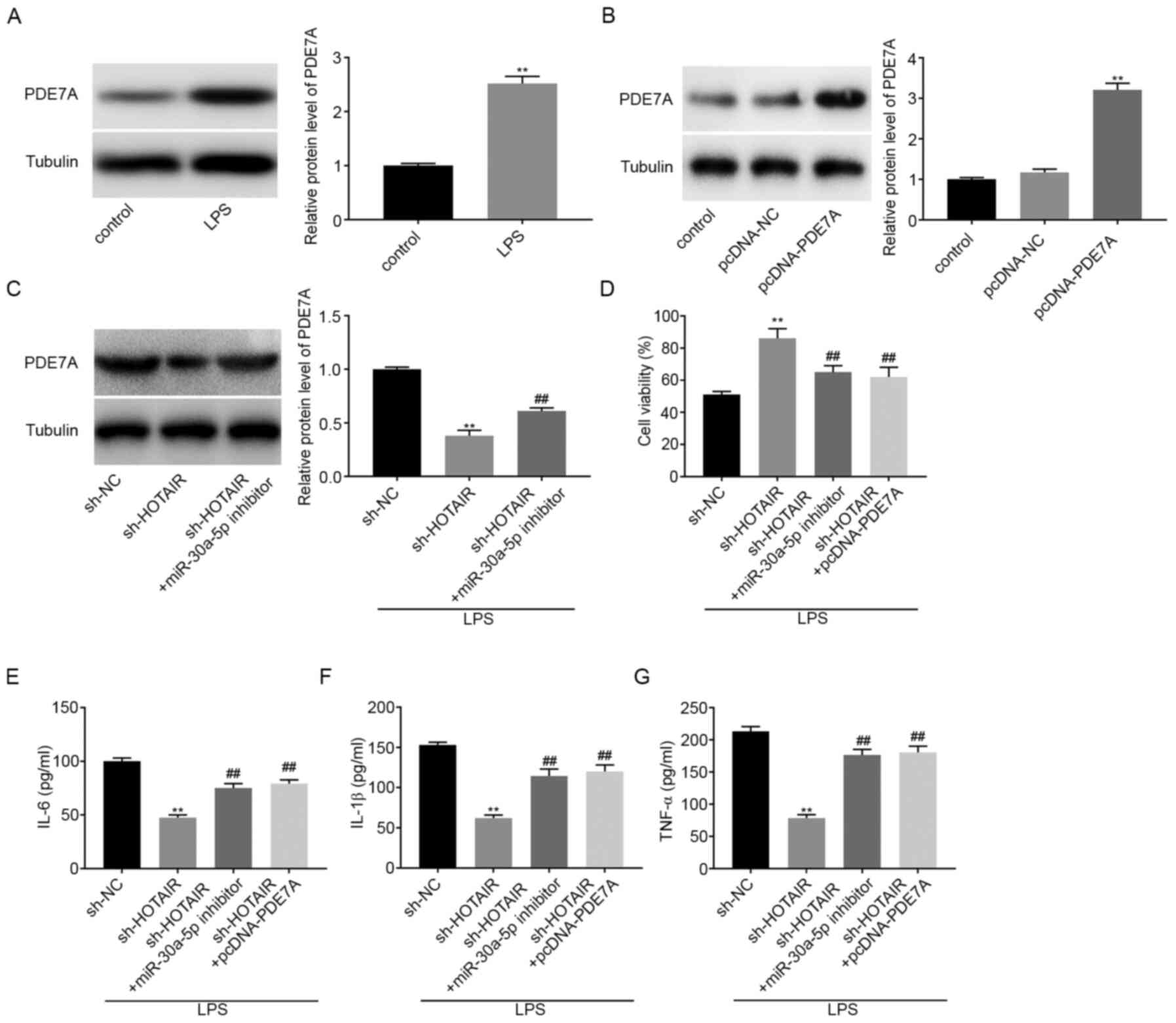
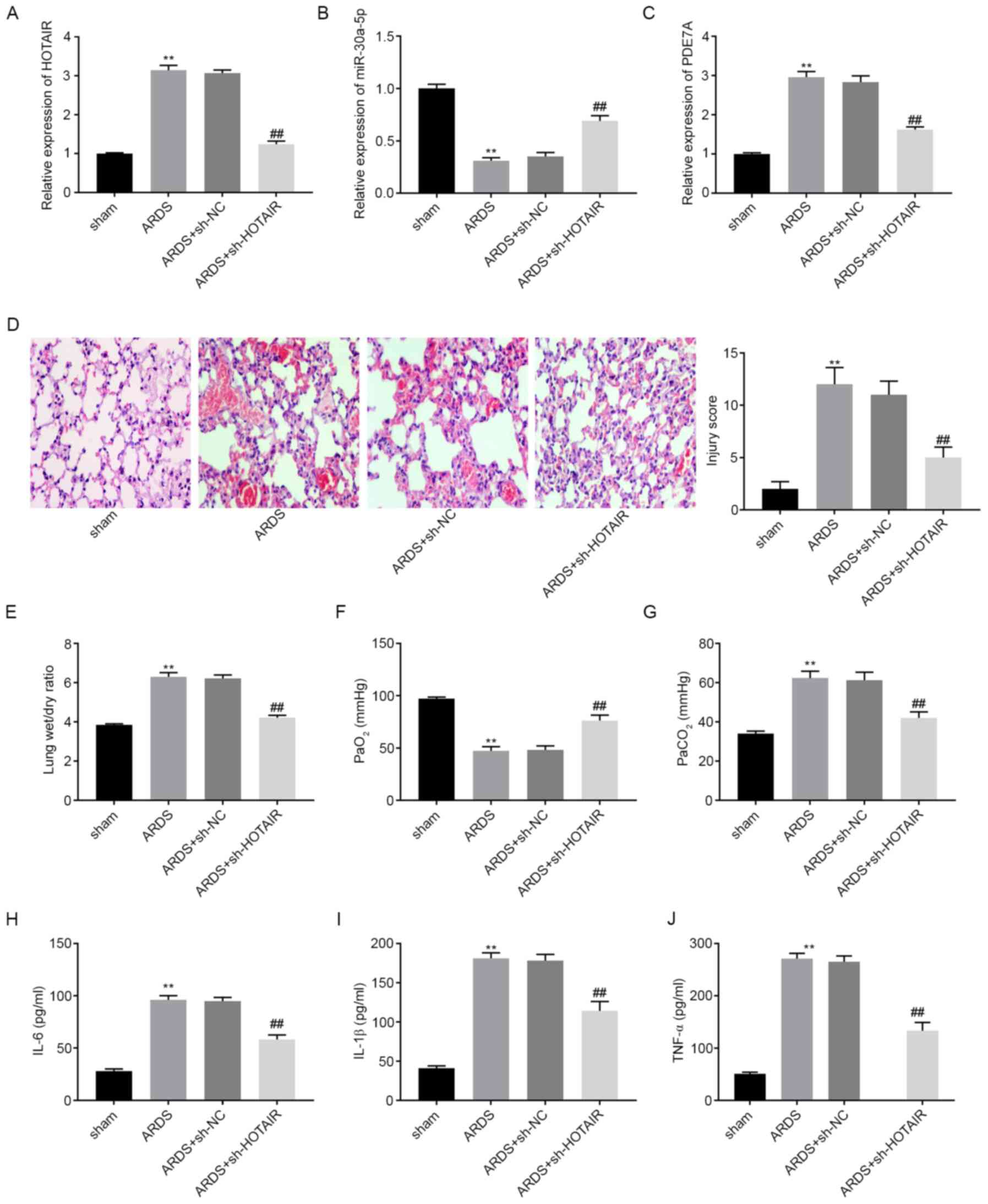
|
1
|
Patel VJ, Roy SB, Mehta HJ, Joo M and
Sadikot RT: Alternative and Natural therapies for acute lung injury
and acute respiratory distress syndrome. Biomed Res Int.
