Introduction
Basosquamous carcinoma (BSC), also called
metatypical basal cell carcinoma, is a subtype of basal cell
carcinoma (BCC), displaying histological features of both BCC and
squamous cell carcinoma (SCC) (1).
This tumor may have a more aggressive behavior that resembles more
an SCC than a BCC in terms of invasiveness and poor prognosis, with
a greater likelihood of recurrence and metastasis (2).
As with any other BCC, it presents as a plaque,
papule, or nodule with an ulcerating potential. Elderly,
fair-skinned white men are the most affected, especially on the
head and neck area (3). Dermoscopy
shows polymorphous vascular structures typical of BCC combined with
scaling, ulceration, white circles and white structureless areas
(4,5). Histopathologically, it is
characterized by islands of basaloid cells mixed with atypical
squamous cells (1). According to
the National Comprehensive Cancer Network (NCCN) Clinical Practice
Guidelines in Oncology, BSC is included in the high-risk BCC
category (2). This is due to the
higher metastatic rate when compared with conventional BCC (5-8.4
vs. <0.1%) (6). These metastases
occur especially in regional lymph nodes and lungs (7). There is also a higher risk of local
recurrence, estimated at 4.5% (8).
This leads to a median survival rate of 6.5 years and an overall
5-year survival rate of 54% in the cases of metastatic disease
(9). First-line treatment for
high-risk BCC, therefore BSC, is Mohs micrographic surgery (MMS)
(2,10,11).
Other surgical methods include standard surgical excision with
postoperative margin assessment (2,10) and
