carcinoma, the addition of IHC
examination provides additional information and establishes the
correct diagnosis in all cases. The use of IHC was available: the
LSAB (HRP) method [labeled streptavidin biotin (LSAB) and
horseradish peroxidase (HRP)] and anti-H. pylori antibodies
were used.
The evolution of patients was analyzed in terms of:
bleeding rate, the presence of other risk factors, the need for
surgical treatment, and the length of hospitalization (influenced
by surgery). Eradication of H. pylori infection was defined
as negative results for the fecal antigen testing after specific
therapy. We decided to retest for H. pylori for all patients
tested, regardless of whether the initial result was positive or
negative. Retesting was performed after discontinuation of proton
pump inhibitors. Testing for H. pylori was performed in
patients with clinical signs of non-variceal UGIB without a
previous history of bleeding.
Results
Testing for H. pylori was consistently
positive in both the noninvasive test and the histological
evaluation, respectively IHC. After histological evaluation and
IHC, of the 166 patients tested for H. pylori and studied,
group A consisted of 96 H. pylori-positive patients and
group B of 70 H. pylori-negative patients. At 30 days after
discharge, all patients were retested. Of the 76 initially negative
patients, 70 (92.1%) remained negative, and 6 patients (7.9%)
became positive (histological examination and IHC confirmed their
positivity for H. pylori). Of the 96 initially positive
patients who received specific treatment, 20 remained positive and
70 patients became negative.
Treatment of H. pylori infection was
initiated during hospitalization, immediately after the bleeding
stopped and continued in the outpatient setting. All patients
received treatment with omeprazole 20 mg twice daily, amoxicillin 1
g twice daily and clarithromycin 500 mg twice daily. We decided on
this treatment scheme for several reasons: i) clarithromycin and
amoxicillin have a low rate of prescription in this area and thus
there is a low rate of resistance; ii) it is an easy treatment
scheme with minimal side effects; and iv) is easy to administer. In
order to prevent possible treatment discontinuation, patients were
asked about the side effects of the therapy and we were assured of
the correct follow-up of this treatment. Evaluation of resistance
to first-line therapy of H. pylori was performed using the
non-invasive test used at diagnosis (detection of specific antigen
in feces). All patients resistant to first-line therapy (22% of
total) were re-evaluated endoscopically and received second-line
therapy.
The second non-invasive test provided information on
identifying 6 more patients who at the first test were
false-negative as well as data on the resistance of the bacterium
in the cases initially treated. We also included in the study 4
patients with a history of gastric resection for gastric ulcer.
Endoscopic evaluation revealed acute anastomotic ulcer and
endoscopic hemostasis was effective.
There was an increased frequency of hemorrhagic
lesions in H. pylori-positive men compared to women with the
same lesions (84.04 vs. 15.96%) In the H. pylori-negative
group, there was also a male prevalence, but with a lower M:F sex
ratio (71.83 vs. 29.17%).
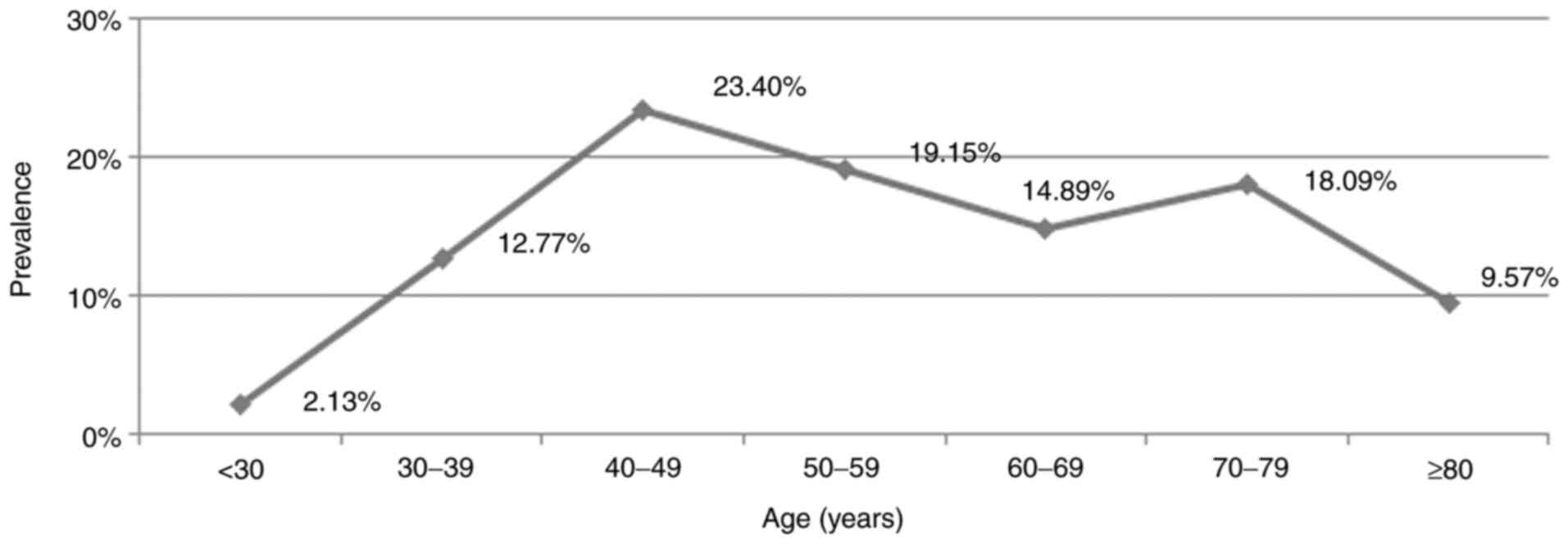
The presence of H. pylori was increased in
the studied group directly proportional to age ≤50 years, and then
decreased slightly in elder age. The most frequent hemorrhagic
lesions caused by H. pylori were in the age segment between
40 and 50 years. In this age segment, >23% of non-variceal UGIB
were directly related to the presence of H. pylori (Fig. 1).