2018(2476824)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bellani G, Laffey JG, Pham T, Fan E,
Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley
DF, et al: Epidemiology, patterns of care, and mortality for
patients with acute respiratory distress syndrome in intensive care
units in 50 countries. JAMA. 315:788–800. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Villar J, Blanco J and Kacmarek RM:
Current incidence and outcome of the acute respiratory distress
syndrome. Curr Opin Crit Care. 22:1–6. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tsai CL, Lin YC, Wang HM and Chou TC:
Baicalein, an active component of Scutellaria baicalensis, protects
against lipopolysaccharide-induced acute lung injury in rats. J
Ethnopharmacol. 153:197–206. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Opitz B, Van Laak V, Eitel J and Suttorp
N: Innate immune recognition in infectious and noninfectious
diseases of the lung. Am J Respir Crit Care Med. 181:1294–1309.
2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Han S and Mallampalli RK: The acute
respiratory distress syndrome: From mechanism to translation. J
Immunol. 194:855–860. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dai L, Zhang G, Cheng Z, Wang X, Jia L,
Jing X, Wang H, Zhang R, Liu M, Jiang T, et al: Knockdown of LncRNA
MALAT1 contributes to the suppression of inflammatory responses by
up-regulating miR-146a in LPS-induced acute lung injury. Connect
Tissue Res. 59:581–592. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang Y, Fu X, Yu B and Ai F: Long
non-coding RNA THRIL predicts increased acute respiratory distress
syndrome risk and positively correlates with disease severity,
inflammation, and mortality in sepsis patients. J Clin Lab Anal.
33(e22882)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhou H, Wang X and Zhang B: Depression of
lncRNA NEAT1 Antagonizes LPS-evoked acute injury and inflammatory
response in alveolar epithelial cells via HMGB1-RAGE signaling.
Mediators Inflamm. 2020(8019467)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Duan G, Song S and Niu S: WITHDRAWN: Long
non-coding RNA HOTAIR promotes LPS-induced inflammatory injury by
down-regulation of microRNA-124 in murine chondrogenic ATDC5 cells.
Life Sci: July 20, 2018 (Epub ahead of print).
|
|
11
|
Obaid M, Udden SMN, Deb P, Shihabeddin N,
Zaki MH and Mandal SS: LncRNA HOTAIR regulates
lipopolysaccharide-induced cytokine expression and inflammatory
response in macrophages. Sci Rep. 8(15670)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wu H, Liu J, Li W, Liu G and Li Z:
LncRNA-HOTAIR promotes TNF-α production in cardiomyocytes of
LPS-induced sepsis mice by activating NF-κB pathway. Biochem
Biophys Res Commun. 471:240–246. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li P, Yao Y, Ma Y and Chen Y: MiR-150
attenuates LPS-induced acute lung injury via targeting AKT3. Int
Immunopharmacol. 75(105794)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cheng D, Zhu C, Liang Y, Xing Y and Shi C:
MiR-424 overexpression protects alveolar epithelial cells from
LPS-induced apoptosis and inflammation by targeting FGF2 via the
NF-κB pathway. Life Sci. 242(117213)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
He R, Li Y, Zhou L, Su X, Pan P and Hu C:
miR-146b overexpression ameliorates lipopolysaccharide-induced
acute lung injury in vivo and in vitro. J Cell Biochem.
120:2929–2939. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhou T and Chen YL: The functional
mechanisms of miR-30b-5p in acute lung injury in children. Med Sci
Monit. 25:40–51. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang Y, Ai H, Fan X, Chen S, Wang Y and
Liu L: Knockdown of long non-coding RNA HOTAIR reverses cisplatin
resistance of ovarian cancer cells through inhibiting
miR-138-5p-regulated EZH2 and SIRT1. Biol Res.