staged excision with circumferential margin assessment (10,12).
External beam radiotherapy (RT) is recommended for patients who
cannot tolerate surgery (10,13).
Case presentation
An 81-year-old male presented with a hemorrhagic
tumor located in the suprasternal notch. He had chronic atrial
fibrillation and was under chronic anticoagulation treatment with
apixaban. There was no personal clinical history and family history
for other tumors, skin or otherwise The tumor had appeared
approximately 12 years ago and had a rapid growth during the
previous year, but the patient did not seek medical care due to the
COVID-19 pandemic crisis at the moment. Physical examination
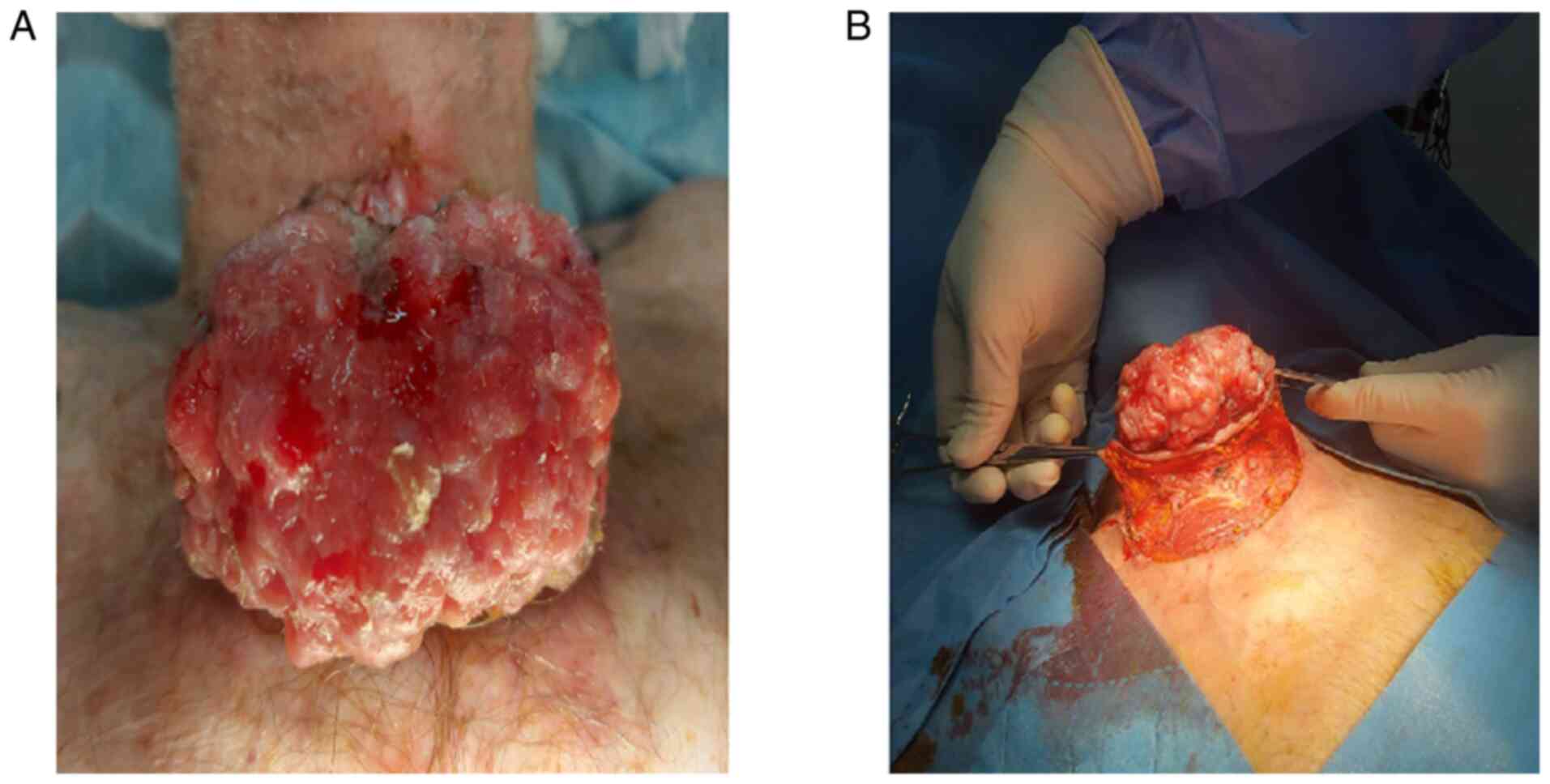
revealed a bleeding vegetating tumor (Fig. 1A), measuring 14x12 cm. The
perilesional skin appeared erythematous and edematous. Clinically,
there was no evidence of lymphadenopathy. The clinical differential
diagnosis included SCC, BCC, Merkel cell carcinoma, amelanotic
melanoma, or cutaneous metastasis of unknown origin. The patient
was controlled using mechanical hemostatic methods such as direct
pressure and gauze pack and local hemostatic agents, while also
stopping the anticoagulant. To further plan the surgical excision,
especially in consideration of the tumor's great size, a whole-body
computed tomography (CT) scan was performed which did not reveal
any metastasis or neoplastic infiltration of the muscle fascia.
Therefore, standard surgical excision with 2-cm margins was
performed with the immediate repair of the surgical defect using
adjacent tissue flaps (Fig. 1B).
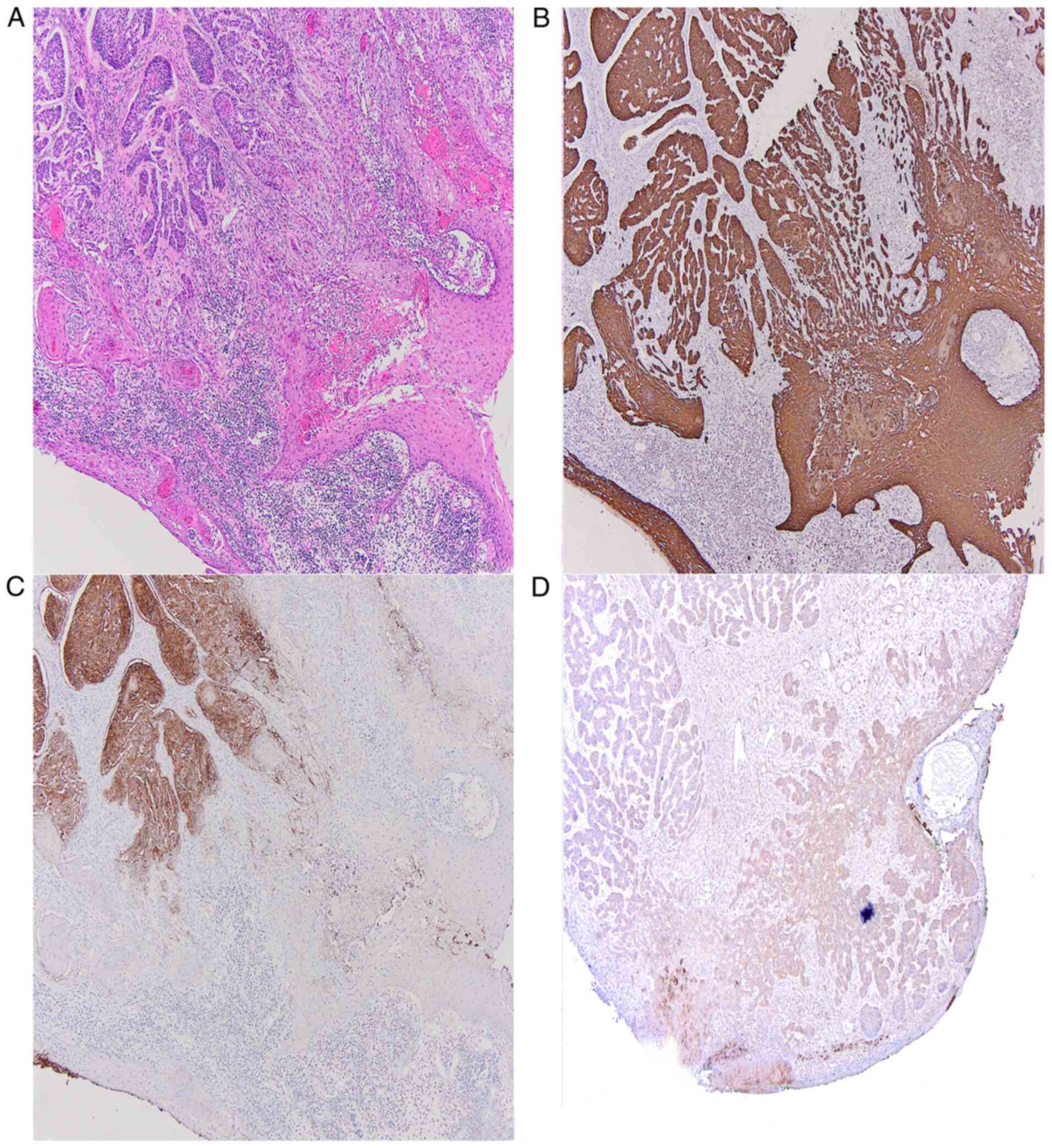