The relationship between NSAID use and H.
pylori infection in patients <50 years of age had a
cumulative effect in causing bleeding. Therefore, the main causes
of lesion bleeding were H. pylori infection and NSAID use.
After the age of 50 years, the main causes included H.
pylori infection and treatment with antiplatelet agents/oral
anticoagulants. In the group of H. pylori-positive patients,
56.5% of patients <50 years of age stated that they had consumed
NSAIDs for >5 days, compared to only 16.67% of H.
pylori-negative patients >50 years of age. Therapies with
antiplatelet agents or oral anticoagulants were the direct causes
of UGIB in 45% of patients with H. pylori-positive
gastro-duodenal lesions >50 years of age. The evolution of a
non-variceal UGIB can be significantly influenced by the value of
coagulation times (modifications of these therapies), at the same
time the transfusion requirement is higher in these patients,
regardless of the presence of H. pylori (Table I).
 | Table IRelationship between NSAID use and
age-related anticoagulants/antiplatelet agents in H.
pylori-positive patients. |
Table I
Relationship between NSAID use and
age-related anticoagulants/antiplatelet agents in H.
pylori-positive patients.
| Age (years) | <50 (%) | >50 (%) |
|---|
| NSAIDs | 56.52 | 16.67 |
| Anticoagulants | 4.35 | 45 |
| No treatment | 39.13 | 38.33 |
In elderly patients with associated comorbidities,
an episode of UGIB can be fatal. We analyzed the effect of NSAID
use associated with alcohol consumption in patients with
hemorrhagic lesions, depending on the presence of H. pylori
and found the following: 28.75% of H. pylori-positive
patients with active bleeding at endoscopic evaluation (Forrest
class IA and IB) were treated with NSAIDs and consumed alcohol
chronically compared to 12.50% of H. pylori-negative
patients (Table II).
 | Table IIDeterminants of bleeding depending on
the presence of H. pylori. |
Table II
Determinants of bleeding depending on
the presence of H. pylori.
| | H.
pylori-negative, with NSAID + alcohol (%) | H.
pylori-positive, without NSAID + alcohol (%) | H.
pylori-positive, with NSAID + alcohol (%) |
|---|
| Active bleeding
lesions | 12.5 | 14.29 | 28.75 |
| No active bleeding
lesions | 87.5 | 85.71 | 71.25 |
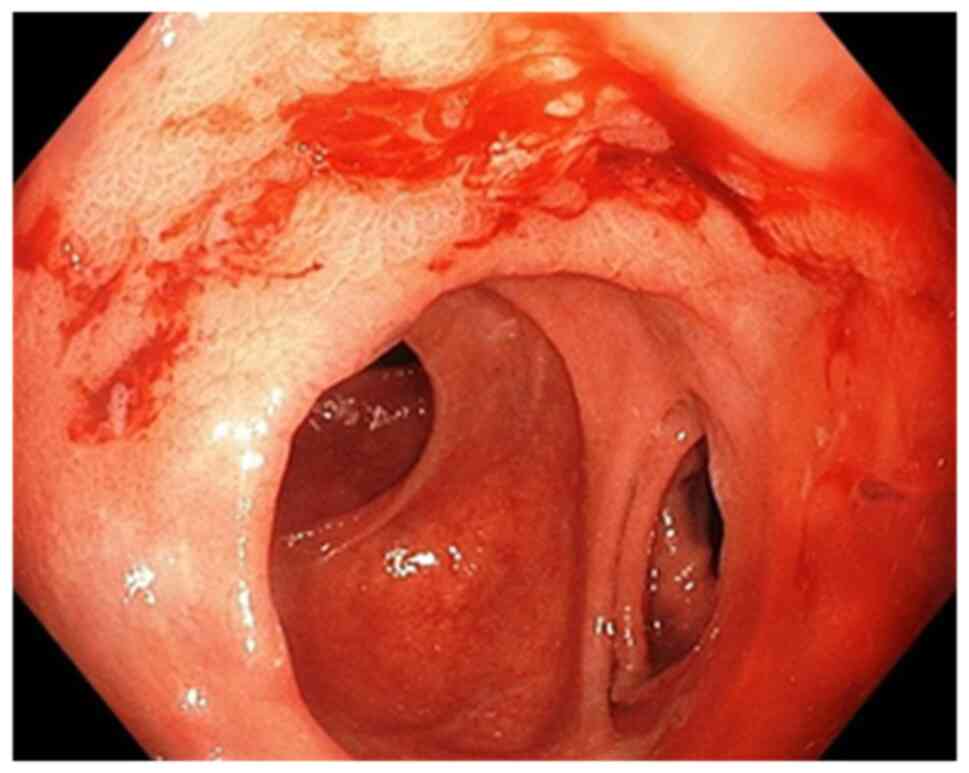
Endoscopic evaluation (early or late) was performed
in all patients tested for H. pylori who had hemorrhagic
lesions and we classified the gastric lesions found on endoscopy as
follows: active bleeding requiring endoscopic or surgical
hemostasis (endoscopic bleeding) and no active
bleeding-supervision. Lesions with active bleeding were classified
as follows: hemorrhagic gastroduodenitis (petechiae and erosions of
the gastroduodenal mucosa), acute gastric ulcer, chronic gastric
ulcer, acute duodenal ulcer, chronic duodenal ulcer and gastric
tumor. We also classified the lesions without active bleeding.