53(18)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wei Z, Chen L, Meng L, Han W, Huang L and
Xu A: LncRNA HOTAIR promotes the growth and metastasis of gastric
cancer by sponging miR-1277-5p and upregulating COL5A1. Gastric
Cancer. 23:1018–1032. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang S, Wang B, Xiao H, Dong J, Li Y, Zhu
C, Jin Y, Li H, Cui M and Fan S: LncRNA HOTAIR enhances breast
cancer radioresistance through facilitating HSPA1A expression via
sequestering miR-449b-5p. Thorac Cancer. 11:1801–1816.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang Y, Gong G, Xu J, Zhang Y, Wu S and
Wang S: Long noncoding RNA HOTAIR promotes breast cancer
development by targeting ZEB1 via sponging miR-601. Cancer Cell
Int. 20(320)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang C, Xu L, Deng G, Ding Y, Bi K, Jin
H, Shu J, Yang J, Deng H, Wang Z and Wang Y: Exosomal HOTAIR
promotes proliferation, migration and invasion of lung cancer by
sponging miR-203. Sci China Life Sci. 63:1265–1268. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Guo Z, Li Q, Han Y, Liang Y, Xu Z and Ren
T: Prevention of LPS-induced acute lung injury in mice by
progranulin. Mediators Inflamm. 2012(540794)2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Smith SJ, Brookes-Fazakerley S, Donnelly
LE, Barnes PJ, Barnette MS and Giembycz MA: Ubiquitous expression
of phosphodiesterase 7A in human proinflammatory and immune cells.
Am J Physiol Lung Cell Mol Physiol. 284:L279–L289. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Petrucci N and De Feo C: Lung protective
ventilation strategy for the acute respiratory distress syndrome.
Cochrane Database Syst Rev. 2013(CD003844)2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sun H, Chen J, Qian W, Kang J, Wang J,
Jiang L, Qiao L, Chen W and Zhang J: Integrated long non-coding RNA
analyses identify novel regulators of epithelial-mesenchymal
transition in the mouse model of pulmonary fibrosis. J Cell Mol
Med. 20:1234–1246. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liang H, Gu Y, Li T, Zhang Y, Huangfu L,
Hu M, Zhao D, Chen Y, Liu S, Dong Y, et al: Integrated analyses
identify the involvement of microRNA-26a in epithelial-mesenchymal
transition during idiopathic pulmonary fibrosis. Cell Death Dis.
5(e1238)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dunkel B: Acute lung injury and acute
respiratory distress syndrome in foals. Equine Vet J. 5:127–133.
2006.
|
|
29
|
Jiang X, Yu M, Zhu T, Lou L, Chen X, Li Q,
Wei D and Sun R: Kcnq1ot1/miR-381-3p/ETS2 axis regulates
inflammation in mouse models of acute respiratory distress
syndrome. Mol Ther Nucleic Acids. 19:179–189. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Guo S, Chen Y, Liu J, Yang J, Yang C,
Zhang T, Jiang K, Wu Z, Shaukat A and Deng G: miR-497a-5p
attenuates lipopolysaccharide-induced inflammatory injury by
targeting IRAK2. J Cell Physiol. 234:22874–22883. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhu S, Zhou Z, Li Z, Shao J, Jiao G, Huang
YE and Lin Y: Suppression of LINC00707 alleviates
lipopolysaccharide-induced inflammation and apoptosis in PC-12
cells by regulated miR-30a-5p/Neurod 1. Biosci Biotechnol Biochem.
83:2049–2056. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Goto M, Tanaka Y, Murakawa M,
Kadoshima-Yamaoka K, Inoue H, Murafuji H, Nagahira A, Kanki S,
Hayashi Y, Nagahira K, et al: Inhibition of phosphodiesterase 7A
ameliorates Concanavalin A-induced hepatitis in mice. Int
Immunopharmacol. 9:1347–1351. 2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kadoshima-Yamaoka K, Goto M, Murakawa M,
Yoshioka R, Tanaka Y, Inoue H, Murafuji H, Kanki S, Hayashi Y,
Nagahira K, et al: ASB16165, a phosphodiesterase 7A inhibitor,
reduces cutaneous TNF-alpha level and ameliorates skin edema in
phorbol ester 12-O-tetradecanoylphorbol-13-acetate-induced skin
inflammation model in mice. Eur J Pharmacol. 613:163–166.
2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yamamoto S, Sugahara S, Naito R, Ichikawa
A, Ikeda K, Yamada T and Shimizu Y: The effects of a novel
phosphodiesterase 7A and -4 dual inhibitor, YM-393059, on
T-cell-related cytokine production in vitro and in vivo. Eur J
Pharmacol. 541:106–114. 2006.PubMed/NCBI View Article : Google Scholar
|