The histopathology examination revealed a large ulcerated
eso-endophytic lesion invading the dermis and the subcutis composed
of atypical basaloid strands and islands commingled with islands of
polygonal atypical squamous cells with abundant eosinophilic
cytoplasm, embedded in a cellular fibrotic stroma and chronic
inflammatory infiltrate. Lymphovascular invasion was present. No
perineural invasion was observed. Circumferential, peripheral and
deep margin assessment was negative. At immunohistochemistry, there
was diffuse positivity for cytokeratin 34betaE12. The basaloid
component stained positive for Ber-EP4 with loss of
immunoreactivity of Ber-EP4 in areas showing squamous
differentiation, whereas epithelial membrane antigen (EMA) and p53
were partially immunoreactive only in the squamous differentiated
areas (Fig. 2). The Ki-67
immunoreactivity was about 60-70%. Based on the data gathered, a
diagnosis of basal cell carcinoma, basosquamous subtype, was made.
The TNM according to the Eighth Edition of the American Joint
Committee on Cancer Classification (AJCC) was pT3Nx (14). Since there was no sign of residual
disease, no further treatment was indicated and follow-up was
recommended.
At six-month follow-up, there was no clinical sign
of local recurrence or metastasis.
Discussion
Non-melanoma skin cancer (NMSC) is the most common
group of cutaneous neoplasms in the Caucasian population with basal
cell carcinoma (BCC) being the prevalent entity, although precise
epidemiologic data are limited, as in most countries there are no
cancer registries that collect data regarding it (15-17).