Endoscopic features have been reported to be useful tools for
diagnosing H. pylori infection in the gastric mucosa.
However, the ability to predict these characteristics can vary
greatly (8) (Table III).
 | Table IIIEndoscopic evaluation of H.
pylori-positive vs. H. pylori-negative patients. |
Table III
Endoscopic evaluation of H.
pylori-positive vs. H. pylori-negative patients.
| | H.
pylori-negative | H.
pylori-positive |
|---|
| Hemorrhagic acute
duodenal ulcer | 6 | 13 |
| Non-hemorrhagic
acute duodenal ulcer | 9 | 9 |
| Hemorrhagic chronic
duodenal ulcer | 11 | 6 |
| Hemorrhagic chronic
duodenal ulcer | 2 | 6 |
| Hemorrhagic acute
gastric ulcer | 9 | 17 |
| Non-hemorrhagic
acute gastric ulcer | 3 | 10 |
| Hemorrhagic chronic
gastric ulcer | 2 | 2 |
| Non-hemorrhagic
chronic gastric ulcer | 4 | 3 |
| Hemobilia | 1 | 1 |
|
Gastroduodenitis | 20 | 20 |
| Gastric tumor | 1 | 7 |
| Undetectable
source | 2 | 2 |
Acute hemorrhagic lesions (gastric, duodenal ulcer
or hemorrhagic gastroduodenitis) were detected more frequently in
the group of positive than negative H. pylori patients (69
vs. 43 cases). At the same time, the rate of acute lesions in the
stomach and duodenum was higher in H. pylori-positive
patients. Chronic hemorrhagic or non-hemorrhagic gastric ulcer was
found in similar proportions in both the negative and positive
patients. Hemorrhagic gastroduodenitis lesions were found in equal
proportions in the studied groups. Evaluation of patients with
active bleeding on endoscopic examination showed that the bleeding
rate was high in the H. pylori-positive patients who were
negative after specific therapy (18.18%) compared to those with
H. pylori-negative lesions (15.79%) and much higher in the
treatment-resistant patients (40%) (Fig. 2).
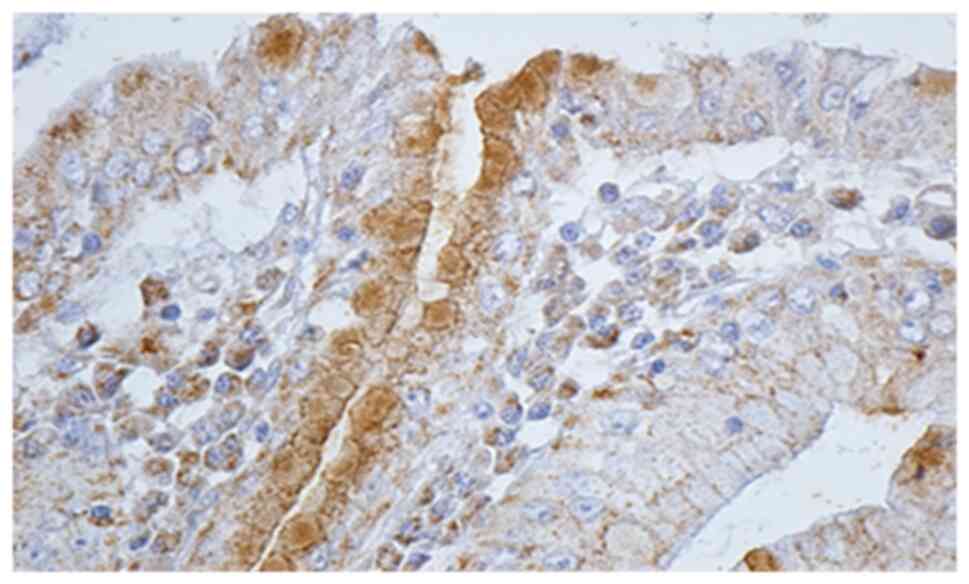
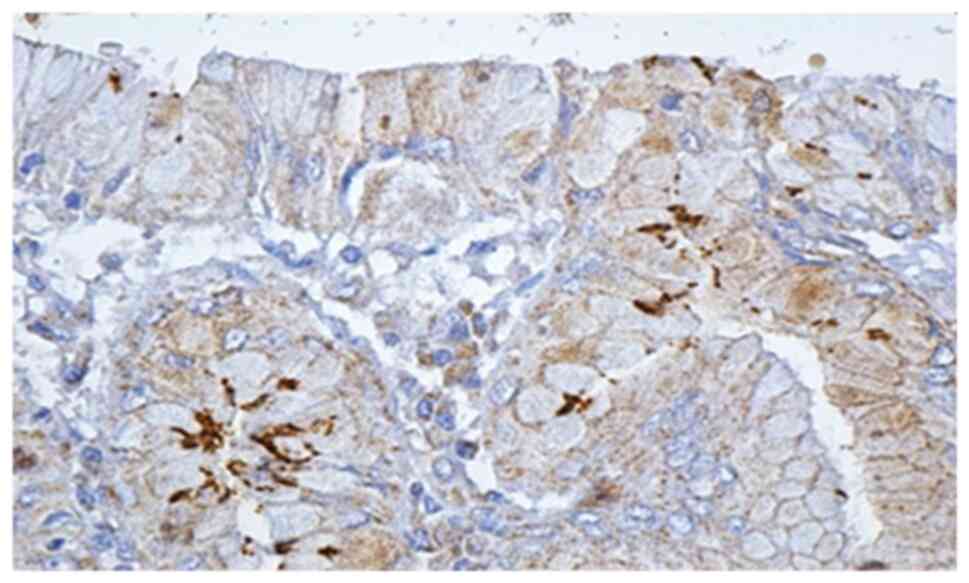
Histological and IHC methods represented the most
specific way of detecting this bacterium. In regards to all
patients for whom histological and noninvasive tests were positive,
IHC methods showed the presence of H. pylori. Moreover, IHC
detected 6 more cases in which the initial noninvasive tests were
negative, and the histological examination detected only 2 cases
out of 6 (Figs. 3 and 4).