However, the NMSC incidence rate is on a steady
increase in the USA, most European countries, and Australia, with
the latter registering the highest growth (15). These trends could be explained in
regards to the inverse association of BCC with the country's
geographic latitude and the Fitzpatrick skin phototype of its
inhabitants (16).
Literature indicates that BCC is a heterogeneous
entity and that specific histological variants can arise in
particular areas, exhibiting different clinical behavior, have a
disparate etiology, and a distinct response to treatment (18). The latest edition of the World
Health Organization (WHO) Classification of Skin Tumors recognizes
the following 10 histological subtypes of BCC: nodular,
superficial, micronodular, infiltrating, sclerosing/morphoeic,
basosquamous, pigmented, fibroepithelial, BCC with sarcomatoid
differentiation, and BCC with adnexal differentiation (1). Although the pathogenesis of
basosquamous carcinoma (BSC) has historically been a subject of
debate, as some authors considered it to be a subtype of BCC and
others an independent tumor with a different evolution, WHO finally
identifies it as a BCC variant (1).
In any case, it is rather rare, and data concerning the precise
percentage are also missing. It is estimated that BSC represents
approximately 1.2 to 2.7% of all malignancies in the NMSC group
(7,19-22).
Ultraviolet radiation (UVR) is known to be the
prevalent risk factor for BCC, SCC, and therefore BSC (23) with a preferential localization on
the sun-exposed areas, especially the head and neck region
(3). Moreover, it appears that BSC
occurs more frequently in men (3,24) in
the six to eight decades of life (24) with a mean age at diagnosis of
72±11.5 years (3).
It is difficult to make a diagnosis of BSC using
only naked eye examination as it resembles other subtypes of BCC.
It presents as a pink or flesh-colored plaque, papule, or nodule.
Ulceration is usually present. The lesion can have a pearly or
translucent feature with telangiectatic vessels being seen within
it (3).
While clinical differentiation from other BCC
variants is demanding, dermoscopy can provide some important hints
allowing a more appropriate treatment planning. The dermoscopic
evaluation of BSC detects features of both BCC and SCC such as
unfocused arborizing vessels, blue-gray blotches, ulceration or
blood crusts, white structures and white structureless areas.
Identification of only one overlapping dermoscopic criterion both
of BCC and SCC is an alarming clue for BSC (4,5).
The definitive diagnosis of BSC is made only by
histopathological examination. The latest edition of the WHO
Classification of Skin Tumors describes BSC as having features of
both BCC and SCC such as islands of basaloid cells mixed with focal
or scattered atypical squamous cells and transition zones
containing cells with features intermediate between the two. The
malignant cells are embedded in a cellular fibrotic stroma. The
basaloid component expresses BerEP4 with a gradual loss of
reactivity towards the squamous cell component. The squamous cells
stain positively for EMA. There is also a high cyclin D1 expression
as well as a low BCL2 expression (1).
Before treatment, assessment of recurrence risk is
mandatory. According to the NCCN Clinical Practice Guidelines in
Oncology concerning the risk of recurrence, there are two main
categories of BCC: the low-risk group and the high-risk group
(2). The following features are
associated with lesions with a low risk of recurrence potential:
immunocompetent patient, no history of radiation therapy at the
site, not a recurrent tumor (a primary tumor); well-defined
clinical borders; <10 mm in diameter in tumors developed on the
cheeks, forehead, scalp, neck, pretibial; <20 mm in diameter in
tumors of the trunk and extremities, excluding pretibia, hands,
feet, nail unit, and ankles; histological subtypes such as nodular
or superficial; the absence of perineural invasion. On the other
hand, lesions with an increased risk for tumor recurrence have the
subsequent characteristics: immunocompromised patients, tumors
located in sites of prior radiation therapy; recurrent tumors;
tumors with ill-defined clinical borders; tumors of any size in
high-risk areas of the face such as the central face, nose, lips,
eyelids, eyebrows, periorbital skin, chin, mandible, ears,
preauricular and postauricular areas, temples or of the hands and
feet; tumors ≥10 mm in diameter in other areas of the head, neck,
and pretibial; tumors ≥20 mm in diameter in all other areas like
trunk and extremities, excluding hands and feet; aggressive
pathologic variants such as morpheaform, sclerosing, mixed
infiltrative, micronodular, basosquamous; the presence of
perineural invasion (2).
BSC is considered an aggressive variant of BCC,
therefore included in the high-risk group, because of the higher
metastatic rate and local recurrence rate. BSC has a metastatic
rate from 5 to 8.4% in comparison with more conventional subtypes
of BCC which have a metastatic rate lower than 0.1% (5). Regional lymph nodes and lungs are the
most frequent sites of metastases (6). Moreover, the recurrence rate is
considered to be higher and estimated at 4.5% (7). Patients with metastatic disease have a
median survival of 6.5 years and an overall 5-year survival rate of
54% (8).
As stated above, BCC treatment is highly influenced
by the recurrence risk potential. First-line therapy for BCC at low
risk of recurrence is standard surgical excision with margins of 4
to 5 mm (10,25). Alternative therapy in select cases
with tissue conservation purposes, especially for lesions located
on the face, is MMS (10,26). Second-line therapies for low-risk
tumors are indicated mainly for superficial BCC in patients who
cannot undergo surgery or prefer to avoid it. This category
includes topical therapies such as imiquimod 5% cream (27) or topical fluorouracil 5% cream or
solution (28,29) and photodynamic therapy (PDT), the
latter being available only in Europe at the moment (30). High-risk tumors, therefore also BSC,
are treated primarily, if available, with MMS (2,10,11).
When MMS cannot be performed because of lack of availability or in
patients who cannot tolerate it, an alternative treatment could be
standard surgical excision with postoperative margin assessment
(2,10). Regarding the appropriate margin
size, it is recommended to use margins wider than 4 to 5 mm,
depending on the localization, although there are no data from
randomized trials addressing the proper margin size for this
category (2). Another option could
be staged excision with circumferential margin assessment, although
is not broadly available and can be less desirable for some
patients (10,12). Finally, external beam RT
administered in a fractionated schedule is an option for older
patients with high-risk BCC who are not candidates for surgery
(10,13). Topical treatments or PDT are not
suitable for this category of patients (2,10).
We presented the case of an 81-year-old-male who was
brought to the Emergency Department of the National Institute of
Pneumology ‘Marius Nasta’ due to uncontrolled hemorrhage of a giant
cutaneous tumor located in the suprasternal notch that was later
diagnosed as BSC. The impressive size of this tumor could be
explained by the aggressive histologic pattern, the high
proliferative rate of the tumor, and neglect for a long period. The
latter was probably the most important pathogenic factor, giving
the fact that the patient had disregarded the tumor's enlargement
for almost 12 years. Moreover, the COVID-19 pandemic increased the
delay. The massive hemorrhage, on the other hand, was probably
caused by the high neovascularization of this type of tumor and
aggravated by the coagulation status of the patient.
In conclusion, cutaneous basosquamous carcinoma is a
basal cell carcinoma subtype that has histopathological
characteristics of both basal and squamous cell carcinoma. It is
considered one of the most aggressive subtypes because of the
high-risk of local invasiveness, high frequency of recurrences, and
metastases. The diagnosis is made almost exclusively by
histopathological examination and the treatment is based mainly on
surgery.
In this article, we presented the case of an
81-year-old-male with an uncontrolled hemorrhage deriving from a
basosquamous carcinoma. As mentioned before, this entity has an
aggressive behavior, presenting with ulceration and infiltration,
but this is the first case report, at least to the best of our
knowledge, that presented as a massive hemorrhage endangering the
patient's life.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Any further information concerning the case report
is available upon request of the corresponding author.
Authors' contributions
All authors had equally contributed to writing and
editing the manuscript. TC contributed in all the stages of the
article; he designed the article and revised the manuscript for
important intellectual content. MMC, EDS and CAP contributed to the
conception of the work, revision of the language and contributed to
drafting the final paper. CS, AVZ, SB and OCD revised the work
critically in light of the literature data. The final manuscript
for publication was approved by all authors.