In 2 of the 6 cases, the IHC assessment consisted of
metaplasia-associated lesions. In one case, the diagnosis of
gastric carcinoma was established by IHC, and in another, the
diagnosis of gastric mucosa-associated lymphoma (MALT) was
established. The patient with gastric carcinoma underwent surgical
operation and underwent a subtotal gastrectomy, and histological
reassessment confirmed the diagnosis of carcinoma. In the case of
MALT lymphoma, a total gastrectomy was performed, and the
histological evaluation and IHC were performed on specimens
directly from the resected piece.
Surgery for hemostasis was needed more frequently in
patients with H. pylori-positive UGIB than in H.
pylori-negative patients. Approximately 16% of patients with
non-variceal UGIB and positive H. pylori results required
surgery to stop the bleeding. In the group of H.
pylori-negative patients, the need for surgery was lower;
respectively 9.7% of patients underwent surgery (suturing or
resection). However, distant mortality is influenced by the
occurrence of bleeding, and in our study, in patients with
non-variceal UGIB due to an H. pylori-positive lesion, it
was lower compared to H. pylori-negative patients. We
considered this difference due to the strict supervision of the
therapy in the case of H. pylori-positive patients and due
to the information of the patients about their disease and its
evolution without treatment.
Discussion
In the present study, we found an increased
frequency of hemorrhagic lesions among men in the group of H.
pylori-positive patients compared to the group of H.
pylori-negative patients (85.04 vs. 70.83%). At the same time,
the frequency of hemorrhagic lesions among H.
pylori-negative women was higher than among the H.
pylori-positive women (29.17 vs. 15.96%). Consistent with these
data, Slăvescu et al conducted a study regarding the
prevalence of H. pylori infection in children in Romania,
and found a higher prevalence among girls (9). H. pylori infection seems to act
convergently with other factors, such as alcohol consumption,
NSAIDs, and smoking, in favor of triggering digestive bleeding.
Another hypothesis that supports these studies showed an increased
frequency of antibiotic resistance of H. pylori in men
compared to women, but dependent on the therapeutic regimen
followed (10). Antibiotic
resistance of H. pylori and poor medical education may also
be factors considered in H. pylori-positive patients who
have a history of gastric ulcer for which surgery has been
performed (11) The persistence of
H. pylori infection, despite gastric resections, still
predisposes to a hemorrhagic complication (12) According to several studies, H.
pylori infection in the remaining stomach appears to play an
important role in the development of gastric cancer (12-14)
even though the prevalence of H. pylori in the remaining
stomach appears to be lower than that in the intact organ (11).
In the present study, the prevalence of H.
pylori infection increased in direct proportion to age ≤50
years, and then a slight decrease was found in elderly patients.
The most common hemorrhagic lesions caused by H. pylori were
in the age segment between 40 and 50 years. Thus, in this age
segment, >23% of the patients with non-variceal UGIB had
gastroduodenal lesions that were directly influenced by the
presence of H. pylori. The prevalence of hemorrhagic
complications caused by H. pylori in the patients studied,
increased sharply in the young age segment (30-50 years), then
gradually decreased, reaching another peak in the age segment 70-79
years. Data from the literature report similar results (15,16).
In the study group, for patients <50 years of age, NSAIDs and
H. pylori infection had a cumulative effect in causing
hemorrhagic lesions. In contrast, after the age of 50 years, the
main causes of bleeding lesions in patients with H.
pylori-positive gastritis were the consumption of NSAIDs and
treatment with antiplatelet agents or oral anticoagulants. An
endoscopic study of Pilotto (17),
performed in 520 patients with peptic hemorrhagic ulcer >65
years (mean age: 81 years), reported that 67% of gastric ulcers and
69% of duodenal ulcers were H. pylori-positive. In addition,
the use of NSAIDs or aspirin, alone or in combination with H.
pylori infection, was reported in 39% of gastric ulcers and in
25% of patients with duodenal ulcers (17). Studies do not take into account the
difference between the use of NSAIDs and the use of low-dose
aspirin (antiplatelet effect). H. pylori is associated with
an increased risk of bleeding in patients under chronic treatment
with AINS or antiplatelet medication (18-20).