Ethics approval and consent to
participate
The patient provided informed written consent prior
to inclusion in the present article. No ethics committee approval
was necessary.
Patient consent for publication
The patient provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests and they have no financial relationships to disclose.
References
|
1
|
Elder DE, Massi D, Scolyer RA and Willemze
R: Basal cell carcinoma. In: WHO Classification of Skin Tumors. 4th
edition. International Agency for Research on Cancer, Lyon, France,
pp26-34, 2018.
|
|
2
|
Bichakjian CK, Olencki T, Aasi SZ, Alam M,
Andersen JS, Berg D, Bowen GM, Cheney RT, Daniels GA, Glass LF, et
al: Basal cell skin cancer, version 1.2016, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 14:574–597.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ciążyńska M, Sławińska M,
Kamińska-Winciorek G, Lange D, Lewandowski B, Reich A, Pabianek M,
Szczepaniak K, Hankiewicz A, Ułańska M, et al: Clinical and
epidemiological analysis of basosquamous carcinoma: Results of the
multicenter study. Sci Rep. 10(18475)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Akay BN, Saral S, Heper AO, Erdem C and
Rosendahl C: Basosquamous carcinoma: Dermoscopic clues to
diagnosis. J Dermatol. 44:127–134. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Giacomel J, Lallas A, Argenziano G,
Reggiani C, Piana S, Apalla Z, Ferrara G, Moscarella E, Longo C and
Zalaudek I: Dermoscopy of basosquamous carcinoma. Br J Dermatol.
169:358–364. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Alam M, Desai S, Nodzenski M, Dubina M,
Kim N, Martini M, Fife D, Reid D, Pirigyi M, Poon E, et al: Active
ascertainment of recurrence rate after treatment of primary basal
cell carcinoma (BCC). J Am Acad Dermatol. 73:323–325.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tan CZ, Rieger KE and Sarin KY:
Basosquamous carcinoma: Controversy, advances, and future
directions. Dermatol Surg. 43:23–31. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wermker K, Roknic N, Goessling K, Klein M,
Schulze HJ and Hallermann C: Basosquamous carcinoma of the head and
neck: Clinical and histologic characteristics and their impact on
disease progression. Neoplasia. 17:301–305. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhu GA, Danial C, Liu A, Li S and Chang
AL: Overall and progression-free survival in metastatic
basosquamous cancer: A case series. J Am Acad Dermatol.
70:1145–1146. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Peris K, Fargnoli MC, Garbe C, Kaufmann R,
Bastholt L, Seguin NB, Bataille V, Marmol VD, Dummer R, Harwood CA,
et al: Diagnosis and treatment of basal cell carcinoma: European
consensus-based interdisciplinary guidelines. Eur J Cancer.
118:10–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ad Hoc Task Force. Connolly SM, Baker DR,
Coldiron BM, Fazio MJ, Storrs PA, Vidimos AT, Zalla MJ, Brewer JD,
Begolka WS, et al: AAD/ACMS/ASDSA/ASMS 2012 appropriate use
criteria for mohs micrographic surgery: A report of the American
academy of dermatology, American college of mohs surgery, American
society for dermatologic surgery association, and the American
society for mohs surgery. J Am Acad Dermatol. 67:531–550.
2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wetzig T, Woitek M, Eichhorn K, Simon JC
and Paasch U: Surgical excision of basal cell carcinoma with
complete margin control: Outcome at 5-year follow-up. Dermatology.
220:363–369. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Likhacheva A, Awan M, Barker CA, Bhatnagar
A, Bradfield L, Brady MS, Buzurovic I, Geiger JL, Parvathaneni U,
Zaky S and Devlin PM: Definitive and postoperative radiation
therapy for basal and squamous cell cancers of the skin: Executive
summary of an American society for radiation oncology clinical
practice guideline. Pract Radiat Oncol. 10:8–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Keohane SG, Proby CM, Newlands C, Motley
RJ, Nasr I and Mohd Mustapa MF: British Association of
Dermatologists (Squamous and Basal Cell Carcinoma Guideline
Development Groups). Slater DN: Royal College of Pathologists (Skin
Cancer Lead). The new 8th edition of TNM staging and its
implications for skin cancer: a review by the British Association
of Dermatologists and the Royal College of Pathologists, U.K. Br J
Dermatol. 179:824–828. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lomas A, Leonardi-Bee J and Bath-Hextall
F: A systematic review of worldwide incidence of nonmelanoma skin
cancer. Br J Dermatol. 166:1069–1080. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Verkouteren JAC, Ramdas KHR, Wakkee M and
Nijsten T: Epidemiology of basal cell carcinoma: Scholarly review.
Br J Dermatol. 177:359–372. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Solovastru LG, Vata D, Statescu L,
Constantin MM and Andrese E: Skin cancer between myth and reality,
yet ethically constrained. Rev Rom Bioet. 12:47–52. 2014.
|
|
18
|
Scrivener Y, Grosshans E and Cribier B:
Variations of basal cell carcinomas according to gender, age,
location and histopathological subtype. Br J Dermatol. 147:41–47.
2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Martin RC II, Edwards MJ, Cawte TG, Sewell
CL and McMasters KM: Basosquamous carcinoma: Analysis of prognostic
factors influencing recurrence. Cancer. 88:1365–1369.
2000.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Garcia C, Poletti E and Crowson AN:
Basosquamous carcinoma. J Am Acad Dermatol. 60:137–143.
2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bowman PH, Ratz JL, Knoepp TG, Barnes CJ
and Finley EM: Basosquamous carcinoma. Dermatol Surg. 29:830–832.
2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Leibovitch I, Huilgol SC, Selva D,
Richards S and Paver R: Basosquamous carcinoma: Treatment with mohs
micrographic surgery. Cancer. 104:170–175. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gallagher RP, Hill GB, Bajdik CD, Fincham
S, Coldman AJ, McLean DI and Threlfall WJ: Sunlight exposure,
pigmentary factors, and risk of nonmelanocytic skin cancer. I.
Basal cell carcinoma. Arch Dermatol. 131:157–163. 1995.PubMed/NCBI
|
|
24
|
Oldbury JW, Wain RAJ, Abas S, Dobson CM
and Iyer SS: Basosquamous carcinoma: A single centre
clinicopathological evaluation and proposal of an evidence-based
protocol. J Skin Cancer. 3(6061395)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gulleth Y, Goldberg N, Silverman RP and
Gastman BR: What is the best surgical margin for a basal cell
carcinoma: A meta-analysis of the literature. Plast Reconstr Surg.
126:1222–1231. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Smeets NW, Krekels GA, Ostertag JU, Essers
BA, Dirksen CD, Nieman FH and Neumann HA: Surgical excision vs
Mohs' micrographic surgery for basal-cell carcinoma of the face:
Randomised controlled trial. Lancet. 364:1766–1772. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bath-Hextall F, Ozolins M, Armstrong SJ,
Colver GB, Perkins W, Miller PS and Williams HC: Surgery versus
Imiquimod for Nodular Superficial basal cell carcinoma (SINS) study
group. Surgical excision versus imiquimod 5% cream for nodular and
superficial basal-cell carcinoma (SINS): A multicentre,
non-inferiority, randomised controlled trial. Lancet Oncol.
15:96–105. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Love WE, Bernhard JD and Bordeaux JS:
Topical imiquimod or fluorouracil therapy for basal and squamous
cell carcinoma: A systematic review. Arch Dermatol. 145:1431–1438.
2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Voiculescu VM, Lisievici CV, Lupu M,
Vajaitu C, Draghici CC, Popa AV, Solomon I, Sebe TI, Constantin MM
and Caruntu C: Mediators of inflammation in topical therapy of skin
cancers. Mediators Inflamm. 10(8369690)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Roozeboom MH, Arits AH, Nelemans PJ and
Kelleners-Smeets NW: Overall treatment success after treatment of
primary superficial basal cell carcinoma: A systematic review and
meta-analysis of randomized and nonrandomized trials. Br J
Dermatol. 167:733–756. 2012.PubMed/NCBI View Article : Google Scholar
|