In the present study, therapies with antiplatelet agents or oral
anticoagulants and H. pylori infection were the most
frequent associations encountered in cases with active bleeding in
the study group. At the same time, in elderly patients with H.
pylori-positive gastritis who started long-term treatment with
NSAIDs, oral antiplatelet agents or anticoagulants, treatment of
the infection significantly reduced the risk of peptic ulcer
(21-23).
Patients >50 who are administered antiplatelet agents or oral
anticoagulants, in addition to performing an upper digestive
endoscopy, should be considered for a non-invasive test for the
diagnosis of H. pylori (21). In our study, the transformation of
non-hemorrhagic gastroduodenal H. pylori-positive lesions
into hemorrhagic lesions was strongly related to alcohol
consumption. We analyzed the effect of NSAID consumption associated
with alcohol consumption depending on the presence of H.
pylori and found that chronic alcohol consumption appeared to
have a similar NSAID effect, so that alcohol consumption without
the association with NSAIDs was considered to cause bleeding in
patients with H. pylori-positive lesions in ~14.29% of
patients; 28.75% of H. pylori-positive patients with active
bleeding at endoscopic evaluation (class IA and IB) were treated
with NSAIDs and were chronic consumers of alcohol compared to
patients with H. pylori-negative hemorrhagic lesions (12.50%
of patients with NSAIDs and alcohol). Studies that looked at the
relationship between H. pylori infection and alcohol
consumption had conflicting results. A multiple logistical study
found that alcohol consumption and active gastritis pathology were
associated with H. pylori infection, and active gastritis
(hemorrhagic or non-hemorrhagic) was associated with chronic
alcohol consumption (24). Recent
studies found an increased prevalence of H. pylori infection
in type 2 diabetes (25,26), one plausible explanation being
related to the changes in microbiota and low grade chronic
inflammation at the level of the gastroduodenal mucosa (27). There is growing evidence of a
bilateral relationship between H. pylori infection and
chronic hepatic diseases (28,29).
On the one hand, the liver plays significant roles in multiple
metabolic pathways, being involved in coagulation and the
trophicity of gastroduodenal mucosa (30). On the other hand, chronic
inflammation caused by H. pylori induces chronic liver
fibrosis (28).
Literature data found evidence that H. pylori
and NSAIDs are major causes of gastroduodenal ulcer, and an
in-depth analysis of the interaction data revealed that the
induction effects of ulcers for both factors were cumulative
(31). Eradication of H.
pylori in chronic NSAID users was found to decrease the
incidence of ulcer disease (2).
Chronic alcohol consumption appears to have a similar effect as
NSAIDs, thus chronic alcohol consumption without an association
with NSAIDs was considered to cause bleeding in patients with H.
pylori-positive lesions in ~14.29% of cases included in group
A. In our study, 18.18% of alcohol consuming H.
pylori-positive patients with active bleeding lesions
(diagnosed endoscopically or surgically) became negative after
specific treatment, while 40% of all these patients remained
positive after therapy.
In the present study, >50% of patients had H.
pylori-positive bleeding lesions. The 30-day retest of the
hemorrhagic episode in all 166 patients showed that out of the 76
initially negative patients, 6 patients became positive. These
results are due to the fact that when patients have acute
gastroduodenal bleeding, most diagnostic tests for H. pylori
infection may show false-negative results (32) and the sensitivity and specificity of
the fecal antigen test is 90% (2).
Non-invasive testing by detecting H. pylori-specific antigen
in feces had increased sensitivity in the diagnosis of infection in
our study, so that out of 166 patients tested, a positive diagnosis
was established with increased accuracy in 70 of the 76 H.
pylori-positive patients. Although histological evaluation and
IHC established the presence of H. pylori in another 6
patients, this non-invasive test had a sensitivity and specificity
of over 90% in assessing the correct diagnosis. This indirect
method of diagnosis is cheaper than other methods and has the
advantage that it can be performed in most medical centers
(1,2). We found that histological examination
of H. pylori lesions is very accurate in providing data on
the degree of atrophy, metaphase or carcinoma, and the addition of
IHC examination brings more information and establishes the correct
diagnosis in all cases. Data from the literature show that
non-invasive methods and histological evaluation of endobiopsies
together establish the diagnosis of H. pylori infection in
>95-97% of cases (33,34). In the patients in the study, the
diagnosis of H. pylori infection was established after
performing non-invasive tests and after histological evaluation in
94% of cases; this share of the diagnosis is similar to other
studies. Highlighting H. pylori in the usual staining was
easier in patients with acute lesions of the gastric mucosa (acute
gastritis), compared to chronic (premalignant lesions: atrophic
gastritis and metaplasia). Specialist studies indicate that when
changes in atrophy occur in the gastric mucosa, a high percentage
of endobioptic samples become negative on histological evaluation
(35), and in areas with
metaplasia, H. pylori is undetectable by conventional
histological staining in most cases, despite serological evidence
(36). In addition, the low
prevalence of H. pylori in antral biopsy specimens with
atrophic mucosa can be explained by an uneven distribution of
bacterial infection resulting from pH-increasing conditions
(37,38). IHC evaluation may be useful in
conditions where no bacteria is visible in the usual H&E or
Giemsa staining, but there is histological evidence of
inflammation; in patients with MALT from post-treatment biopsy
specimens (to ensure that eradication therapy was successful) and
in forms in which H. pylori cannot be conclusively
identified (32). Routine IHC
evaluation of this pathology is questionable due to the high cost
and need for specialized personnel; however, it can be considered
due to the accuracy of the diagnosis and the accuracy of
identification of associated lesions (atrophic gastritis,
metaplasia, dysplasia, carcinoma, lymphoma) (39).
Aggression and resistance of H. pylori to
antibiotic therapy is correlated positively with an increased rate
of active bleeding. Evaluation of patients with active bleeding on
endoscopic examination showed that the bleeding rate was high in
treatment-resistant H. pylori-positive patients (40%),
compared to those who were negative after specific therapy
(18.18%). In contrast, we found that the frequency of hemorrhagic
lesions was much lower in those with H. pylori-negative
lesions (15.79%) compared to H. pylori-positive patients.
Literature data show that before the eradication of H.
pylori, 20-25% of patients with peptic ulcer disease developed
complications such as hemorrhage, perforation or stenosis (40) and it is estimated that ~1/3 of H.
pylori-positive patients with hemorrhagic ulcer will develop
recurrent bleeding in the next 1-2 years in the absence of testing
and eradication of H. pylori (41). The increased resistance of H.
pylori to antibiotics is also correlated with the higher rate
of bleeding at the distance of the hemorrhagic episode, despite PPI
therapy. Endoscopic evaluation should be performed in all patients
with hemorrhagic lesions for diagnostic or therapeutic purposes,
but also to obtain biopsies for histological or IHC diagnosis of
H. pylori (2). In our study,
acute hemorrhagic lesions (gastric, duodenal ulcer or hemorrhagic
gastroduodenitis) were found more frequently in the group of H.
pylori-positive patients than in H. pylori-negative ones
(69 vs. 43 cases). Acute hemorrhagic lesions are more common in
H. pylori-positive patients, so acute hemorrhagic duodenal
ulcer and acute hemorrhagic gastric ulcer are more common in these
patients than in H. pylori-negative patients (41,42).
Chronic hemorrhagic endoscopic lesions (chronic duodenal ulcer,
chronic gastric ulcer), as well as non-hemorrhagic ones, seem to be
more common in H. pylori-negative patients. Hemorrhage may
occur more frequently in the colonization phase associated with
acute H. pylori gastritis compared to chronic lesions. At
the same time, tumor lesions were much more common in H.
pylori-positive patients (7 vs. 1 patient). Data from the
literature have shown that endoscopic features have been reported
to be useful tools for diagnosing H. pylori infection in the
gastric mucosa. However, the ability to predict these
characteristics can vary greatly (8).
In the present study, the need for surgery for
hemostasis was more common in patients with UGIB with H.
pylori-positive gastroduodenal lesions, compared to H.
pylori-negative patients. Approximately 16% of patients with
non-variceal and H. pylori-positive UGIB required surgery to
stop the bleeding (16 vs. 9.7%). Regarding the type of surgery, in
the case of H. pylori-positive patients with UGIB, resection
was needed in several cases, compared to the group of patients with
H. pylori-negative hemorrhagic lesions. At the same time,
according to other studies, postoperative complications appear more
frequently in patients who undergo resection (gastrectomies,
duodenopancreatectomies), compared to patients in whom suturing is
performed (16,43-46).
Postoperative adherences are significant causes of long term
morbidity, causing episodes of abdominal pain and bowel obstruction
(47).
The outcome of patients with UGIB depends very much
on the need for surgery for hemostasis. The need for hemostasis
surgery in the case of non-variceal UGIB is the most constant
indicator of prolonged hospitalization and high financial cost, and
the prophylaxis of these episodes of bleeding is the simplest
modality of treatment. Thus, the presence of H. pylori
significantly contributes to the increase in hospital stay and
treatment costs in these patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
DGP, CVO, MEC, DS, CMe, MC and BN led the
conception and design of this study. BS, DNF, VDB, MST, CMe MEC,
MD, LCT, IDV, and BN were responsible for the data collection and
analysis. CMi, DNF, VDB, MC, LCT and DS were in charge of reviewing
the data and drafting the manuscript. DGP, BS, DS, CMi, MST and BN
revised critical perspectives for important intellectual content.
The final version was read and approved by all the authors.
Ethics approval and consent to
participate
The study was conducted according to the World
Medical Association Declaration of Helsinki, using a protocol
approved by the local Bioethics Committee from County Emergency
Clinical Hospital (Craiova, Romania). All patients previously
signed an informed written consent concerning the hospitalization
and research investigation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Obleagă CV, Vere CC, Vîlcea ID, Ciorbagiu
MC, Moraru E and Mirea CS: Helicobacter pylori: Types of
diseases, diagnosis, treatment and causes of therapeutic failure. J
Mind Med Sci. 3:156–161. 2016.
|
|
2
|
Kuipers EJ, Thijs JC and Festen HP: The
prevalence of Helicobacter pylori in peptic ulcer disease.
Aliment Pharmacol Ther. 9 (Suppl 20):S59–S69. 1995.PubMed/NCBI
|
|
3
|
Hentschell E, Brandstätter G, Dragosics B,
Hirschl AM, Nemec H, Schütze K, Taufer M and Wurzer H: Effect of
ranitidine and amoxycillin plus metronidazole on the eradication of
Helicobacter pylori and the recurrence of duodenal ulcer. N
Engl J Med. 328:308–312. 1993.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gisbert JP, Khorrami S, Carballo F, Calvet
X, Gene E and Dominguez-Munoz E: Meta-analysis: Helicobacter
pylori eradication therapy vs. antisecretory non-eradication
therapy for the prevention of recurrent bleeding from peptic ulcer.
Aliment Pharmacol Ther. 19:617–629. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Guo CG, Cheung KS, Zhang F, Chan EW, Chen
L, Wong ICK and Leung WK: Delay in retreatment of Helicobacter
pylori infection increases risk of upper gastrointestinal
bleeding. Clin Gastroenterol Hepatol. 19:314–322.e2.
2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gisbert JP and Abraira V: Accuracy of
Helicobacter pylori diagnostic tests in patients with
bleeding peptic ulcer: A systematic review and meta-analysis. Am J
Gastroenterol. 101:848–863. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Stolte M and Meining A: The updated Sydney
system: Classification and grading of gastritis as the basis of
diagnosis and treatment. Can J Gastroenterol. 15:591–598.
2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mao T, Wang Y, Yin F, Zhao Q, Yang L, Ding
X and Tian Z: Association of endoscopic features of gastric mucosa
with Helicobacter pylori infection in Chinese patients.
Gastroenterol Res Pract. 2016(6539639)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Slăvescu KC, Șarban C, Pîrvan A, Gheban D,
Mărgescu C and Miu N: Prevalence of Helicobacter pylori
infection in children with gastritis and peptic ulcer disease in
north-western and central Romania. Med Pharmacy Rep. 85:457–462.
2012.
|
|
10
|
Megraud F: H. pylori antibiotic
resistance: Prevalence, importance and advances in testing. Gut.
53:1374–1384. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Park S and Chun HJ: Helicobacter
pylori infection following partial gastrectomy for gastric
cancer. World J Gastroenterol. 20:2765–2770. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Testerman TL and Morris J: Beyond the
stomach: An updated view of Helicobacter pylori
pathogenesis, diagnosis, and treatment. World J Gastroenterol.
20:12781–12808. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wroblewski LE, Peek RM Jr and Wilson KT:
Helicobacter pylori and gastric cancer: Factors that
modulate disease risk. Clin Microbiol Rev. 23:713–739.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Conteduca V, Sansonno D, Lauletta G, Russi
S, Ingravallo G and Dammacco F: H. pylori infection and
gastric cancer: State of the art (Review). Int J Oncol. 42:5–18.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Alberto P and Franceschi M:
Helicobacter pylori infection in older people. World J
Gastroenterol. 20:6364–6373. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Constantin VD, Paun S, Ciofoaia VV, Budu V
and Socea B: Multimodal management of upper gastrointestinal
bleeding caused by stress gastropathy. J Gastrointestin Liver Dis.
18:279–284. 2009.PubMed/NCBI
|
|
17
|
Pilotto A: Aging and upper
gastrointestinal disorders. Best Pract Res Clin Gastroenterol. 18
(Suppl):S73–S81. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Venerito M, Schneider C, Costanzo R, Breja
R, Röhl FW and Malfertheiner P: Contribution of Helicobacter
pylori infection to the risk of peptic ulcer bleeding in
patients on nonsteroidal anti-inflammatory drugs, antiplatelet
agents, anticoagulants, corticosteroids and selective serotonin
reuptake inhibitors. Aliment Pharmacol Ther. 47:1464–1471.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pilotto A and Franceschi M:
Helicobacter pylori infection in older people. World J
Gastroenterol. 20:6364–6373. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lanas A, Dumonceau JM, Hunt RH, Fujishiro
M, Scheiman JM, Gralnek IM, Campbell HE, Rostom A, Villanueva C and
Sung JJY: Non-variceal upper gastrointestinal bleeding. Nat Rev Dis
Primers. 4(18020)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pilotto A, Franceschi M, Leandro G, Paris
F, Cascavilla L, Longo MG, Niro V, Andriulli A, Scarcelli C and Di
Mario F: Proton-pump inhibitors reduce the risk of uncomplicated
peptic ulcer in elderly either acute or chronic users of
aspirin/non-steroidal anti-inflammatory drugs. Aliment Pharmacol
Ther. 20:1091–1097. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Narayanan M, Reddy KM and Marsicano E:
Peptic ulcer disease and Helicobacter pylori infection. Mo
Med. 115:219–224. 2018.PubMed/NCBI
|
|
23
|
Zapata-Colindres JC, Zepeda-Gómez S,
Montaño-Loza A, Vázquez-Ballesteros E, De Jesús Villalobos J and
Valdovinos-Andraca F: The association of Helicobacter pylori
infection and nonsteroidal anti-inflammatory drugs in peptic ulcer
disease. Can J Gastroenterol. 20:277–280. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang L, Eslick GD, Xia HH, Wu C, Phung N
and Talley NJ: Relationship between alcohol consumption and active
Helicobacter pylori infection. Alcohol Alcohol. 45:89–94.
2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hosseininasab Nodoushan SA and Nabavi A:
The interaction of Helicobacter pylori infection and type 2
diabetes mellitus. Adv Biomed Res. 8(15)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
He C, Yang Z and Lu NH: Helicobacter
pylori infection and diabetes: Is it a myth or fact? World J
Gastroenterol. 20:4607–4617. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Suceveanu AI, Stoian AP, Parepa I, Voinea
C, Hainarosie R, Manuc D, Nitipir C, Mazilu L and Suceveanu AP: Gut
microbiota patterns in obese and type 2 diabetes (T2D) patients
from romanian black sea coast region. Rev Chim. 69:2260–2267.
2018.
|
|
28
|
Okushin K, Tsutsumi T, Ikeuchi K, Kado A,
Enooku K, Fujinaga H, Moriya K, Yotsuyanagi H and Koike K:
Helicobacter pylori infection and liver diseases:
Epidemiology and insights into pathogenesis. World J Gastroenterol.
24:3617–3625. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tsai CE, Liang CM, Lee CH, Kuo YH, Wu KL,
Chiu YC, Tai WC and Chuah SK: First-line Helicobacter pylori
eradication among patients with chronic liver diseases in Taiwan.
Kaohsiung J Med Sci. 32:397–402. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gheorghe G, Stoian AP, Gaman M, Socea B,
Neagu TP, Stanescu AMA, Bratu OG, Mischianu DLD, Suceveanu AI and
Diaconu CC: The Benefits and Risks of Antioxidant Treatment in
Liver Diseases. Rev Chim. 70(651)2019.
|
|
31
|
Huan JQ, Sridhar S and Hunt RH: Role of
Helicobacter pylori infection and nonsteroidal
anti-inflammatory drugs in peptic-ulcer disease: A meta-analysis.
Lancet. 359:14–22. 2002.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Braden B: Diagnosis of Helicobacter
pylori infection. BMJ. 344(828)2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee JY and Kim N: Diagnosis of
Helicobacter pylori by invasive test: Histology. Ann Transl
Med. 3(10)2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Alius C, Tudor C, Badiu CD, Dascalu AM,
Smarandache CG, Sabau AD, Tanasescu C, Balasescu SA and Serban D:
Indocyanine green-enhanced colorectal surgery-between being
superfluous and being a game-changer. Diagnostics (Basel).
10(742)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kokkola A, Rautelin H, Puolakkainen P,
Sipponen P, Färkkilä M, Haapiainen R and Kosunen TU: Diagnosis of
Helicobacter pylori infection in patients with atrophic
gastritis: Comparison of histology, 13C-urea breath test, and
serology. Scand J Gastroenterol. 35:138–141. 2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Loffeld RJ, Stobberingh E, Flendrig JA and
Arends JW: Helicobacter pylori in gastric biopsy specimens.
Comparison of culture, modified giemsa stain, and
immunohistochemistry. A retrospective study. J Pathol. 165:69–73.
1991.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Testoni PA, Colombo E, Cattani L, Longhi
M, Bagnolo F, Lella F, Buizza M and Scelsi R: Helicobacter
pylori serology in chronic gastritis with antral atrophy and
negative histology for Helicobacter-like organisms. J Clin
Gastroenterol. 22:182–185. 1996.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kang HY, Kim N, Park YS, Hwang JH, Kim JW,
Jeong SH, Lee DH, Jung HC and Song IS: Progression of atrophic
gastritis and intestinal metaplasia drives Helicobacter
pylori out of the gastric mucosa. Dig Dis Sci. 51:2310–2315.
2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Park YH and Kim N: Review of atrophic
gastritis and intestinal metaplasia as a premalignant lesion of
gastric cancer. J Cancer Prev. 20:25–40. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Penston JG: A decade of experience with
long-term continuous treatment of peptic ulcers with H2-receptor
antagonists. Aliment Pharmacol Ther. 7 (Suppl 2):S27–S33.
1993.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Laine LA: Helicobacter pylori and
complicated ulcer disease. Am J Med. 100 (Suppl):S52–S59.
1996.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Campuzano-Maya G: Hematologic
manifestations of Helicobacter pylori infection. World J
Gastroenterol. 20:12818–12838. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Obleagă CV, Vere CC, Pătraşcu AM, Moraru
E, Crafciuc AV, Foarfă MC, Mogoantă SŞ, Streba CT, Bondari S,
Paitici Ş, et al: Severe upper gastrointestinal bleeding determined
by a gastric lymphoma associated with Helicobacter
pylori-positive atrophic gastritis. Rom J Morphol Embryol.
58:611–617. 2017.PubMed/NCBI
|
|
44
|
Obleagă CV, Vere CC, Mogoanţa S, Firuț C,
Meșina C, Ciorbagiu MC, Mirea CS and Vîlcea ID: Upper
gastrointestinal bleeding-initial manifestation of pancreatic head
carcinoma. Curr Health Sci J. 43:236–240. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Serban D, Vancea G, Balasescu SA, Socea B,
Tudor C and Dascalu AM: Informed consent in all surgical
specialties: From legal obligation to patient satisfaction. Rom J
Leg Med. 28:317–321. 2020.
|
|
46
|
Constantin VD, Socea B, Sireţeanu G and
Popa F: Epidemiological aspects and risk factors in the outcome of
variceal eso-gastric bleeding at cirrhosis patients. J Appl Quant
Meth. 3:316–324. 2008.
|
|
47
|
Fometescu SG, Costache M, Coveney A,
Oprescu SM, Serban D and Savlovschi C: Peritoneal fibrinolytic
activity and adhesiogenesis. Chirurgia (Bucur). 108:331–340.
2013.PubMed/NCBI
|